Complexes of the Antibiotic Drug Oxolinic Acid with Fe(III), Zn(II), Ca(II), and Mg(II) Ions: Preparation, Characterization, and In Vitro Evaluation of Biological Activity
Abstract
1. Introduction
- (i)
- Chemistry:This part aimed to prepare the complexes of OA molecule with four metal ions [i.e., Fe(III), Zn(II), Ca(II), Mg(II)] in neutral media (pH 7–8) with a 1:1 metal-to-OA stoichiometry at 60–70 °C and to characterize the generated OA complexes with several physicochemical techniques: Fourier-transform infrared (FT-IR), nuclear magnetic resonance (1H NMR), ultraviolet/visible (UV-visible) spectroscopies, and CHN elemental analyzer.
- (ii)
- Morphology:This part aimed to observe the phase purity and visualize the surface morphology of the generated OA complexes by X-ray powder diffractometry (XRD), and scanning and transmission electron microscopies (SEM and TEM, respectively).
- (iii)
- Biology:This part aimed to assay the antimicrobial properties (i.e., antibacterial and antifungal) of the OA complexes in vitro using the Kirby–Bauer disc diffusion assay method with five bacterial and three fungal microbes. The screening data of the standard drugs (streptomycin and ketoconazole) were used for comparison.
2. Experiments
2.1. Chemicals and Instrumentation
2.2. Synthesis of OA Complexes
2.3. Biological Evaluation
3. Results and Discussion
3.1. Chemistry
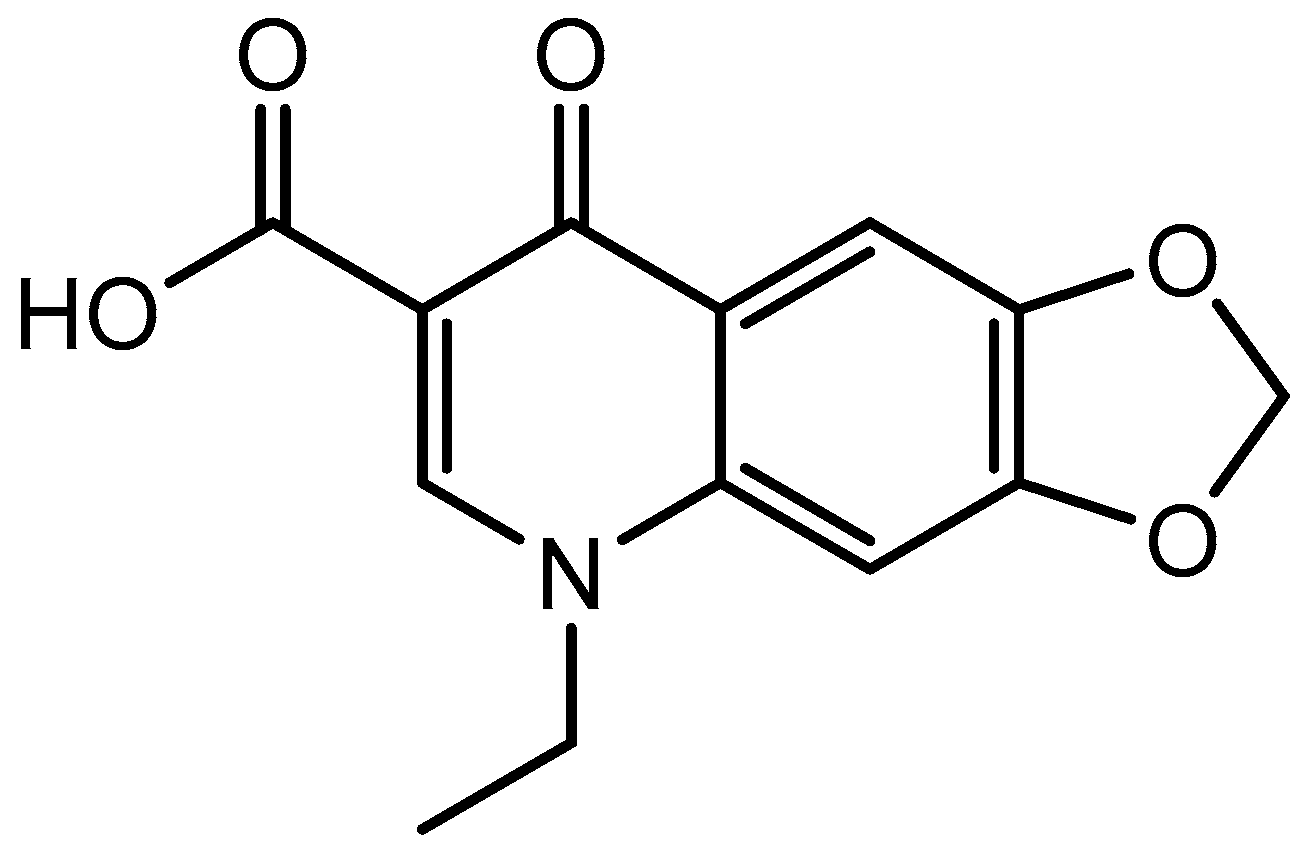
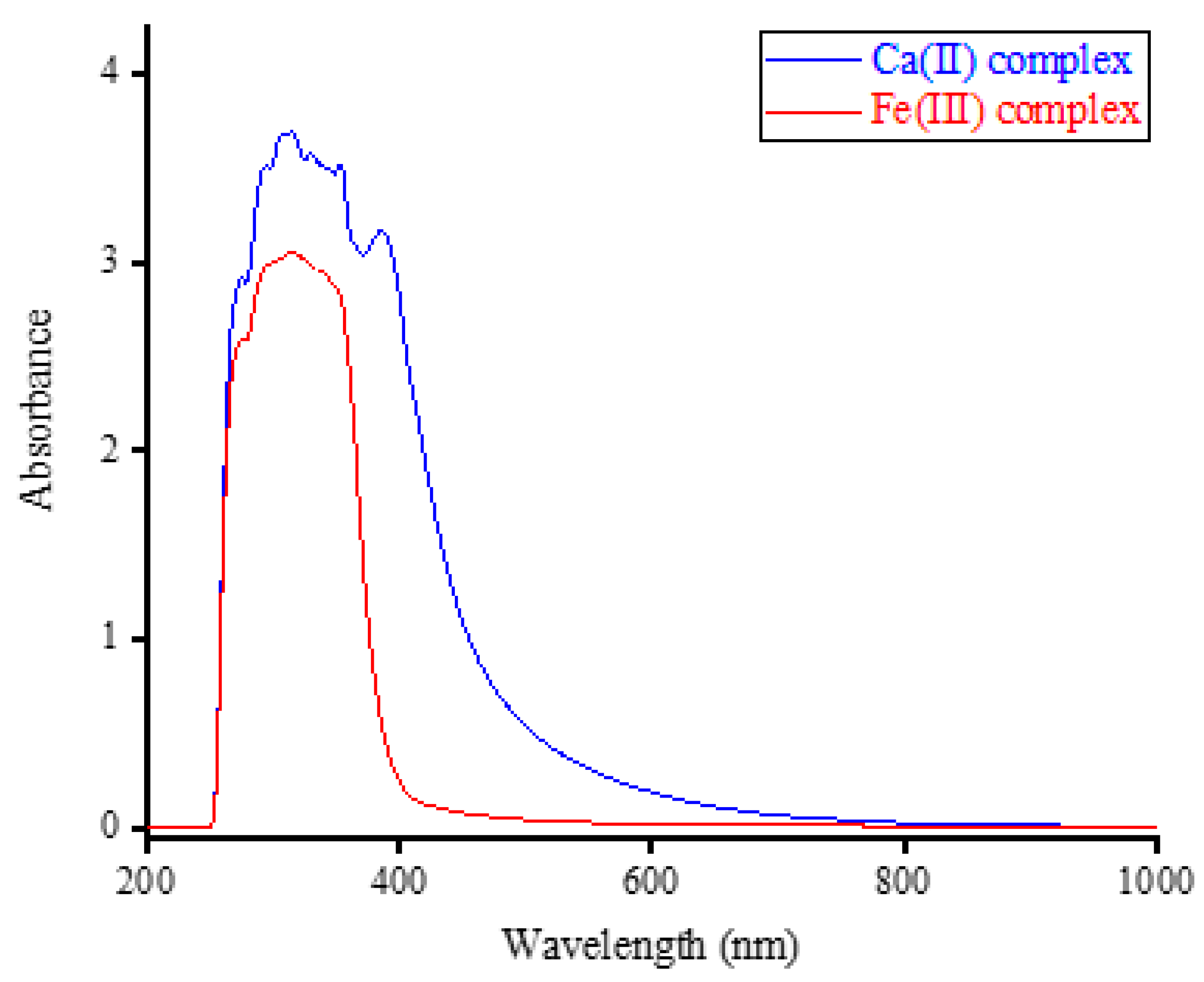
3.1.1. CHN Analysis and UV-Visible Spectral Results
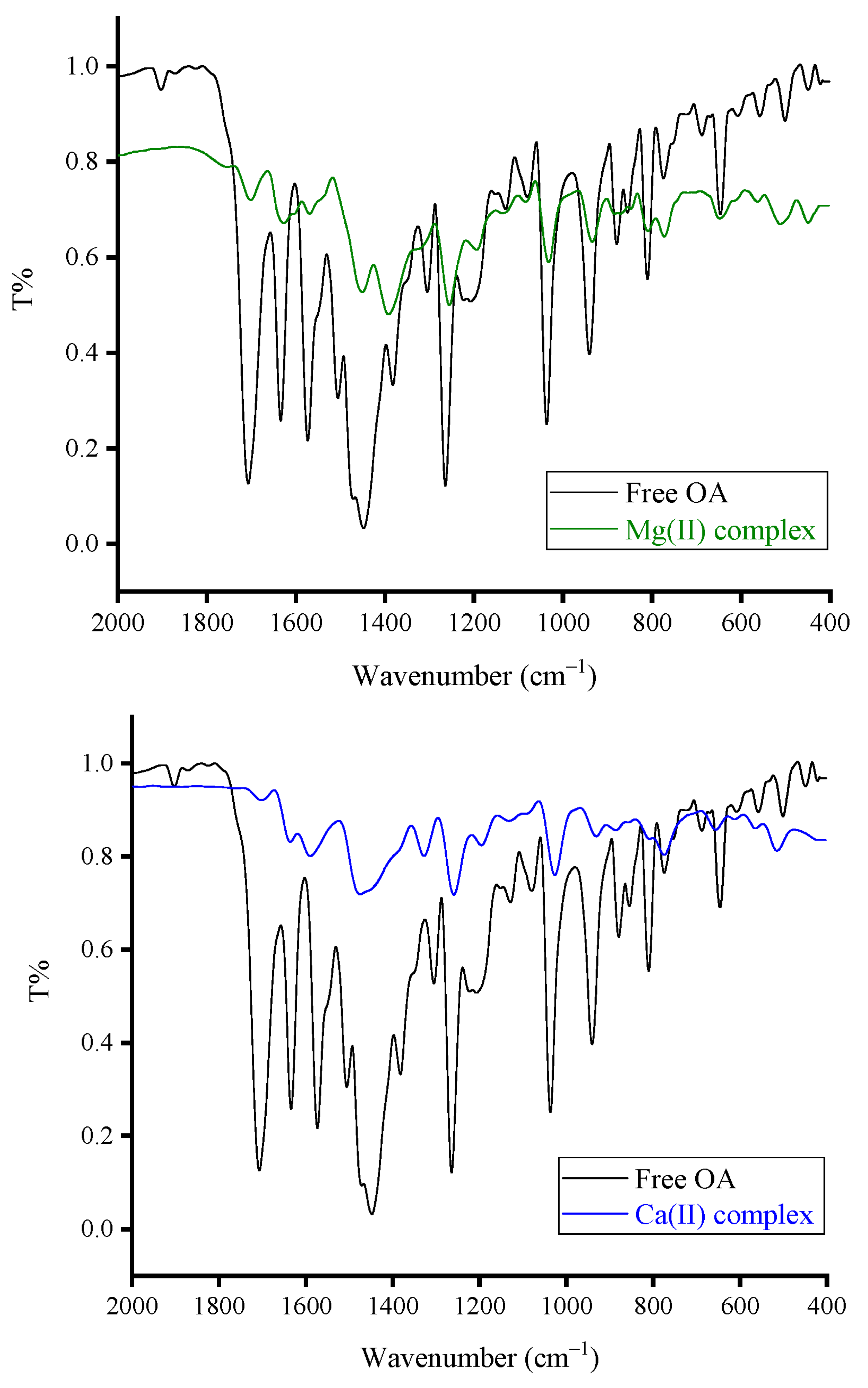
3.1.2. FT-IR Spectral Results
- (i)
- The vibrations of ν(O–H) resulted from the –COOH group in the OA molecule, and moisture water produced a medium-intensity absorption band located at 3418 cm−1;
- (ii)
- The vibrations of νasym(C–H) and νsym(C–H) resulted from the –CH3, –CH2, and –CH moieties produced three medium-intensity absorption bands resonated at 3063, 2982, and 2933 cm−1;
- (iii)
- The vibrations of ν(C=O)COOH originated a very strong and sharp band at 1710 cm−1. The vibrations of ν(C=O)pyridone and ν(C=C) generated strong and sharp bands at 1634 and 1579 cm−1, respectively [25];
- (iv)
- The vibrations of δrock(CH3) and δsciss(CH2) produced a very strong and intense absorption band at 1450 cm−1. This band has two shoulder bands at 1510 and 1377 cm−1;
- (v)
- The vibrations of δrock(CH2) were responsible for the strong and sharp absorption band that appeared at 1262 cm−1;
- (vi)
- Absorptions at 1210 and 1128 cm−1 could be referred to as the νasym(C–N) and νsym(C–N) vibrations, respectively. Absorptions at 1086 and 1040 cm−1 could be due to the ν(O–CH2–O) vibrations [26].
- (i)
- Disappearance of the band due to the ν(C=O)COOH vibrations. This band resonated at 1710 cm−1 in the FT-IR spectrum of free OA, but it was absent in the FT-IR spectra of Fe(III), Zn(II), and Ca(II) complexes. The intensity of this band was greatly decreased in the spectrum of the Mg(II) complex;
- (ii)
- Shifting of the band due to the ν(C=O)pyridone vibrations. This band was resonated at 1634 cm−1 in the FT-IR spectrum of free OA, but it was shifted in the FT-IR spectra of Fe(III), Zn(II), Ca(II), and Mg(II) complexes to 1597, 1616, 1598, and 1613 cm−1, respectively;
- (iii)
- Appearance of new medium-intensity absorption band at 503, 500, 515, and 512 cm−1 in the spectra of Fe(III), Zn(II), Ca(II), and Mg(II), respectively, and could be referred to as the ν(M–O) vibrations [26].
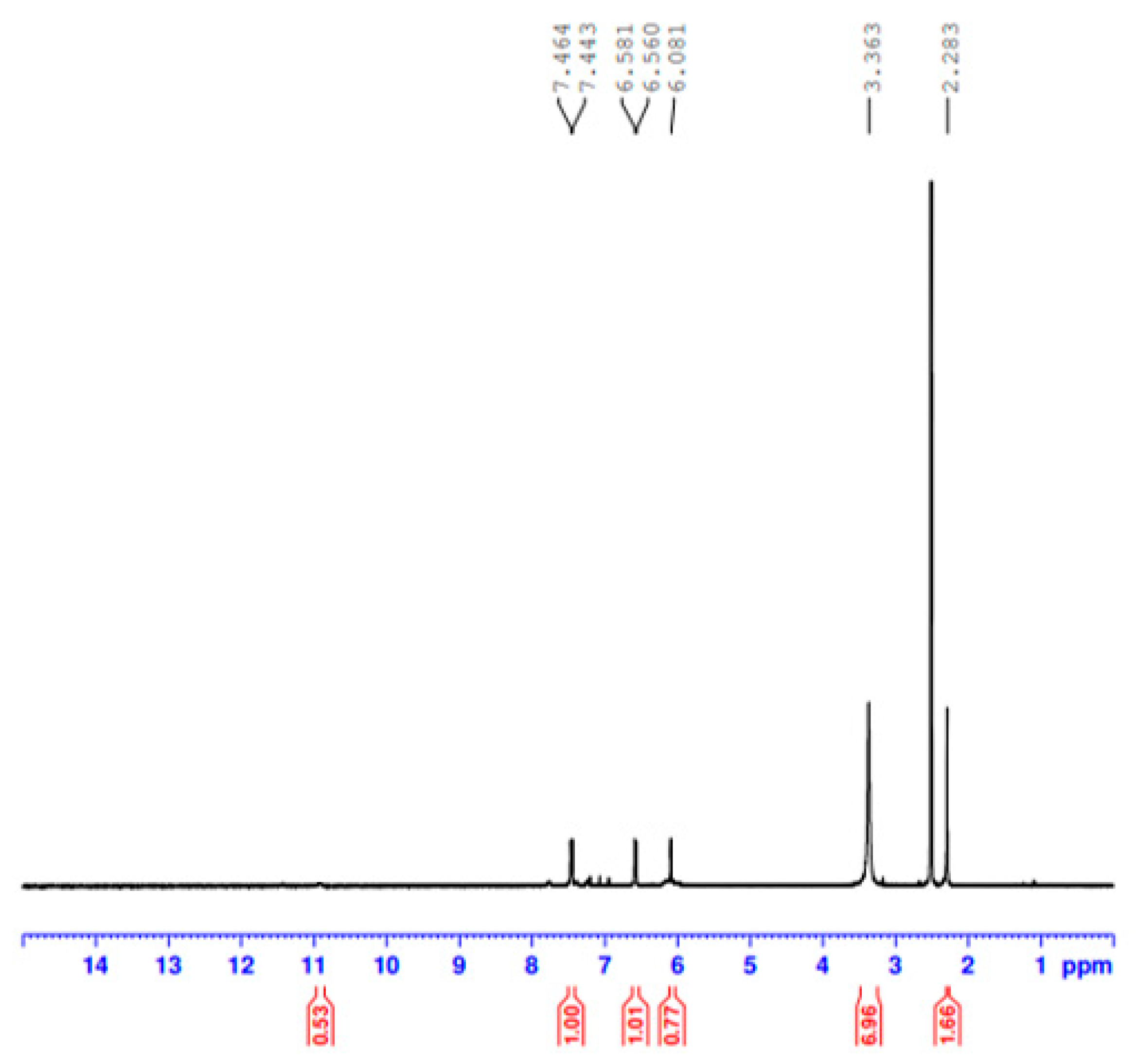
3.1.3. H NMR Spectral Results
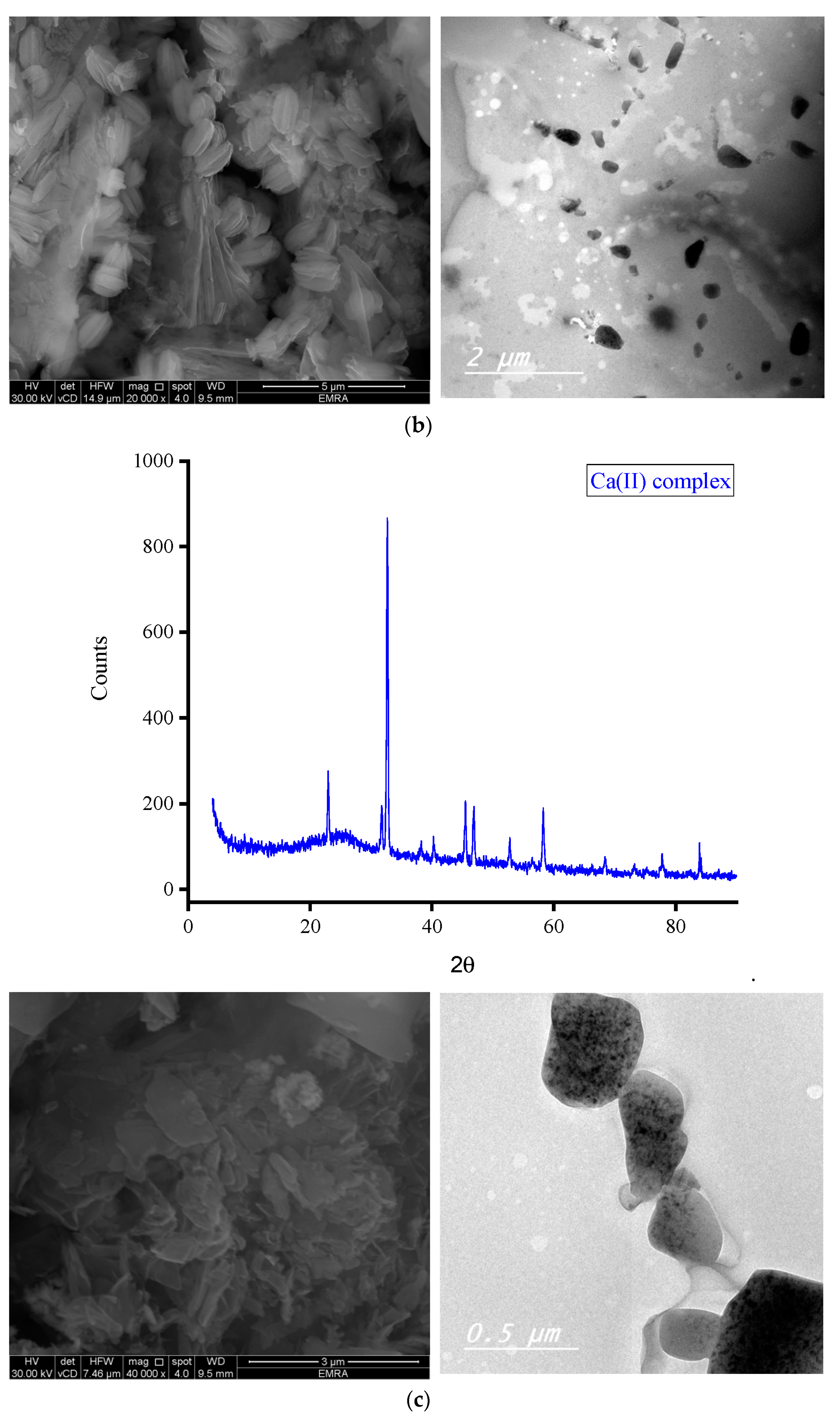
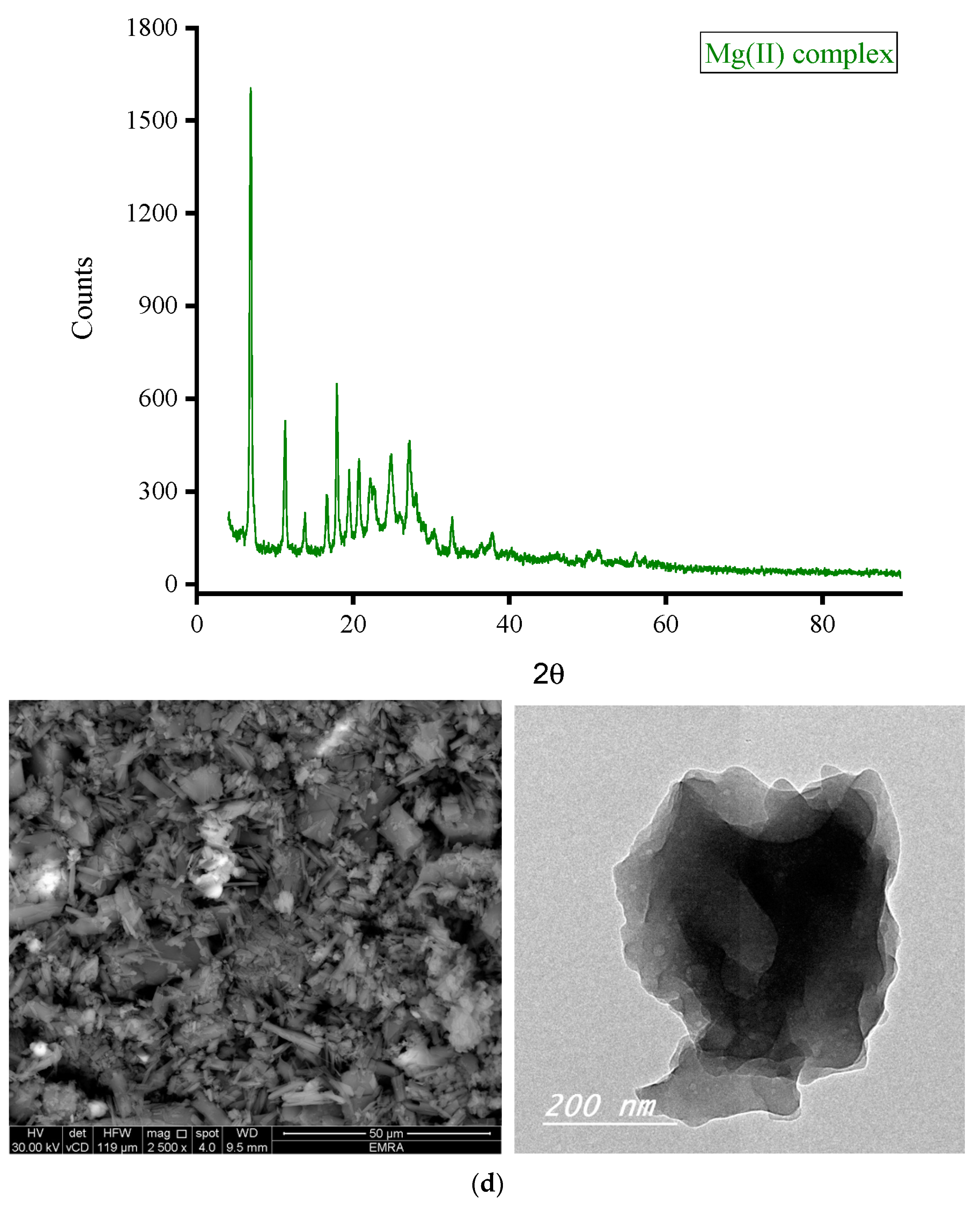
3.2. Morphology
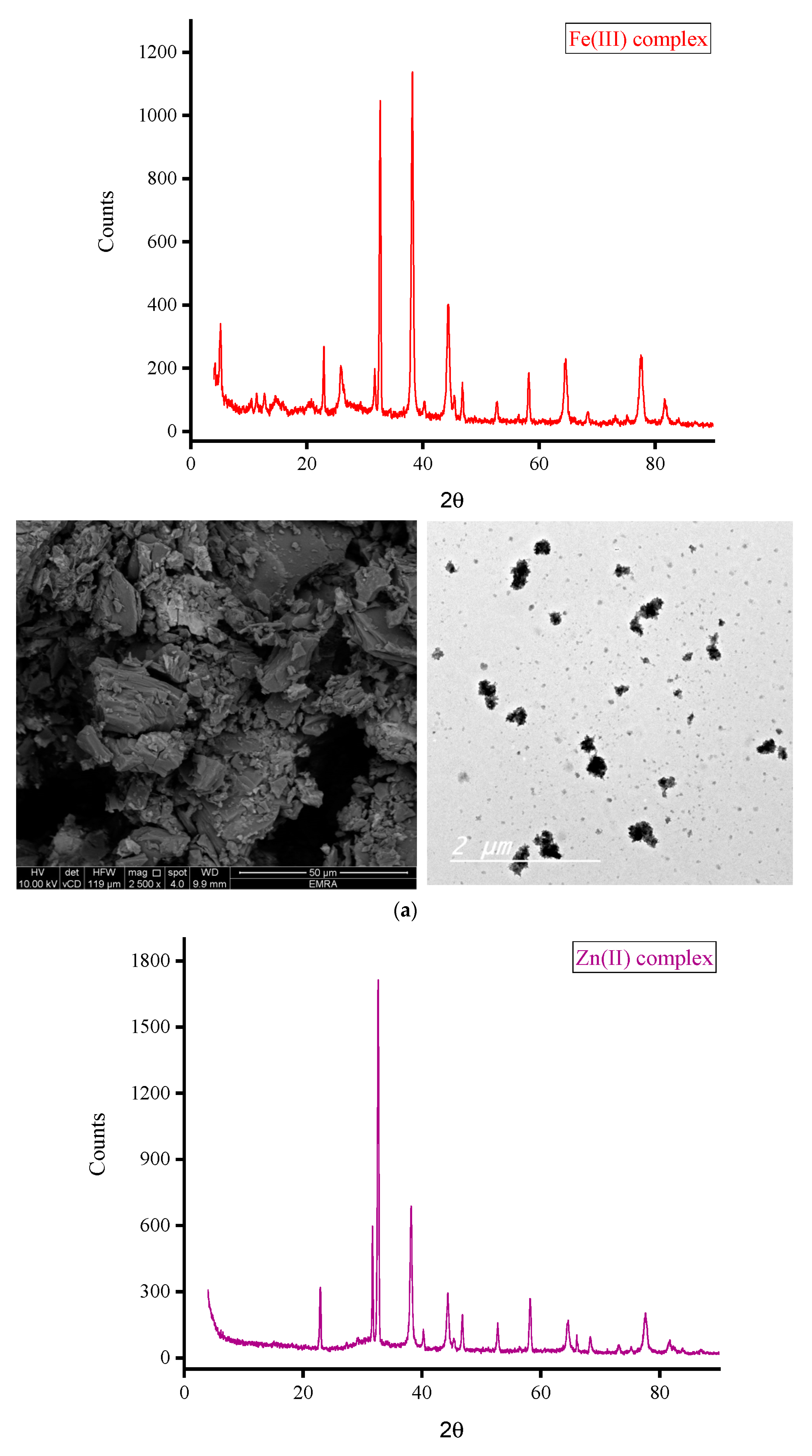
3.2.1. XRD Analysis
- (i)
- XRD patterns
- (ii)
- XRD spectral data
| Bragg’s law: | d = λ/2sin θ |
| Debye–Scherrer’s law: | D = 0.94λ/β cos θ |
3.2.2. Microscopes
3.3. Antimicrobial
3.3.1. Antibacterial Screening
3.3.2. Antifungal Screening
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tella, A.C.; Obaleye, J.A.; Olawale, M.D.; Ngororabanga, J.M.V.; Ogunlaja, A.S.; Bourne, S.A. Synthesis, crystal structure, and density functional theory study of a zinc(II) complex containing terpyridine and pyridine-2,6-dicarboxylic acid ligands: Analysis of the interactions with amoxicillin. Comptes Rendus Chim. 2018, 22, 3–12. [Google Scholar] [CrossRef]
- Eichhorn, G.L.; Marzilli, L.G. Advances in Inorganic Biochemistry Models in Inorganic Chemistry; PTR Prentice-Hall, Inc.: Hoboken, NJ, USA, 1994. [Google Scholar]
- Hughes, M.N. The Inorganic Chemistry of Biological Processes, 2nd ed.; Wiley: Chichester, UK, 1984. [Google Scholar]
- Alessio, E. Bioinorganic Medicinal Chemistry; Wiley-VCH Verlag GmbH and Co. KgaA: Weinheim, Germany, 2011. [Google Scholar]
- Trudu, F.; Amato, F.; Vaňhara, P.; Pivetta, T.; Peña-Méndez, E.; Havel, J. Coordination compounds in cancer: Past, present and perspectives. J. Appl. Biomed. 2015, 13, 79–103. [Google Scholar] [CrossRef]
- Singh, U.; Malla, A.M.; Bhat, I.A.; Ahmad, A.; Bukhari, M.N.; Bhat, S.; Anayutullah, S.; Hashmi, A.A. Synthesis, molecular docking and evaluation of antifungal activity of Ni (II), Co (II) and Cu (II) complexes of porphyrin core macromolecular ligand. Microb. Pathog. 2016, 93, 172. [Google Scholar] [CrossRef]
- Netalkar, P.P.; Netalkar, S.P.; Revankar, V.K. Transition metal complexes of thiosemicarbazone: Synthesis, structures and invitro antimicrobial studies. Polyhedron 2015, 100, 215. [Google Scholar] [CrossRef]
- Ragheb, M.A.; Eldesouki, M.A.; Mohamed, M.S. DNA binding, photo-induced DNA cleavage and cytotoxicity studies of lomefloxacin and its transition metal complexes. Spectrochim. Acta A 2015, 138, 585. [Google Scholar] [CrossRef]
- Khan, T.-M.; Gul, N.S.; Lu, X.; Wei, J.-H.; Liu, Y.-C.; Sun, H.; Liang, H.; Orvig, C.; Chen, Z.-F. In vitro and in vivo anti-tumor activity of two gold(III) complexes with isoquinoline derivatives as ligands. Eur. J. Med. Chem. 2019, 163, 333–343. [Google Scholar] [CrossRef]
- Cao, Q.; Li, Y.; Freisinger, E.; Qin, P.Z.; Sigel, R.K.O.; Mao, Z.-W. G-quadruplex DNA targeted metal complexes acting as potential anticancer drugs. Inorg. Chem. Front. 2017, 4, 10–32. [Google Scholar] [CrossRef]
- Tavares, T.T.; Azevedo, G.C.; Garcia, A.; Carpanez, A.G.; Lewer, P.M.; Paschoal, D.; Müller, B.L.; Santos, H.F.D.; Matos, R.C.; Silva, H.; et al. Gold(I) complexes with aryl-thiosemicarbazones: Molecular modeling; synthesis, cytotoxicity and TrxR inhibition. Ployhedron 2017, 132, 95–104. [Google Scholar] [CrossRef]
- Qin, Q.-P.; Wang, S.-L.; Tan, M.-X.; Liu, Y.-C.; Meng, T.; Zou, B.-Q.; Liang, H. Synthesis of two platinum(II) complexes with 2-methyl-8-quinolinol derivatives as ligands and study of their antitumor activities. Eur. J. Med. Chem. 2019, 161, 334–342. [Google Scholar] [CrossRef]
- Meng, T.; Tang, S.-F.; Qin, Q.-P.; Liang, Y.-L.; Wu, C.-X.; Wang, C.-Y.; Yan, H.-T.; Dong, J.-X.; Liu, Y.-C. Evaluation of the effect of iodine substitution of 8-hydroxyquinoline on its platinum(II) complex: Cytotoxicity, cell apoptosis and telomerase inhibition. Med. Chem. Commun. 2016, 7, 1802–1811. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, G.; Zhang, Z.; Li, F.; Li, J.; Liang, F. Two hydrazone copper(II) complexes: Synthesis; crystal structure; cytotoxicity; action mechanism. RSC Adv. 2016, 6, 36077–36084. [Google Scholar] [CrossRef]
- Fricker, S.P. Metal based drugs: From serendipity to design. Dalton Trans. 2007, 43, 4903. [Google Scholar] [CrossRef]
- Jurowska, A.; Jurowski, K.; Szklarzewicz, J.; Buszewski, B.; Kalenik, T.; Piekoszewski, W. Molybdenum Metallopharmaceuticals Candidate Compounds-The “Renaissance” of Molybdenum Metallodrugs? Cur. Med. Chem. 2016, 23, 3322. [Google Scholar] [CrossRef]
- Efthimiadou, E.K.; Thomadaki, H.; Sanakis, Y.; Raptopoulou, C.P.; Katsaros, N.; Scorilas, A.; Karaliota, A.; Psomas, G.J. Structure and biological properties of the copper (II) complex with the quinolone antibacterial drug N-propyl-norfloxacin and 2, 2′-bipyridine. Inorg. Biochem. 2007, 101, 64. [Google Scholar] [CrossRef] [PubMed]
- Vieira, L.M.M.; de Almeida, M.V.; Lourenço, M.C.S.; Bezerra, F.A.F.M.; Fontes, A.P.S. Synthesis and antitubercular activity of palladium and platinum complexes with fluoroquinolones. Eur. J. Med. Chem. 2009, 44, 4107–4111. [Google Scholar] [CrossRef] [PubMed]
- Bauer, A.W.; Kirby, W.M.; Sherris, C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Biemer, J.J. Antimicrobial susceptibility testing by the Kirby-Bauer disc diffusion method. Ann. Clin. Lab. Sci. 1973, 3, 135–140. [Google Scholar]
- Serrano, M.C.; Ramírez, M.; Morilla, D.; Valverde, A.; Chávez, M.; Espinel-Ingroff, A.; Claro, R.; Fernández, A.; Almeida, C.; Martín-Mazuelos, E. A comparative study of the disc diffusion method with the broth microdilution and Etest methods for voriconazole susceptibility testing of Aspergillus spp. J. Antimicrob. Chemo-Ther. 2004, 53, 739–742. [Google Scholar] [CrossRef]
- Neugebauer, U.; Szeghalmi, A.; Schmitt, M.; Kiefer, W.; Popp, J.; Holzgrabe, U. Vibrational spectroscopic characterization of fluoroquinolones. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2005, 61, 1505–1517. [Google Scholar] [CrossRef]
- Allan, J.R.; Baird, N.D.; Kassyk, A.L. Some first row transition metal complexes of nicotinamide and nicotinic acid. J. Therm. Anal. 1979, 16, 79–90. [Google Scholar] [CrossRef]
- Öztirk, Ö.F.; Şekerci, M.; Özdemir, E. Preparation of complexes of 1-amino-6,7-O-cyclohexylidene-4-azaheptane with transition metal acetates. Russ. J. Gen. Chem. 2006, 76, 33–36. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared Spectra of Inorganic and Coordination Compounds, 2nd ed.; Wiley Interscience, John Wiley & Sons: New York, NY, USA, 1970. [Google Scholar]
- El-Megharbel, S.M.; Hegab, M.S.; Manaaa, E.A.; Al-Humaidif, J.Y.; Refat, M.S. Synthesis and physicochemical characterizations of coordination between palladium(ii) metal ions with floroquinolone drugs as medicinal model against cancer cells: Novel metallopharmaceuticals. New J. Chem. 2018, 42, 9709–9719. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hussien, M.A.; Refat, M.S. In-Situ Copper(II) Complexes of Some Quinolone Drug Ligands Were Discussed for Their Molecular Structures: Synthesis in Binary Solvent. J. Comput. Theoret. Nanosc. 2017, 14, 561–576. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Pearson Education Limited: London, UK, 2014. [Google Scholar]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Material; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Estermann, M.A.; David, W.I.F. Structure Determination from Powder Diffraction Data (SDPD); David, W.I.F., Shankland, K., Mccusker, I.B., Baerlocher, C., Eds.; Oxford Science Publications: New York, NY, USA, 2002. [Google Scholar]


| Microbe | Abbreviation |
|---|---|
| (A) Gram-negative bacterial species | |
| Pseudomonas aeruginosa | P. aeruginosa |
| Escherichia coli | E. coli |
| (B) Gram-positive bacterial species | |
| Staphylococcus aureus | S. aureus |
| Streptococcus pneumoniae | S. pneumoniae |
| Bacillus subtilis | B. subtilis |
| (C) Fungal species | |
| Candida albicans | C. albicans |
| Penicillium sp. | - |
| Aspergillus niger | A. niger |
| Complex OA- | Elemental Results (%) Found (Calculated) | |||||
|---|---|---|---|---|---|---|
| Carbon | Hydrogen | Nitrogen | Chlorine | Water | Metal | |
| -Fe(III) | 33.78 (34.00) | 4.16 (3.92) | 3.21 (3.05) | 15.30 (15.45) | 15.48 (15.69) | 12.05 (12.17) |
| -Zn(II) | 37.70 (37.58) | 3.77 (3.85) | 3.26 (3.37) | 8.73 (8.54) | 13.23 (13.01) | 15.60 (15.75) |
| -Ca(II) | 44.22 (44.10) | 3.30 (3.39) | 4.07 (3.96) | 10.26 (10.02) | 5.21 (5.09) | 11.52 (11.33) |
| -Mg(II) | 45.98 (46.16) | 3.72 (3.55) | 4.30 (4.14) | 10.75 (10.49) | 5.26 (5.33) | 9.57 (7.19) |
| Complex | 2θ; (°) | θ; (°) | d-Spacing Value; (Å) | β; FWHM (rad.) | Particle Size (nm) |
|---|---|---|---|---|---|
| Fe(III) complex | 38.105 | 19.053 | 2.35966 | 0.00698 | 21.95 |
| Zn(II) complex | 32.544 | 16.272 | 2.73156 | 0.00576 | 26.19 |
| Ca(II) complex | 32.667 | 16.334 | 2.73899 | 0.00567 | 26.76 |
| Mg(II) complex | 6.977 | 3.489 | 12.6580 | 0.00524 | 27.69 |
| Sample | Gram-Negative Bacterial Species | Gram-Positive Bacterial Species | |||
|---|---|---|---|---|---|
| P. aeruginosa | E. coli | S. aureus | S. pneumoniae | B. subtilis | |
| DMSO (−control) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Streptomycin (+control) | 27.0 | 22.0 | 20.0 | 17.0 | 18.0 |
| Fe(III) complex | 12.0 | 11.0 | 9.0 | 3.0 | 4.0 |
| Zn(II) complex | 23.0 | 20.0 | 18.0 | 15.0 | 19.0 |
| Ca(II) complex | 28.0 | 21.0 | 22.0 | 18.0 | 20.0 |
| Mg(II) complex | 12.0 | 14.0 | 11.0 | 9.0 | 3.0 |
| Sample | Fungal Strains | ||
|---|---|---|---|
| C. albicans | Penicillium sp. | A. niger | |
| DMSO (−control) | 0.0 | 0.0 | 0.0 |
| Ketoconazole (+control) | 21.0 | 21.0 | 18.0 |
| Fe(III) complex | 12.0 | 8.0 | 8.0 |
| Zn(II) complex | 22.0 | 15.0 | 17.0 |
| Ca(II) complex | 23.0 | 20.0 | 20.0 |
| Mg(II) complex | 12.0 | 11.0 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almehizia, A.A.; Al-Omar, M.A.; Naglah, A.M.; Bhat, M.A.; Alanazi, F.S.; Alotaibi, F.A.; Refat, M.S.; Adam, A.M.A. Complexes of the Antibiotic Drug Oxolinic Acid with Fe(III), Zn(II), Ca(II), and Mg(II) Ions: Preparation, Characterization, and In Vitro Evaluation of Biological Activity. Crystals 2023, 13, 1012. https://doi.org/10.3390/cryst13071012
Almehizia AA, Al-Omar MA, Naglah AM, Bhat MA, Alanazi FS, Alotaibi FA, Refat MS, Adam AMA. Complexes of the Antibiotic Drug Oxolinic Acid with Fe(III), Zn(II), Ca(II), and Mg(II) Ions: Preparation, Characterization, and In Vitro Evaluation of Biological Activity. Crystals. 2023; 13(7):1012. https://doi.org/10.3390/cryst13071012
Chicago/Turabian StyleAlmehizia, Abdulrahman A., Mohamed A. Al-Omar, Ahmed M. Naglah, Mashooq A. Bhat, Fhdah S. Alanazi, Fatimah A. Alotaibi, Moamen S. Refat, and Abdel Majid A. Adam. 2023. "Complexes of the Antibiotic Drug Oxolinic Acid with Fe(III), Zn(II), Ca(II), and Mg(II) Ions: Preparation, Characterization, and In Vitro Evaluation of Biological Activity" Crystals 13, no. 7: 1012. https://doi.org/10.3390/cryst13071012
APA StyleAlmehizia, A. A., Al-Omar, M. A., Naglah, A. M., Bhat, M. A., Alanazi, F. S., Alotaibi, F. A., Refat, M. S., & Adam, A. M. A. (2023). Complexes of the Antibiotic Drug Oxolinic Acid with Fe(III), Zn(II), Ca(II), and Mg(II) Ions: Preparation, Characterization, and In Vitro Evaluation of Biological Activity. Crystals, 13(7), 1012. https://doi.org/10.3390/cryst13071012










