Abstract
A new ethylene derivative was synthesized as a precursor for the [3+2] cycloaddition (32CA) reaction to access a novel spirooxindole embodied with benzimidazole with a pyridine spacer. The chalcone derivatives 3a–j is obtained with condensation of the acetyl derivative with aryl aldehydes. The one-pot multi-component reaction of the ethylene derivative, 5-Cl-isatin, and octahydroindole-2-carboxylic acid enables the construction of a highly functionalized quaternary center spirooxindole scaffold in a high chemical yield. A study using the Molecular Electron Density Theory (MEDT) explains the complete regio- and stereoselectivity of the reaction, resulting in the exclusive formation of the ortho/endo-cycloadduct under kinetic control. The low activation Gibbs free energy is the result of the supernucleophilic character of the in situ-generated azomethine ylide and the strong electrophilic character of the ethylene derivatives.
1. Introduction
The skeleton of a spirooxindole core structure is present in many natural alkaloids and attracts many synthetic chemists because this privileged structure plays an important role in drug discovery and medication enhancement [1,2,3,4,5]. There are diverse synthetic tools that are accessible and reproducible for the construction of chiral spirooxindole frameworks with pharmacological relevant targets [6,7,8,9,10]. Spirooxindoles are a prominent class of compounds that possess many medication targets including treatment of cancer [11,12,13,14,15], anti-inflammatory [16], SARS-CoV-2 [17,18], anti-diabetics [19,20,21], and others [22,23,24,25,26,27]. This rigid spirocyclic scaffold has an outstanding ability for the physicochemical properties’ improvement compared to other mono-cyclic structures [28]. Therefore, the construction of spirooxindoles based on chiral quaternary centers has attracted significant attention from many researchers and remains a challenging task.
Several synthetic approaches have the ability to access spirooxindoles; among them are the use of nano-catalysis [29], organocatalyst oxidative annulations [30,31,32,33], microwave irradiations [34], sonochemical strategy [35], NHC (N-Heterocyclic carbene) catalyst-mediated [3+2] cycloaddition annulation [36], and transition metal catalysts [37,38] (e.g., Ru, Rh, Pd, etc.) that require a specific olefin or indolinone-based alkene moiety in the precursor structure. A mild condition, facile, and eco-friendly approach to compose the spirooxindoles are still required to a great extent.
The one-pot multi-component [3+2] cycloaddition (32CA) reaction is among the most versatile eco-friendly and atom-economy strategies that enable the synthesis of spirooxindole-based chiral functionality. Many spirooxindole molecules were developed based on this approach and were discovered to be competitive and highly effective for their valuable anti-disease pharmacological potential.
Azomethine ylides (AYs) are highly reactive intermediates in 32CA reactions, resulting in the formation of diverse hetero/carbocyclic molecules, particularly spirooxindoles and others [39,40]. These three-atom components (TACs) have been extensively studied and explored in many total syntheses of biologically active natural as well as synthetic products from 32CA reactions. Recent Molecular Electron Density Theory (MEDT) [41] studies of the chemical reactivity of these TACs suggested that these organic species may present pseudodiradical, pseudo(mono)radical, carbenoid, and even zwitterionic natures depending on the substitution, and consequently, the term of “1,3-dipole” is not justified for these reactions [42].
In this work, we designed a new olefin for the 32CA reaction, which is crucial for accessing the desired spirooxindoles. The mechanism of the 32CA reaction is studied from the perspective of MEDT.
2. Materials and Methods
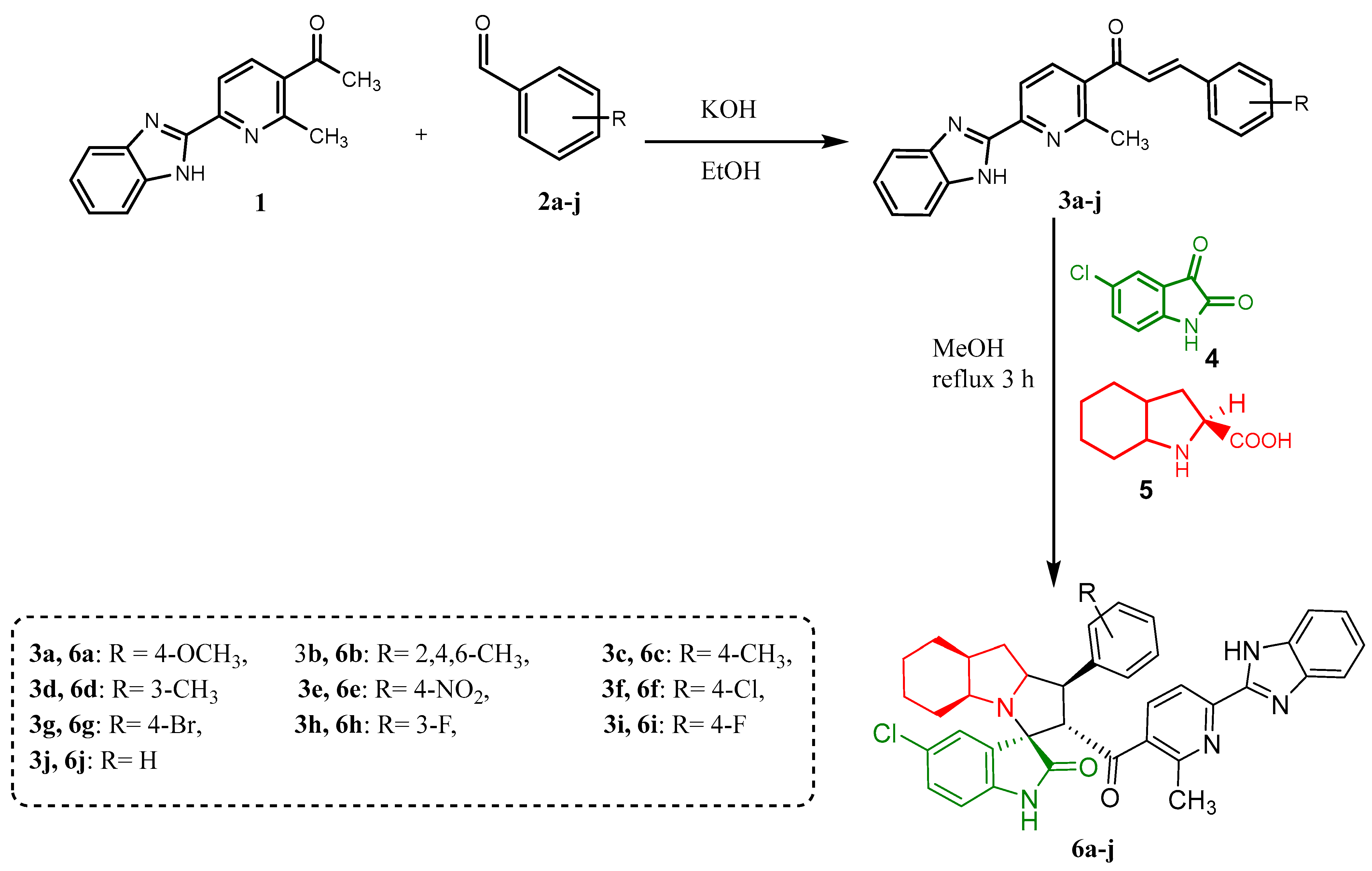
2.1. Synthesis of Chalcones (3a–j) and Spiro-Compounds (6a–j)
2.1.1. Synthesis of Chalcones (3a–j)
The arylaldehyde derivative 2a–j (2 mmol) was added to ketone derivative 1 (0.5 g, 2 mmol) in an ethanol solution of potassium hydroxide (40 mmol of potassium hydroxide in 40 mL of ethanol). The reaction mixture was subsequently stirred at room temperature for 12 h and neutralized with a solution of 30% acetic acid, leading to a precipitate. It was filtered, dried, and recrystallized in EtOH to give compounds 3a–j.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(4-methoxyphenyl)prop-2-en-1-one 3a
- 1H-NMR (DMSO-d6, 400 MHz) δ 13.03 (1H, NH, s), 8.28 (1H, Py-H, d, J = 8.1 Hz), 8.20 (1H, Py-H, d, J = 8.1 Hz), 7.79 (2H, ArH, d, J = 8.8 Hz), 7.74 (1H, ArH, d, J = 8.1 Hz), 7.61 (1H, ArH, d, J = 7.3 Hz), 7.56 (1H, =CH, d, J = 16.1 Hz), 7.35 (1H, COCH, d, J = 16.1 Hz), 7.26 (2H, ArH, m), 7.01 (2H, ArH, d, J = 8.8 Hz), 3.81 (3H, OCH3, s), 2.71 (3H, CH3, s); 13C-NMR (DMSO-d6, 100 MHz) δ 194.1, 162.3, 156.9, 150.7, 149.4, 146.6, 144.6, 138.01, 135.6, 135.0, 131.6, 127.5, 124.0, 122.7, 120.0, 119.1, 115.1, 112.9, 56.0, 24.0; Anal. for C23H19N3O2; Calcd: C, 74.78; H, 5.18; N, 11.37; Found: C, 75.15; H, 4.89; N, 11.14.
- 1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-mesitylprop-2-en-1-one 3b
- 1H-NMR (DMSO-d6, 500 MHz) δ 12.99 (1H, NH, s), 8.22 (1H, Py-H, d, J = 8.0 Hz), 8.15 (1H, Py-H, d, J = 8.0 Hz), 7.68 (1H, ArH, d, J = 7.9 Hz), 7.64 (1H, =CH, d, J = 16.4 Hz,), 7.56 (1H, ArH, d, J = 7.9 Hz), 7.23 (1H, ArH, t, J = 7.5 Hz), 7.18 (1H, ArH, t, J = 7.5 Hz), 6.92 (1H, COCH, d, J = 16.4 Hz), 6.89 (2H, ArH, s), 2.70 (3H, CH3, s), 2.28 (6H, CH3, s), 2.19 (3H, CH3, s); 13C-NMR (DMSO-d6, 126 MHz) δ 194.1, 165.4, 157.4, 149.5, 144.6, 139.3, 138.2, 137.6, 135.8, 134.5, 131.0, 129.7, 124.0, 122.7, 120.0, 119.0, 112.9, 23.8, 20.3; Anal. for C25H23N3O; Calcd: C, 78.71; H, 6.08; N, 11.02; Found: C, 78.75; H, 6.05; N, 11.07.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(p-tolyl)prop-2-en-1-one 3c
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.64 (1H, NH, s), 8.30 (1H, Py-H, d, J = 8.0 Hz), 8.23 (1H, Py-H, d, J = 8.0 Hz), 7.72 (2H, ArH, d, J = 7.9 Hz), 7.69–7.65 (2H, ArH, m), 7.58 (1H, =CH, d, J = 16.0 Hz), 7.45 (1H, COCH, d, J = 16.0 Hz), 7.29–7.22 (4H, ArH, m), 2.72 (3H, CH3, s), 2.33 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 194.0, 158.1, 155.4, 152.2, 150.1, 146.5, 140.9, 138.2, 134.6, 133.0, 130.8, 129.9, 125.8, 122.6, 120.0, 118.9, 24.1, 21.5; Anal. for C23H19N3O; Calcd: C, 78.16; H, 5.42; N, 11.89; Found: C, 78.21; H, 5.37; N, 11.85.
- 1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(m-tolyl)prop-2-en-1-one 3d
- 1H-NMR (DMSO-d6, 500 MHz) δ 13.00 (1H, NH, s), 8.25 (1H, Py-H, d, J = 8.1 Hz), 8.21 (1H, Py-H, d, J = 8.1 Hz), 7.63 (1H, ArH, d, J = 2.0 Hz), 7.57 (2H, ArH, d, J = 7.8 Hz), 7.53 (1H, =CH, d, J = 16.0 Hz), 7.45 (1H, COCH, d, J = 16.0 Hz), 7.30 (1H, ArH, t, J = 7.6 Hz), 7.24 (2H, ArH, d, J = 7.8 Hz), 7.22–7.18 (2H, ArH, m), 2.69 (3H, CH3, s), 2.30 (3H, CH3, s); 13C-NMR (DMSO-d6, 126 MHz) δ 194.0, 157.2, 150.6, 149.5, 146.5, 138.8, 138.2, 136.7, 135.8, 134.8, 134.6, 132.25, 129.9, 129.4, 127.9, 126.8, 126.1, 123.7, 122.7, 119.1, 112.9, 24.1, 21.4; Anal. for C23H19N3O; Calcd: C, 78.16; H, 5.42; N, 11.89; Found: C, 78.20; H, 5.36; N, 11.86.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(4-nitrophenyl)prop-2-en-1-one 3e
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.85 (1H, NH, s), 8.35 (1H, Py-H, d, J = 8.1 Hz), 8.30 (1H, Py-H, d, J = 8.1 Hz), 8.25 (1H, ArH, d, J = 8.1 Hz), 8.10 (2H, ArH, d, J = 8.8 Hz), 7.79–7.58 (4H, ArH, CH=CH, m), 7.34–7.17 (3H, ArH, m), 2.76 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 200.2, 157.8, 150.5, 149.8, 138.7, 133.9, 130.5, 123.4, 119.1, 24.5; Anal. for C22H16N4O3; Calcd: C, 68.74; H, 4.20; N, 14.58; Found: C, 68.12; H, 4.01; N, 13.98.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(4-chlorophenyl)prop-2-en-1-one 3f
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.71 (1H, NH, s), 8.53 (1H, Py-H, d, J = 8.1 Hz), 8.37 (1H, Py-H, d, J = 8.1 Hz), 8.14–8.05 (2H, ArH, m), 8.02–7.96 (1H, ArH, m), 7.91 (2H, ArH, d, J = 8.1 Hz), 7.83 (1H, ArH, d, J = 6.6 Hz), 7.70 (2H, ArH, d, J = 7.3 Hz), 7.42 (1H, ArH, d, J = 4.4 Hz), 7.38 (1H, ArH, d, J = 4.4 Hz), 2.74 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 193.8, 157.4, 150.6, 149.67, 144.7, 138.4, 136.1, 135.4, 134.4, 133.9, 131.2, 129.9, 128.8, 126.0, 123.2, 119.2, 23.9; Anal. for C22H16ClN3O; Calcd: C, 70.68; H, 4.31; N, 11.24; Found: C, 71.21; H, 4.13; N, 11.91.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(4-bromophenyl)prop-2-en-1-one 3g
- 1H-NMR (DMSO-d6, 400 MHz) δ 13.04 (1H, NH, s), 8.42 (1H, Py-H, d, J = 8.1 Hz), 8.27 (1H, Py-H, d, J = 8.1 Hz), 7.73 (2H, d, J = 8.1 Hz), 7.64 (1H, d, J = 8.8 Hz), 7.61-755 (3H, m), 7.41 (1H, d, J = 5.9 Hz), 7.26 (3H, m), 2.60 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 193.8, 157.3, 150.5, 149.6, 144.8, 138.4, 136.3, 134.4, 134.2, 132.8, 132.5, 131.8, 131.4, 126.9, 124.9, 122.7, 120.0, 119.0, 112.9, 24.2; Anal. for C22H16BrN3O; Calcd: C, 63.17; H, 3.86; N, 10.05; Found: C, 63.85; H, 3.74; N, 10.12.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(3-fluorophenyl)prop-2-en-1-one 3h
- 1H-NMR (400 MHz, DMSO-d6) δ 12.91 (1H, NH, s), 8.50 (1H, Py-H, d, J = 8.1 Hz), 8.39 (1H, Py-H, d, J = 8.1 Hz), 8.27 (1H, ArH, d, J = 7.3 Hz), 8.00 (1H, d, J = 8.1 Hz), 7.79 (2H, d, J = 9.5 Hz), 7.54–7.43 (3H, m), 7.31 (2H, d, J = 8.1 Hz), 6.79 (1H, s), 2.75 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 193.78, 164.9, 159.0, 157.5, 150.6, 150.2, 150.1, 149.7, 144.7, 138.5, 137.4, 136.1, 134.3, 127.5, 126.5, 125.2, 119.1, 112.7, 25.0; Anal. for C22H16FN3O; Calcd: C, 73.94; H, 4.51; N, 11.76; Found: C, 73.19; H, 4.23; N, 11.44.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-(4-fluorophenyl)prop-2-en-1-one 3i
- 1H-NMR (DMSO-d6, 400 MHz) δ 13.06 (1H, NH, s), 8.30 (1H, Py-H, d, J = 8.1 Hz), 8.26 (1H, Py-H, d, J = 8.1 Hz), 7.92 (2H, ArH, dd, J = 8.8, 5.1 Hz), 7.74 (1H, ArH, d, J = 9.5 Hz), 7.63 (1H, ArH, d, J = 7.3 Hz), 7.49 (1H, CH=, d, J = 16.1 Hz), 7.34 (1H, =CH, d, J = 16.1 Hz), 7.27 (4H, ArH, m), 2.73 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 193.9, 165.4, 163.0, 157.3, 150.6, 149.6, 145.1, 144.6, 138.3, 135.6, 134.6, 132.5, 131.6, 126.2, 124.0, 122.6, 120.0, 119.1, 116.6, 115.3, 112.0, 24.2; Anal. for C22H16FN3O; Calcd: C, 73.94; H, 4.51; N, 11.76; Found: C, 73.29; H, 4.31; N, 11.84.
- (E)-1-(6-(1H-Benzo[d]imidazol-2-yl)-2-methylpyridin-3-yl)-3-phenylprop-2-en-1-one 3j
- 1H-NMR (DMSO-d6, 400 MHz) δ 13.07 (1H, NH, s), 8.30 (1H, Py-H, d, J = 8.1 Hz), 8.26 (1H, Py-H, d, J = 8.1 Hz), 7.86–7.81 (2H, ArH, m), 7.75 (1H, ArH, d, J = 8.1 Hz), 7.65–7.59 (2H, ArH, =CH(β), m), 7.52 (1H, =CH(α), d, J = 16.1 Hz), 7.45 (2H, ArH, d, J = 5.9 Hz), 7.29–7.21 (3H, ArH, m), 2.74 (3H, CH3, s); 13C-NMR (DMSO-d6, 101 MHz) δ 194.1, 157.3, 150.6, 149.56, 146.4, 144.6, 138.3, 135.6, 134.9, 134.6, 131.6, 129.6, 129.5, 128.8, 126.3, 124.1, 122.7, 120.1, 119.1, 112.9, 24.2; Anal. for C22H17N3O; Calcd: C, 77.86; H, 5.05; N, 12.38; Found: C, 77.81; H, 5.09; N, 12.42.
2.1.2. Synthesis of Spiro-Oxindole Derivatives (6a–j)
Enones 3a–j (0.5 mmol), 5-chloro isatin (91 mg, 0.5 mmol), and octahydroindole-2-carboxylic acid (84.62 mg, 0.5 mmol) were dissolved in 20 mL of dry MeOH in a 100 mL round-bottom flask. Then, the reaction mixture was heated for 3 h at 60–65 °C. After the reaction was completed, as monitored with thin-layer chromatography (TLC), the desired spiro-oxindole derivatives 6a–j was purified by flash column chromatography by using n-hexane/ethyl acetate (4:2).
- Spiro-oxindole derivative 6a
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.94 (s, 1H, NH), 10.12 (s, 1H, NH), 8.11 (m, 2H, Py-H), 7.73 (d, J = 7.3 Hz, 1H, ArH), 7.56 (d, J = 8.1 Hz, 1H, ArH), 7.54–7.50 (m, 2H, ArH), 7.49 (s, 1H, ArH), 7.33–7.17 (m, 3H, ArH), 6.89 (dd, J = 7.0, 4.8 Hz, 2H, ArH), 6.55 (d, J = 8.8 Hz, 1H, ArH), 5.06 (d, J = 11.7 Hz, 1H, COCH), 4.06–3.91 (m, 1H), 3.84 (t, J = 11.0 Hz, 1H), 3.72 (s, 3H, OCH3), 3.53–3.37 (m, 1H, aliphatic-H), 3.15–2.96 (m, 1H, aliphatic-H), 2.08 (dt, J = 11.0, 5.5 Hz, 1H, aliphatic-H), 2.03–1.90 (m, 1H, aliphatic-H), 1.85 (s, 3H, CH3), 1.59–1.41 (m, 3H, aliphatic-H), 1.37–1.22 (m, 2H, aliphatic-H), 0.90–0.66 (m, 3H, aliphatic-H); 13C-NMR (DMSO-d6, 101 MHz) δ 198.4, 179.8, 158.6, 157.9, 150.3, 149.8, 144.5, 141.5, 138.8, 135.5, 132.0, 131.8, 130.7, 130.2, 129.2, 128.2, 126.3, 126.1, 124.2, 122.8, 120.0, 118.5, 114.4, 112.9, 111.8, 71.4, 65.8, 57.2, 56.6, 55.6, 51.4, 41.7, 36.7, 28.1, 27.9, 24.9, 24.7, 22.8, 21.6, 19.8, 19.1; Anal. for C39H36ClN5O3; Calcd: C, 71.17; H, 5.51; N, 10.64; Found: C, 70.95; H, 4.12; N, 11.24.
- Spiro-oxindole derivative 6b
- 1H-NMR (DMSO-d6, 500 MHz) δ 12.88 (s, 1H, NH), 10.17 (s, 1H, NH), 8.06 (d, J = 8.1 Hz, 1H, Py-H), 7.71 (d, J = 8.1 Hz, 1H, Py-H), 7.68 (d, J = 7.9 Hz, 1H, ArH), 7.51 (d, J = 7.2 Hz, 1H, ArH), 7.25–7.17 (m, 4H, ArH), 6.79 (d, J = 16.4 Hz, 2H, ArH), 6.49 (d, J = 8.2 Hz, 1H, ArH), 5.43 (d, J = 12.1 Hz, 1H, COCH), 4.42–4.33 (m, 1H), 4.21–4.13 (m, 1H), 3.01 (d, J = 4.5 Hz, 1H, aliphatic-H), 2.68 (s, 3H, CH3), 2.63 (s, 3H, CH3), 2.12 (s, 3H, CH3), 2.08 (q, J = 5.7 Hz, 1H, aliphatic-H), 1.86 (s, 3H, CH3), 1.76 (dd, J = 15.3, 11.8 Hz, 1H, aliphatic-H), 1.52 (dd, J = 10.8, 5.9 Hz, 2H, aliphatic-H), 1.48–1.40 (m, 2H, aliphatic-H), 1.29–1.21 (m, 2H, aliphatic-H), 0.95–0.89 (m, 1H, aliphatic-H), 0.85–0.80 (m, 1H, aliphatic-H), 0.74 (d, J = 12.7 Hz, 1H, aliphatic-H); 13C-NMR (DMSO-d6, 126 MHz) δ 199.0, 179.9, 161.5, 158.0, 150.1, 149.8, 144.4, 141.6, 138.6, 138.4, 135.9, 135.7, 135.5, 131.8, 131.7, 130.2, 130.0, 129.8, 127.6, 126.5, 126.1, 124.2, 123.5, 122.8, 120.0, 118.6, 112.9, 112.0, 71.9, 67.8, 64.6, 57.3, 47.4, 41.8, 38.1, 28.3, 27.6, 27.3, 24.7, 23.0, 22.3, 21.7, 20.8, 19.9; Anal. for C41H40ClN5O2; Calcd: C, 73.47; H, 6.02; N, 10.45; Found: C, 73.42; H, 6.07; N, 10.40.
- Spiro-oxindole derivative 6c
- 1H-NMR (DMSO-d6, 500 MHz) δ 12.90 (s, 1H, NH), 10.07 (s, 1H, NH), 8.09–8.04 (m, 2H, Py-H), 7.65 (ddd, J = 29.4, 5.9, 3.3 Hz, 1H, ArH), 7.53 (s, 1H, ArH), 7.48 (d, J = 2.3 Hz, 1H, ArH), 7.42 (d, J = 8.2 Hz, 2H, ArH), 7.24 (dd, J = 8.3, 2.2 Hz, 1H, ArH), 7.23–7.17 (m, 2H, ArH), 7.09 (d, J = 8.1 Hz, 2H, ArH), 6.51 (d, J = 8.2 Hz, 1H, ArH), 5.05 (d, J = 11.8 Hz, 1H, COCH), 3.95–3.89 (m, 1H), 3.85–3.79 (m, 1H), 3.02 (d, J = 4.3 Hz, 1H, aliphatic-H), 2.22 (s, 3H, CH3), 2.03 (ddd, J = 9.3, 5.6, 3.6 Hz, 1H, aliphatic-H), 1.95–1.90 (m, 1H, aliphatic-H), 1.80 (s, 3H, CH3), 1.49–1.44 (m, 2H, aliphatic-H), 1.41–1.37 (m, 1H, aliphatic-H), 1.25 (dd, J = 9.6, 3.6 Hz, 2H, aliphatic-H), 0.96–0.89 (m, 1H, aliphatic-H), 0.87–0.77 (m, 2H, aliphatic-H), 0.67 (d, J = 10.5 Hz, 1H, aliphatic-H); 13C-NMR (DMSO-d6, 126 MHz) δ 198.3, 179.7, 172.6, 157.8, 150.2, 149.8, 141.5, 138.8, 136.9, 136.2, 132.0, 130.2, 129.6, 128.0, 126.3, 126.1, 118.5, 111.8, 71.4, 71.3, 65.6, 57.1, 51.7, 41.8, 36.6, 29.5, 28.1, 27.8, 24.9, 22.7, 21.6, 21.2, 19.7, 14.5; Anal. for C39H36ClN5O2; Calcd: C, 72.94; H, 5.65; N, 10.91; Found: C, 72.22; H, 5.14; N, 11.10.
- Spiro-oxindole derivative 6d
- 1H-NMR (DMSO-d6, 500 MHz) δ 12.90 (s, 1H, NH), 10.07 (s, 1H, NH), 8.10 (d, J = 8.1 Hz, 1H, PyH), 8.06 (d, J = 8.1 Hz, 1H, PyH), 7.68 (d, J = 8.0 Hz, 1H, ArH), 7.52 (d, J = 7.6 Hz, 1H, ArH), 7.38 (t, J = 2.0 Hz, 1H, ArH), 7.33 (d, J = 7.9 Hz, 1H, ArH), 7.24 (dd, J = 8.4, 2.2 Hz, 2H, ArH), 7.18 (m, 3H, ArH), 6.98 (d, J = 8.8 Hz, 1H, ArH), 6.50 (d, J = 8.2 Hz, 1H, ArH), 5.07 (d, J = 12.0 Hz, 1H, COCH), 3.97–3.92 (m, 1H), 3.84–3.80 (m, 1H), 3.02 (d, J = 4.3 Hz, 1H, aliphatic-H), 2.28 (s, 3H, CH3), 2.07–2.04 (m, 1H, aliphatic-H), 1.93 (td, J = 5.9, 5.1, 2.2 Hz, 1H, aliphatic-H), 1.80 (s, 3H, CH3), 1.47 (dd, J = 11.7, 6.5 Hz, 2H, aliphatic-H), 1.39 (d, J = 5.1 Hz, 1H, aliphatic-H), 1.27–1.21 (m, 3H, aliphatic-H), 0.93 (dt, J = 13.0, 3.2 Hz, 1H, aliphatic-H), 0.84–0.81 (m, 1H, aliphatic-H), 0.68 (d, J = 10.8 Hz, 1H, aliphatic-H); 13C-NMR (DMSO-d6, 126 MHz) δ 198.4, 179.7, 157.9, 150.2, 149.8, 144.5, 141.4, 139.9, 138.9, 138.1, 135.5, 131.9, 130.2, 128.8, 128.8, 128.0, 127.8, 126.3, 126.1, 125.2, 122.75, 120.0, 118.4, 112.9, 111.8, 71.33, 65.7, 57.2, 52.1, 41.8, 36., 34.02, 29.5, 28.1, 27.8, 24.9, 22.7, 21.6, 19.7, 18.0; Anal. for C39H36ClN5O2; Calcd: C, 72.94; H, 5.65; N, 10.91; Found: C, 72.92; H, 5.61; N, 10.89.
- Spiro-oxindole derivative 6e
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.96 (s, 1H, NH), 10.16 (s, 1H, NH), 8.24 (d, J = 8.1 Hz, 1H, Py-H), 8.20 (d, J = 8.8 Hz, 2H, ArH), 8.12 (d, J = 8.1 Hz, 1H, Py-H), 7.96 (d, J = 8.1 Hz, 2H, ArH), 7.73 (d, J = 7.3 Hz, 1H, ArH), 7.65 (s, 1H, ArH), 7.57 (d, J = 7.3 Hz, 1H, ArH), 7.26 (m, 3H, ArH), 6.55 (d, J = 8.1 Hz, 1H, ArH), 5.26 (d, J = 11.7 Hz, 1H, COCH), 4.14 (t, J = 11.0 Hz, 1H), 4.02 (d, J = 7.3 Hz, 1H), 3.07 (d, J = 3.7 Hz, 1H, aliphatic-H), 2.12–2.01 (m, 2H, aliphatic-H), 1.84 (s, 3H, CH3), 1.48 (dd, J = 11.4, 6.2 Hz, 2H, aliphatic-H), 1.42 (dd, J = 8.4, 4.8 Hz, 1H, aliphatic-H), 1.33–1.23 (m, 2H, aliphatic-H), 1.10–1.04 (m, 1H, aliphatic-H), 1.01–0.93 (m, 1H, aliphatic-H), 0.86 (d, J = 12.5 Hz, 1H, aliphatic-H), 0.72 (d, J = 11.7 Hz, 1H, aliphatic-H); 13C-NMR (DMSO-d6, 101 MHz) δ 198.3, 179.5, 157.9, 150.2, 149.9, 148.4, 147.0, 144.5, 141.5, 138.8, 135.5, 131.7, 129.8, 129.8, 127.7, 126.3, 126.1, 124.1, 124.0, 123.2, 120.6, 118.0, 113.0, 111.9, 71.3, 57.2, 51.9, 46.0, 41.7, 38.7, 35.9, 28.0, 24.9, 19.8; Anal. for C38H33ClN6O4; Calcd: C, 67.80; H, 4.94; N, 12.48; Found: C, 68.10; H, 4.21; N, 12.16.
- Spiro-oxindole derivative 6f
- 1H-NMR (DMSO-d6, 400 MHz) δ 12.94 (s, 1H, NH), 10.12 (s, 1H, NH), 8.17 (d, J = 8.1 Hz, 1H, Py-H), 8.10 (d, J = 8.1 Hz, 1H, Py-H), 7.73 (d, J = 7.3 Hz, 1H, ArH), 7.65 (d, J = 8.1 Hz, 2H, ArH), 7.58–7.54 (m, 3H, ArH), 7.50 (s, 1H, ArH), 7.28 (d, J = 8.1 Hz, 3H, ArH), 6.54 (d, J = 8.1 Hz, 1H, ArH), 5.12 (d, J = 11.7 Hz, 1H, COCH), 4.01–3.95 (m, 2H), 3.06 (d, J = 3.7 Hz, 1H, aliphatic-H), 2.11–2.08 (m, 1H, aliphatic-H), 1.98 (d, J = 9.5 Hz, 1H, aliphatic-H), 1.83 (s, 3H, CH3), 1.51 (d, J = 6.6 Hz, 2H, aliphatic-H), 1.27 (d, J = 9.5 Hz, 2H, aliphatic-H), 1.05 (d, J = 5.9 Hz, 1H, aliphatic-H), 0.97 (d, J = 13.2 Hz, 2H, aliphatic-H), 0.84 (d, J = 3.7 Hz, 1H, aliphatic-H), 0.71 (d, J = 12.5 Hz, 1H, aliphatic-H); Anal. for C38H33Cl2N5O2; Calcd: C, 68.88; H, 5.02; N, 10.57; Found: C, 68.12; H, 4.93; N, 11.05.
- Spiro-oxindole derivative 6g
- 1H-NMR (CDCl3, 500 MHz) δ 8.82 (s, 1H, NH), 8.18 (d, J = 7.3 Hz, 1H), 7.76 (s, 1H, NH), 7.58 (s, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.40 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 8.6 Hz, 1H), 7.26 (dd, J = 6.1, 2.9 Hz, 2H), 7.22 (d, J = 2.1 Hz, 1H), 7.19 (d, J = 8.9 Hz, 1H), 6.58 (d, J = 8.6 Hz, 1H), 4.96 (d, J = 10.9 Hz, 1H, COCH), 4.32–4.23 (m, 1H), 3.69 (t, J = 10.7 Hz, 1H), 3.08 (d, J = 4.0 Hz, 1H), 1.95 (s, 3H, CH3), 1.73 (td, J = 12.0, 6.1 Hz, 2H), 1.55 (d, J = 7.2 Hz, 1H), 1.53 (d, J = 6.9 Hz, 1H), 1.42–1.35 (m, 2H), 1.27 (d, J = 4.9 Hz, 1H), 1.01 (dd, J = 29.6, 14.0 Hz, 2H), 0.93–0.89 (m, 1H), 0.80 (d, J = 15.9 Hz, 1H); 13C-NMR (CDCl3, 126 MHz) δ 197.4, 180.9, 159.3, 139.5, 138.1, 132.0, 130.0, 129.7, 127.9, 127.7, 125.9, 124.2, 121.1, 118.4, 111.7, 71.73, 71.01, 66.99, 57.6, 52.8, 41.9, 37.7, 31.0, 29.8, 29.5, 28.3, 27.64, 24.7, 23.0, 19.7; Anal. for C38H33BrClN5O2; Calcd: C, 64.55; H, 4.70; N, 9.90; Found: C, 64.78; H, 4.80; N, 10.15.
- Spiro-oxindole derivative 6h
- 1H-NMR (CDCl3, 500 MHz) δ 8.91 (s, 1H, NH), 8.11 (d, J = 7.7 Hz, 1H), 7.73 (s, 1H, NH), 7.59 (d, J = 14.0 Hz, 2H), 7.32–7.27 (m, 3H), 7.27 (d, J = 4.7 Hz, 1H), 7.21 (d, J = 2.3 Hz, 2H), 7.16 (dd, J = 8.2, 2.2 Hz, 2H), 6.93–6.90 (m, 1H), 6.54 (d, J = 8.7 Hz, 1H), 4.96 (d, J = 11.6 Hz, 1H, COCH), 4.31–4.25 (m, 1H), 4.23–4.15 (m, 1H), 3.08 (d, J = 4.6 Hz, 1H), 1.96 (s, 3H, CH3), 1.79–1.72 (m, 3H), 1.66–1.61 (m, 2H), 1.57 (d, J = 3.0 Hz, 1H), 1.09–1.05 (m, 1H), 1.02–0.97 (m, 1H), 0.93 (d, J = 7.2 Hz, 2H), 0.84 (d, J = 3.0 Hz, 1H); 13C-NMR (CDCl3, 126 MHz) δ 197.0, 183.4, 161.1, 149.2, 144.4, 139.0, 129.9, 127.2, 125.7, 123.7, 119.0, 115.4, 111.1, 72.0, 71.8, 71.0, 57.6, 46.4, 41.9, 41.0, 37.7, 32.2, 29.3, 28.5, 28.3, 27.8, 27.6, 26.5, 24.4, 24.0, 23.1, 20.9, 19.8, 17.7, 17.6, 17.38, 14.73; Anal. for C38H33ClFN5O2; Calcd: C, 70.64; H, 5.15; N, 10.84; Found: C, 71.02; H, 4.93; N, 11.14.
- Spiro-oxindole derivative 6i
- 1H NMR (DMSO-d6, 500 MHz) δ 12.90 (s, 1H, NH), 10.07 (s, 1H, NH), 8.13 (d, J = 8.2 Hz, 1H, Py-H), 8.06 (d, J = 8.2 Hz, 1H, Py-H), 7.68 (d, J = 7.8 Hz, 1H, ArH), 7.61 (dd, J = 9.0, 5.5 Hz, 2H, ArH), 7.52 (d, J = 9.1 Hz, 2H, ArH), 7.26–7.22 (m, 2H, ArH), 7.18 (td, J = 7.6, 7.2, 1.5 Hz, 1H, ArH), 7.13–7.08 (m, 2H, ArH), 6.50 (d, J = 8.2 Hz, 1H, ArH), 5.08 (d, J = 11.8 Hz, 1H, COCH), 3.94–3.86 (m, 2H), 3.01 (s, 1H), 2.05 (q, J = 4.7 Hz, 1H), 1.96–1.92 (m, 1H), 1.79 (s, 3H, CH3), 1.48–1.43 (m, 2H), 1.29–1.17 (m, 4H), 1.13 (t, J = 7.1 Hz, 1H), 1.06–1.02 (m, 1H), 0.67 (d, J = 13.0 Hz, 1H); 13C-NMR (DMSO-d6, 126 MHz) δ 198.4, 179.6, 162.6, 160.7, 157.9, 150.2, 149.8, 144.4, 141.5, 138.9, 136.1, 135.5, 131.9, 130.2, 130.1, 130.0, 128.0, 126.2, 126.1, 124.1, 122.8, 120.0, 118.4, 115.7, 115.5, 112.9, 111.8, 71.3, 71.3, 65.6, 57.1, 51.2, 41.8, 36.3, 31.5, 28.1, 27.8, 24.9, 22.7, 22.6, 19.7, 14.6, 14.5; Anal. for C38H33ClFN5O2; Calcd: C, 70.64; H, 5.15; N, 10.84; Found: C, 70.60; H, 5.11; N, 10.89.
- Spiro-oxindole derivative 6j
- 1H-NMR (DMSO-d6, 400 MHz) δ 13.00 (s, 1H, NH), 10.18 (s, 1H, NH), 8.17 (d, J = 8.1 Hz, 1H, Py-H), 8.14 (d, J = 8.1 Hz, 1H, Py-H), 7.74 (d, J = 8.1 Hz, 1H, ArH), 7.63–7.54 (m, 4H, ArH), 7.39–7.17 (m, 6H, ArH), 6.57 (d, J = 8.1 Hz, 1H, ArH), 5.16 (d, J = 11.7 Hz, 1H, COCH), 4.06–3.97 (m, 1H), 3.92 (t, J = 10.6 Hz, 1H), 3.08 (d, J = 3.7 Hz, 1H), 2.06 (dt, J = 11.0, 5.9 Hz, 1H), 1.97 (q, J = 6.6, 5.9 Hz, 1H), 1.87 (s, 3H, CH3), 1.55–1.39 (m, 3H), 1.38–1.24 (m, 2H), 1.10–1.02 (m, 1H), 0.91 (dt, J = 38.8, 13.9 Hz, 2H), 0.73 (d, J = 11.7 Hz, 1H); 13C-NMR (DMSO-d6, 101 MHz) δ 198.4, 179.8, 158.0, 150.3, 149.9, 144.5, 141.5, 140.0, 138.9, 135.5, 132.0, 130.2, 129.0, 128.2, 128.0, 127.2, 126.3, 126.2, 124.2, 122., 120.1, 118.5, 112.9, 111.9, 71.5, 71.4, 65.7, 57.2, 52.2, 41.8, 36.7, 28.1, 27.8, 25.0, 22.8, 19.8; Anal. for C38H34ClN5O2; Calcd: C, 72.66; H, 5.46; N, 11.15; Found: C, 72.57; H, 5.42; N, 11.19.
2.2. Computational Protocol
“The ωB97X-D [43] functional, together with the standard 6-311G (d,p) [44] basis set, was used throughout this MEDT study. Solvent effects of methanol were taken into account by full optimization of the gas-phase structures at the same computational level using the polarizable continuum model (PCM) [45,46] in the framework of the self-consistent reaction field (SCRF) [47,48,49]. The global electron density transfer (GEDT) [50] values were computed using the equation GEDT(f) = Σqf, where q is the natural charges [51,52] of the atoms belonging to one of the two frameworks (f) at the TS geometries. Global and local Conceptual DFT (CDFT) indices [53,54] were calculated using the equations given in reference [54]. The Gaussian 16 suite of programs was used to perform the calculations [55]. Molecular geometries were visualized using the GaussView program” [56].
3. Results and Discussion
3.1. Experimental Characterization
A variety of chalcones were prepared using acetyl derivative 1 previously reported by our research group [57] followed by the [3+2] cycloaddition reactions to obtain the desired spiro-compounds 6a–j. The general synthetic strategy for the formation of the desired compounds is shown in Scheme 1. Chalcones 3a–j were synthesized from 1-(6-(1H-benzo[d]imidazole-2-yl)-2-methylpyridin-3-yl)ethan-1-one 1. This ketone was then condensed with aryl aldehyde derivatives (2a–j) using the Claisen–Schmidt reaction. The neutralization of the reaction mixture with dilute acetic acid followed by recrystallization gave compounds 3a–j with yields ranging between 65 and 95%. The structures of the final products were confirmed on the basis of spectral studies. IR, 1H-NMR, 13C-NMR, and an elemental analysis were used to characterize all the newly synthesized compounds. The IR spectrum of (E)-1-(6-(1H-benzo[d]imidazole-2-yl)-2-methylpyridin-3-yl)-3-(4-methoxyphenyl)prop-2-en-1-one (3a) showed a strong absorption band at 3428 cm−1 corresponding to benzimidazole NH. A sharp absorption at 1592 cm−1 corresponds to carbonyl stretching. The 1H-NMR spectrum showed a singlet peak at δ 13.03 ppm, which was assigned to benzimidazole NH. The two doublet peaks at δ 8.28 and 8.20 ppm correspond to pyridine CH/CH. The doublet peaks for α,β-unsaturated protons appear at δ 7.56 (CHβ)/7.35 (CHα) ppm, and the singlet peak at δ 3.81 ppm corresponds to the protons of the methoxy group (OCH3). The 13C-NMR spectrum showed a peak at δ 194.10 ppm, which was assigned to the carbonyl group (C=O), while all other peaks for carbons are observed in their expected region, which supported its structure.
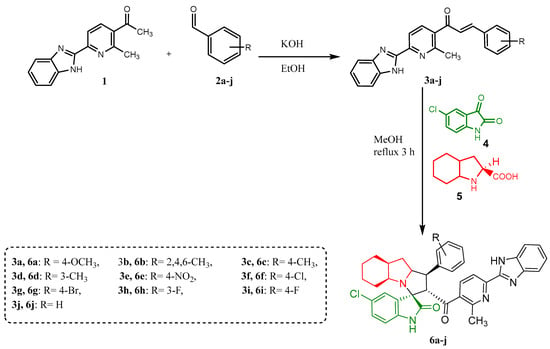
Scheme 1.
Synthesis of chalcones (3a–j) and spiro-compounds (6a–j).
The synthesis of spiro-derivatives (6a–j) from the three-component reaction was achieved via a 32CA protocol (Scheme 1). The reaction of enones (3a–j) with 5-chloro isatin, and octahydroindole-2-carboxylic acid, was carried out at 60 °C in MeOH for 3 h to produce the target compounds in good to moderate yields. The structure of the synthesized spiro-compounds was confirmed through spectroscopic analyses. For instance, the IR spectrum for (3a) showed specific signals for the functional groups such as 3436 cm−1 for NH and 1729 cm−1 and 1690 cm−1 for the two carbonyl groups (C=O). The 1H-NMR data for compound (3a) confirm its structure; the peaks at δ 12.94 and 10.12 ppm refer to NH in benzimidazole and isatin, respectively, in the region of 8.11 until 6.55 ppm related to aromatic protons; the one at δ 3.72 ppm belongs to the methoxy group, while those from 5.06 to 0.66 ppm indicate the aliphatic protons. The 13C-NMR spectrum also supports the proposed structure. The two peaks at δ 198.41 and 179.79 ppm were assigned to carbonyl groups (C=O), and a spiro-carbon peak appears at δ 71.38 ppm, while all other peaks for carbons are observed in their expected region.
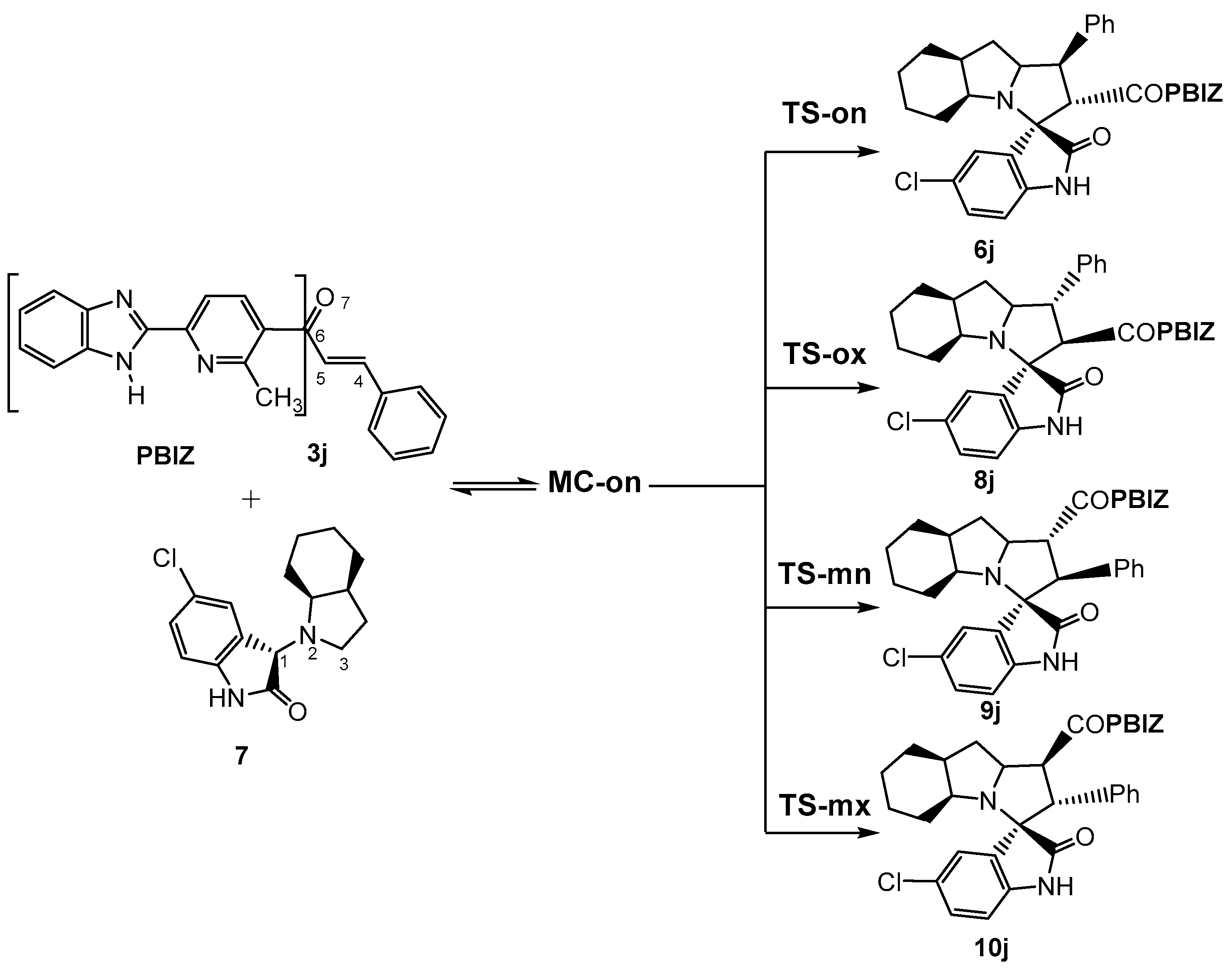
3.2. MEDT Study of the 32CA Reaction between AY 7 and Ethylene 3j
In order to understand the experimental outcomes, the 32CA reaction between AY 7 and ethylene 3j is theoretically studied in this section within the MEDT [41].
3.2.1. Analysis of Conceptual DFT (CDFT) Reactivity Indicators
The reactivity indicators defined within the CDFT [53,54] have demonstrated to be useful tools to predict and understand reactivity in polar reactions [58]. The global reactivity indices, namely, the electronic chemical potential μ, chemical hardness η, electrophilicity ω, and nucleophilicity N, for AY 7 and ethylene 3j are gathered in Table 1.

Table 1.
ωB97X-D/6-311G (d,p) electronic chemical potential μ, chemical hardness η, electrophilicity ω, and nucleophilicity N indices, in eV, of AY 7 and ethylene 3j.
The electronic chemical potential μ [59] of AY 7, μ = −3.15 eV, is higher than that of ethylene 3j, μ = −4.41 eV, indicating that in a polar 32CA reaction, the GEDT [50] will take place from AY 7, acting as a nucleophile, to ethylene 3j, acting as an electrophile. Thus, the studied 32CA reaction is classified as of a forward electron density flux (FEDF) [60].
AY 7 has an electrophilicity ω index [61] of 0.71 eV, which allows for classifying it as a moderate electrophile based on the electrophilicity scale [54,62], and a nucleophilicity N index [63] of 4.77 eV, which allows for categorizing it as a strong nucleophile based on the nucleophilicity scale [54,62]. The very strong nucleophilic character of AY 7, higher than 4.0 eV, indicates that it is a supernucleophile [58,62]. On the other hand, ethylene 3j has electrophilicity ω and nucleophilicity N indices of 1.36 and 3.43 eV, respectively, thus being classified as a strong electrophile and as a moderate nucleophile.
The supernucleophilic character of AY 7 together with the strong electrophilic character of ethylene 3j suggest that the present 32CA reaction of FEDF will be highly polar [58], which is known to enhance reaction rates.
3.2.2. Study of the Competitive Reaction Paths
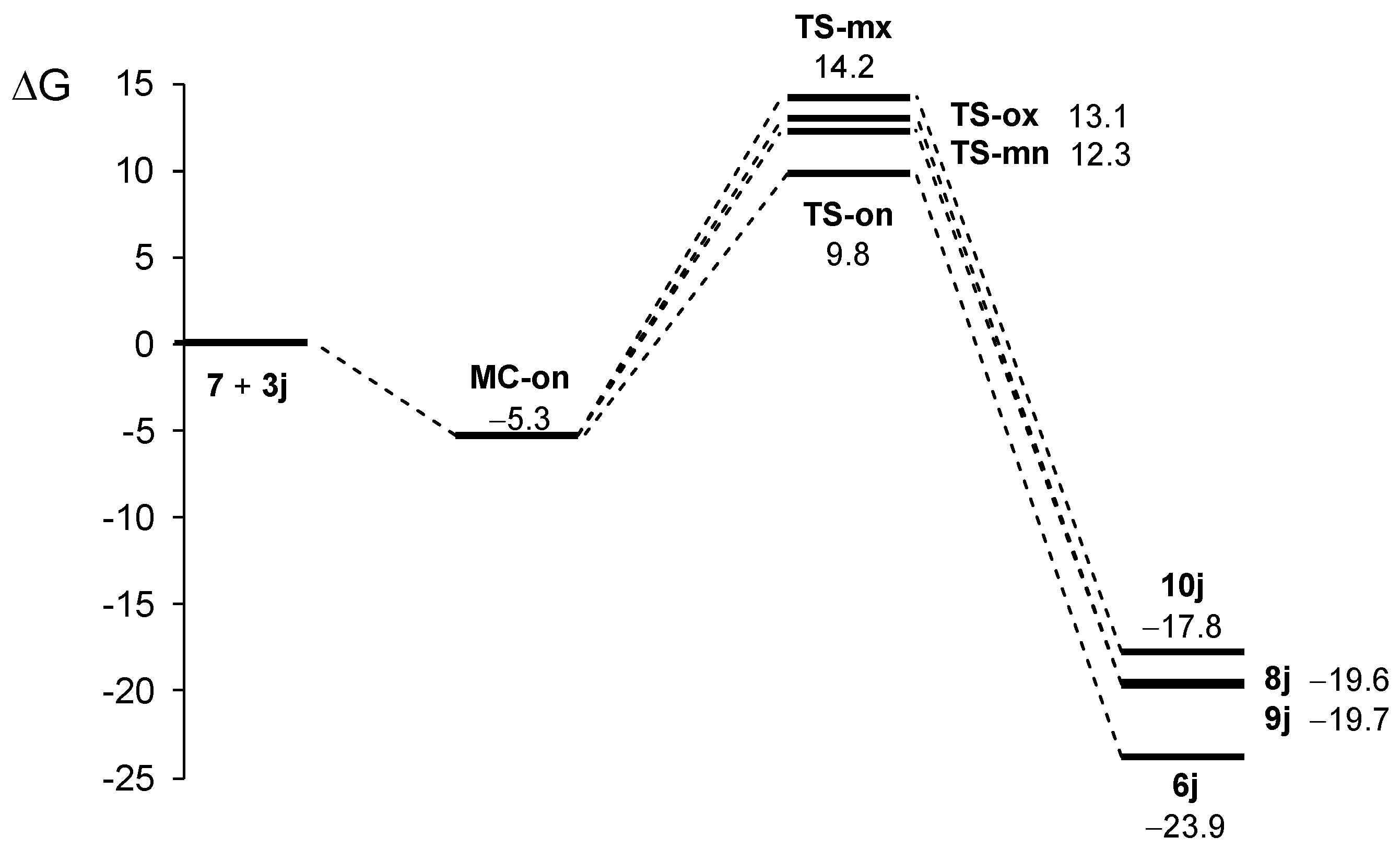
Owing to the non-symmetry of the reagents, the 32CA reaction between AY 7 and ethylene 3j can take place along two ortho/meta-regioisomeric reaction paths and two endo/exo-stereoisomeric paths (see Scheme 2). Note that as the octahydroindole substituent of AY 7 hinders one of its two diastereoisomeric faces, only the less hindered approach has been studied. The Gibbs free energy profiles corresponding to the four competitive reaction paths are represented in Figure 1, while full thermodynamic data are given in Table S1 in the Supplementary Material.
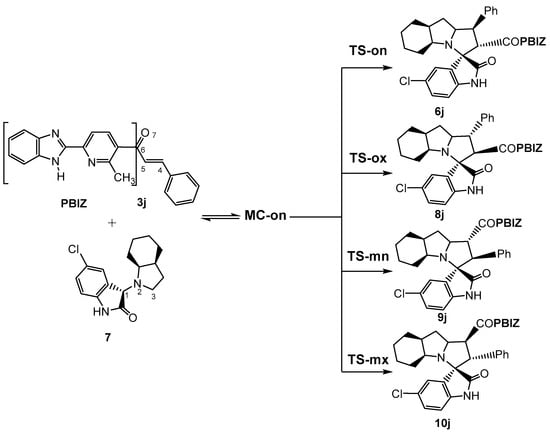
Scheme 2.
The reaction of AY 7 with ethylene derivative 3j by cycloaddition reaction (32CA); A competitive reaction paths.
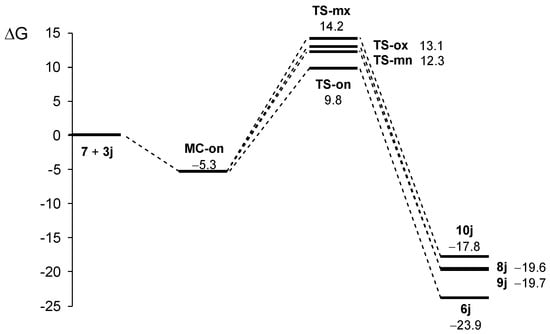
Figure 1.
ωB97X-D/6-311G (d,p) Gibbs free energy profile, in kcal·mol−1, for the 32CA reaction of AY 7 with ethylene 3j in methanol at 60 °C.
The stationary points located in the four reaction paths show that this 32CA reaction follows a one-step mechanism. For each channel, a molecular complex (MC) strongly stabilized by weak intermolecular interactions between the two reagents was found. Given the thermodynamic equilibrium between them, only the most stable one, MC-on, was considered the energy reference to obtain relative energies. Formation of this MC is exergonic by 5.3 kcal·mol−1 (see Figure 2). Considering the presence of MC-on, the activation Gibbs free energies of the selected isomeric paths range between 15.0 (TS-on) and 19.4 (TS-mx) kcal·mol−1, while reaction Gibbs free energies are found between −17.8 (10j) and −23.9 (6j) kcal·mol−1. The high exergonic characteristic of the reaction indicates that it is irreversible under the experimental conditions and, therefore, the product of a kinetic control will be obtained. In this sense, the Eyring–Polanyi kinetics equation [64] yields a product percentage relation of 97 (6j):0.7 (8j):2.2 (9j):0.1 (10j), indicating that the reaction is completely ortho/endo-selective via TS-on, leading to 6j exclusively. These results are fully consistent with the experimental data.
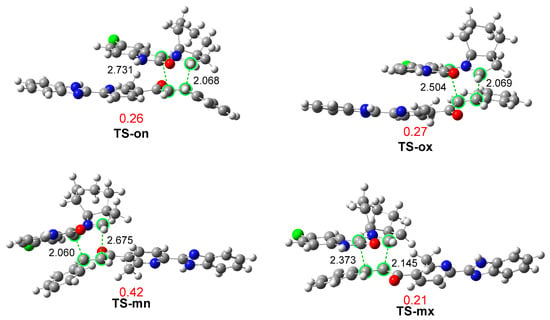
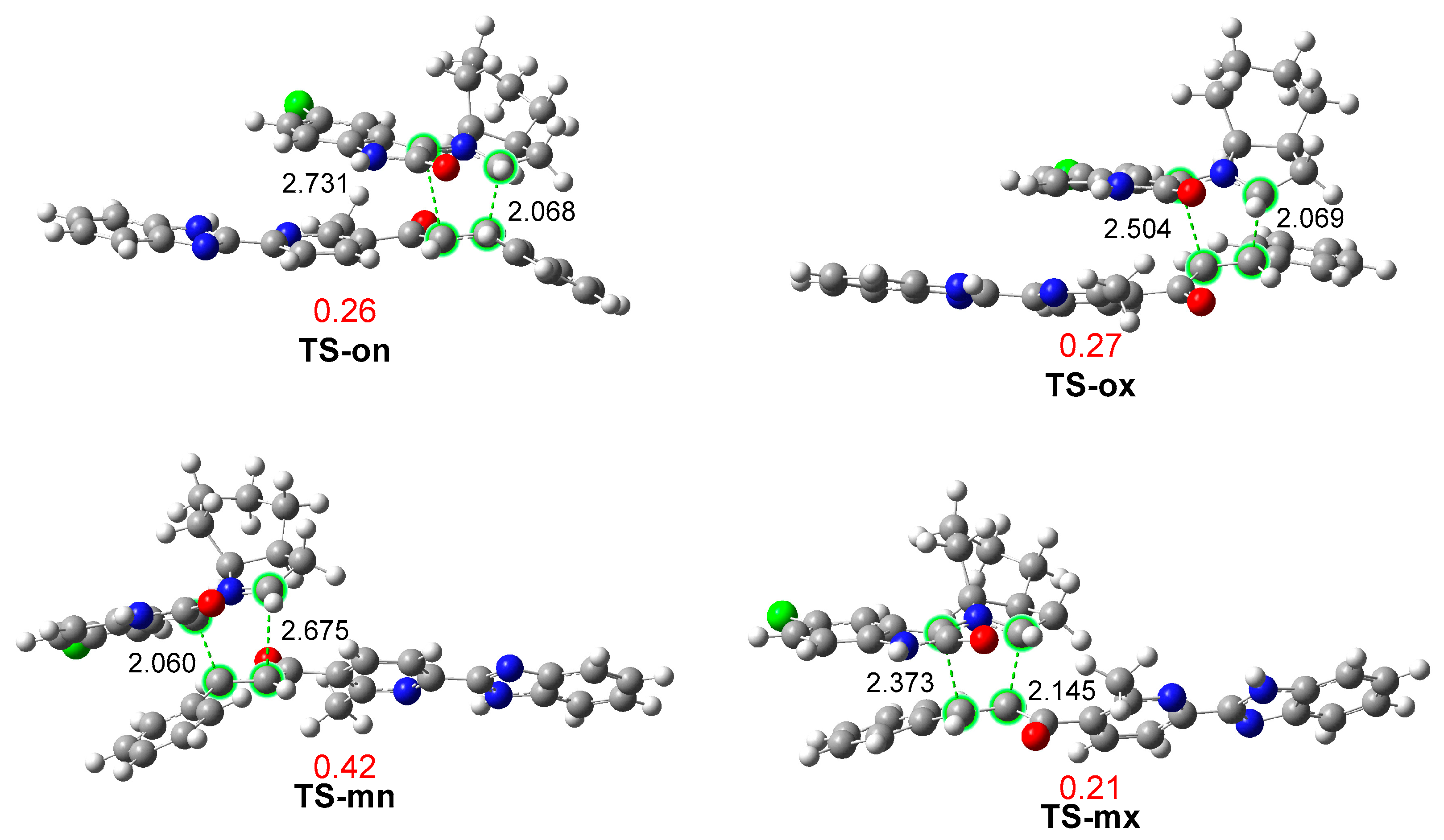
Figure 2.
ωB97X-D/6-311G (d,p) optimized geometries in methanol of the TSs involved in the 32CA reaction of AY 7 with ethylene 3j. Distances are expressed in angstroms, Å, while GEDT values, in red, are given in average number of electrons, e.
The optimized geometries of the four TSs in methanol are displayed in Figure 2. The C3(1)–C4 and C1(3)–C5 distances at the four TSs indicate that, except for the most unfavorable TS-mx, the other three TSs correspond to asynchronous C–C single bond formation processes in which the shorter C–C distance involves the most electrophilic β-conjugated C4 carbon of ethylene 3j. The most favorable TS-on, with C3–C4 and C1–C5 distances of 2.068 and 2.731 Å, respectively, is the most asynchronous one. An analysis of the intrinsic reaction coordinate (IRC) path [65] from the highly asynchronous TS-on towards CA-on indicates that the formation of the second C1–C5 single bond begins when the first C3–C4 single bond is completely formed (see Figure S1 in Supplementary Material). Consequently, the present 32CA reaction takes place through a non-concerted two-stage one-step mechanism [66].
Finally, an analysis of GEDT [50] at the most favorable TS-on allows quantifying the polar characteristic of this 32CA reaction. GEDT values lower than 0.05 e correspond to non-polar processes, while values higher than 0.20 e characterize polar processes. The GEDT values at the four TSs are given in Figure 2. The GEDT at TS-on is 0.26 e. This high value, which is a consequence of the supernucleophilic character of AY 7 and the strong electrophilic character of ethylene 3j (see Table 1), corroborates the highly polar character of this 32CA reaction, which accounts for its low activation Gibbs free energy of 15.0 kcal·mol−1 via TS-on. The direction of the flux of the electron density, from AY 7 to ethylene 3j, consolidates the classification of this 32CA reaction as FEDF [60], as predicted with the analysis of the CDFT indicators.
4. Conclusions
A new series of spirooxindoles based on benzimidazole with a pyridine spacer was synthesized in a high yield via a 32CA reaction approach using a wide range of reagents with varying substitutions. The desired compounds were obtained with full regio- and stereoselectivity, as confirmed by the Molecular Electronic Density Theory (MEDT) study of the 32CA reaction, with reagents containing the simplest substitution (R = H) as a case study reference.
The activation Gibbs free energy of the reaction via the most favorable TS-on is 15.0 kcal·mol−1—the reaction being strongly exergonic with 23.9 kcal·mol−1. The MEDT study accounts for the total ortho/endo-selectivity, as TS-on is 3.3 and 2.5 kcal·mol−1 lower in energy than the corresponding exo- and meta-TSs, respectively. No diastereoisomer of the final products is detected, due to the presence of the octahydroindole substituent at AY 7, which hinders one of its two diastereoisomeric faces. The low energy barrier of the wide range of reactions reported herein is the result of the supernucleophilic character of the reactive AY 7 generated in situ and the strong electrophilic character of the α,β-unsaturated carbonyl compounds, which render these 32CA reactions of FEDF highly polar. This favors bond formation through a non-concerted two-stage one-step mechanism in which the first single bond formation involves the hexahydroindole carbon of AY 7 and the β-conjugated carbon of ethylene derivative 3j.
Given the well-known pharmacological applications of spirocyclic compounds, the new products reported herein could be useful for drug discovery application, which will be considered in the near future by our research group.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13071085/s1, IUPAC name for the spiro-oxindole derivatives; Table S1: Full thermodynamic data. Figure S1: IRC of the most favorable endo/exo-reaction path via TS-on. Cartesian coordinates, electronic energies, and imaginary frequencies of the stationary points involved in the 32CA reaction of AY 7 with ethylene derivative 3j.
Author Contributions
Conceptualization, A.B.; methodology, S.A. and A.S.A.; software, M.R.-G.; validation, S.A., A.S.A. and M.R.-G.; formal analysis, S.A. and A.S.A.; investigation, S.A. and A.S.A.; resources, A.B.; data curation, A.B. and M.R.-G.; writing—original draft preparation, A.B. and M.R.-G.; writing—review and editing, A.B. and M.R.-G.; supervision, A.B. and A.M.A.-M.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Deputyship for Research & Innovation, “Ministry of Education” in Saudi Arabia for funding this research work through the project number IFKSUOR3–128-1. This work has also been supported by the Ministry of Science and Innovation (MICINN) of the Spanish Government, through the project PID2019-110776GB-I00 (AEI/FEDER, UE).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, L.M.; Qu, R.Y.; Yang, G.F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef]
- Galliford, C.V.; Scheidt, K.A. Pyrrolidinyl-spirooxindole natural products as inspirations for the development of potential therapeutic agents. Angew. Chem. Int. Ed. 2007, 46, 8748–8758. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.S.; Girgis, A.S.; Aziz, M.N.; Bekheit, M.S. Spirooxindole: A Versatile Biologically Active Heterocyclic Scaffold. Molecules 2023, 28, 618. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M. Recent advances in the synthesis of biologically active spirooxindoles. Tetrahedron 2014, 70, 9735–9757. [Google Scholar] [CrossRef]
- Molteni, G.; Silvani, A. Spiro-2-oxindoles via 1, 3-dipolar cycloadditions. A decade update. Eur. J. Org. Chem. 2021, 2021, 1653–1675. [Google Scholar] [CrossRef]
- Nasri, S.; Bayat, M.; Mirzaei, F. Recent strategies in the synthesis of spiroindole and spirooxindole scaffolds. Top. Curr. Chem. 2021, 379, 25. [Google Scholar] [CrossRef]
- Xia, M.; Ma, R.Z. Recent progress on routes to spirooxindole systems derived from isatin. J. Heterocycl. Chem. 2014, 51, 539–554. [Google Scholar] [CrossRef]
- Babu, S.A.; Padmavathi, R.; Aslam, N.A.; Rajkumar, V. Recent developments on the synthesis and applications of natural products-inspired spirooxindole frameworks. Stud. Nat. Prod. Chem. 2015, 46, 227–339. [Google Scholar]
- Pavlovska, T.L.; Redkin, R.G.; Lipson, V.V.; Atamanuk, D.V. Molecular diversity of spirooxindoles. Synthesis and biological activity. Mol. Divers. 2016, 20, 299–344. [Google Scholar] [CrossRef]
- Ball-Jones, N.R.; Badillo, J.J.; Franz, A.K. Strategies for the enantioselective synthesis of spirooxindoles. Org. Biomol. Chem. 2012, 10, 5165–5181. [Google Scholar] [CrossRef]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Alamary, A.S.; Haukka, M.; Abu-Serie, M.M.; Dömling, A.; Mazyed, E.A.; Badria, F.A.; El-Senduny, F.F. Novel spirooxindole based benzimidazole scaffold: In vitro, nanoformulation and in vivo studies on anticancer and antimetastatic activity of breast adenocarcinoma. Bioorg. Chem. 2022, 129, 106124. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; El-Senduny, F.F.; Badria, F.A.; Elshaier, Y.A.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Barakat, A.; Abu-Serie, M.M.; Ali, M.; Al-Majid, A.M.; Ashraf, S.; Zia, K.; Ul-Haq, Z.; Al-Dhfyan, A.; Abdel-Aziz, H.A.; El-Faham, A.; et al. Synthesis, In Vitro and in Cell Study of a New Spirooxindoles-Based N-Alkylated Maleimides Targeting HER2/3 Signaling Pathway. Polycycl. Aromat. Compd. 2022, 43, 1–25. [Google Scholar] [CrossRef]
- Lotfy, G.; Aziz, Y.M.A.; Said, M.M.; El Sayed, H.; El Sayed, H.; Abu-Serie, M.M.; Teleb, M.; Dömling, A.; Barakat, A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 117, 105427. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Y.M.A.; Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Soliman, S.; Abu-Serie, M.M.; Teleb, M.; Yousuf, S.; Dömling, A. Design, synthesis, chemical and biochemical insights on to novel hybrid spirooxindoles-based p53-MDM2 inhibitors with potential Bcl2 signaling attenuation. Front. Chem. 2021, 9, 735236. [Google Scholar] [CrossRef]
- Kumar, R.S.; Antonisamy, P.; Almansour, A.I.; Arumugam, N.; Periyasami, G.; Altaf, M.; Kim, H.R.; Kwon, K.B. Functionalized spirooxindole-indolizine hybrids: Stereoselective green synthesis and evaluation of anti-inflammatory effect involving TNF-α and nitrite inhibition. Eur. J. Med. Chem. 2018, 152, 417–423. [Google Scholar] [CrossRef]
- Barakat, A.; Mostafa, A.; Ali, M.; Al-Majid, A.M.; Domingo, L.R.; Kutkat, O.; Moatasim, Y.; Zia, K.; Ul-Haq, Z.; Elshaier, Y.A. Design, synthesis and in vitro evaluation of spirooxindole-based phenylsulfonyl moiety as a candidate anti-SAR-CoV-2 and MERS-CoV-2 with the Implementation of combination studies. Int. J. Mol. Sci. 2022, 23, 11861. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.M.; Lotfy, G.; Ali, M.; Mostafa, A.; Elshaier, Y.A. Drug repurposing of lactoferrin combination in a nanodrug delivery system to combat severe acute respiratory syndrome coronavirus-2 infection. Dr. Sulaiman Al Habib Med. J. 2021, 3, 104–112. [Google Scholar] [CrossRef]
- Toumi, A.; Boudriga, S.; Hamden, K.; Sobeh, M.; Cheurfa, M.; Askri, M.; Knorr, M.; Strohmann, C.; Brieger, L. Synthesis, antidiabetic activity and molecular docking study of rhodanine-substitued spirooxindole pyrrolidine derivatives as novel α-amylase inhibitors. Bioorg. Chem. 2021, 106, 104507. [Google Scholar] [CrossRef]
- Nivetha, N.; Martiz, R.M.; Patil, S.M.; Ramu, R.; Sreenivasa, S.; Velmathi, S. Benzodioxole grafted spirooxindole pyrrolidinyl derivatives: Synthesis, characterization, molecular docking and anti-diabetic activity. RSC Adv. 2022, 12, 24192–24207. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H.A. Spiroindolone analogues as potential hypoglycemic with dual inhibitory activity on α-amylase and α-glucosidase. Molecules 2019, 24, 2342. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Chen, H.; Wold, E.A.; Shi, P.Y.; Zhou, J. Therapeutic potential of spirooxindoles as antiviral agents. ACS Infect. Dis. 2016, 2, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Ali, M.; Altowyan, M.S.; Islam, M.S.; Alamary, A.S.; Ashraf, S.; Ul-Haq, Z. Synthesis of a new class of spirooxindole–benzo [b] thiophene-based molecules as acetylcholinesterase inhibitors. Molecules 2020, 25, 4671. [Google Scholar] [CrossRef]
- Saraswat, P.; Jeyabalan, G.; Hassan, M.Z.; Rahman, M.U.; Nyola, N.K. Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moities. Synth. Commun. 2016, 46, 1643–1664. [Google Scholar] [CrossRef]
- Mu, J.; Xie, X.; Xiong, S.; Zhang, Y.; Wang, Y.; Zhao, Q.; Zhu, H.; Huang, W.; He, G. Discovery of spirooxindole–ferrocene hybrids as novel MDM2 inhibitors. Chin. Chem. Lett. 2021, 32, 1897–1901. [Google Scholar] [CrossRef]
- Yu, B.; Yu, D.Q.; Liu, H.M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Antonchick, A.P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Highly enantioselective synthesis and cellular evaluation of spirooxindoles inspired by natural products. Nat. Chem. 2010, 2, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.J.; Tice, C.M. The utilization of spirocyclic scaffolds in novel drug discovery. Expert Opin. Drug Discov. 2016, 11, 831–834. [Google Scholar] [CrossRef]
- Deng, J.; Mo, L.P.; Zhao, F.Y.; Zhang, Z.H.; Liu, S.X. One-pot, three-component synthesis of a library of spirooxindole-pyrimidines catalyzed by magnetic nanoparticle supported dodecyl benzenesulfonic acid in aqueous media. ACS Comb. Sci. 2012, 14, 335–341. [Google Scholar] [CrossRef]
- Cheng, D.; Ishihara, Y.; Tan, B.; Barbas, C.F., III. Organocatalytic asymmetric assembly reactions: Synthesis of spirooxindoles via organocascade strategies. ACS Catal. 2014, 4, 743–762. [Google Scholar] [CrossRef]
- Sansinenea, E.; Martínez, E.F.; Ortiz, A. Organocatalytic synthesis of chiral spirooxindoles with quaternary stereogenic centers. Eur. J. Org. Chem. 2020, 2020, 5101–5118. [Google Scholar] [CrossRef]
- Xu, P.W.; Cui, X.Y.; Yu, J.S.; Zhou, J. Spirooxindoles: Synthesis via Organocatalytic Processes. In Spiro Compounds: Synthesis and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 103–160. [Google Scholar] [CrossRef]
- Voituriez, A.; Pinto, N.; Neel, M.; Retailleau, P.; Marinetti, A. An organocatalytic [3+2] cyclisation strategy for the highly enantioselective synthesis of spirooxindoles. Chem.–Eur. J. 2010, 16, 12541–12544. [Google Scholar] [CrossRef] [PubMed]
- Katowah, D.F.; Hassaneen, H.M.; Farghaly, T.A. Novel Spiro-pyrrolizidine-Oxindole and Spiropyrrolidine-Oxindoles: Green synthesis under Classical, Ultrasonic, and microwave conditions and Molecular docking simulation for antitumor and type 2 diabetes. Arab. J. Chem. 2022, 15, 103930. [Google Scholar] [CrossRef]
- Dabiri, M.; Tisseh, Z.N.; Bahramnejad, M.; Bazgir, A. Sonochemical multi-component synthesis of spirooxindoles. Ultrason. Sonochem. 2011, 18, 1153–1159. [Google Scholar] [CrossRef]
- Pavithra, T.; Devi, E.S.; Maheswari, C.U. Recent Advances in N-Heterocyclic Carbene Catalyzed Oxidative Cyclization for the Formation of Heterocycles. Asian J. Org. Chem. 2021, 10, 1861–1883. [Google Scholar] [CrossRef]
- Saranya, P.V.; Neetha, M.; Aneeja, T.; Anilkumar, G. Transition metal-catalyzed synthesis of spirooxindoles. RSC Adv. 2021, 11, 7146–7179. [Google Scholar] [CrossRef]
- Hanhan, N.V.; Ball-Jones, N.R.; Tran, N.T.; Franz, A.K. Catalytic asymmetric [3+2] annulation of allylsilanes with isatins: Synthesis of spirooxindoles. Angew. Chem. Int. Ed. 2012, 51, 989–992. [Google Scholar] [CrossRef]
- Das, T.; Jana, R.; Dubey, S.; Pal, A.; Roy, S.; Sasmal, S.; Tamrakar, A. Recent advances on (3+2) cycloaddition of Azomethine Ylide. New J. Chem. 2023, 47, 8997–9034. [Google Scholar]
- Panda, S.S.; Aziz, M.N.; Stawinski, J.; Girgis, A.S. Azomethine Ylides—Versatile Synthons for Pyrrolidinyl-Heterocyclic Compounds. Molecules 2023, 28, 668. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef] [PubMed]
- Rios-Gutierrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Hehre, M.J.; Radom, L.; Schleyer, P.v.R.; Pople, J. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular interactions in solution: And overview of methods based on continuous distributions of the solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Simkin, B.Y.; Sheikhet, I.I. Quantum Chemical and Statistical Theory of Solutions—Computational Approach; Ellis Horwood: London, UK, 1995. [Google Scholar]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Lett. 1996, 255, 327–335. [Google Scholar] [CrossRef]
- Cances, E.; Mennucci, B.; Tomasi, J. A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Domingo, L.R. A new C–C bond formation model based on the quantum chemical topology of electron density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the conceptual density functional indices to organic chemistry reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.; Cheeseman, J.R.; Scalmani, G.; Barone, V.P.G.A.; Petersson, G.A.; Nakatsuji, H.J.R.A.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Alshahrani, S.; Soliman, S.M.; Alamary, A.S.; Al-Majid, A.M.; Haukka, M.; Yousuf, S.; Barakat, A. Synthesis of Enaminones-Based Benzo[d]imidazole Scaffold: Characterization and Molecular Insight Structure. Crystals 2020, 10, 955. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. Application of Reactivity Indices in the Study of Polar Diels–Alder Reactions in Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; Liu, S., Ed.; WILEY-VCH GmbH: Weinheim, Germany, 2022; Volume 2, pp. 481–502. [Google Scholar]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. Sci. Rad. 2023, 2, 1. [Google Scholar] [CrossRef]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Saz Sousa, A.; Domingo, L.R. Electrophilicity and nucleophilicity scales at different DFT computational levels. J. Phys. Org. Chem. 2023, 36, e4503. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Pérez, P. Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions. A theoretical study. J. Org. Chem. 2008, 73, 4615–4624. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Domingo, L.R.; Sáez, J.A.; Zaragozá, R.J.; Arnó, M. Understanding the Participation of Quadricyclane as Nucleophile in Polar Cycloadditions toward Electrophilic Molecules. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).