Abstract
Context and objective: A novel method of fabricating probiotic nanowhiskers—using pure cheese as a source of probiotics, sans metal/chemical surfactants—is reported in the present study. Materials and methods: This was followed by an extensive characterization; FTIR spectroscopy, X-ray diffraction, particle size measurements, and transmission electron microscopy. Thermal analysis via differential scanning calorimetry (DSC) and n screening of the volatile compounds via gas chromatography/mass spectroscopy (GC-MS) was used to assess the purity of the nano-crystalline whiskers. Additionally, the anti-oxidant status and the metal-chelating effect of the nanowhiskers was evaluated in Wistar rats exposed to cadmium chloride hydrate (70 ppm) for 35 days. Group I was the positive control and groups II and III were exposed to Cd, with group III being treated with the cheese nanowhiskers (100 mL/L) in drinking water. Results: The nanoparticles were 112 nm in size (PDI 0.484) with the illustrated whisker/elongated shape being crystalline in nature. Lipid peroxidation was significantly enhanced followed by a marked bioaccumulation of Cd in the target organs. Discussion: Co-treatment with cheese nanowhiskers led to a marked reversal in the Cd-induced modulations in the endpoints evaluated. Conclusions: It is suggested that a dietary intervention in the form of a nano-probiotic supplement such as cheese is a prospective remedy for heavy metal toxicity/oxidative damage, being safe and efficacious.
1. Introduction
Cadmium (Cd), a toxic heavy metal, is a naturally occurring environmental pollutant that mainly emanates from agricultural and industrial sources [1]. Humans are exposed to Cd either via the environment or occupational exposure. Exposure to cadmium in humans is a global health concern. Chronic adverse effects of Cd exposure include the physiological dysfunction of various systems. This heavy metal is also recognized for its carcinogenicity in humans [2]. Bioaccumulation of Cd is well recognized and is taken up into the GI tract via several membrane transporters including MT (metallothionein) [3].
Lech and Sadlik [4] reported that in a decade-long examination of different tissues in a non-poisoned population, the highest levels of the metal were found in the kidneys and liver, as they are the two target organs where it is primarily distributed [2]. Animal studies in the past have also demonstrated that chronic exposure to Cd causes disarray in the distribution of essential bio-elements such as Zn and Cu. This subsequently leads to retention of these elements in the liver and kidneys and a parallel reduction in their levels in the serum [3]. Numerous strategies have been proposed recently to alleviate heavy metal contamination; however, most of them are expensive or are not environmentally sound. Conventionally, chelating agents have been used as a treatment for heavy metal intoxication including Cd toxicity. Chelators are usually administered intraperitoneally or subcutaneously, which can subsequently have deleterious side effects on organs. There have been several studies on the use of phytochemicals as metal chelators, which are a safer alternative means to counteract Cd intoxication [2,5,6,7,8]. Thus, it is imperative to look for effective dietary interventions against cadmium toxicity. The major advantage of a dietary strategy in chelation therapy is that the nutritional constituents can be affordably included in the daily diet, and are bioavailable and safe. At the current point, post COVID-19, the consumption of dairy and plant-based foods has been encouraged and supported by scientific research. This is due to their beneficial effects on the inflammatory and oxidative state, which contributes to the maintenance of general health and to alleviating pathological conditions [9]. In addition, ‘functional foods’ have gained traction as a therapeutic approach to combatting ailments and disease. Probiotics are a class of such functional foods which have garnered attention in recent times [2,10]. Probiotics are best defined as living, non-pathogenic microorganisms that enhance the host the gut flora and confer health benefits. Microbial spp. referred to as probiotics include species of Bifidobacterium and Lactobacilli, along with yeast Saccharomyces [11]. Previous studies have reported the curative effects of probiotics against Cd toxicity and the associated oxidative stress and bioaccumulation [9,12]. Cheeses are a good source of probiotic bacteria, primarily members of the genera Lactobacillus and Bifidobacterium. Despite the extended maturation/ripening process and shelf life of cheese, probiotic counts in cheese appear to be appreciable and stable for months [9]. In addition, recently, probiotic properties have been observed in bacteria belonging to the genera, Propionibacteriumas and Enterococcus, present in in cheeses [13]. Cheese is an ideal delivery system for viable probiotics, attributed to its higher pH, larger buffering ability, and firm consistency. In addition, the probiotic microbes in cheese provide conferred protection during storage and passage through the peptic system due to the high fat content in cheese [13]. Furthermore, amongst the tested dairy foods in a study by Basilicata et al. [14], buffalo ricotta cheese digest demonstrated a significant inhibition of reactive-oxygen-species (ROS)-release in peroxide-treated intestinal epithelial cells. Thus, the peptides in buffalo ricotta cheese could be potential functional foods that could attenuate the commencement of certain gastrointestinal diseases. Thus, the development of innovative formulations/products of probiotic and prebiotic foods is imperative to enable effective probiotic delivery to the biological system.
Nanotechnology is a futuristic science which has the potential to revolutionize the food industry with novel approaches to enhancing the quality, bioavailability, and safety of food coupled with improved stability, as nano formulations limit the degradation of the active bio-constituents in the biological milieu. Previous literature has reported on the use of probiotics in nano systems which have been effectively used as therapy against oxidative stress, inflammation, and other aspects of metal toxicity [9,15]. Hence, the development of a novel dietary nano formulation has been promoted as an effective nutritional therapeutic strategy.
On this premise, the present study reports a novel fabrication of probiotic nanowhiskers using cheese powder and an evaluation of its antioxidant potential against cadmium-induced toxicity in a rat model.
2. Materials and Methods
2.1. Synthesis of Nanowhiskers
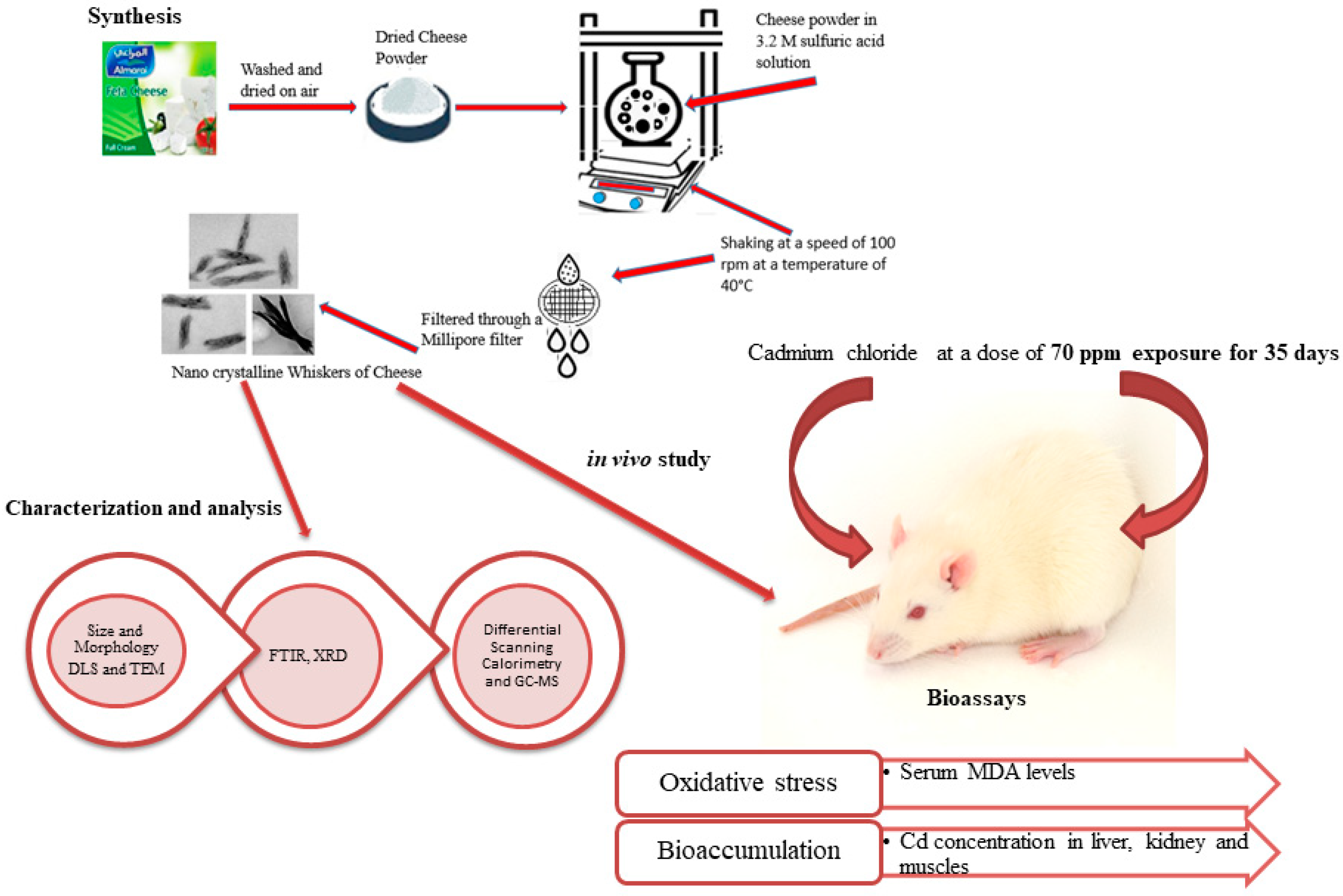
The nanowhiskers were synthesized using feta cheese from a local supermarket in Riyadh City, Saudi Arabia, as described by Virk et al. [16] (Figure 1).
Figure 1.
Schematic illustration of the experimental design.
2.2. Characterization of Nanowhiskers
- (i)
- The size and morphology of nanoparticles was assessed using a Zeta sizer (ZEN 3600, MALVERN, United Kingdom) and transmission electron microscopy (TEM, JEM-1400, JEOL, Tokyo, Japan).
- (ii)
- The constituent functional groups in the samples were mapped using Fourier transform infrared (FTIR) spectroscopy (Perkin-Elmer FTIR spectrum BX, Waltham, MA, USA).
The spectra were recorded in the range from 4400 to 400 cm−1.
- (iii)
- Diffraction patterns were recorded using a PAN Analytical XPert PRO (Amsterdam, The Netherlands) operated at 40 mA and 45 kV using CuK radiation (0.15406 nm).
The crystallographic information was recorded in a range from 0° to 100°.
- (iv)
- The differential scanning calorimetry (DSC) curves for both bulk cheese and nanowhiskers were obtained using a differential scanning calorimeter (DSC-4000 Perkin Elmer, Waltham, MA, USA) equipment.
- (v)
- The constituent volatile compounds in the bulk cheese and the nanowhiskers were analyzed by gas chromatography-mass spectrometry. The sample was injected into a gas chromatograph with a mass detector (GC-MS; Agilent 6890 Series GC system with Agilent 5973 mass selective detector; Agilent, Santa Clara, CA, USA). The injector temperature was 250 °C, and the desorption time was 10 min.
Volatile aromatic compounds were separated on a Dbwax column (60 m × 0.32 mm i.d. × 0.5-μm film thickness). The initial oven temperature was at 32 °C for 4 min followed by a programmed increase in phases (32 °C to 80 °C at 3 °C per minute, 80 °C to 200 °C at 4 °C per minute and finally held for 5 min at 200 °C). Helium served as the carrier gas over the whole 17-min run, at a 1 mL/min flow rate.
2.3. Chemicals and Techniques for Bioassays
For the exposure study, cadmium salt—cadmium chloride hemimonohydrate (Cd Cl2 .1/2 H2O)—was procured from Techno Pharmchem, Haryana (Bahadurgarh, India). Serum MDA levels were measured using high-performance liquid chromatography (HPLC) following a standard method according to Al-Enazi et al. [8]. Samples of liver, kidneys, and muscle were mixed with 65% HNO3 and 30% H2O2 in a microwave digester (Milestone, Italy) and Cd concentration was assessed using an atomic absorption spectrophotometer [8]. The final concentration of Cd (ng/g) was computed using a calibration curve with the standards.
2.4. Experimental Design
The exposure study was approved by the local animal ethics committee (IRB number: PSAU−2018-Para-830/PI). Adult male Wistar rats weighing 150 ± 10 g, were procured from the Animal House Facility at King Saud University, Riyadh. After acclimation to the laboratory conditions (22 ± 2 °C and 12 h light/dark cycle) for a week, rats were divided into three experimental groups consisting of 6 rats. Commercial feed was given to the rats with ad libitum tap water.
Rats were given the following required doses via oral gavage. Group I, the control group, received normal saline. Group II received 70 ppm of CdCl2 in saline [17]. Group III was administered CdCl2 (70 ppm) and cheese nanoparticles (1 mL). After an exposure period of five weeks, samples of blood were collected from all rats to prepare serum for the determination of MDA levels. Samples of liver, kidney and muscle tissues were excised out after dissection to determine the cadmium concentration. Until further analysis, all samples of serum and tissues were stored at −80 °C.
3. Results and Discussion
3.1. Characterization of Nanowhiskers
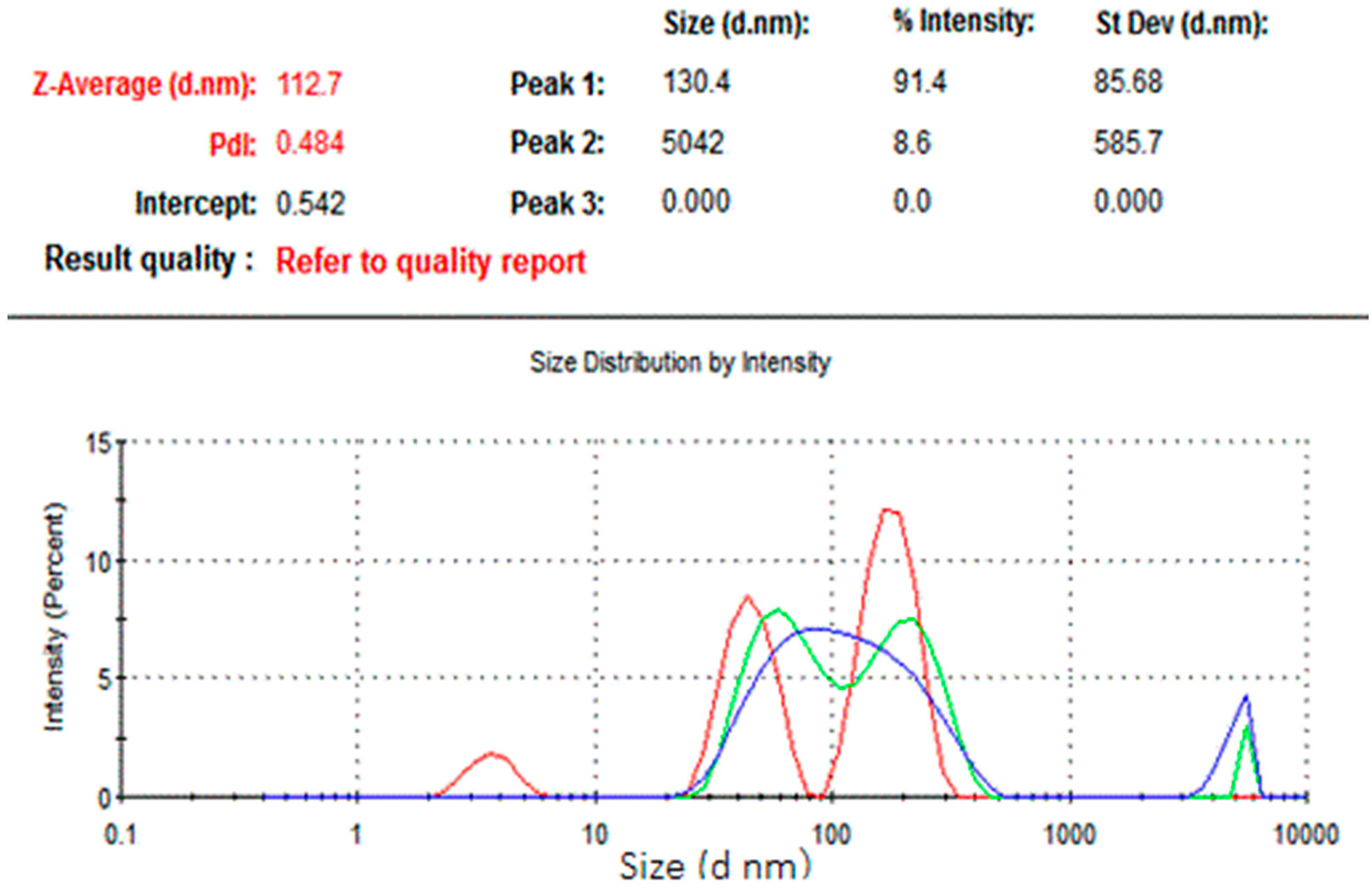
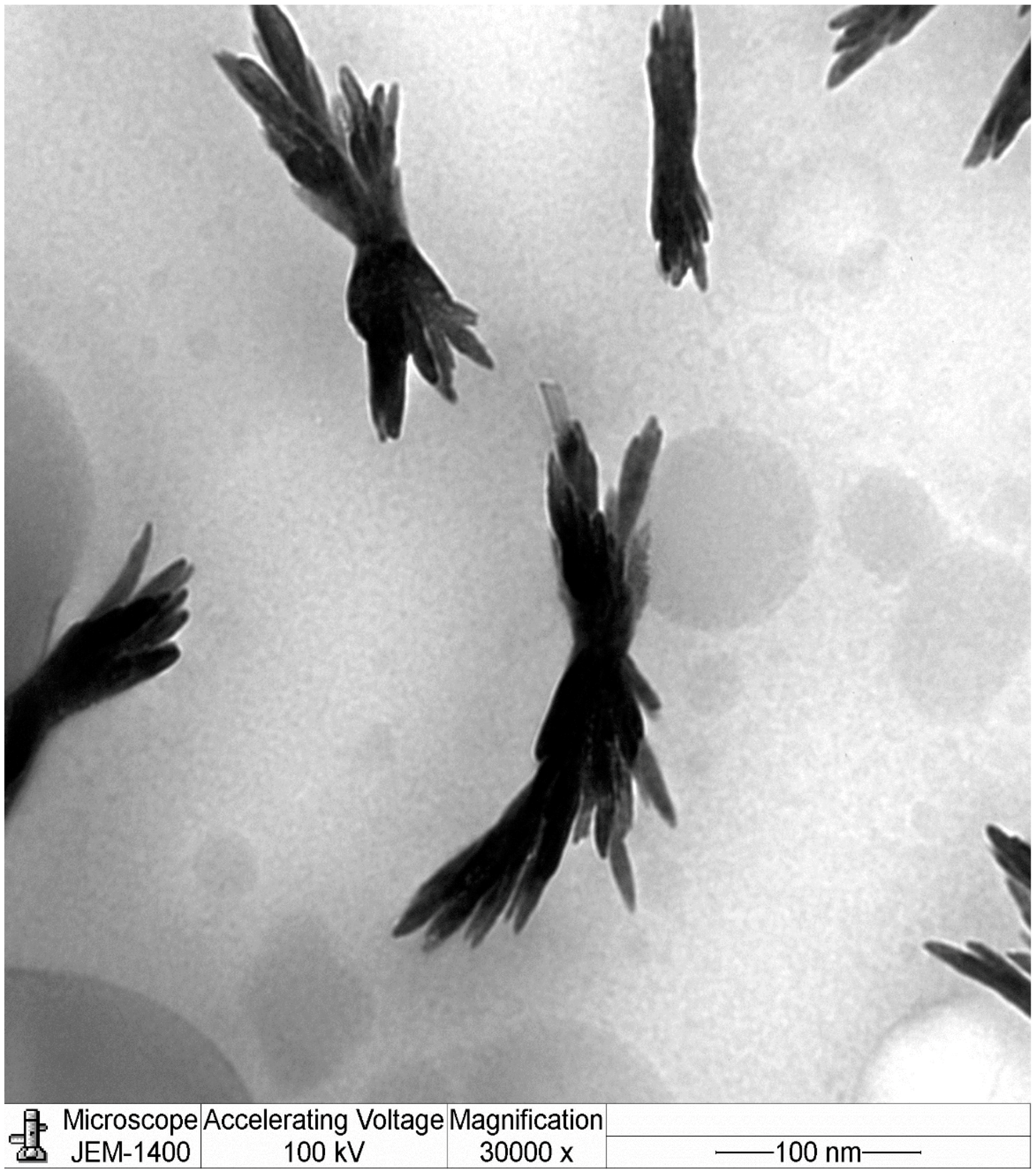
The results of dynamic light scattering (DLS) analysis showed the average size of the NCh (cheese nanowhiskers) to be 112.7 nm (Figure 2), larger than the nanoscale which was acquired from TEM images, as the DLS measures the “hydrodynamic diameter” [18]. Accordingly, the actual diameter of these particles could possibly be smaller than this. In addition, the polydispersity index was 0.484, which indicates that the particles had no homogeneity in the formulation. Figure 3 shows the TEM micrograph of the as-synthesized NCh. The synthesized nanoparticles demonstrated individually well-dispersed, whisker-like/elongated, rod-shaped morphotypes. It was notable that synthesized particles were observed as bundles of closely packed, rod-shaped particles. This was possibly due to the nano-aggregation during formation of nanowhiskers.
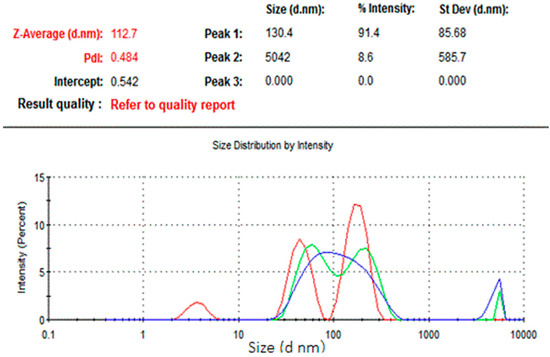
Figure 2.
Size distribution of the nanoparticles.
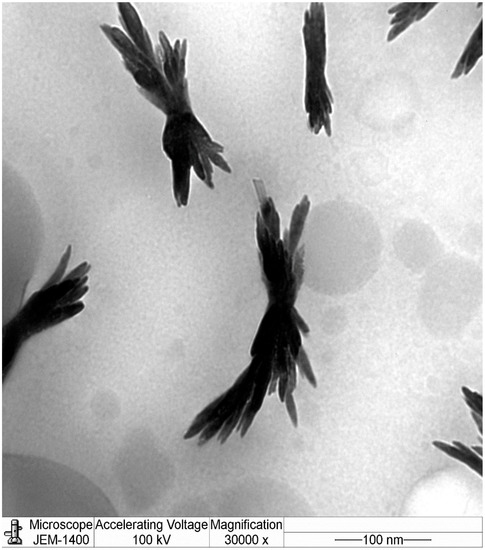
Figure 3.
Electron micrographs of the nanowhiskers of cheese (NCh).
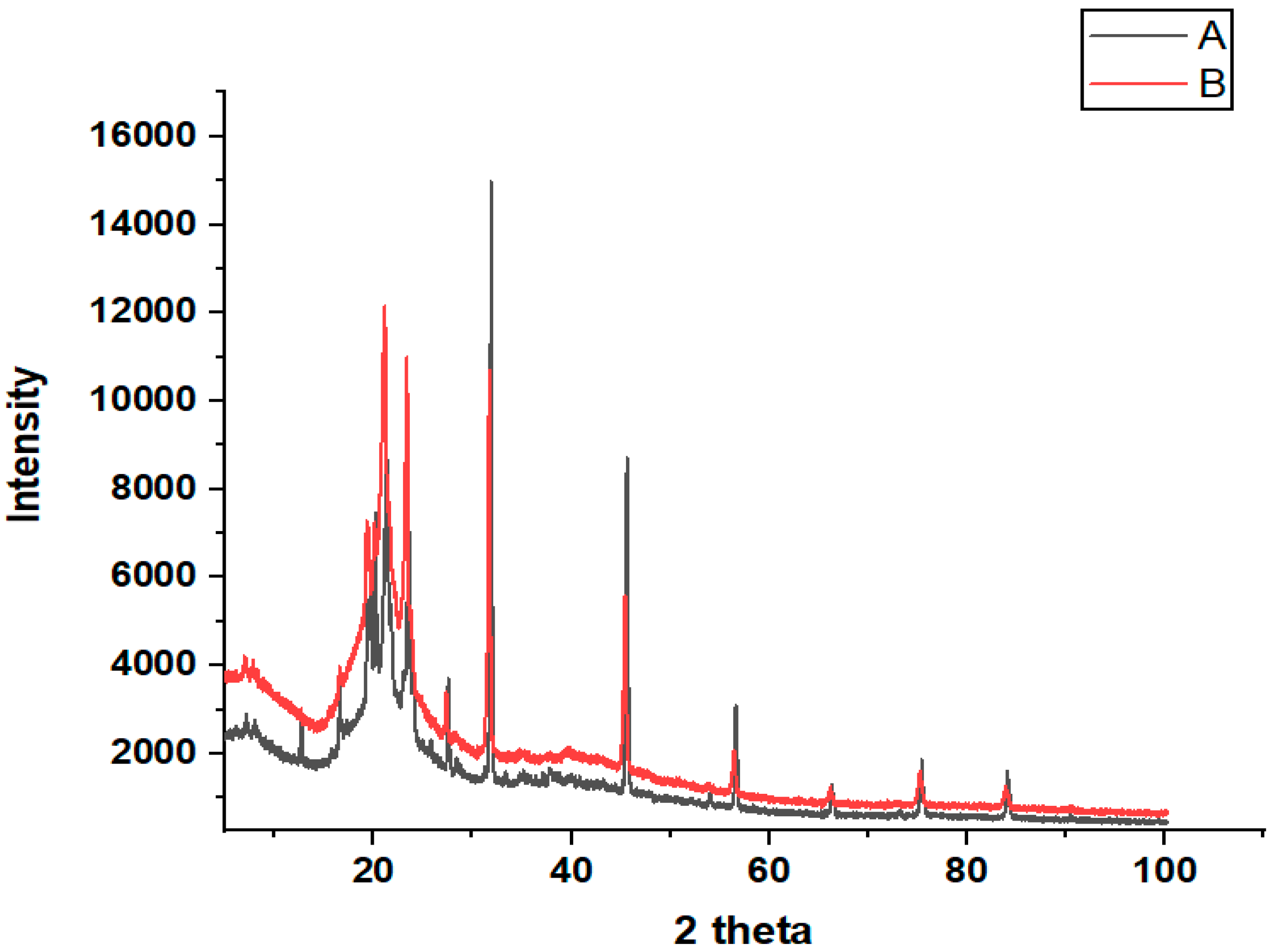
XRD analysis was carried out to determine the crystal structure and phase composition of the prepared sample. The XRD diffractograms of the bulk cheese (Ch) (A) and nanowhiskers of cheese (NCh) (B) are illustrated in Figure 4. Two-dimensional X-ray diffraction peaks at 21.09°, 23.29, and 45.62°, as well as the highest peak at 31.85° correspond to the main crystalline phase in these samples, which represent and confirm the presence of calcium hydroxyapatite, which is the glue holding the protein mesh of caseins and fats together in cheese [19]. The diffraction line of NCh at 20 theta has a higher density than bulk cheese (Ch). On other hand, no shift in the XRD peaks and patterns was observed between the NCh and bulk cheese throughout the angular range (10–100°), indicating that the as-synthesized nanowhiskers were in the pure crystalline phase. Furthermore, the observed diffraction peaks illustrate the crystalline nature of the nanowhiskers in the size range, while the broadened peaks are associated with the nano-scaling. However, the predominance of the peaks indicate a unidirectional reorientation of the nanoparticle grains as opposed to the random orientation of grains in the bulk state [20].
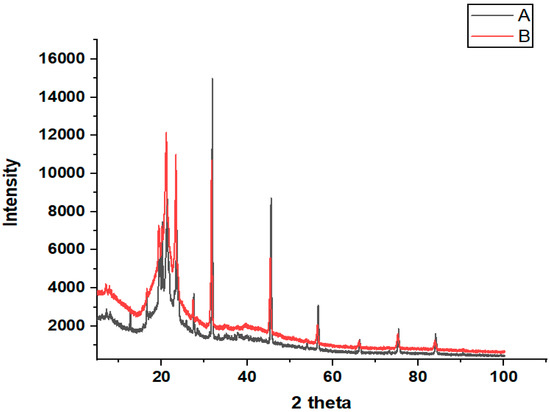
Figure 4.
X-ray diffractograms of (A) bulk cheese (Ch) and (B) nanowhiskers (NCh) of cheese.
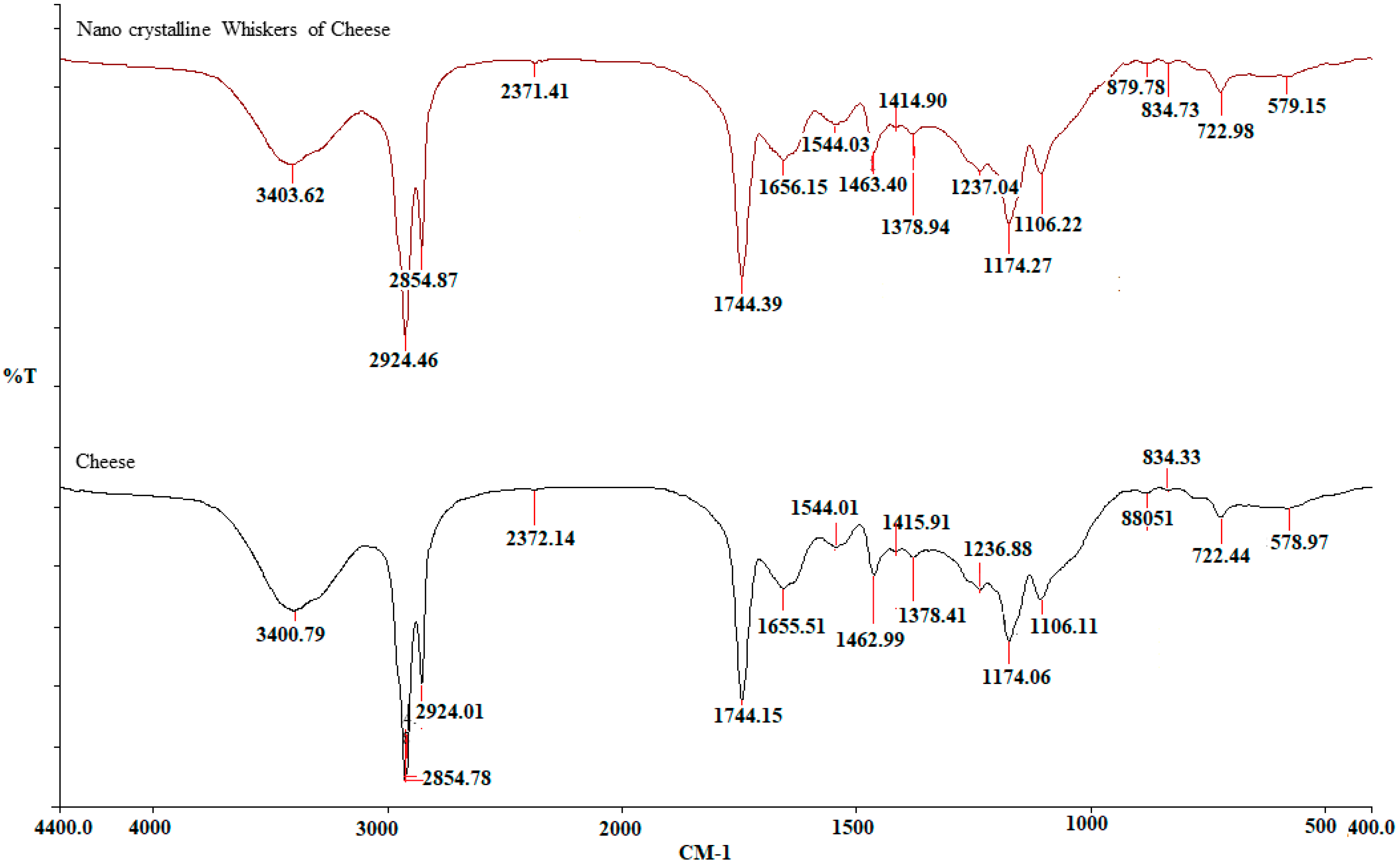
The (FT-IR) spectra of cheese nanoparticles and bulk cheese are depicted in Figure (Figure 5; Table 1). The comparison of the spectra of Ch and ChNPs reveals that there are no differences in the positions and magnitudes of the absorption bands between the two samples. The FT-IR spectra map the constituent biomolecules in the samples. Sakkas et al. [21] reported that the milk component of cheese contains phospholipids. The basic structure of the phospholipid molecule includes glycerol bonded to two fatty acids and one phosphate group via ester linkages. The peaks at 1700–1600 cm−1 could be ascribed to the carbonyl group and/or carboxylic acids, or esters of fatty acids or peptides. The peak corresponding to 1400–1500 cm−1 is assigned to proteins, and/or peptides, acidic amino acids/amines, and/or lipids. The peaks at 1106.11 cm−1 and 1106.22 cm−1 correspond with the P-O-C asymmetric stretching [22,23,24].
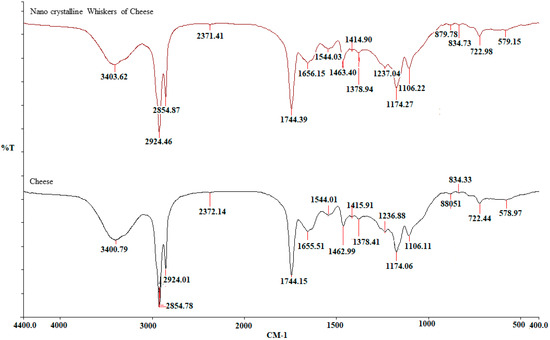
Figure 5.
Fourier transform infrared spectroscopy spectra of bulk cheese and nanowhiskers of cheese.

Table 1.
Major peaks in the FTIR spectra of both Ch and ChNPs.
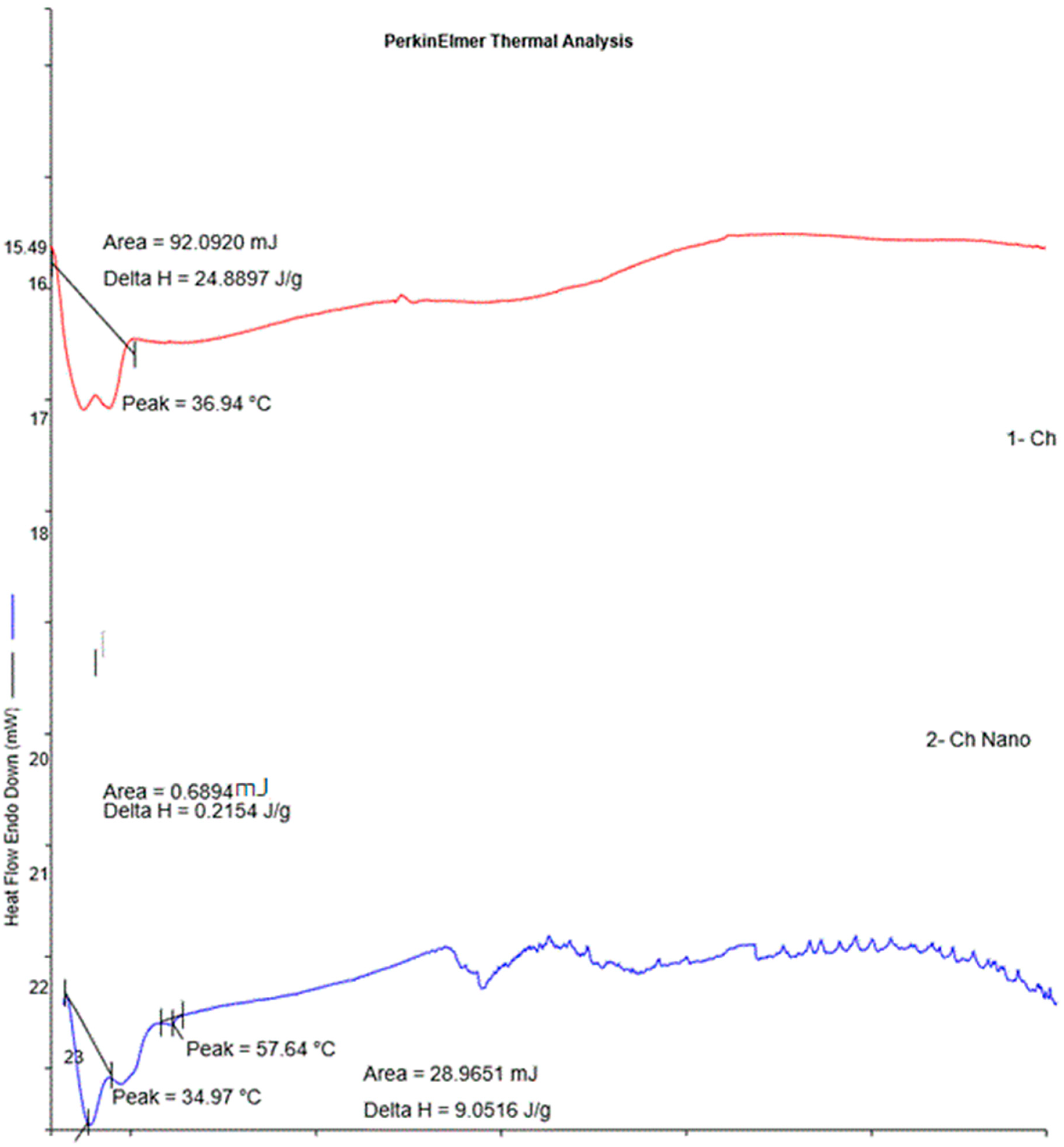
DSC is a thermo-analytical technique used to observe the process of crystallization. DSC thermograms of the bulk cheese and the nanowhiskers showed interaction between the components of the cheese and slight changes during nano-formulation (Figure 6). The DSC of bulk cheese exhibited an endothermic peak at 36.94 °C and the nanowhiskers demonstrated endothermic peaks at about 34.97 °C and 57.64 °C, ascribed to the evaporation of absorbed water. It has been reported that endothermic peaks are correlated with a loss of water through evaporation associated with hydrophilic groups, while exothermic peaks illustrate degradation based on dehydration, depolymerization reactions, and chain scission [25]. There were many exothermic peaks observed in a noisy pattern in the thermograms of the nanowhiskers after 150 °C. This could be attributed to the onset of new chemical interactions on nanonization, leading to crystallization [25,26].
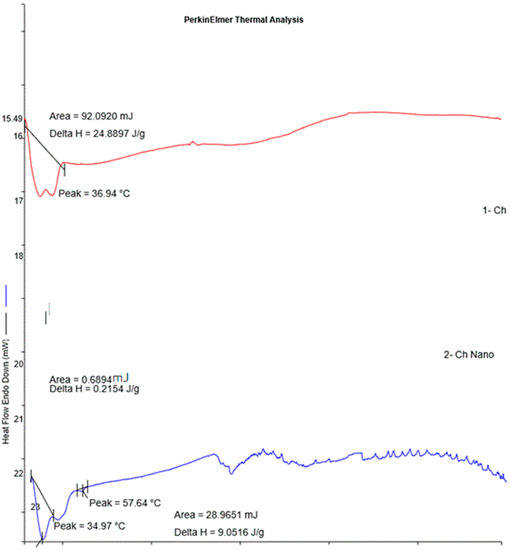
Figure 6.
DSC thermograms of Ch and NCh.
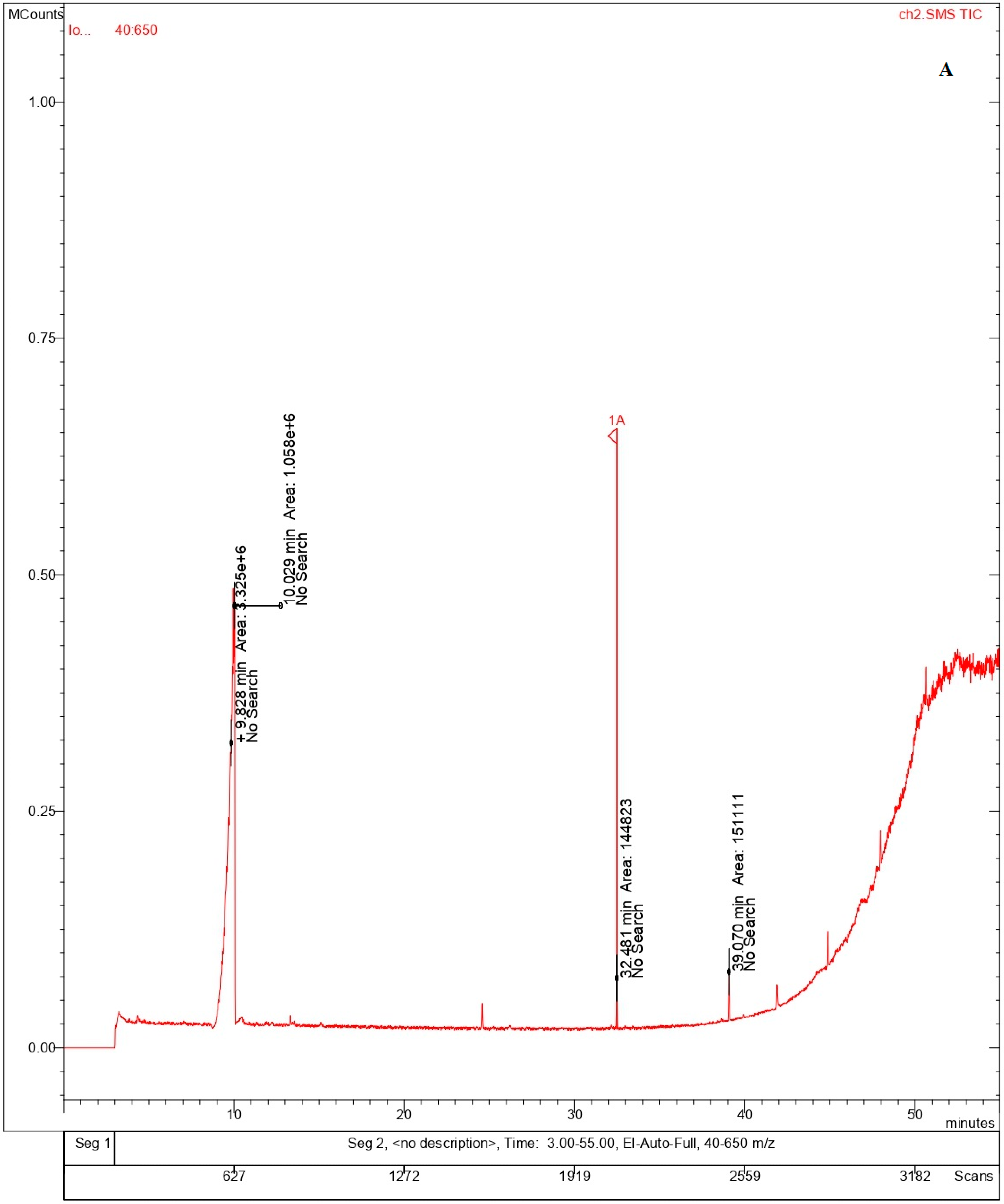
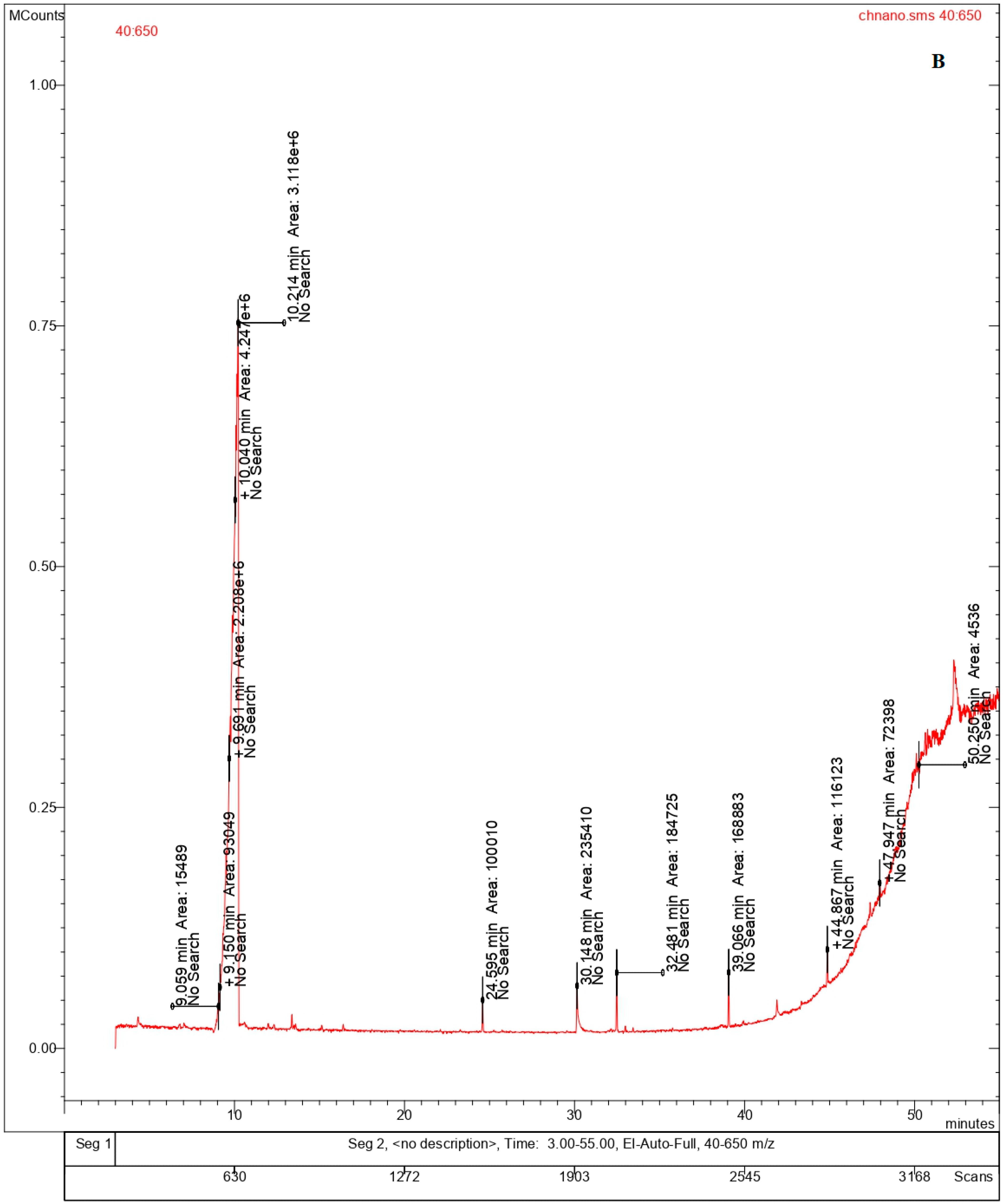
Volatile organic compounds (VOC) are important constituents of different variety of cheeses, as they contribute to the sensory characteristics of the cheeses based on its ripening process. The volatile compounds identified in the bulk cheese and nano whisker samples consisted primarily of alcohols and alkaloids, as illustrated by the GC-MS chromatograms (Table 2, Figure 7A,B) [27]. Similar compounds were detected in both the Ch and ChN samples, ascribed to different retention times. The fatty alcohols contribute to the organoleptic properties of the cheese, while the natural alkaloids are known for their antioxidant, antibacterial, and antifungal properties [28,29] that enhances the quality of the cheese. The composition of fatty acids in milk and cheese varies by geography and management practices. It has been reported that microbes alter the appearance and aroma of different cheeses during the ripening process, via proteolysis and lipolysis, resulting in the production of free amino acids (FAAs) and free fatty acids (FFAs). The microbial metabolism of FFAs results in the formation of varied compounds, including esters, alcohols, ketones, thioesters, etc., that contribute to the aroma of the cheese [30,31]. This is in line with the volatile compounds reported in the present study, which were mainly fatty alcohols (n-tridecanol-1-ol, undecanol, and pentadecanol) derived from fatty acids. The natural alkaloid, rescinnamine, reported both in the bulk and nanowhiskers, comes from the Rauwolfia serpentina plant, which is known for its constituent alkaloids, exhibiting a wide range of pharmacological benefits [32].

Table 2.
Volatile content in analyzed bulk cheese and nanowhiskers.
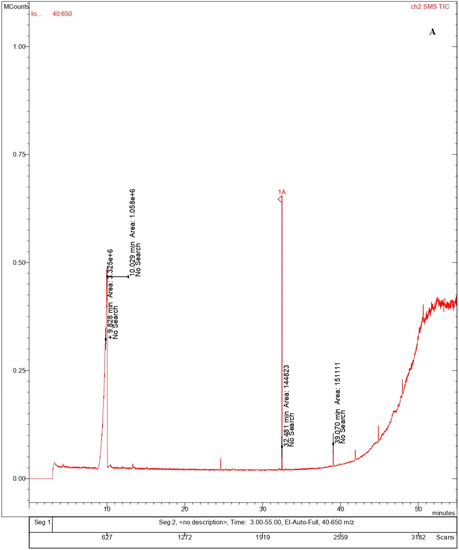
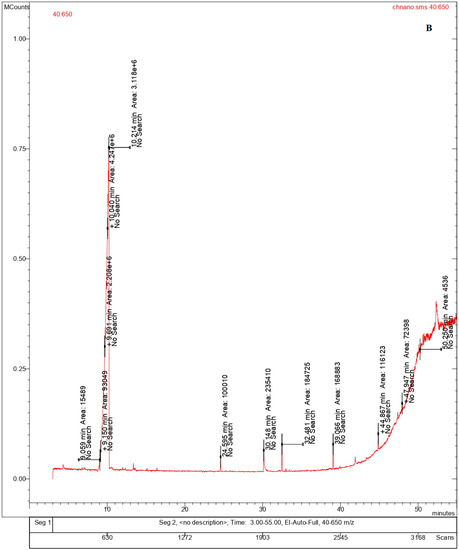
Figure 7.
Chromatograms showing the volatile components of (A) bulk cheese and (B) nanowhiskers via GC-MS.
3.2. Bioassays
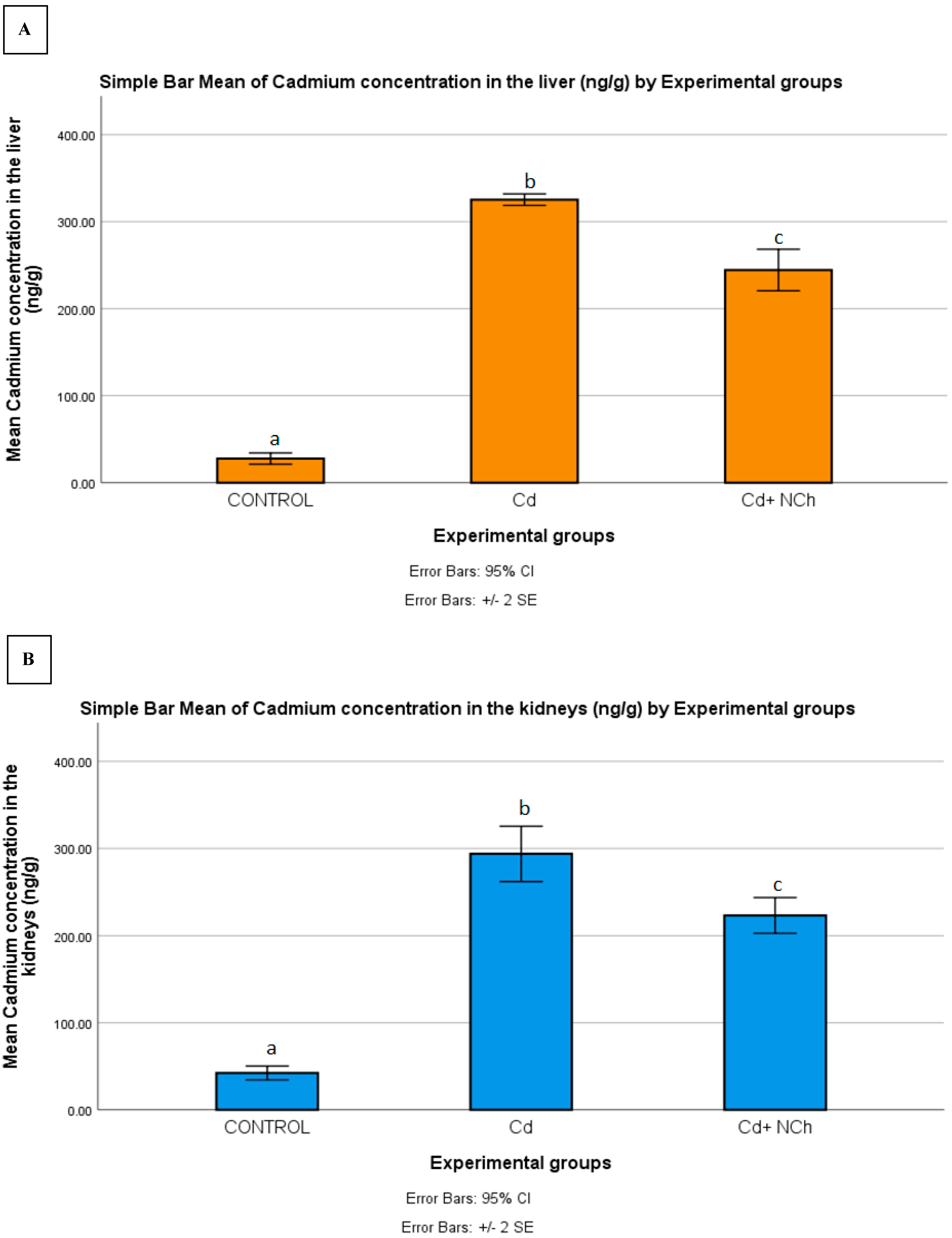
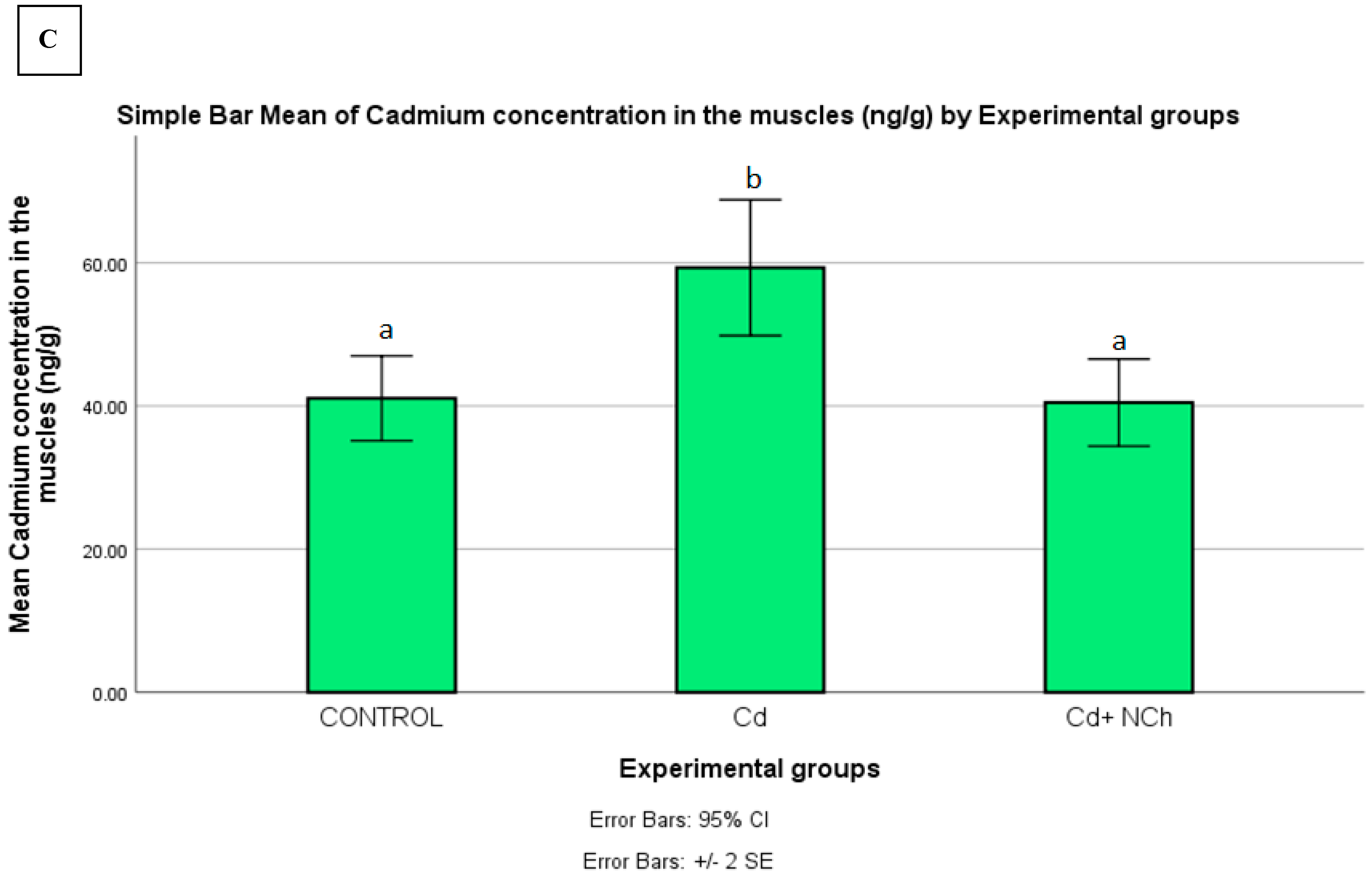
Serum MDA levels increased significantly (p ≤ 0.05) in the rats exposed to Cd (1.50 ± 0.19 nmol/mL) in comparison to the control group (0.80 ± 0.02 nmol/mL). Co-administration of NCh (0.95± 0.06 nmol/mL) significantly (p ≤ 0.05) reduced the MDA levels (Table 3). The enhanced levels of serum MDA in the present study indicated lipid peroxidation, a commonly used biomarker of oxidative stress. To date, several mechanisms for the mode of cadmium toxicity have been postulated, including a disarray in pro-oxidant–antioxidant balance which elicits oxidative stress. The oxidative damage via the overproduction of free radicals during cadmium metabolism in the body is recognized as a major factor in cadmium toxicity [2,33]. Additionally, the significant increase in MDA levels was coupled with the enhanced bioaccumulation of the metal in the target organs. Following the 5-week period of exposure to CdCl2, there was a significant (p ≤ 0.05) increase in Cd concentration in the liver (325.260 ± 3.2957 ng/g), kidneys (293.840 ± 15.806 ng/g), and muscle (59.320 ± 4.749 ng/g) of the rats as compared to the control group (Figure 8A–C). Treatment with the cheese nanowhiskers (NCh) significantly (p ≤ 0.05) reduced the Cd levels in all the abovementioned target tissues in comparison to the group exposed only to Cd (Figure 8A–C).

Table 3.
MDA levels in the serum.
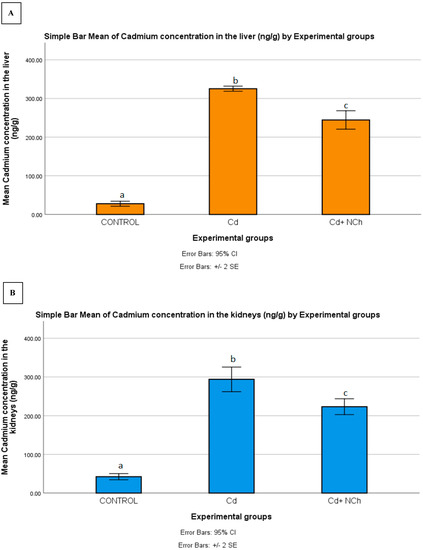
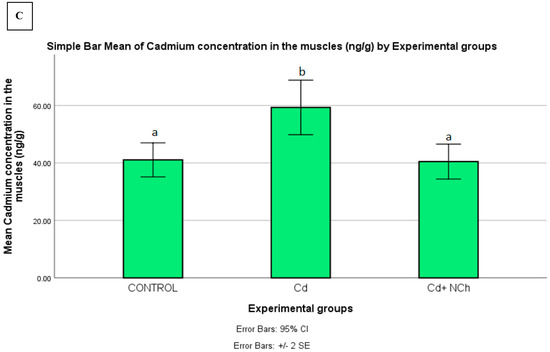
Figure 8.
Mean ± SE of Cd concentration in the (A) liver, (B) kidneys, and (C) muscle of rats from all experimental groups. Different letters indicant a significant difference (p ≥ 0.05).
It has been postulated that exposure to xenobiotics/heavy metals including Cd in animals/humans induces oxidative stress that leads to necrosis/apoptosis of enterocytes, activating inflammation and thereby disrupting the tight junctions in the gut, causing higher absorption and bioaccumulation of the toxins [12,34,35,36]. The key results of the current study are in line with a study by Djurasevic et al. [37] that revealed that co-administration of probiotics with Cd in a rat model decreased the metal concentration in blood, liver, and kidneys, and attenuated the liver function modulations and histopathological changes in the liver and kidney induced by Cd exposure. In line with the results of the bioassays in the present study, a previous study on occupational heavy metal exposure in industry workers showed a reduction in heavy metal load with probiotic consumption in the form of yoghurt, with a parallel in enhanced levels of anti-inflammatory cytokines and CAT activity and reduced levels of MDA in the sera [38]. However, studies do report that these adverse effects could possibly be attenuated by antioxidants [12,39]. The antioxidant status of milk and dairy products is attributed to a complex balance of varied constituent components in milk/milk products. In fact, fractions of milk including whey, casein, and lactoferrin are recognized for their antioxidant properties [40], which aid in alleviating lipid peroxidation [41]. There has been limited research on the antioxidant capacity of cheeses; however, the interest in this field has been increasing in current times. Although the production affects the total antioxidant content of cheese, fat-soluble vitamins are mostly responsible [40,41] for this content. The process of cheese-ripening involves a number of biochemical and chemical changes. Additionally, Trolox equivalent antioxidant capacity (TEAC) is primarily linked to milk caseins, and depends on the rate of formation of soluble antioxidant peptides in the proteolysis of casein during cheese-ripening [42,43]. Khan et al. [44] reported that peptides formed as proteolytic products from β-casein and αS1-casein exhibit potential radical-scavenging activity and that further ripening of cheese involves secondary proteolysis which results in the formation of other peptides, demonstrating potent antioxidant status. Furthermore, Revilla et al. [45] evaluated the antioxidant capacity of 224 cheese samples and concluded that the total antioxidant capacity of cheese was positively correlated with the content of some antioxidant constituents—total protein content and retinol, in addition to the total fat content and minerals. Additionally, probiotics have been reported to be effective in metal chelation in a literature survey by Bhattacharya [46], which showed that four different types of probiotic microbes exhibited a marked amelioration of lead toxicity in experimental pre-clinical studies. Furthermore, other studies have shown that probiotics provide a protective effect against the disruption of the intestinal barrier by reducing intestinal permeability and alleviating oxidative stress in animals [9,12]. The detoxification mechanism of probiotics on heavy metals occurs via the metal ions binding to the bacterial the cell wall, followed by bioaccumulation within the bacteria [47]. In addition, probiotic bacteria lower the toxicity of the metal by converting it into less toxic forms. For instance, Lactobacillus bacteria convert methylated mercury to its inorganic form (Hg2+) and then to Hg0, showing poor gastrointestinal absorption [48]. The carboxyl group of proteins and the hydroxyl group of peptidoglycans of probiotics are mainly involved in metal chelation [48,49,50].
Furthermore, the modification of the gut microbiome has been postulated to contribute to heavy metal stress in animals and humans. It has been observed that heavy metal exposure results in increased levels of serum indices which are primarily associated with enhanced antioxidant defense due to oxidative stress. This further supports the fact that the antioxidant status of probiotics is the fundamental mechanism responsible for the ameliorative effect against metal toxicity [38].
Overall, the outcomes of the present study are in line with an earlier study that reported the ameliorative effect of commercial probiotics and their nano-formulation against Cd-induced oxidative stress in rats [10]. When nanotechnology meets gut health, it brings about a futuristic approach to human well-being through dietary intervention. The key findings of the present study demonstrate the relevance of exploring novel nanonization approaches to probiotics and the utility of these nano-probiotics in their sustained delivery, availability, and stability in the biological milieu. Since there is a paucity of experimental data on nano-probiotics in the scientific literature, it is imperative to encourage research in this field with regard to effectiveness, bioavailability, and safety aspects.
4. Conclusions
The present study has successfully reported the synthesis of non-metallic cheese nanowhiskers using a common dietary source of probiotics, in keeping with the essence of clean technology and eco-efficiency. Additionally, the nanowhiskers also demonstrated a marked alleviation in Cd-induced toxicity, oxidative stress and decreased gut permeability, leading to reduced bioaccumulation. Thus, the findings of the current study, backed by previous literature, demonstrate the value of probiotics with potent antioxidant capacities in counteracting heavy metal toxicity and the associated oxidative damage. Probiotic-based gut remediation in the form of nano-formulations is a workable and promising therapeutic technique to combat oxidative stress/disease.
Author Contributions
Conceptualization, M.A.A. and P.V.; data curation, T.B.; formal analysis, M.A.A., P.V. and F.A.-A.; funding acquisition, M.M.A.; investigation, K.M.O.O.; methodology, M.A.A., P.V. and F.A.-A.; project administration, M.M.A. and T.B.; resources, M.M.A., A.A.H. and F.A.; software, A.A.H., A.W.A. and K.M.O.O.; supervision, A.A.H. and A.W.A.; validation, M.M.A.; visualization, A.W.A. and F.A.; writing—original draft, P.V. and M.A.A.; writing—review and editing, M.A.A. and A.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0047).
Data Availability Statement
All data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Bhattacharya, S. The Role of Probiotics in the Amelioration of Cadmium Toxicity. Biol. Trace Elem. Res. 2020, 197, 440–444. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Lech, T.; Sadlik, J.K. Cadmium concentration in human autopsy tissues. Biol. Trace Elem. Res. 2017, 179, 172–177. [Google Scholar] [CrossRef]
- Bhattacharya, S. The role of medicinal plants and natural products in amelioration of cadmium toxicity. Orient. Pharm. Exp. Med. 2018, 18, 177–186. [Google Scholar] [CrossRef]
- Bhattacharya, S. Medicinal plants and natural products can play a significant role in mitigation of mercury toxicity. Interdiscip. Toxicol. 2018, 11, 247–254. [Google Scholar] [CrossRef]
- Alharbi, N.; Elobeid, M.; Virk, P. Protective Effect of Quercetin Treatment against Cadmium-Induced Oxidative Stress in a Male Rat Model. Pak. J. Zool. 2019, 51, 2287. [Google Scholar] [CrossRef]
- Al-Ghamdi, N.A.M.; Virk, P.; Hendi, A.; Awad, M.; Elobeid, M. Antioxidant potential of bulk and nanoparticles of naringenin against cadmium-induced oxidative stress in Nile tilapia, Oreochromis niloticus. Green Process. Synth. 2021, 10, 392–402. [Google Scholar] [CrossRef]
- Torres, S.; Contreras, L.; Verón, H.; Isla, M.I. Chapter 10—Prospects of dairy and vegetables-based food products in human health: Current status and future directions. In Research and Technological Advances in Food Science; Prakash, B., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 243–267. [Google Scholar]
- Al-Enazi, A.M.M.; Virk, P.; Hindi, A.; Awad, M.A.; Elobeid, M.; Qindeel, R. Protective effect of probiotic bacteria and its nanoformulation against cadmium-induced oxidative stress in male Wistar rat. J. King Saud. Univ. Sci. 2020, 32, 3045–3051. [Google Scholar] [CrossRef]
- Foligné, B.; Daniel, C.; Pot, B. Probiotics from research to market: The possibilities, risks and challenges. Curr. Opin. Microbiol. 2013, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Tian, F.; Zhao, J.; Zhang, H.; Narbad, A.; Chen, W. Oral administration of probiotics inhibits absorption of the heavy metal cadmium by protecting the intestinal barrier. Appl. Environ. Microbiol. 2016, 82, 4429–4440. [Google Scholar] [CrossRef]
- Plessas, S.; Bosnea, L.; Alexopoulos, A.; Bezirtzoglou, E. Potential effects of probiotics in cheese and yogurt production: A review. Eng. Life Sci. 2012, 12, 433–440. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Adesso, S.; Ostacolo, C.; Sala, M.; Sommella, E.; Scala, M.C.; Messore, A.; Autore, G.; Marzocco, S.; et al. Antioxidant Properties of Buffalo-Milk Dairy Products: A β-Lg Peptide Released after Gastrointestinal Digestion of Buffalo Ricotta Cheese Reduces Oxidative Stress in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2018, 19, 1955. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.G.; Abdelfattah-Hassan, A.; Eldoumani, H.; Elazab, S.T.; Mohamed, S.A.; Metwally, A.S.; El-Shetry, E.S.; Saleh, A.A.; ElSawy, N.A.; Ibrahim, D. Probiotics-loaded nanoparticles attenuated colon inflammation, oxidative stress, and apoptosis in colitis. Sci. Rep. 2022, 12, 5116. [Google Scholar] [CrossRef]
- Virk, P.; Awad, M.A.; Elobeid, M.A.; Ortashi, K.M.O.; Hendi, A.A. Fabrication of Probiotics Nanowhiskers Using Cheese. U.S. Patent 10,517,905 B1, 31 December 2019. [Google Scholar]
- Jama, A.M.; Mitić-Ćulafić, D.; Kolarević, S.; Đurašević, S.F.; Knežević-Vukčević, J. Protective effect of probiotic bacteria against cadmium-induced genotoxicity in rat hepatocytes In vivo and In Vitro. Arch. Biol. Sci. 2012, 64, 1197–1206. [Google Scholar] [CrossRef]
- Lim, J.; Yeap, S.P.; Che, H.X.; Low, S.C. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res. Lett. 2013, 8, 381. [Google Scholar] [CrossRef]
- Grigoraviciute-Puroniene, I.; Zarkov, A.; Tsuru, H.; Ishikawa, K.; Kareiva, A. A novel synthetic approach for the calcium hydroxyapatite from the food products. J. Solgel. Sci. Technol. 2019, 91, 63–71. [Google Scholar] [CrossRef]
- Sen, P.; Ghosh, J.; Abdullah, A.; Kumar, P. Preparation of Cu, Ag, Fe and Al nanoparticles by the exploding wire technique. J. Chem. Sci. 2003, 115, 499–508. [Google Scholar] [CrossRef]
- Sakkas, L.; Pappas, C.S.; Moatsou, G. FT-MIR Analysis of water-soluble extracts during the ripening of sheep milk cheese with different phospholipid content. Dairy 2021, 2, 530–541. [Google Scholar] [CrossRef]
- Karoui, R.; Mouazen, A.M.; Dufour, É.; Pillonel, L.; Picque, D.; Bosset, J.-O.; De Baerdemaeker, J. Mid-infrared spectrometry: A tool for the determination of chemical parameters in Emmental cheeses produced during winter. Lait 2006, 86, 83–97. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; John Wiley and Sons: West Sussex, UK, 2001; pp. 229–338. [Google Scholar]
- Alatini, E.; Sakkas, L.; Moatsou, G. Use of sweet sheep buttermilk in the manufacture of reduced-fat sheep milk cheese. Int. Dairy J. 2021, 120, 105079. [Google Scholar]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Marinho, M.T.; Bersot, L.D.; Nogueira, A.; Colman, T.A.D.; Schnitzler, E. Antioxidant effect of dehydrated rosemary leaves in ripened semi-hard cheese: A study using coupled TG–DSC–FTIR (EGA). LWT-Food.Sci.Technol. 2015, 63, 1023–1028. [Google Scholar] [CrossRef]
- Surat, P. Presenting GC-MS Results in a Publication. News-Medical. Available online: https://www.news-medical.net/life-sciences/Presenting-GC-MS-Results-in-a-Publication.aspx.2019 (accessed on 27 February 2023).
- Harisaranraj, R.; Suresh, K.; Saravana Babu, S.; Achudhan, V.V. Phytochemical based strategies for pathogen control and antioxidant capacities of Rauwolfia serpentina extracts. Recent Res. Sci. Technol. 2009, 1, 67–73. [Google Scholar]
- Stelios, K.; Paraskevi, S.; Theophilos, M.; Aikaterini, G. Study of organic acids, volatile fraction and caseins of a new Halloumi-type cheese during ripening in whey brine. Int. J. Food Sci. Technol. 2009, 44, 297–304. [Google Scholar] [CrossRef]
- Eisenstecken, D.; Stanstrup, J.; Robatscher, P.; Huck, C.W.; Oberhuber, M. Fatty acid profiling of bovine milk and cheese from six European areas by GC-FID and GC-MS. Int. J. Dairy Technol. 2021, 74, 215–224. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef]
- Lobay, D. Rauwolfia in the Treatment of Hypertension. Integr. Med. 2015, 14, 40–46. [Google Scholar]
- Das, A.; Roy, A.; Das, R.; Bhattacharya, S.; Haldar, P.K. Naringenin alleviates cadmium-induced toxicity through the abrogation of oxidative stress in Swiss albino mice. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 161–169. [Google Scholar] [CrossRef]
- Hyun, J.S.; Satsu, H.; Shimizu, M. Cadmium induces Interleukin-8 production via NF-_B activation in the human intestinal epithelial cell, Caco-2. Cytokine 2007, 37, 26–34. [Google Scholar] [CrossRef]
- Basuroy, S.; Sheth, P.; Kuppuswamy, D.; Balasubramanian, S.; Ray, R.M.; Rao, R.K. Expression of kinase-inactive c-Src delays oxidative stress induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 2003, 278, 11916–11924. [Google Scholar] [CrossRef] [PubMed]
- Banan, A.; Choudhary, S.; Zhang, Y.; Fields, J.; Keshavarzian, A. Ethanol-induced barrier dysfunction and its prevention by growth factorsNin human intestinal monolayers: Evidence for oxidative and cytoskeletalNmechanisms. J. Pharmacol. Exp. Ther. 1999, 291, 1075–1085. [Google Scholar]
- Djurasevic, S.; Jama, A.; Jasnic, N.; Vujovic, P.; Jovanovic, M.; Mitic-Culafic, D.; Knezevic-Vukcevic, J.; Cakic-Milosevic, M.; Ilijevic, K.; Djordjevic, J. The Protective Effects of Probiotic Bacteria on Cadmium Toxicity in Rats. J. Med. Food. 2017, 20, 189–196. [Google Scholar] [CrossRef]
- Feng, P.; Yang, J.; Zhao, S.; Ling, Z.; Han, R.; Wu, Y.; Salama, E.S.; Kakade, A.; Khan, A.; Jin, W.; et al. Human supplementation with Pediococcus acidilactici GR-1 decreases heavy metals levels through modifying the gut microbiota and metabolome. npj Biofilms Microbiomes 2022, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Martín, A.R.; Villegas, I.; La Casa, C.; de la Lastra, C.A. Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem. Pharmacol. 2004, 67, 1399–1410. [Google Scholar] [CrossRef]
- Steijns, J.M.; van Hooijdonk, A.C. Occurrence, structure, biochemical properties and technological characteristics of lactoferrin. Br. J. Nutr. 2000, 84, S11–S17. [Google Scholar] [CrossRef]
- Lindmark-Månsson, H.; Åkesson, B. Antioxidative factors in milk. Br. J. Nutr. 2000, 84, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant activity of Cheddar cheeses at different stages of ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. Effect of genetic type on antioxidant activity of Caciocavallo cheese during ripening. J. Dairy Sci. 2015, 98, 3690–3694. [Google Scholar] [CrossRef]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ullah, R.; Ajmal, M.; Jaspa, M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids Health Dis. 2019, 18, 41. [Google Scholar] [CrossRef]
- Revilla, I.; González-Martín, M.I.; Vivar-Quintana, A.M.; Blanco-López, M.A.; Lobos-Ortega, I.A.; Hernández-Hierro, J.M. Antioxidant capacity of different cheeses: Affecting factors and prediction by near infrared spectroscopy. J. Dairy Sci. 2016, 99, 5074–5082. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S. Probiotics against alleviation of lead toxicity: Recent advances. Interdiscip. Toxicol. 2019, 12, 89–92. [Google Scholar] [CrossRef]
- Bhakta, J.N.; Ohnishi, K.; Munekage, Y.; Iwasaki, K.; Wei, M.Q. Characterization of lactic acid bacteria-based probiotics as potential heavy metal sorbents. J. Appl. Microbiol. 2012, 112, 1193–1206. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Megeed, R.M. Probiotics: A Promising Generation of Heavy Metal detoxification. Biol. Trace Elem. Res. 2021, 199, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Ninkov, M.; Popov Aleksandrov, A.; Demenesku, J.; Mirkov, I.; Mileusnic, D.; Petrovic, A.; Grigorov, I.; Zolotarevski, L.; Tolinacki, M.; Kataranovski, D.; et al. Toxicityof oral cadmium intake: Impact on gut immunity. Toxicol. Lett. 2015, 237, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Qu, D.; Feng, S.; Yu, Y.; Yu, L.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Oral supplementation of lead intolerant intestinal microbes protects against lead (Pb) toxicity in mice. Front. Microbiol. 2020, 10, 3161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).