Mimicking Natural-Colored Photonic Structures with Cellulose-Based Materials
Abstract
1. Soft Matter and Structural Color: Short Introduction
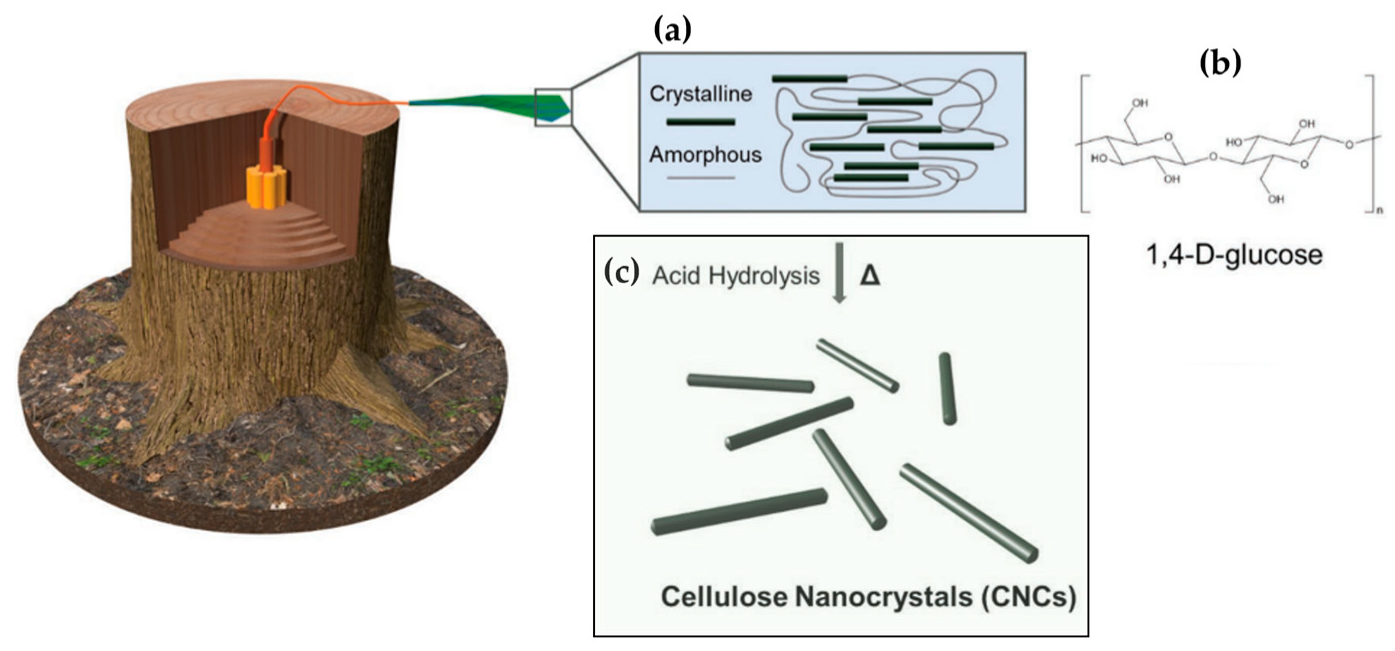
2. Cellulose and Cellulose Nanocrystals: Short Overview
3. Photonic Structures in Nature

4. Liquid Crystalline Phases in Cellulose: Short Overview
5. Photonic Structures in Cellulose-Based Materials
6. Cellulose-Based Composite Materials with Structural Color

7. Light Responsive and Color-Stimuli-Responsive Cellulose-Based Materials
8. Tapping the Engineering and Industrial Potential
9. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Niu, S.; Li, B.; Mu, Z.; Yang, M. Excellent Structure-Based Multifunction of Morpho Butterfly Wings: A Review. J. Bionic. Eng. 2015, 12, 170–189. [Google Scholar] [CrossRef]
- Kinoshita, S. Front Matter. In Structural Colors in the Realm of Nature; World Scientific: Singapore, 2008; Volume 136, pp. i–xiii. [Google Scholar]
- Tadepalli, S.; Slocik, J.M.; Gupta, M.K.; Naik, R.R.; Singamaneni, S. Bio-Optics and Bio-Inspired Optical Materials. Chem. Rev. 2017, 117, 12705–12763. [Google Scholar] [CrossRef]
- de Gennes, P.-G.; Prost, J. The Physics of Liquid Crystals; International Series of Monographs on Physics; Oxford University Press: Oxford, UK, 1993; Volume 83. [Google Scholar]
- Kléman, M.; Lavrentovich, O.D. Soft Matter Physics: An Introduction; Springer: New York, NY, USA, 2003. [Google Scholar]
- Yang, D.K.; Wu, S.T. Fundamentals of Liquid Crystal Devices, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Liu, P.; Wang, J.; Qi, H.; Koddenberg, T.; Xu, D.; Liu, S.; Zhang, K. Biomimetic Confined Self-Assembly of Chitin Nanocrystals. Nano Today 2022, 43, 101420. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887–4926. [Google Scholar] [CrossRef]
- Fernández-Rico, C.; Chiappini, M.; Yanagishima, T.; de Sousa, H.; Aarts, D.G.A.L.; Dijkstra, M.; Dullens, R.P.A. Shaping Colloidal Bananas to Reveal Biaxial, Splay-Bend Nematic, and Smectic Phases. Science 2020, 369, 950–955. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Savin, T. Recent Advances in the Biomimicry of Structural Colours. Chem. Soc. Rev. 2016, 45, 6698–6724. [Google Scholar] [CrossRef]
- George, J.; S N, S. Cellulose Nanocrystals: Synthesis, Functional Properties, and Applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Borges, J.P.; Canejo, J.P.; Fernandes, S.N.; Brogueira, P.; Godinho, M.H. Cellulose-Based Liquid Crystalline Composite Systems. In Nanocellulose Polymer Nanocomposites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 215–235. [Google Scholar]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Frey-Wyssling, A. The Fine Structure of Cellulose Microfibrils. Science 1954, 119, 80–82. [Google Scholar] [CrossRef]
- Hamad, W. On the Development and Applications of Cellulosic Nanofibrillar and Nanocrystalline Materials. Can. J. Chem. Eng. 2008, 84, 513–519. [Google Scholar] [CrossRef]
- Kaushik, M.; Fraschini, C.; Chauve, G.; Putaux, J.-L.; Moores, A. Transmission Electron Microscopy for the Characterization of Cellulose Nanocrystals. In The Transmission Electron. Microscope—Theory and Applications; InTech: Gurugram, India, 2015. [Google Scholar]
- Available online: https://Commons.Wikimedia.Org/Wiki/File:Cellulose_Sessel.Svg (accessed on 1 June 2023).
- Tran, A.; Boott, C.E.; MacLachlan, M.J. Understanding the Self-Assembly of Cellulose Nanocrystals—Toward Chiral Photonic Materials. Adv. Mater. 2020, 32, 1905876. [Google Scholar] [CrossRef]
- Rånby, B.G. The Colloidal Properties of Cellulose Micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Rånby, B.G. The Cellular Micelles. TAPPI J. 1952, 35, 53–58. [Google Scholar]
- Revol, J.-F.; Godbout, L.; Gray, D.G. Solidifi Ed Liquid Crystals of Cellulose with Optically Variable Properties. U.S. Patent No 5,629,055, 13 May 1997. [Google Scholar]
- Dong, X.M.; Revol, J.-F.; Gray, D.G. Effects of Microcrystallite Preparation Conditions on the Formation of Colloid Crystals of Cellulose. Cellulose 1998, 5, 19–32. [Google Scholar] [CrossRef]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal Self-Ordering of Cellulose Microfibrils in Aqueous Suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Sassi, J.; Chanzy, H. Ultrastructure Aspects of the Acetylation of Cellulose. Cellulose 1995, 2, 111–127. [Google Scholar] [CrossRef]
- Guo, J.-X.; Gray, D.G. Lyotropic Cellulosic Liquid Crystals. In Cellulosic Polymers, Blends and Composites; Gilbert, R.D., Ed.; Hanser: Cincinnati, OH, USA, 1994; pp. 25–45. [Google Scholar]
- Cavaille, J.; Chanzy, H.; Fleury, E.; Sassi, J. Surface-Modifi Ed Cellulose Microfi Brils, Method for Making the Same, and Use Thereof as a Filler in Composite Materials. U.S. Patent No 6,117,545, 12 September 2000. [Google Scholar]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and Chiral-Nematic Self-Ordering of Cellulose Microcrystals Suspensions in Nonpolar Solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric Stabilization of a Cellulose Microcrystal Suspension by Poly(Ethylene Glycol) Grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Hanna, M.; Biby, G.; Miladinov, V. Production of Microcrystalline Cellulose by Reactive Extraction. U.S. Patent No 6,228,213, 8 May 2001. [Google Scholar]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 1–49. [Google Scholar]
- Tonoli, G.H.D.; Teixeira, E.M.; Corrêa, A.C.; Marconcini, J.M.; Caixeta, L.A.; Pereira-da-Silva, M.A.; Mattoso, L.H.C. Cellulose Micro/Nanofibres from Eucalyptus Kraft Pulp: Preparation and Properties. Carbohydr. Polym. 2012, 89, 80–88. [Google Scholar] [CrossRef]
- Shishehbor, M.; Zavattieri, P.D. Effects of Interface Properties on the Mechanical Properties of Bio-Inspired Cellulose Nanocrystal (CNC)-Based Materials. J. Mech. Phys. Solids 2019, 124, 871–896. [Google Scholar] [CrossRef]
- Fu, Y.; Tippets, C.A.; Donev, E.U.; Lopez, R. Structural Colors: From Natural to Artificial Systems. WIREs Nanomed. Nanobiotechnol. 2016, 8, 758–775. [Google Scholar] [CrossRef]
- Burg, S.L.; Parnell, A.J. Self-Assembling Structural Colour in Nature. J. Phys. Condens. Matter 2018, 30, 413001. [Google Scholar] [CrossRef]
- Datta, B.; Spero, E.F.; Martin-Martinez, F.J.; Ortiz, C. Socially-Directed Development of Materials for Structural Color. Adv. Mater. 2022, 34, 2100939. [Google Scholar] [CrossRef]
- Tan, A.; Ahmad, Z.; Vukusic, P.; Cabral, J.T. Multifaceted Structurally Coloured Materials: Diffraction and Total Internal Reflection (TIR) from Nanoscale Surface Wrinkling. Molecules 2023, 28, 1710. [Google Scholar] [CrossRef]
- Whitney, H.M.; Kolle, M.; Andrew, P.; Chittka, L.; Steiner, U.; Glover, B.J. Floral Iridescence, Produced by Diffractive Optics, Acts As a Cue for Animal Pollinators. Science 2009, 323, 130–133. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Bhushan, B.; Koch, K. Plant Surfaces: Structures and Functions for Biomimetic Innovations. Nanomicro Lett. 2017, 9, 23. [Google Scholar] [CrossRef]
- de Premorel, G.; Giurfa, M.; Andraud, C.; Gomez, D. Higher Iridescent-to-Pigment Optical Effect in Flowers Facilitates Learning, Memory and Generalization in Foraging Bumblebees. Proc. R. Soc. B Biol. Sci. 2017, 284, 20171097. [Google Scholar] [CrossRef]
- Gegear, R.J.; Burns, J.G. The Birds, the Bees, and the Virtual Flowers: Can Pollinator Behavior Drive Ecological Speciation in Flowering Plants? Am. Nat. 2007, 170, 551–566. [Google Scholar] [CrossRef]
- Vignolini, S.; Moyroud, E.; Glover, B.J.; Steiner, U. Analysing Photonic Structures in Plants. J. R. Soc. Interface 2013, 10, 20130394. [Google Scholar] [CrossRef]
- Antoniou Kourounioti, R.L.; Band, L.R.; Fozard, J.A.; Hampstead, A.; Lovrics, A.; Moyroud, E.; Vignolini, S.; King, J.R.; Jensen, O.E.; Glover, B.J. Buckling as an Origin of Ordered Cuticular Patterns in Flower Petals. J. R. Soc. Interface 2013, 10, 20120847. [Google Scholar] [CrossRef]
- Kevan, P.G.; Lane, M.A. Flower Petal Microtexture Is a Tactile Cue for Bees. Proc. Natl. Acad. Sci. USA 1985, 82, 4750–4752. [Google Scholar] [CrossRef]
- Huang, X.; Hai, Y.; Xie, W.-H. Anisotropic Cell Growth-Regulated Surface Micropatterns in Flower Petals. Theor. Appl. Mech. Lett. 2017, 7, 169–174. [Google Scholar] [CrossRef]
- Vukusic, P. Evolutionary Photonics with a Twist. Science 2009, 325, 398–399. [Google Scholar] [CrossRef]
- Chang, Y.; Middleton, R.; Ogawa, Y.; Gregory, T.; Steiner, L.M.; Kovalev, A.; Karanja, R.H.N.; Rudall, P.J.; Glover, B.J.; Gorb, S.N. Cell Wall Composition Determines Handedness Reversal in Helicoidal Cellulose Architectures of Pollia condensata Fruits. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Vignolini, S.; Rudall, P.J.; Rowland, A.V.; Reed, A.; Moyroud, E.; Faden, R.B.; Baumberg, J.J.; Glover, B.J.; Steiner, U. Pointillist Structural Color in Pollia Fruit. Proc. Natl. Acad. Sci. USA 2012, 109, 15712–15715. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hu, B.; Song, J.; Chu, Z.; Wu, W. Bioinspired Multiscale Wrinkling Patterns on Curved Substrates: An Overview. Nanomicro Lett. 2020, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.W. Structural Colors in Insects. I. J. Phys. Chem. 1926, 30, 383–395. [Google Scholar] [CrossRef]
- Mason, C.W. Structural Colors in Insects. II. J. Phys. Chem. 1927, 31, 321–354. [Google Scholar] [CrossRef]
- Jacucci, G.; Vignolini, S.; Schertel, L. The Limitations of Extending Nature’s Color Palette in Correlated, Disordered Systems. Proc. Natl. Acad. Sci. USA 2020, 117, 23345–23349. [Google Scholar] [CrossRef]
- Roberts, N.W.; Marshall, N.J.; Cronin, T.W. High Levels of Reflectivity and Pointillist Structural Color in Fish, Cephalopods, and Beetles. Proc. Natl. Acad. Sci. USA 2012, 109. [Google Scholar] [CrossRef]
- Fan, X.; Zheng, X.; An, T.; Li, X.; Leung, N.; Zhu, B.; Sui, T.; Shi, N.; Fan, T.; Zhao, Q. Light Diffraction by Sarcomeres Produces Iridescence in Transmission in the Transparent Ghost Catfish. Proc. Natl. Acad. Sci. USA 2023, 120, e2219300120. [Google Scholar] [CrossRef]
- Medina, J.M.; Díaz, J.A.; Vukusic, P. Classification of Peacock Feather Reflectance Using Principal Component Analysis Similarity Factors from Multispectral Imaging Data. Opt. Express 2015, 23, 10198. [Google Scholar] [CrossRef]
- Okazaki, T. Ultraviolet Reflectance Structures of Peacock Feathers. Zoolog. Sci. 2018, 35, 421–426. [Google Scholar] [CrossRef]
- Eliason, C.M.; Shawkey, M.D. Rapid, Reversible Response of Iridescent Feather Color to Ambient Humidity. Opt. Express 2010, 18, 21284. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Y.; Wang, Z.; Xu, Q.; Zhang, L. Study on the Microstructure and Its Coloration Mechanism of Peacock Feather by the FDTD Method. J. Phys. Conf. Ser. 2020, 1549, 032036. [Google Scholar] [CrossRef]
- Okazaki, T. Structural Color Expression Due to Specular Reflection from Bird Feathers. FORMA 2022, 37, 5–12. [Google Scholar] [CrossRef]
- Liao, S.-F.; Yao, C.-Y.; Lee, C.-C. Measuring and Modeling the Inconspicuous Iridescence of Formosan Blue Magpie’s Feather (Urocissacaerulea). Appl. Opt. 2015, 54, 4979. [Google Scholar] [CrossRef] [PubMed]
- Jeon, D.-J.; Ji, S.; Lee, E.; Kang, J.; Kim, J.; D’Alba, L.; Manceau, M.; Shawkey, M.D.; Yeo, J.-S. How Keratin Cortex Thickness Affects Iridescent Feather Colours. R. Soc. Open. Sci. 2023, 10. [Google Scholar] [CrossRef]
- Doucet, S.M.; Shawkey, M.D.; Hill, G.E.; Montgomerie, R. Iridescent Plumage in Satin Bowerbirds: Structure, Mechanisms and Nanostructural Predictors of Individual Variation in Colour. J. Exp. Biol. 2006, 209, 380–390. [Google Scholar] [CrossRef]
- Kertész, K.; Bálint, Z.; Piszter, G.; Horváth, Z.E.; Biró, L.P. Multi-Instrumental Techniques for Evaluating Butterfly Structural Colors: A Case Study on Polyommatus Bellargus (Rottemburg, 1775) (Lepidoptera: Lycaenidae: Polyommatinae). Arthropod. Struct. Dev. 2021, 61, 101010. [Google Scholar] [CrossRef]
- Ghiradella, H. Light and Color on the Wing: Structural Colors in Butterflies and Moths. Appl. Opt. 1991, 30, 3492. [Google Scholar] [CrossRef]
- Kinoshita, S.; Yoshioka, S.; Kawagoe, K. Mechanisms of Structural Colour in the Morpho Butterfly: Cooperation of Regularity and Irregularity in an Iridescent Scale. Proc. R. Soc. Lond. B Biol. Sci. 2002, 269, 1417–1421. [Google Scholar] [CrossRef] [PubMed]
- Noh, M.Y.; Muthukrishnan, S.; Kramer, K.J.; Arakane, Y. Cuticle Formation and Pigmentation in Beetles. Curr. Opin. Insect Sci. 2016, 17, 1–9. [Google Scholar] [CrossRef]
- Barrows, F.P.; Bartl, M.H. Photonic Structures in Biology: A Possible Blueprint for Nanotechnology. Nanomater. Nanotechnol. 2014, 4, 1. [Google Scholar] [CrossRef]
- Scalet, J.M.; Sprouse, P.A.; Schroeder, J.D.; Dittmer, N.; Kramer, K.J.; Kanost, M.R.; Gehrke, S.H. Temporal Changes in the Physical and Mechanical Properties of Beetle Elytra during Maturation. Acta Biomater. 2022, 151, 457–467. [Google Scholar] [CrossRef]
- Vincent, J.F.V.; Wegst, U.G.K. Design and Mechanical Properties of Insect Cuticle. Arthropod. Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Hernández-Jiménez, M.; Azofeifa, D.E.; Libby, E.; Barboza-Aguilar, C.; Solís, Á.; Arce-Marenco, L.; García-Aguilar, I.; Hernández, A.; Vargas, W.E. Qualitative Correlation between Structural Chirality through the Cuticle of Chrysina Aurigans Scarabs and Left-Handed Circular Polarization of the Reflected Light. Opt. Mater. Express 2014, 4, 2632. [Google Scholar] [CrossRef]
- Shevtsova, E.; Hansson, C.; Janzen, D.H.; Kjærandsen, J. Stable Structural Color Patterns Displayed on Transparent Insect Wings. Proc. Natl. Acad. Sci. USA 2011, 108, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ingram, A.L.; Deparis, O.; Boulenguez, J.; Kennaway, G.; Berthier, S.; Parker, A.R. Structural Origin of the Green Iridescence on the Chelicerae of the Red-Backed Jumping Spider, Phidippus Johnsoni (Salticidae: Araneae). Arthropod. Struct. Dev. 2011, 40, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.A.; Berthier, V.; Wilts, B.D.; Cambourieux, M.-C.; Bennet, P.; Pollès, R.; Teytaud, O.; Centeno, E.; Biais, N.; Moreau, A. Evolutionary Algorithms Converge towards Evolved Biological Photonic Structures. Sci. Rep. 2020, 10, 12024. [Google Scholar] [CrossRef]
- Prum, R.O.; Torres, R.H. Structural Colouration of Mammalian Skin: Convergent Evolution of Coherently Scattering Dermal Collagen Arrays. J. Exp. Biol. 2004, 207, 2157–2172. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bhushan, B. Structure and Mechanical Properties of Beetle Wings: A Review. RSC Adv. 2012, 2, 12606. [Google Scholar] [CrossRef]
- Simonis, P.; Vigneron, J.P. Structural Color Produced by a Three-Dimensional Photonic Polycrystal in the Scales of a Longhorn Beetle: Pseudomyagrus Waterhousei (Coleoptera: Cerambicidae). Phys. Rev. E 2011, 83, 011908. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.-L.; Hsu, W.-L.; Chiang, Y.-W. Trapping Structural Coloration by a Bioinspired Gyroid Microstructure in Solid State. ACS Nano 2018, 12, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Burresi, M.; Cortese, L.; Pattelli, L.; Kolle, M.; Vukusic, P.; Wiersma, D.S.; Steiner, U.; Vignolini, S. Bright-White Beetle Scales Optimise Multiple Scattering of Light. Sci. Rep. 2014, 4, 6075. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Compton_scattering (accessed on 26 May 2023).
- Available online: https://bmet.fandom.com/wiki/Diffraction (accessed on 26 May 2023).
- Available online: https://en.wikipedia.org/wiki/Polarization_%28physics%29 (accessed on 26 May 2023).
- Available online: https://www.e-education.psu.edu/mcl-optpro/book/export/html/858 (accessed on 26 May 2023).
- Moud, A.A.; Moud, A.A. Flow and assembly of cellulose nanocrystals (CNC): A bottom-up perspective—A review. Int. J. Biol. Macromol. 2023, 232, 123391. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef]
- Gil, U. Mechanical Properties of Cellulose Nanocrystal Thin Films; McMaster University: Hamilton, ON, USA, 2017. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of Reaction Conditions on the Properties and Behavior of Wood Cellulose Nanocrystal Suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an Acetic Ferment Which Forms Cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Song, H.; Li, J.; Feng, Y.; Shi, Z.; Wang, X.; Lin, Q. Synthesis of Bacterial Cellulose and Bacterial Cellulose Nanocrystals for Their Applications in the Stabilization of Olive Oil Pickering Emulsion. Food Hydrocoll. 2017, 72, 127–135. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Produced in Sugar Beet Molasses and Cheese Whey Media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Belton, P.S.; Tanner, S.F.; Cartier, N.; Chanzy, H. High-Resolution Solid-State Carbon-13 Nuclear Magnetic Resonance Spectroscopy of Tunicin, an Animal Cellulose. Macromolecules 1989, 22, 1615–1617. [Google Scholar] [CrossRef]
- Dunlop, M.J.; Clemons, C.; Reiner, R.; Sabo, R.; Agarwal, U.P.; Bissessur, R.; Sojoudiasli, H.; Carreau, P.J.; Acharya, B. Towards the Scalable Isolation of Cellulose Nanocrystals from Tunicates. Sci. Rep. 2020, 10, 19090. [Google Scholar] [CrossRef] [PubMed]
- Chanthathamrongsiri, N.; Petchsomrit, A.; Leelakanok, N.; Siranonthana, N.; Sirirak, T. The Comparison of the Properties of Nanocellulose Isolated from Colonial and Solitary Marine Tunicates. Heliyon 2021, 7, e07819. [Google Scholar] [CrossRef]
- Martins, A.F. Os Cristais Líquidos. Independent 1991, 7, 253. [Google Scholar]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2017; ISBN 9781315272801. [Google Scholar]
- Hamad, W.Y. Cellulose Nanocrystals: Properties, Production and Applications; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- de Vries, H. Rotatory Power and Other Optical Properties of Certain Liquid Crystals. Acta Crystallogr. 1951, 4, 219–226. [Google Scholar] [CrossRef]
- Almeida, A.P.C.; Canejo, J.P.; Fernandes, S.N.; Echeverria, C.; Almeida, P.L.; Godinho, M.H. Cellulose-Based Biomimetics and Their Applications. Adv. Mater. 2018, 30, 1703655. [Google Scholar] [CrossRef]
- Rojas, O.J. Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Stein, P.; Finkelmann, H. Chirality in Liquid Crystal Elastomers. In Chirality in Liquid Crystals; Springer: Berlin/Heidelberg, Germany, 2000; pp. 433–446. [Google Scholar]
- Fernandes, S.N.; Geng, Y.; Vignolini, S.; Glover, B.J.; Trindade, A.C.; Canejo, J.P.; Almeida, P.L.; Brogueira, P.; Godinho, M.H. Structural Color and Iridescence in Transparent Sheared Cellulosic Films. Macromol. Chem. Phys. 2013, 214, 25–32. [Google Scholar] [CrossRef]
- Fernandes, S.N.; Almeida, P.L.; Monge, N.; Aguirre, L.E.; Reis, D.; de Oliveira, C.L.P.; Neto, A.M.F.; Pieranski, P.; Godinho, M.H. Mind the Microgap in Iridescent Cellulose Nanocrystal Films. Adv. Mater. 2017, 29, 1603560. [Google Scholar] [CrossRef]
- Flory, P.J. Statistical Thermodynamics of Semi-Flexible Chain Molecules. Proc. R. Soc. Lond. A Math. Phys. Sci. 1956, 234, 60–73. [Google Scholar] [CrossRef]
- Gray, D.G. Cellulose Nanocrystal Research; A Personal Perspective. Carbohydr. Polym. 2020, 250, 116888. [Google Scholar] [CrossRef]
- Gray, D.G. Chemical Characteristics of Cellulosic Liquid Crystals. Faraday Discuss. Chem. Soc. 1985, 79, 257. [Google Scholar] [CrossRef]
- Fujisawa, H.; Ryu, M.; Lundgaard, S.; Linklater, D.P.; Ivanova, E.P.; Nishijima, Y.; Juodkazis, S.; Morikawa, J. Direct Measurement of Temperature Diffusivity of Nanocellulose-Doped Biodegradable Composite Films. Micromachines 2020, 11, 738. [Google Scholar] [CrossRef]
- Malshe, A.; Bapat, S.; Rajurkar, K.; Melkote, S. Biological Strategies from Natural Structures for Resilience in Manufacturing. CIRP J. Manuf. Sci. Technol. 2021, 34, 146–156. [Google Scholar] [CrossRef]
- McDougal, A.; Miller, B.; Singh, M.; Kolle, M. Biological Growth and Synthetic Fabrication of Structurally Colored Materials. J. Opt. 2019, 21, 073001. [Google Scholar] [CrossRef]
- Shatkin, J.A.; Wegner, T.H.; Bilek, E.M.; Cowie, J. Market Projections of Cellulose Nanomaterial-Enabled Products—Part 1: Applications. TAPPI J. 2014, 13, 9–16. [Google Scholar] [CrossRef]
- Vignolini, S.; Gregory, T.; Kolle, M.; Lethbridge, A.; Moyroud, E.; Steiner, U.; Glover, B.J.; Vukusic, P.; Rudall, P.J. Structural Colour from Helicoidal Cell-Wall Architecture in Fruits of Margaritaria nobilis. J. R. Soc. Interface 2016, 13, 20160645. [Google Scholar] [CrossRef]
- Parker, R.M.; Guidetti, G.; Williams, C.A.; Zhao, T.; Narkevicius, A.; Vignolini, S.; Frka-Petesic, B. The Self-Assembly of Cellulose Nanocrystals: Hierarchical Design of Visual Appearance. Adv. Mater. 2018, 30, 1704477. [Google Scholar] [CrossRef]
- Tan, K.; Heo, S.; Foo, M.; Chew, I.M.; Yoo, C. An Insight into Nanocellulose as Soft Condensed Matter: Challenge and Future Prospective toward Environmental Sustainability. Sci. Total Environ. 2019, 650, 1309–1326. [Google Scholar] [CrossRef] [PubMed]
- Revol, J.-F.; Godbout, L.; Gray, D.G. Solid Self-Assembled Films of Cellulose with Chiral Nematic Order and Optically Variable Properties. J. Pulp Pap. Sci. 1998, 24, 146–149. [Google Scholar]
- Wang, C.; Tang, C.; Wang, Y.; Shen, Y.; Qi, W.; Zhang, T.; Su, R.; He, Z. Chiral Photonic Materials Self-Assembled by Cellulose Nanocrystals. Curr. Opin. Solid State Mater. Sci. 2022, 26, 101017. [Google Scholar] [CrossRef]
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Tactoid Annealing Improves Order in Self-Assembled Cellulose Nanocrystal Films with Chiral Nematic Structures. Langmuir 2018, 34, 646–652. [Google Scholar] [CrossRef]
- Dionne, G.F.; Allen, G.A.; Haddad, P.R.; Ross, C.A.; Lax, B. Circular Polarization and Nonreciprocal Propagation in Magnetic Media. Linc. Lab. J. 2005, 15, 323–340. [Google Scholar]
- Frka-Petesic, B.; Vignolini, S. So Much More than Paper. Nat. Photonics 2019, 13, 365–367. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-L.; Bay, M.M.; Vadrucci, R.; Barty-King, C.H.; Peng, J.; Baumberg, J.J.; De Volder, M.F.L.; Vignolini, S. Roll-to-Roll Fabrication of Touch-Responsive Cellulose Photonic Laminates. Nat. Commun. 2018, 9, 4632. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, R.; Deutschman, C.P.; Han, L.; Pope, M.A.; Tam, K.C. Cellulose Nanocrystals in Smart and Stimuli-Responsive Materials: A Review. Mater. Today Adv. 2020, 5, 100055. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Hyun Park, J.; Scalia, G.; Bergström, L. Cellulose Nanocrystal-Based Materials: From Liquid Crystal Self-Assembly and Glass Formation to Multifunctional Thin Films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- Dumanli, A.G.; Kamita, G.; Landman, J.; van der Kooij, H.; Glover, B.J.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Controlled, Bio-inspired Self-Assembly of Cellulose-Based Chiral Reflectors. Adv. Opt. Mater. 2014, 2, 646–650. [Google Scholar] [CrossRef]
- Tran, A.; Hamad, W.Y.; MacLachlan, M.J. Fabrication of Cellulose Nanocrystal Films through Differential Evaporation for Patterned Coatings. ACS Appl. Nano Mater. 2018, 1, 3098–3104. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, P.; Nan, F.; Zhou, L.; Zhang, J. Tuning the Iridescence of Chiral Nematic Cellulose Nanocrystal Films with a Vacuum-Assisted Self-Assembly Technique. Biomacromolecules 2014, 15, 4343–4350. [Google Scholar] [CrossRef]
- Nguyen, T.-D.; Hamad, W.Y.; MacLachlan, M.J. Tuning the Iridescence of Chiral Nematic Cellulose Nanocrystals and Mesoporous Silica Films by Substrate Variation. Chem. Commun. 2013, 49, 11296. [Google Scholar] [CrossRef]
- O’Keeffe, O.; Wang, P.-X.; Hamad, W.Y.; MacLachlan, M.J. Boundary Geometry Effects on the Coalescence of Liquid Crystalline Tactoids and Formation of Topological Defects. J. Phys. Chem. Lett. 2019, 10, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Tardy, B.L.; Mattos, B.D.; Greca, L.G.; Kämäräinen, T.; Klockars, K.W.; Rojas, O.J. Tessellation of Chiral-Nematic Cellulose Nanocrystal Films by Microtemplating. Adv. Funct. Mater. 2019, 29, 1808518. [Google Scholar] [CrossRef]
- Beck, S.; Bouchard, J.; Chauve, G.; Berry, R. Controlled Production of Patterns in Iridescent Solid Films of Cellulose Nanocrystals. Cellulose 2013, 20, 1401–1411. [Google Scholar] [CrossRef]
- Dumanli, A.G.; van der Kooij, H.M.; Kamita, G.; Reisner, E.; Baumberg, J.J.; Steiner, U.; Vignolini, S. Digital Color in Cellulose Nanocrystal Films. ACS Appl. Mater. Interfaces 2014, 6, 12302–12306. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.; Ma, Z.; Tian, D.; Gu, M.; Lin, F. Structure–Color Mechanism of Iridescent Cellulose Nanocrystal Films. RSC Adv. 2014, 4, 39322–39331. [Google Scholar] [CrossRef]
- Jativa, F.; Schütz, C.; Bergström, L.; Zhang, X.; Wicklein, B. Confined Self-Assembly of Cellulose Nanocrystals in a Shrinking Droplet. Soft Matter 2015, 11, 5374–5380. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Bouchard, J.; Berry, R. Controlling the Reflection Wavelength of Iridescent Solid Films of Nanocrystalline Cellulose. Biomacromolecules 2011, 12, 167–172. [Google Scholar] [CrossRef]
- De La Cruz, J.A.; Liu, Q.; Senyuk, B.; Frazier, A.W.; Peddireddy, K.; Smalyukh, I.I. Cellulose-Based Reflective Liquid Crystal Films as Optical Filters and Solar Gain Regulators. ACS Photonics 2018, 5, 2468–2477. [Google Scholar] [CrossRef]
- Caligiuri, V.; Tedeschi, G.; Palei, M.; Miscuglio, M.; Martin-Garcia, B.; Guzman-Puyol, S.; Hedayati, M.K.; Kristensen, A.; Athanassiou, A.; Cingolani, R. Biodegradable and Insoluble Cellulose Photonic Crystals and Metasurfaces. ACS Nano 2020, 14, 9502–9511. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.S.; Grolman, D.L.; Karim, A.; Gilman, J.W. What Do We Still Need to Understand to Commercialize Cellulose Nanomaterials. Green Mater. 2015, 3, 53–58. [Google Scholar] [CrossRef]
- Pan, J.; Hamad, W.; Straus, S.K. Parameters Affecting the Chiral Nematic Phase of Nanocrystalline Cellulose Films. Macromolecules 2010, 43, 3851–3858. [Google Scholar] [CrossRef]
- Meda, R.S.; Jain, S.; Singh, S.; Verma, C.; Nandi, U.; Maji, P.K. Novel Lagenaria Siceraria Peel Waste Based Cellulose Nanocrystals: Isolation and Rationalizing H-Bonding Interactions. Ind. Crops Prod. 2022, 186, 115197. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Singh, S.; Meda, R.S.; Jain, S.; Maji, P.K. Structural and Morphological Exploration of Cellulose Nanocrystals Extracted from Lignocellulosic Waste Biomass of Brassica Nigra (Mustard Straw). Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Rani, A.; Kumari, A.; Thakur, M.; Mandhan, K.; Chandel, M.; Sharma, A. Bionanocomposite Synthesized from Nanocellulose Obtained from Agricultural Biomass as Raw Material. In Biorenewable Nanocomposite Materials, Vol. 1: Electrocatalysts and Energy Storage; American Chemical Society: Washington, DC, USA, 2022; pp. 47–74. [Google Scholar]
- Raza, M.; Abu-Jdayil, B.; Banat, F.; Al-Marzouqi, A.H. Isolation and Characterization of Cellulose Nanocrystals from Date Palm Waste. ACS Omega 2022, 7, 25366–25379. [Google Scholar] [CrossRef] [PubMed]
- Gray, D. Recent Advances in Chiral Nematic Structure and Iridescent Color of Cellulose Nanocrystal Films. Nanomaterials 2016, 6, 213. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.E.S.; Chagas, R.; Fernandes, S.N.; Pieranski, P.; Selinger, R.L.B.; Godinho, M.H. Travelling Colourful Patterns in Self-Organized Cellulose-Based Liquid Crystalline Structures. Commun. Mater. 2021, 2, 79. [Google Scholar] [CrossRef]
- Wilts, B.D.; Dumanli, A.G.; Middleton, R.; Vukusic, P.; Vignolini, S. Invited Article: Chiral Optics of Helicoidal Cellulose Nanocrystal Films. APL Photonics 2017, 2, 040801. [Google Scholar] [CrossRef]
- Trindade, A.C.; Carreto, M.; Helgesen, G.; Knudsen, K.D.; Puchtler, F.; Breu, J.; Fernandes, S.; Godinho, M.H.; Fossum, J.O. Photonic Composite Materials from Cellulose Nanorods and Clay Nanolayers. Eur. Phys. J. Spec. Top. 2020, 229, 2741–2755. [Google Scholar] [CrossRef]
- Kumar, A.; Cruz, C.; Figueirinhas, J.L.; Sebastião, P.J.; Trindade, A.C.; Fernandes, S.N.; Godinho, M.H.; Fossum, J.O. Water Dynamics in Composite Aqueous Suspensions of Cellulose Nanocrystals and a Clay Mineral Studied through Magnetic Resonance Relaxometry. J. Phys. Chem. B 2021, 125, 12787–12796. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive Photonic Hydrogels Based on Nanocrystalline Cellulose. Angew. Chem. Int. Ed. 2013, 52, 8912–8916. [Google Scholar] [CrossRef]
- Yurtsever, A.; Wang, P.-X.; Priante, F.; Morais Jaques, Y.; Miyazawa, K.; MacLachlan, M.J.; Foster, A.S.; Fukuma, T. Molecular Insights on the Crystalline Cellulose-Water Interfaces via Three-Dimensional Atomic Force Microscopy. Sci. Adv. 2022, 8. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Lu, W.; Qiu, L.; Meng, Z.; Qiao, Y. Thermal and Stress Tension Dual-Responsive Photonic Crystal Nanocomposite Hydrogels. RSC Adv. 2019, 9, 21202–21205. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent Advances in Nanocellulose Composites with Polymers: A Guide for Choosing Partners and How to Incorporate Them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Ni, Z.; Yang, C.; Ni, Y.; Lv, Y. Gelatin-Oxidized Nanocellulose Hydrogels Suitable for Extrusion-Based 3D Bioprinting. Processes 2022, 10, 2216. [Google Scholar] [CrossRef]
- Han, C.; Wang, X.; Ni, Z.; Ni, Y.; Huan, W.; Lv, Y.; Bai, S. Effects of Nanocellulose on Alginate/Gelatin Bio-Inks for Extrusion-Based 3D Printing. Bioresources 2020, 15, 7357–7373. [Google Scholar] [CrossRef]
- Szymkowiak, J.K.; Walters, C.M.; Hamad, W.Y.; MacLachlan, M.J. Tuning the Properties of Chiral Nematic Mesoporous (Organo)Silica Through Thiol-Ene Click Chemistry. Eur. J. Inorg. Chem. 2022, 2022, e202200218. [Google Scholar] [CrossRef]
- Terpstra, A.S.; Arnett, L.P.; Manning, A.P.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Iridescent Chiral Nematic Mesoporous Organosilicas with Alkylene Spacers. Adv. Opt. Mater. 2018, 6, 1800163. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.; Wang, Q.; Zhao, Y.; Shang, L. Cholesteric Cellulose Liquid Crystal Ink for Three-Dimensional Structural Coloration. Proc. Natl. Acad. Sci. USA 2022, 119. [Google Scholar] [CrossRef]
- Giese, M.; Khan, M.K.; Hamad, W.Y.; MacLachlan, M.J. Imprinting of Photonic Patterns with Thermosetting Amino-Formaldehyde-Cellulose Composites. ACS Macro. Lett. 2013, 2, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Lizundia, E.; Nguyen, T.-D.; Vilas, J.L.; Hamad, W.Y.; MacLachlan, M.J. Chiroptical, Morphological and Conducting Properties of Chiral Nematic Mesoporous Cellulose/Polypyrrole Composite Films. J. Mater. Chem. A Mater. 2017, 5, 19184–19194. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, D.; Jia, H.; Lei, K.; Zheng, Z.; Wang, X. Humidity and Heat Dual Response Cellulose Nanocrystals/Poly(N-Isopropylacrylamide) Composite Films with Cyclic Performance. ACS Appl. Mater. Interfaces 2019, 11, 39192–39200. [Google Scholar] [CrossRef] [PubMed]
- Walters, C.M.; Boott, C.E.; Nguyen, T.-D.; Hamad, W.Y.; MacLachlan, M.J. Iridescent Cellulose Nanocrystal Films Modified with Hydroxypropyl Cellulose. Biomacromolecules 2020, 21, 1295–1302. [Google Scholar] [CrossRef]
- Chen, J.; Ling, Z.; Wang, X.; Ping, X.; Xie, Y.; Ma, H.; Guo, J.; Yong, Q. All Bio-Based Chiral Nematic Cellulose Nanocrystals Films under Supramolecular Tuning by Chitosan/Deacetylated Chitin Nanofibers for Reversible Multi-Response and Sensor Application. Chem. Eng. J. 2023, 466, 143148. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, Z.; Chen, J.; Luo, Y.; Li, L.; Liu, K.; Ding, S.; Li, H.; Liu, M.; Zhou, C. Photocurable Liquid Crystal Hydrogels with Different Chargeability and Tunable Viscoelasticity Based on Chitin Whiskers. Carbohydr. Polym. 2023, 301, 120299. [Google Scholar] [CrossRef]
- Basta, A.A.H.; Lotfy, V.; Micky, J.; Salem, A.M. Selective Route for Enhancing Liquid Crystal-Based Hydroxylpropyl Cellulose by Esterification. Pigment. Resin. Technol. 2023, 52, 285–298. [Google Scholar] [CrossRef]
- Querejeta-Fernández, A.; Chauve, G.; Methot, M.; Bouchard, J.; Kumacheva, E. Chiral Plasmonic Films Formed by Gold Nanorods and Cellulose Nanocrystals. J. Am. Chem. Soc. 2014, 136, 4788–4793. [Google Scholar] [CrossRef]
- Xia, K.; Zheng, X.; Wang, Y.; Zhong, W.; Dong, Z.; Ye, Z.; Zhang, Z. Biomimetic Chiral Photonic Materials with Tunable Metallic Colorations Prepared from Chiral Melanin-like Nanorods for UV Shielding, Humidity Sensing, and Cosmetics. Langmuir 2022, 38, 8114–8124. [Google Scholar] [CrossRef]
- Qi, F.; Jeong, K.-J.; Gong, J.; Tang, Z. Modulation of Nano-Superstructures and Their Optical Properties. Acc. Chem. Res. 2022, 55, 2425–2438. [Google Scholar] [CrossRef]
- Schlesinger, M.; Giese, M.; Blusch, L.K.; Hamad, W.Y.; MacLachlan, M.J. Chiral Nematic Cellulose–Gold Nanoparticle Composites from Mesoporous Photonic Cellulose. Chem. Commun. 2015, 51, 530–533. [Google Scholar] [CrossRef] [PubMed]
- Bast, L.K.; Klockars, K.W.; Greca, L.G.; Rojas, O.J.; Tardy, B.L.; Bruns, N. Infiltration of Proteins in Cholesteric Cellulose Structures. Biomacromolecules 2021, 22, 2067–2080. [Google Scholar] [CrossRef] [PubMed]
- Mehranfar, A.; Khavani, M.; Mofrad, M.R.K. Adsorption Process of Various Antimicrobial Peptides on Different Surfaces of Cellulose. ACS Appl. Bio Mater. 2023, 6, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, P.; Gandier, J.; Nonappa; Wagermaier, W.; Miserez, A.; Penttilä, M. Bioinspired Functionally Graded Composite Assembled Using Cellulose Nanocrystals and Genetically Engineered Proteins with Controlled Biomineralization. Adv. Mater. 2021, 33, 2102658. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Chen, J.; Ling, Z.; Guo, J.; Huang, J.; Ma, J.; Jin, Z. Chiral Nematic Cellulose Nanocrystal Films Cooperated with Amino Acids for Tunable Optical Properties. Polymers 2021, 13, 4389. [Google Scholar] [CrossRef]
- Aalbers, G.J.W.; Boott, C.E.; D’Acierno, F.; Lewis, L.; Ho, J.; Michal, C.A.; Hamad, W.Y.; MacLachlan, M.J. Post-Modification of Cellulose Nanocrystal Aerogels with Thiol–Ene Click Chemistry. Biomacromolecules 2019, 20, 2779–2785. [Google Scholar] [CrossRef]
- Xu, Y.-T.; Walters, C.M.; D’Acierno, F.; Hamad, W.Y.; Michal, C.A.; MacLachlan, M.J. Cellulose Nanocrystal Chiral Nematic Composites with Wet Mechanical Adaptability. Chem. Mater. 2022, 34, 4311–4319. [Google Scholar] [CrossRef]
- Andrew, L.J.; Walters, C.M.; Hamad, W.Y.; MacLachlan, M.J. Coassembly of Cellulose Nanocrystals and Neutral Polymers in Iridescent Chiral Nematic Films. Biomacromolecules 2023, 24, 896–908. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Zhai, S.; Sugiyama, J.; Pan, M.; Shi, J.; Lu, H. Dual Response of Photonic Films with Chiral Nematic Cellulose Nanocrystals: Humidity and Formaldehyde. ACS Appl. Mater. Interfaces 2020, 12, 17833–17844. [Google Scholar] [CrossRef]
- Peng, N.; Huang, D.; Gong, C.; Wang, Y.; Zhou, J.; Chang, C. Controlled Arrangement of Nanocellulose in Polymeric Matrix: From Reinforcement to Functionality. ACS Nano 2020, 14, 16169–16179. [Google Scholar] [CrossRef]
- Ling, Z.; Chen, J.; Wang, X.; Shao, L.; Wang, C.; Chen, S.; Guo, J.; Yong, Q. Nature-Inspired Construction of Iridescent CNC/Nano-Lignin Films for UV Resistance and Ultra-Fast Humidity Response. Carbohydr. Polym. 2022, 296, 119920. [Google Scholar] [CrossRef]
- Duan, R.; Lu, M.; Tang, R.; Guo, Y.; Zhao, D. Structural Color Controllable Humidity Response Chiral Nematic Cellulose Nanocrystalline Film. Biosensors 2022, 12, 707. [Google Scholar] [CrossRef]
- Kaschuk, J.J.; Al Haj, Y.; Rojas, O.J.; Miettunen, K.; Abitbol, T.; Vapaavuori, J. Plant-Based Structures as an Opportunity to Engineer Optical Functions in Next-Generation Light Management. Adv. Mater. 2022, 34, 2104473. [Google Scholar] [CrossRef]
- Tran, K.T.M.; Nguyen, T.D. Lithography-Based Methods to Manufacture Biomaterials at Small Scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Kasani, S.; Curtin, K.; Wu, N. A Review of 2D and 3D Plasmonic Nanostructure Array Patterns: Fabrication, Light Management and Sensing Applications. Nanophotonics 2019, 8, 2065–2089. [Google Scholar] [CrossRef]
- Wolfberger, A.; Petritz, A.; Fian, A.; Herka, J.; Schmidt, V.; Stadlober, B.; Kargl, R.; Spirk, S.; Griesser, T. Photolithographic Patterning of Cellulose: A Versatile Dual-Tone Photoresist for Advanced Applications. Cellulose 2015, 22, 717–727. [Google Scholar] [CrossRef]
- Espinha, A.; Dore, C.; Matricardi, C.; Alonso, M.I.; Goñi, A.R.; Mihi, A. Hydroxypropyl Cellulose Photonic Architectures by Soft Nanoimprinting Lithography. Nat. Photonics 2018, 12, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Chu, G.; Camposeo, A.; Vilensky, R.; Vasilyev, G.; Martin, P.; Pisignano, D.; Zussman, E. Printing Flowers? Custom-Tailored Photonic Cellulose Films with Engineered Surface Topography. Matter 2019, 1, 988–1000. [Google Scholar] [CrossRef]
- Chu, G.; Qu, D.; Camposeo, A.; Pisignano, D.; Zussman, E. When Nanocellulose Meets Diffraction Grating: Freestanding Photonic Paper with Programmable Optical Coupling. Mater. Horiz. 2020, 7, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Khakalo, A.; Mäkelä, T.; Johansson, L.-S.; Orelma, H.; Tammelin, T. High-Throughput Tailoring of Nanocellulose Films: From Complex Bio-Based Materials to Defined Multifunctional Architectures. ACS Appl. Bio Mater. 2020, 3, 7428–7438. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, T.; Hokkanen, A.; Sneck, A.; Ruotsalainen, T.; Khakalo, A.; Tammelin, T. Vapour-Assisted Roll-to-Roll Nanoimprinting of Micropillars on Nanocellulose Films. Microelectron. Eng. 2020, 225, 111258. [Google Scholar] [CrossRef]
- Mäkelä, T.; Kainlauri, M.; Willberg-Keyriläinen, P.; Tammelin, T.; Forsström, U. Fabrication of Micropillars on Nanocellulose Films Using a Roll-to-Roll Nanoimprinting Method. Microelectron. Eng. 2016, 163, 1–6. [Google Scholar] [CrossRef]
- Mäkelä, T.; Haatainen, T.; Ahopelto, J. Roll-to-Roll Printed Gratings in Cellulose Acetate Web Using Novel Nanoimprinting Device. Microelectron. Eng. 2011, 88, 2045–2047. [Google Scholar] [CrossRef]
- Daqiqeh Rezaei, S.; Dong, Z.; You En Chan, J.; Trisno, J.; Ng, R.J.H.; Ruan, Q.; Qiu, C.-W.; Mortensen, N.A.; Yang, J.K.W. Nanophotonic Structural Colors. ACS Photonics 2021, 8, 18–33. [Google Scholar] [CrossRef]
- He, Y.-D.; Zhang, Z.-L.; Xue, J.; Wang, X.-H.; Song, F.; Wang, X.-L.; Zhu, L.-L.; Wang, Y.-Z. Biomimetic Optical Cellulose Nanocrystal Films with Controllable Iridescent Color and Environmental Stimuli-Responsive Chromism. ACS Appl. Mater. Interfaces 2018, 10, 5805–5811. [Google Scholar] [CrossRef] [PubMed]
- Anusuyadevi, P.R.; Shanker, R.; Cui, Y.; Riazanova, A.V.; Järn, M.; Jonsson, M.P.; Svagan, A.J. Photoresponsive and Polarization-Sensitive Structural Colors from Cellulose/Liquid Crystal Nanophotonic Structures. Adv. Mater. 2021, 33, 2101519. [Google Scholar] [CrossRef]
- Zhu, S.; Tang, Y.; Lin, C.; Liu, X.Y.; Lin, Y. Recent Advances in Patterning Natural Polymers: From Nanofabrication Techniques to Applications. Small Methods 2021, 5, 2001060. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M. Emerging Applications of Stimuli-Responsive Polymer Materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Li, Z.; Yang, X.; Li, W.; Liu, H. Stimuli-Responsive Cellulose Paper Materials. Carbohydr. Polym. 2019, 210, 350–363. [Google Scholar] [CrossRef]
- Moreno, A.; Sipponen, M.H. Lignin-Based Smart Materials: A Roadmap to Processing and Synthesis for Current and Future Applications. Mater. Horiz. 2020, 7, 2237–2257. [Google Scholar] [CrossRef]
- Cao, X.; Peng, X.; Zhong, L.; Sun, R. Multiresponsive Hydrogels Based on Xylan-Type Hemicelluloses and Photoisomerized Azobenzene Copolymer as Drug Delivery Carrier. J. Agric. Food Chem. 2014, 62, 10000–10007. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Lin, Q.; Tai, Y.-A.A.; Wang, Y. Applications of Cellulose Nanomaterials in Stimuli-Responsive Optics. J. Agric. Food Chem. 2020, 68, 12940–12955. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Huang, C.; Huang, H. Recent Advances in Structural Color Display of Cellulose Nanocrystal Materials. Appl. Mater. Today 2021, 22, 100912. [Google Scholar] [CrossRef]
- Wang, Q.; Ji, C.; Sun, J.; Zhu, Q.; Liu, J. Structure and Properties of Polylactic Acid Biocomposite Films Reinforced with Cellulose Nanofibrils. Molecules 2020, 25, 3306. [Google Scholar] [CrossRef]
- Pinto, L.F.V.; Kundu, S.; Brogueira, P.; Cruz, C.; Fernandes, S.N.; Aluculesei, A.; Godinho, M.H. Cellulose-Based Liquid Crystalline Photoresponsive Films with Tunable Surface Wettability. Langmuir 2011, 27, 6330–6337. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, H.; Li, G.; Wang, C.; Huang, Y.; Liu, R. Synthesis and Photosensitivity of Azobenzene Functionalized Hydroxypropylcellulose. RSC Adv. 2013, 3, 15909. [Google Scholar] [CrossRef]
- Filpponen, I.; Sadeghifar, H.; Argyropoulos, D.S. Photoresponsive Cellulose Nanocrystals. Nanomater. Nanotechnol. 2011, 1, 7. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Morozova, S.M. Sustainable optics? A critical insight into biopolymer-enabled optics. Tetrahedron Green Chem. 2023, 1, 100005. [Google Scholar] [CrossRef]
- Ai, L.; Liu, H.; Liu, R.; Song, H.; Song, Z.; Nie, M.; Waterhouse, G.I.N.; Lu, S. Dual Sensitivity of Spiropyran-Functionalized Carbon Dots for Full Color Conversions. Sci. China Chem. 2022, 65, 2274–2282. [Google Scholar] [CrossRef]
- Jin, K.; Ji, X.; Yang, T.; Zhang, J.; Tian, W.; Yu, J.; Zhang, X.; Chen, Z.; Zhang, J. Facile Access to Photo-Switchable, Dynamic-Optical, Multi-Colored and Solid-State Materials from Carbon Dots and Cellulose for Photo-Rewritable Paper and Advanced Anti-Counterfeiting. Chem. Eng. J. 2021, 406, 126794. [Google Scholar] [CrossRef]
- Chu, L.; Zhang, X.; Niu, W.; Wu, S.; Ma, W.; Tang, B.; Zhang, S. Hollow Silica Opals/Cellulose Acetate Nanocomposite Films with Structural Colors for Anti-Counterfeiting of Banknotes. J. Mater. Chem. C Mater. 2019, 7, 7411–7417. [Google Scholar] [CrossRef]
- Ma, W.; Kou, Y.; Zhao, P.; Zhang, S. Bioinspired Structural Color Patterns Derived from 1D Photonic Crystals with High Saturation and Brightness for Double Anti-Counterfeiting Decoration. ACS Appl. Polym. Mater. 2020, 2, 1605–1613. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Gong, Y.-B.; Ma, S.; Wang, Y.-H.; Gan, L.; Huang, J. Antistatic Structural Color and Photoluminescent Membranes from Co-Assembling Cellulose Nanocrystals and Carbon Nanomaterials for Anti-Counterfeiting. Chin. J. Polym. Sci. 2020, 38, 1061–1071. [Google Scholar] [CrossRef]
- Choi, J.; Hua, M.; Lee, S.Y.; Jo, W.; Lo, C.; Kim, S.; Kim, H.; He, X. Hydrocipher: Bioinspired Dynamic Structural Color-Based Cryptographic Surface. Adv. Opt. Mater. 2020, 8, 1901259. [Google Scholar] [CrossRef]
- Lv, H.; Wang, S.; Wang, Z.; Meng, W.; Han, X.; Pu, J. Fluorescent Cellulose-Based Hydrogel with Carboxymethyl Cellulose and Carbon Quantum Dots for Information Storage and Fluorescent Anti-Counterfeiting. Cellulose 2022, 29, 6193–6204. [Google Scholar] [CrossRef]
- Li, D.; Yuan, J.; Cheng, Q.; Wei, P.; Cheng, G.J.; Chang, C. Additive Printing of Recyclable Anti-Counterfeiting Patterns with Sol–Gel Cellulose Nanocrystal Inks. Nanoscale 2021, 13, 11808–11816. [Google Scholar] [CrossRef]
- Chang, T.; Wang, B.; Yuan, D.; Wang, Y.; Smalyukh, I.; Zhou, G.; Zhang, Z. Cellulose Nanocrystal Chiral Photonic Micro-Flakes for Multilevel Anti-Counterfeiting and Identification. Chem. Eng. J. 2022, 446, 136630. [Google Scholar] [CrossRef]
- Nawaz, H.; Chen, S.; Li, X.; Zhang, X.; Zhang, X.; Wang, J.-Q.; Xu, F. Cellulose-Based Environment-Friendly Smart Materials for Colorimetric and Fluorescent Detection of Cu2+/Fe3+ Ions and Their Anti-Counterfeiting Applications. Chem. Eng. J. 2022, 438, 135595. [Google Scholar] [CrossRef]
- Hong, W.; Yuan, Z.; Chen, X. Structural Color Materials for Optical Anticounterfeiting. Small 2020, 16, 1907626. [Google Scholar] [CrossRef]
- Tittl, A. Tunable Structural Colors on Display. Light Sci. Appl. 2022, 11, 155. [Google Scholar] [CrossRef]
- Available online: htttps://Www.Photonics.Com/Articles/Tunable_Approach_to_Structural_Color_Powers/A67188 (accessed on 15 May 2023).
- Espinha, A.; Guidetti, G.; Serrano, M.C.; Frka-Petesic, B.; Dumanli, A.G.; Hamad, W.Y.; Blanco, Á.; López, C.; Vignolini, S. Shape Memory Cellulose-Based Photonic Reflectors. ACS Appl. Mater. Interfaces 2016, 8, 31935–31940. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Cheng, Z.; Wang, B.; Zeng, J.; Xu, J.; Li, J.; Gao, W.; Chen, K. Chiral Photonic Liquid Crystal Films Derived from Cellulose Nanocrystals. Small 2021, 17, 2007306. [Google Scholar] [CrossRef]
- Ghiradella, H. Coloration. In Encyclopedia of Insects; Elsevier: Amsterdam, The Netherlands, 2009; pp. 213–220. [Google Scholar]
- Eremeeva, E.; Sergeeva, E.; Neterebskaia, V.; Morozova, S.; Kolchanov, D.; Morozov, M.; Chernyshov, I.; Milichko, V.; Vinogradov, A. Printing of Colorful Cellulose Nanocrystalline Patterns Visible in Linearly Polarized Light. ACS Appl. Mater. Interfaces 2020, 12, 45145–45154. [Google Scholar] [CrossRef] [PubMed]
- Umani, V. Colors in Architecture: Matter and Communication Tool. In Colour and Colorimetry Multidisciplinary Contributions Vol. XVII; AIC Association Internationale de la Couleur: Florence, Italy, 2022. [Google Scholar]
- Körner, A.; Born, L.; Bucklin, O.; Suzuki, S.; Vasey, L.; Gresser, G.T.; Menges, A.; Knippers, J. Integrative Design and Fabrication Methodology for Bio-Inspired Folding Mechanisms for Architectural Applications. Comput. Aided Des. 2021, 133, 102988. [Google Scholar] [CrossRef]
- An, B.; Xu, M.; Sun, J.; Sun, W.; Miao, Y.; Ma, C.; Luo, S.; Li, J.; Li, W.; Liu, S. Cellulose Nanocrystals-Based Bio-Composite Optical Materials for Reversible Colorimetric Responsive Films and Coatings. Int. J. Biol. Macromol. 2023, 233, 123600. [Google Scholar] [CrossRef]
- Droguet, B.E.; Liang, H.-L.; Frka-Petesic, B.; Parker, R.M.; De Volder, M.F.L.; Baumberg, J.J.; Vignolini, S. Large-Scale Fabrication of Structurally Coloured Cellulose Nanocrystal Films and Effect Pigments. Nat. Mater. 2022, 21, 352–358. [Google Scholar] [CrossRef]
- Wei, J.; Zhang, G.; Dong, J.; Wang, H.; Guo, Y.; Zhuo, X.; Li, C.; Liang, H.; Gu, S.; Li, C. Facile, Scalable Spray-Coating of Stable Emulsion for Transparent Self-Cleaning Surface of Cellulose-Based Materials. ACS Sustain. Chem. Eng. 2018, 6, 11335–11344. [Google Scholar] [CrossRef]
- Teisala, H.; Tuominen, M.; Kuusipalo, J. Superhydrophobic Coatings on Cellulose-Based Materials: Fabrication, Properties, and Applications. Adv. Mater. Interfaces 2014, 1, 1300026. [Google Scholar] [CrossRef]
- Chen, Z.; Xiao, P.; Zhang, J.; Tian, W.; Jia, R.; Nawaz, H.; Jin, K.; Zhang, J. A Facile Strategy to Fabricate Cellulose-Based, Flame-Retardant, Transparent and Anti-Dripping Protective Coatings. Chem. Eng. J. 2020, 379, 122270. [Google Scholar] [CrossRef]
- de Amorim, J.D.P.; de Souza, K.C.; Duarte, C.R.; da Silva Duarte, I.; de Assis Sales Ribeiro, F.; Silva, G.S.; de Farias, P.M.A.; Stingl, A.; Costa, A.F.S.; Vinhas, G.M. Plant and Bacterial Nanocellulose: Production, Properties and Applications in Medicine, Food, Cosmetics, Electronics and Engineering. A Review. Environ. Chem. Lett. 2020, 18, 851–869. [Google Scholar] [CrossRef]
- Yıldırım, F.F.; Yavas, A.; Avinc, O. Bacteria Working to Create Sustainable Textile Materials and Textile Colorants Leading to Sustainable Textile Design. In Sustainability in the Textile and Apparel Industries: Sustainable Textiles, Clothing Design and Repurposing; Springer: Berlin/Heidelberg, Germany, 2020; pp. 109–126. [Google Scholar]
- Benkhaya, S.; M’ rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Yavuz, G.; Zille, A.; Seventekin, N.; Souto, A.P. Structural Coloration of Chitosan Coated Cellulose Fabrics by Electrostatic Self-Assembled Poly (Styrene-Methyl Methacrylate-Acrylic Acid) Photonic Crystals. Carbohydr. Polym. 2018, 193, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Wu, S.; Lv, P.; Ke, H.; Huang, F.; Wei, Q. Chameleon-Inspired Iridescent Structural Color Textiles with Reversible Multiple Stimulus-Responsive Functions. Chem. Eng. J. 2022, 433, 134410. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quelhas, A.R.; Trindade, A.C. Mimicking Natural-Colored Photonic Structures with Cellulose-Based Materials. Crystals 2023, 13, 1010. https://doi.org/10.3390/cryst13071010
Quelhas AR, Trindade AC. Mimicking Natural-Colored Photonic Structures with Cellulose-Based Materials. Crystals. 2023; 13(7):1010. https://doi.org/10.3390/cryst13071010
Chicago/Turabian StyleQuelhas, Ana Rita, and Ana Catarina Trindade. 2023. "Mimicking Natural-Colored Photonic Structures with Cellulose-Based Materials" Crystals 13, no. 7: 1010. https://doi.org/10.3390/cryst13071010
APA StyleQuelhas, A. R., & Trindade, A. C. (2023). Mimicking Natural-Colored Photonic Structures with Cellulose-Based Materials. Crystals, 13(7), 1010. https://doi.org/10.3390/cryst13071010





