Aqueous Chemical Synthesis of Nanosized ZnGa2O4 Using Mild Reaction Conditions: Effect of pH on the Structural, Morphological, Textural, Electronic, and Photocatalytic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Materials
2.3. Titration Study
2.4. Photocatalysis Experiments
2.5. Total Organic Carbon
2.6. Materials Characterization
3. Results and Discussion
3.1. Microstructure
3.2. Titration Curve
3.3. Reaction Mechanism Proposal
3.4. SEM Analysis
3.5. Electronic Properties
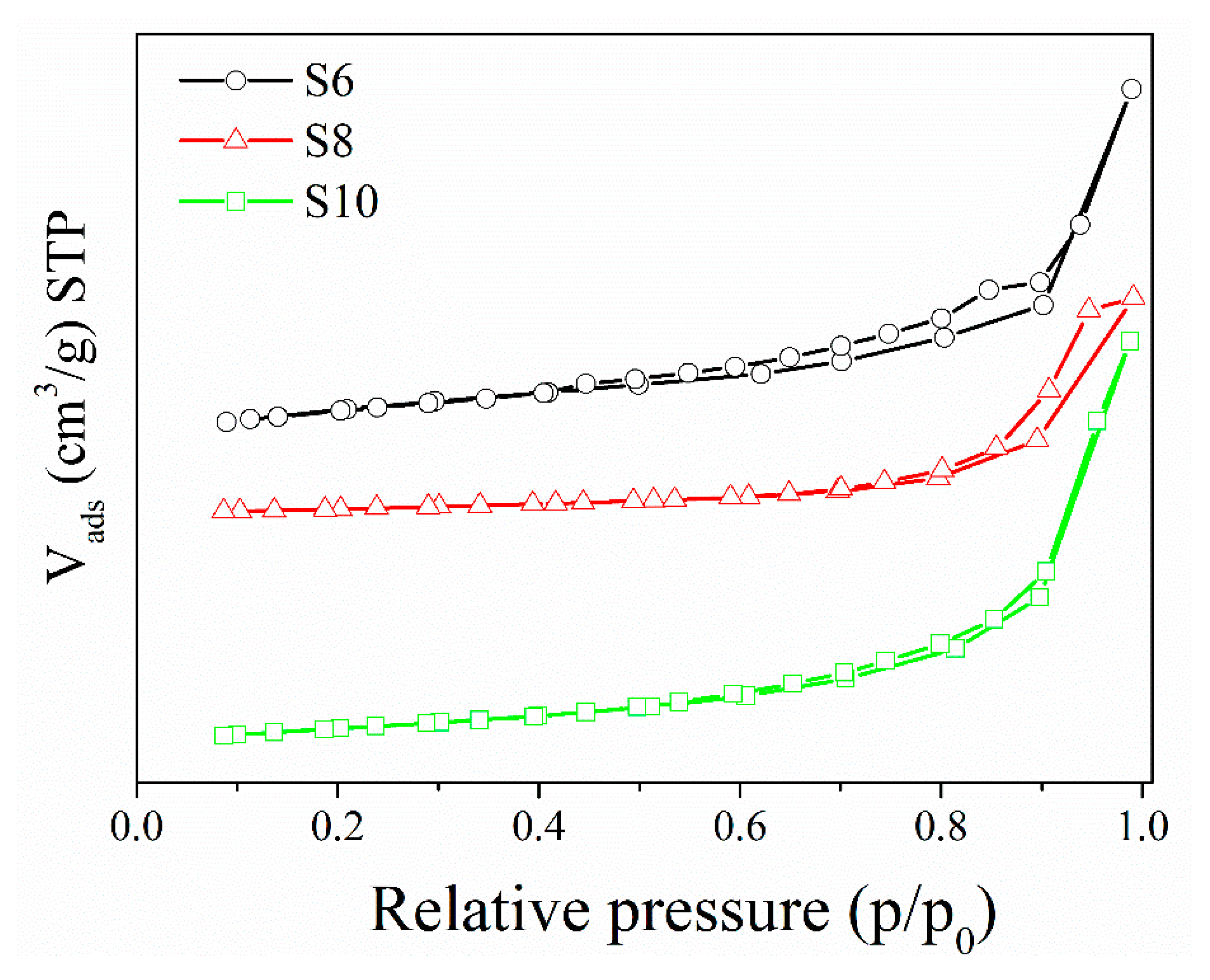
3.6. Textural Properties
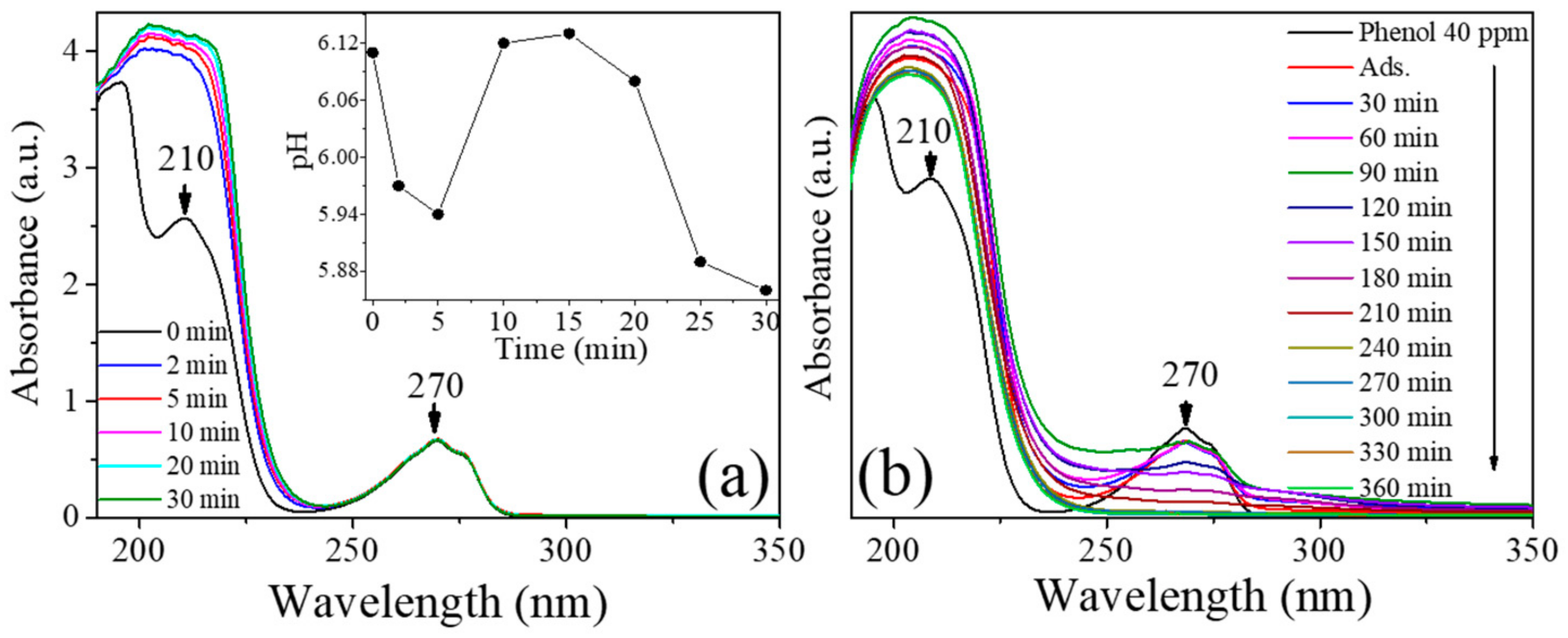
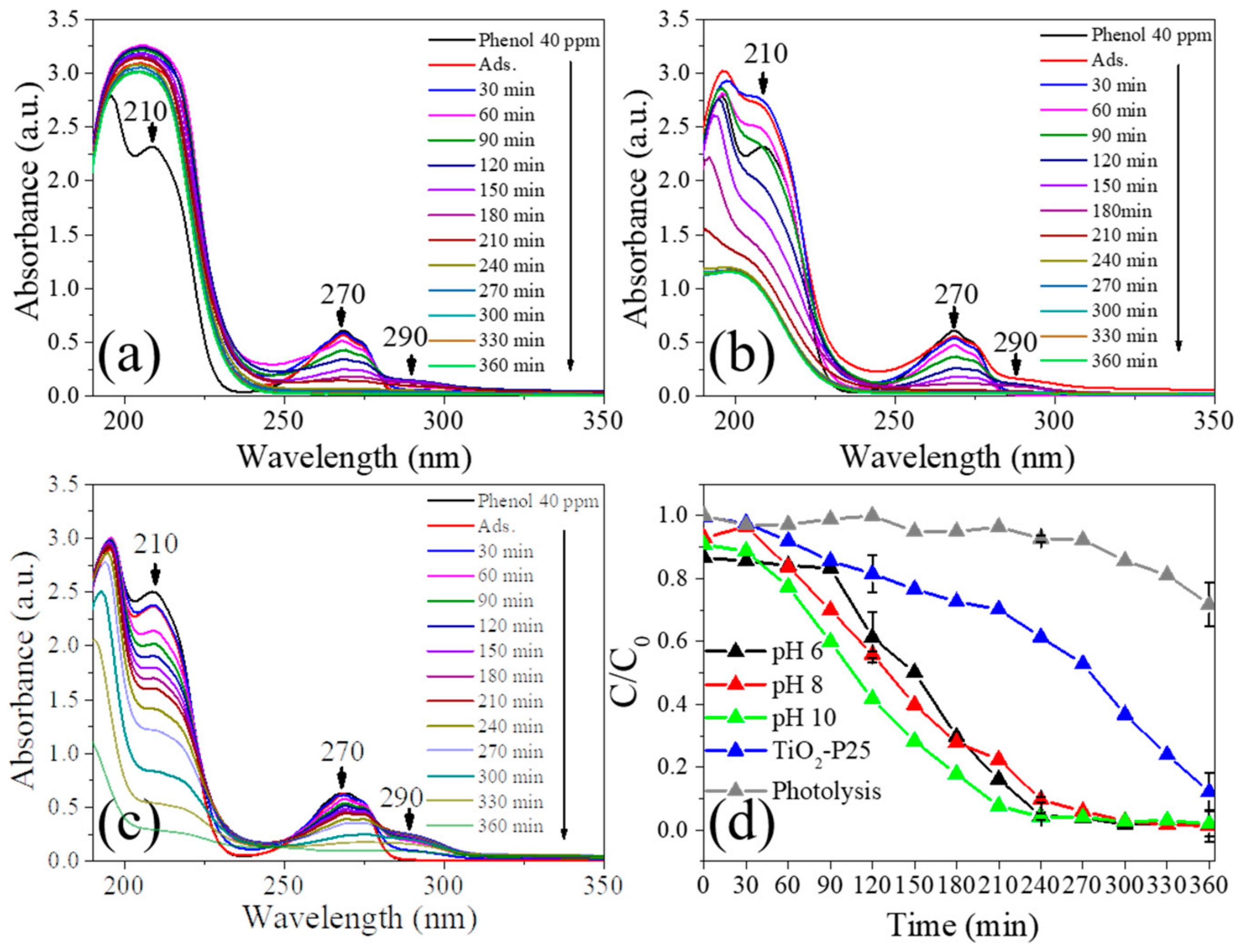
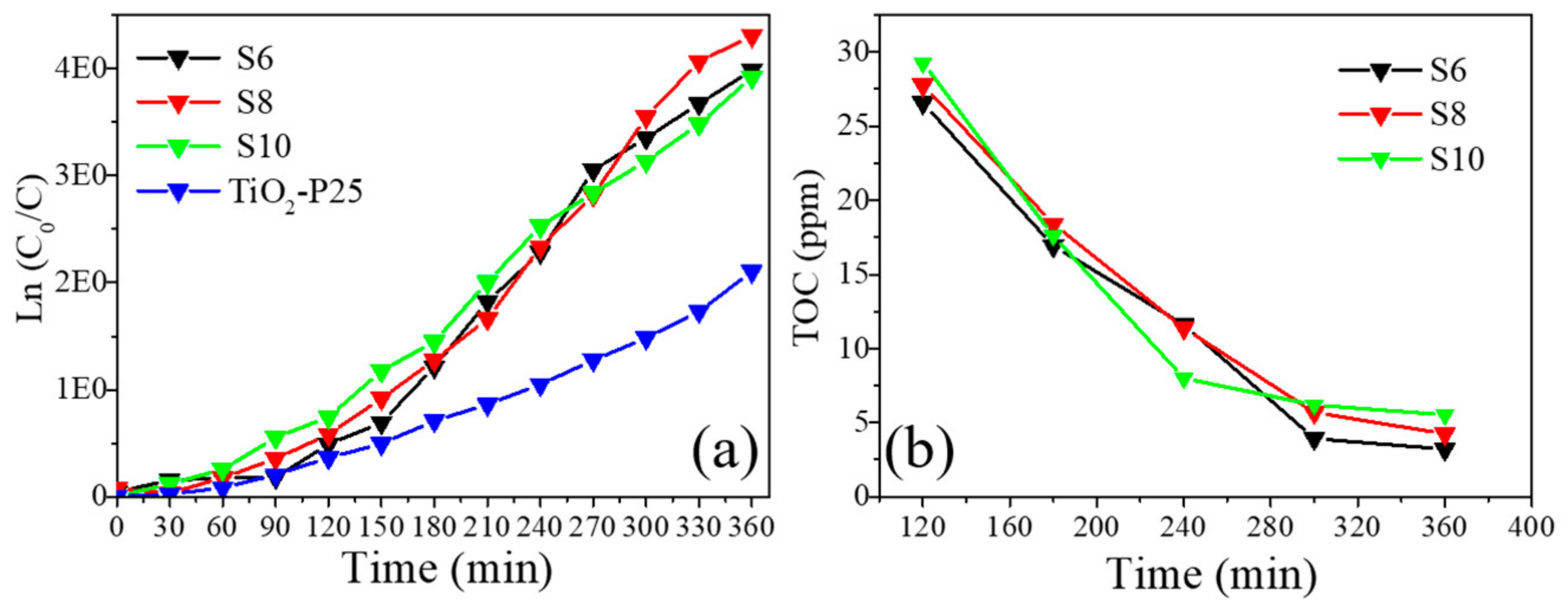
3.7. Photocatalytic Activity
3.8. TOC Results
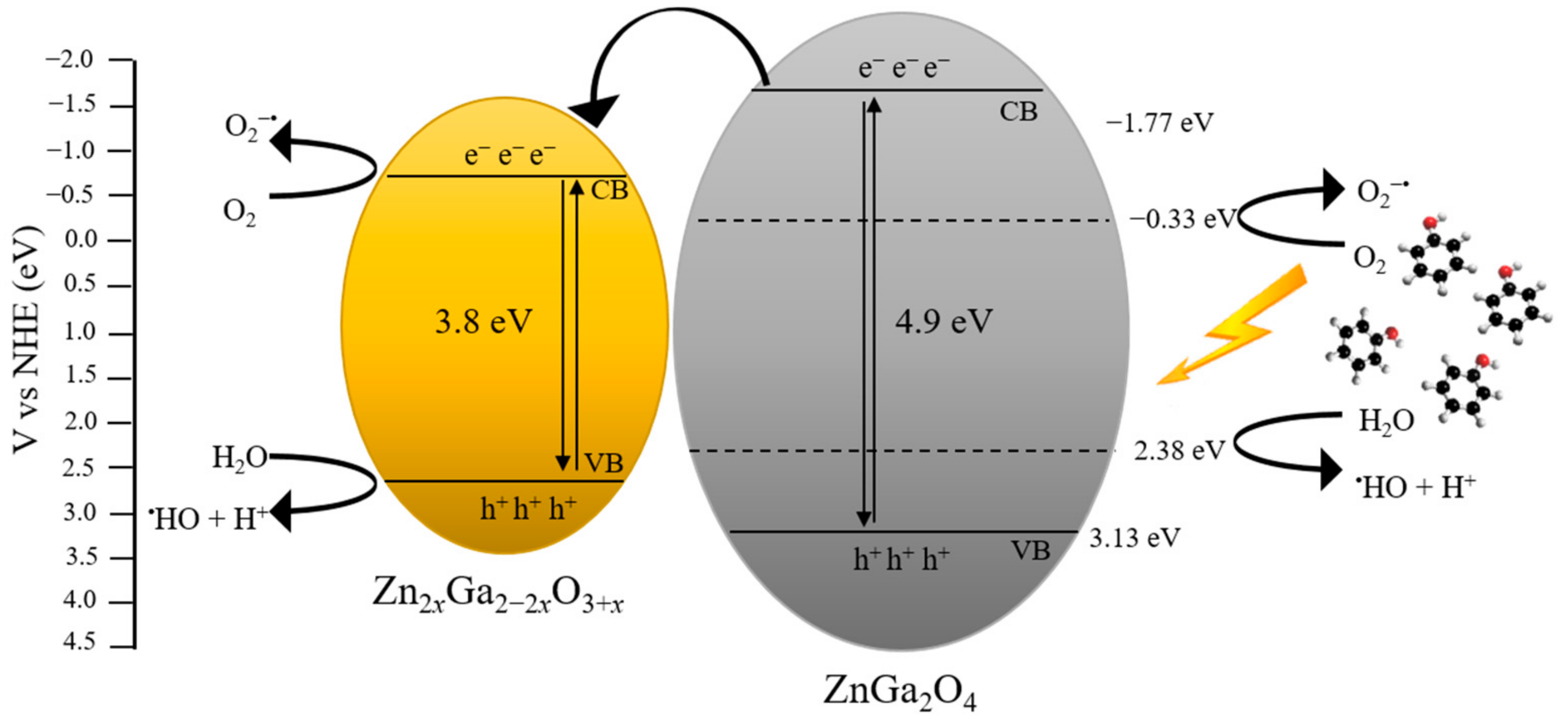
3.9. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boy, J.; Handwerg, M.; Mitdank, R.; Galazka, Z.; Fischer, S. Charge carrier density, mobility, and Seebeck coefficient of melt-grown bulk ZnGa2O4 single crystals. AIP Adv. 2010, 10, 055005. [Google Scholar] [CrossRef]
- Chi, Z.; Tarntair, F.-G.; Frègnaux, M.; Wu, W.-Y.; Sartel, C.; Madaci, I.; Chapon, P.; Sallet, V.; Dumont, Y.; Pérez-Tomás, A.; et al. Bipolar self-doping in ultra-wide band-gap spinel ZnGa2O4. Mater. Today Phys. 2021, 20, 100466. [Google Scholar]
- Zeng, C.; Hu, T.; Hou, N.; Liu, S.; Gao, W.; Cong, R.; Yang, T. Photocatalytic pure water splitting activities for ZnGa2O4 synthesized by various methods. Mater. Res. Bull. 2015, 61, 481–485. [Google Scholar] [CrossRef]
- Yang, J.; Sun, X.; Yang, W.; Zhu, M.; Shi, J. The Improvement of Coralline-Like ZnGa2O4 by Cocatalysts for the Photocatalytic Degradation of Rhodamine B. Catalysts 2020, 10, 221. [Google Scholar] [CrossRef]
- Nunes da Silva, M.; Miranda de Carvalho, J.; Carvalho de Abreu Fantini, M.; Chiavacci, L.A.; Bourgaux, C. Nanosized ZnGa2O4:Cr3+ Spinels as Highly Luminescent Materials for Bioimaging. ACS Appl. Nano Mater. 2019, 2, 6918–6927. [Google Scholar] [CrossRef]
- Safeera, T.A.; Jhons, N.; Krishna, K.M.; Sreenivasan, P.V.; Reshmi, R.; Anila, E.I. Zinc gallate and its starting materials in solid state reaction route: A comaparative study. Mater. Chem. Phys. 2016, 181, 21–25. [Google Scholar] [CrossRef]
- Hirano, M. Hydrothermal synthesis and characterization of ZnGa2O4 spinel fine particles. J. Mater. Chem. 2000, 10, 469–472. [Google Scholar] [CrossRef]
- Lee, J.-H.; Park, H.-J.; Yoo, J.; Kim, B.-W.; Lee, J.C.; Park, S. Characteristics of nano-sized ZnGa2O4 phosphor prepared by solution combustion method and solid state reaction method. J. Eur. Ceram. Soc. 2007, 27, 965–968. [Google Scholar] [CrossRef]
- Cheng, L.-C.; Wu, M.-R.; Huang, C.-Y.; Juang, T.-K.; Liu, P.-L.; Horng, R.-H. Effect of Defects on the Properties of ZnGa2O4 Thin-Film Transistors. ACS Appl. Electron. Mater. 2019, 1, 253–259. [Google Scholar] [CrossRef]
- Hirano, M.; Okumura, S.; Hasegawa, Y.; Inagaki, M. Direct precipitation of spinel type oxide ZnGa2O4 from aqueous solutions at low temperature below 90 °C. Int. J. Inorg. Mater. 2001, 3, 797–801. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Lee, K.; Kirchgeorg, R.; Hahn, R.; Schmuki, P. Self-organization and zinc doping of Ga2O3 nanoporous architecture: A potential nano-photogenerator for hydrogen. Electrochem. Commun. 2013, 35, 112–115. [Google Scholar] [CrossRef]
- Sakata, Y.; Matsuda, Y.; Yanagida, T.; Hirata, K.; Imamura, H.; Teramura, K. Effect of Metal Ion Addition in a Ni Supported Ga2O3 Photocatalyst on the Photocatalytic Overall Splitting of H2O. Catal. Lett. 2008, 125, 22–26. [Google Scholar] [CrossRef]
- Shrestha, N.K.; Bui, H.T.; Lee, T.; Noh, Y.-Y. Interfacial engineering of nanoporous architectures in Ga2O3 film towards self-aligned tubular nanostructure with an enhanced photocatalytic activity on water splitting. Langmuir 2018, 34, 4575–4583. [Google Scholar] [CrossRef]
- Ikarashi, K.; Sato, J.; Kobayashi, H.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalysis for Water Decomposition by RuO2-Dispersed ZnGa2O4 with d10 Configuration. J. Phys. Chem. B 2002, 106, 9048–9053. [Google Scholar] [CrossRef]
- Sharifi, F.; Mahmoodi, Z.; Abbasi, S.M.; Najafi, A.; Khalaj, G. Synthesis and characterization of mesoporous TiC nanopowder/nanowhisker with low residual carbon processed by sol-gel method. J. Mater. Res. Technol. 2023, 22, 2462–2472. [Google Scholar] [CrossRef]
- López-Salinas, E.; Torres-García, E.; García-Sánchez, M. Thermal behavior of hydrotalcite-like [Mg1−xGax(OH)2](CO3)x/2mH2O as a function of gallium content. J. Phys. Chem. Solids 1997, 58, 919–925. [Google Scholar] [CrossRef]
- Ross, G.J.; Kodama, H. Properties of a Synthetic Magnesium-Aluminum Carbonate Hydroxide and Its Relationship to Magnesium-Aluminum Double Hydroxide, Manasseite and Hydrotalcite. Am. Mineral. 1967, 52, 1036–1047. [Google Scholar]
- Jasrotia, R.; Suman Verma, A.; Verma, R.; Ahmed, J.; Kumar Godara, S.; Kumar, G.; Mehtab, A.; Ahmad, T.; Kalia, S. Photocatalytic dye degradation efficiency and reusability of Cu-substituted Zn-Mg spinel nanoferrites for wastewater remediation. J. Water Process. Eng. 2001, 48, 2214–7144. [Google Scholar] [CrossRef]
- Thomas, G.S.; Kamath, P.V. The layered double hydroxide (LDH) of Zn with Ga: Synthesis and reversible thermal behaviour. Solid State Sci. 2006, 8, 1181–1186. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, L.; Zuo, J.; Sun, D.; Jiang, C.; Fu, Y. Zinc-rich Ga1-xZnxN1-xOx solid solutions with tunable composition prepared from a constant-pH coprecipitation method. IOP Conf. Ser. Mater. Sci. Eng. 2007, 207, 012006. [Google Scholar] [CrossRef]
- Iida, Y.; Masaharu, O. Characterization of Zinc Gallate Phosphors. Anal. Sci. 2001, S17, i1145–i1147. [Google Scholar]
- Cavani, F.; Trifiro, F.; Vaccari, A. Hydrotalcite-type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Safeera, T.A.; Khanal, R.; Medvedeva, J.E.; Martinez, A.I.; Vinitha, G.; Anila, E.I. Low temperature synthesis and characterization of zinc gallate quantum dots for optoelectronic applications. J. Alloys Compd. 2001, 740, 567–573. [Google Scholar] [CrossRef]
- Bahri, Z.; Rezai, B.; Kowsari, E. Selective separation of gallium from zinc using flotation: Effect of solution pH value and the separation mechanism. Miner. Eng. 2016, 86, 104–113. [Google Scholar] [CrossRef]
- Boclair, J.W.; Braterman, P.S. Layered Double Hydroxide Stability. 1. Relative Stabilities of Layered Double Hydroxides and Their Simple Counterparts. Chem. Mater. 2001, 11, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Boclair, J.W.; Braterman, P.S.; Jiang j Lou, S.; Yarberry, F. Layered Double Hydroxide Stability. 2. Formation of Cr(III)-Containing Layered Double Hydroxides Directly from Solution. Chem. Mater. 1991, 11, 303–307. [Google Scholar] [CrossRef]
- Turner, R.C.; Brydon, J.E. Formation of Double Hydroxides and the Titration of Clays. Science 1962, 136, 1052–1054. [Google Scholar] [CrossRef]
- Chung, Y.; Lee, C.-W. Electrochemistry of Gallium. J. Electrochem. Sci. Technol. 2013, 4, 1–18. [Google Scholar]
- McMahon, M.E.; Santucci, R.J., Jr.; Scully, J.R. Advanced chemical stability diagrams to predict the formation of complex zinc compounds in a cloride enviroment. RSC Adv. 2019, 9, 19905. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Zhang, Y.; Hahn, S.H.; Kim, E.J. From Zn(OH)2 to ZnO: A study on the mechanism of phase transformation. Cryst. Eng. Comm. 2011, 13, 6024. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Vedel, J. Supersaturated Zincate Solutions: A Study of the Decomposition Kinetics. J. Electrochem. Soc. 1991, 138, 2538–2542. [Google Scholar] [CrossRef]
- Stepanova, L.N.; Belskaya, O.B.; Salanov, A.N.; Serkova, A.N.; Likholobov, V.A. SEM study of the surface morphology and chemical composition of the MgAl- and MgGa-layered hydroxides in different steps of platinum catalysts Pt/Mg(Al, Ga)Ox synthesis. Appl. Clay Sci. 2018, 157, 267–273. [Google Scholar] [CrossRef]
- Yuan, Y.; Du, W.; Qian, X. ZnxGa2O3+x (0 ≤ x ≤ 1) solid solution nanocrystals: Tunable composition and optical properties. J. Mater. Chem. 2012, 22, 653–659. [Google Scholar] [CrossRef]
- Zak, K.; Razali, A.; Majid, A.; Binti, W.H.; Majid, D. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar]
- Xu, S.-M.; Pan, T.; Dou, Y.-B.; Yan, H.; Zhang, S.-T.; Ning, F.-Y.; Shi, W.-Y.; Wei, M. Theoretical and Experimental Study on MII MIII -Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Kawamura, S.; Puscasu, M.C.; Yoshida, Y.; Izumi, Y.; Carja, G. Tailoring assemblies of plasmonic silver/gold and zinc–gallium layered double hydroxides for photocatalytic conversion of carbon dioxide using UV–visible light. Appl. Catal. A Gen. 2015, 504, 238–247. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Ruiz-López, I.I.; de Ruiz-Peralta, M.L.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated method for the determination of the band gap energy of pure and mixed powder samples using diffuse reflectance spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef] [PubMed]
- Chikoidze, E.; Sartel, C.; Madaci, I.; Mohamed, H.; Vilar, C.; Ballesteros, B.; Belarre, F.; del Corro, E.; Vales-Castro, P.; Sauthier, G.; et al. p-Type Ultrawide-Band-Gap Spinel ZnGa2O4: New Perspectives for Energy Electronic. Cryst. Growth Des. 2020, 20, 2535–2546. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and its Applications, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Cho, S.; Jung, S.-H.; Lee, K.-H. Morphology-Controlled Growth of ZnO Nanostructures Using Microwave Irradiation: From Basic to Complex Structures. J. Phys. Chem. C 2008, 112, 12769–12776. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Roquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids Principles, Methodology and Applications; Chapter 7; Academic Press: Cambridge, MA, USA, 1999; p. 204. [Google Scholar]
- Yang, X.; Ma, J.; Guo, R.; Fan, K.; Xue, P.; Wang, X.; Sun, H.; Yang, Q.; Lai, X. Ordered mesoporous ZnGa2O4 for photocatalytic hydrogen evolution. Mater. Chem. Front. 2021, 5, 5790–5797. [Google Scholar] [CrossRef]
- Hirano, M.; Sakaida, N. Hydrothermal Synthesis and Low-Temperature Sintering of Zinc Gallate Spinel Fine Particles. J. Am. Ceram. Soc. 2002, 85, 1145–1150. [Google Scholar] [CrossRef]
- Aluker, N.L.; Lavrentieva, A.L.; Suzdaltseva, Y.M. Direct Optical Research Methods in the Analytics of Phenol. Opt. Spectrosc. 2020, 128, 422–428. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Lan, X.; Chen, Z.; Wang, T. Photocatalytic performance of ZnGa2O4 for degradation of methylene blue and its improvement by doping with Cd. Catal. Commun. 2010, 11, 1104–1108. [Google Scholar] [CrossRef]
- Seftel, M.; Puscasu, M.C.; Mertens, M.; Cool, P.; Carja, G. Assemblies of nanoparticles of CeO2–ZnTi-LDHs and their derived mixed oxides as novel photocatalytic systems for phenol degradation. Appl. Catal. B Environ. 2014, 150–151, 157–166. [Google Scholar] [CrossRef]
- Valdés, C.; Alzate-Morales, J.; Osorio, E.; Villaseñor, J.; Navarro-Retamal, C. A characterization of the two-step reaction mechanism of phenol decomposition by a Fenton reaction. Chem. Phys. Lett. 2015, 640, 16–22. [Google Scholar] [CrossRef]
- Trinh, D.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Investigation of Intermediate Compounds of Phenol in Photocatalysis Process. Inter. J. Chem. Eng. Appl. 2016, 7, 273–276. [Google Scholar] [CrossRef]
- Ren, Y.-Z.; Wu, Z.-L.; Franke, M.; Braeutigam, P.; Ondruschka, B.; Comeskey, D.J.; King, P.M. Sonoelectrochemical degradation of phenol in aqueous solutions. Ultrason. Sonochem. 2013, 20, 715–721. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, C.; Zhang, X.-X.; Liu, Y.-N.; Yan, J.-H. Preparation and photocatalytic performance of ZnO/ZnGa2O4 composite microspheres. J. Cent. South Univ. 2016, 23, 3092–3099. [Google Scholar] [CrossRef]
- Tian, Q.; Ren, S.; Cai, Z.; Chen, C.; Zheng, Y.; Zhuang, J. Glucose-Mediated Synthesis of Hierarchical Porous ZnGa2O4 Microspheres for Effective Photocatalytic Removal of Aromatic and Arsenic Pollutants. Catalysts 2019, 9, 828. [Google Scholar] [CrossRef]

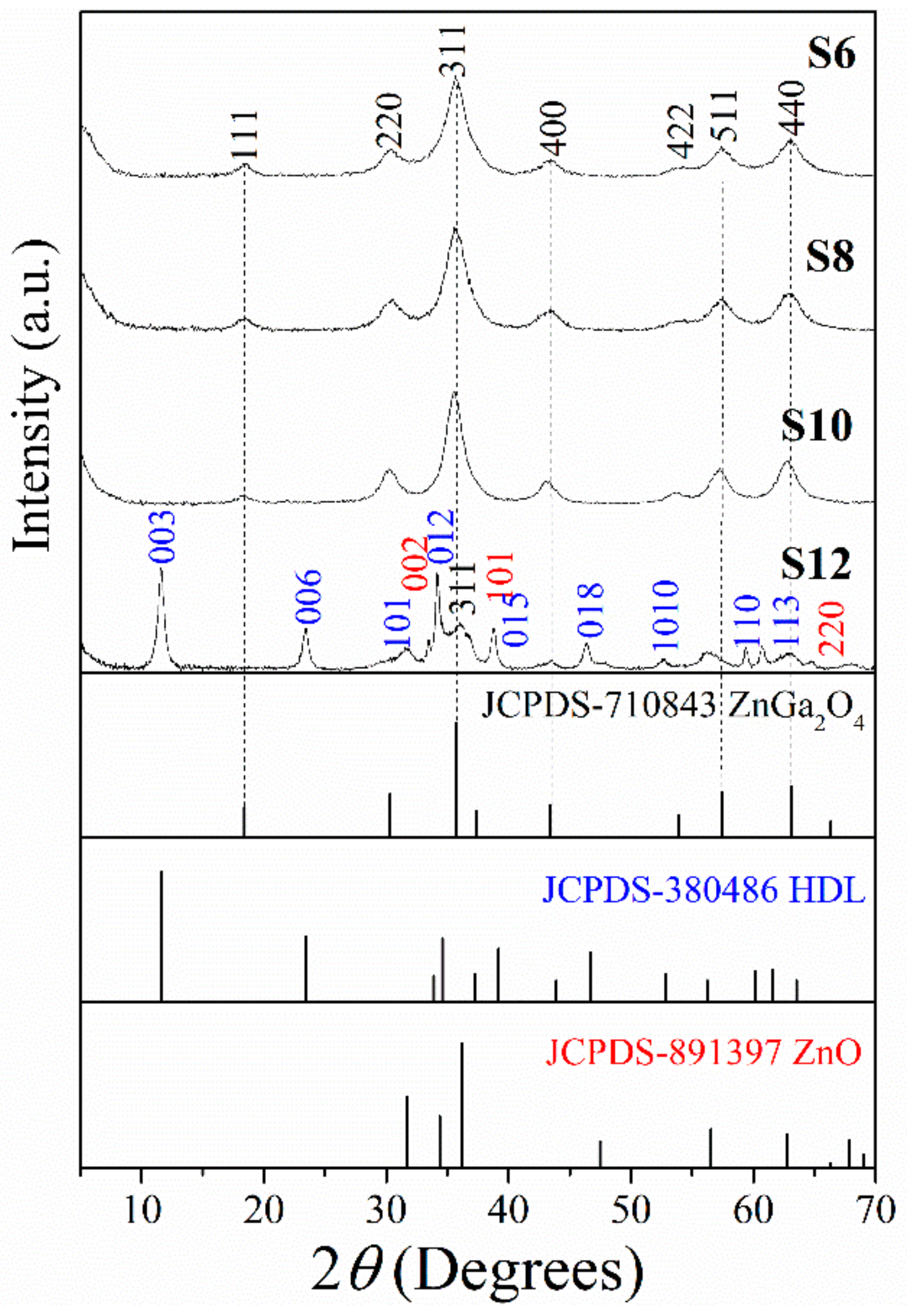
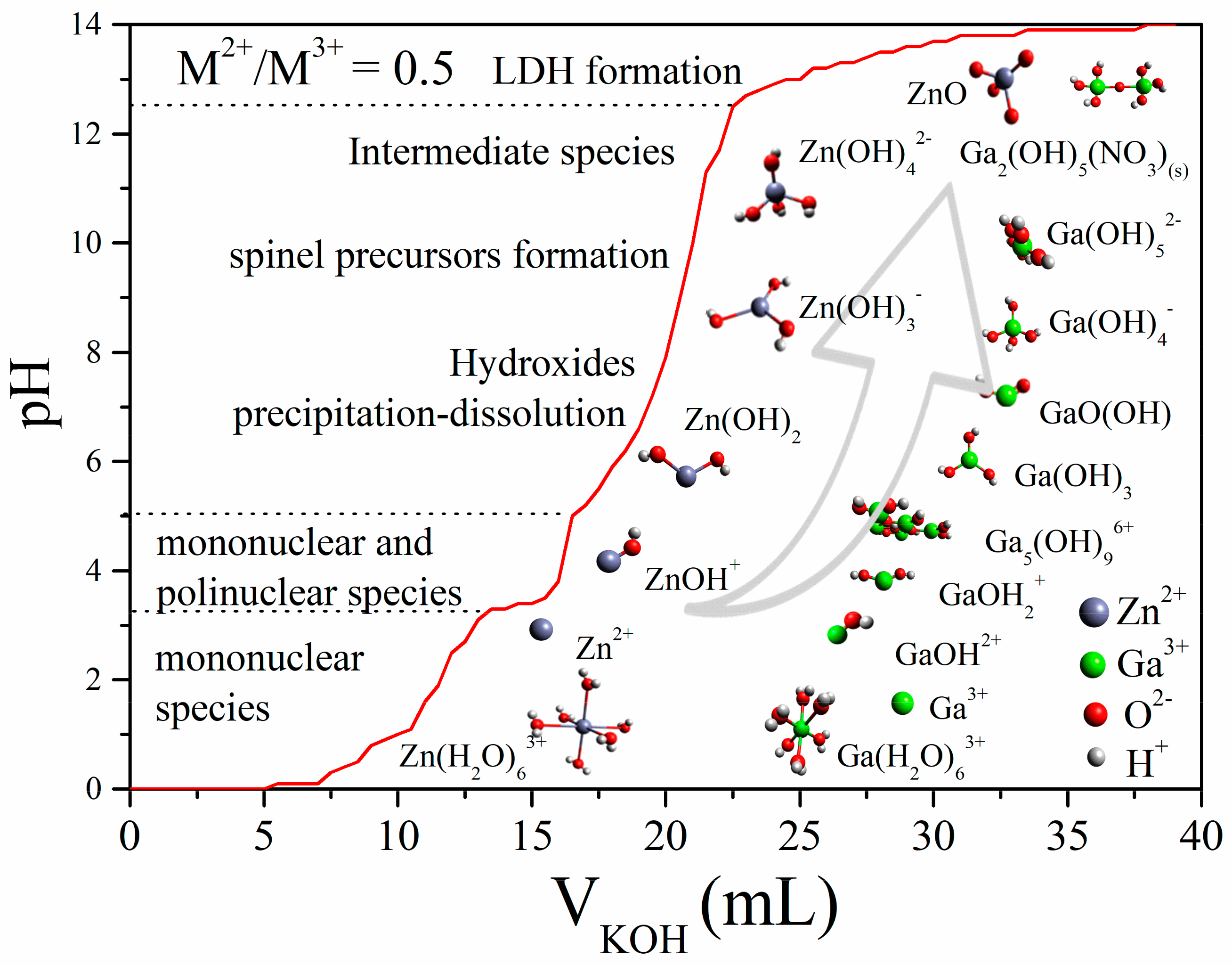

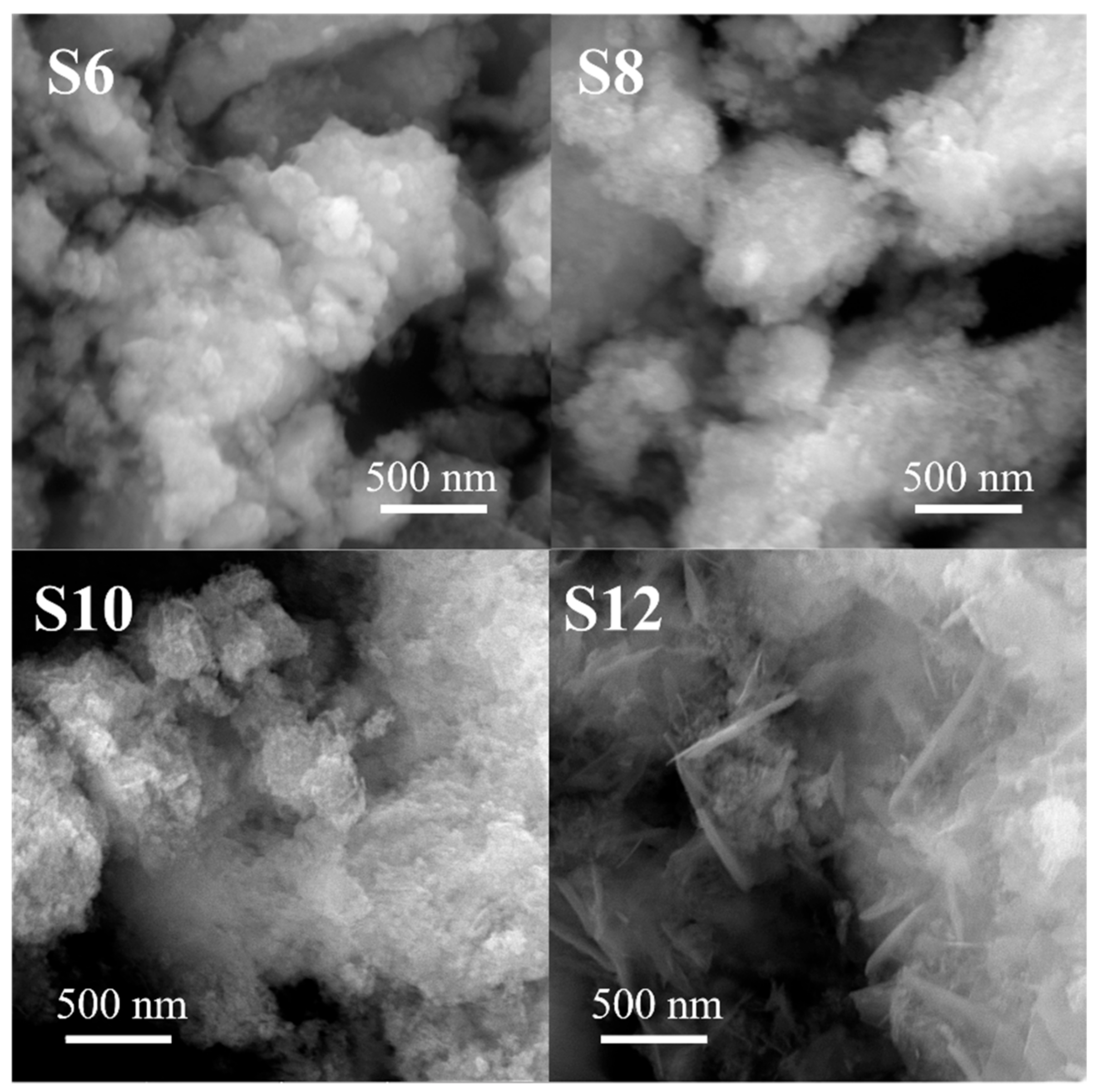
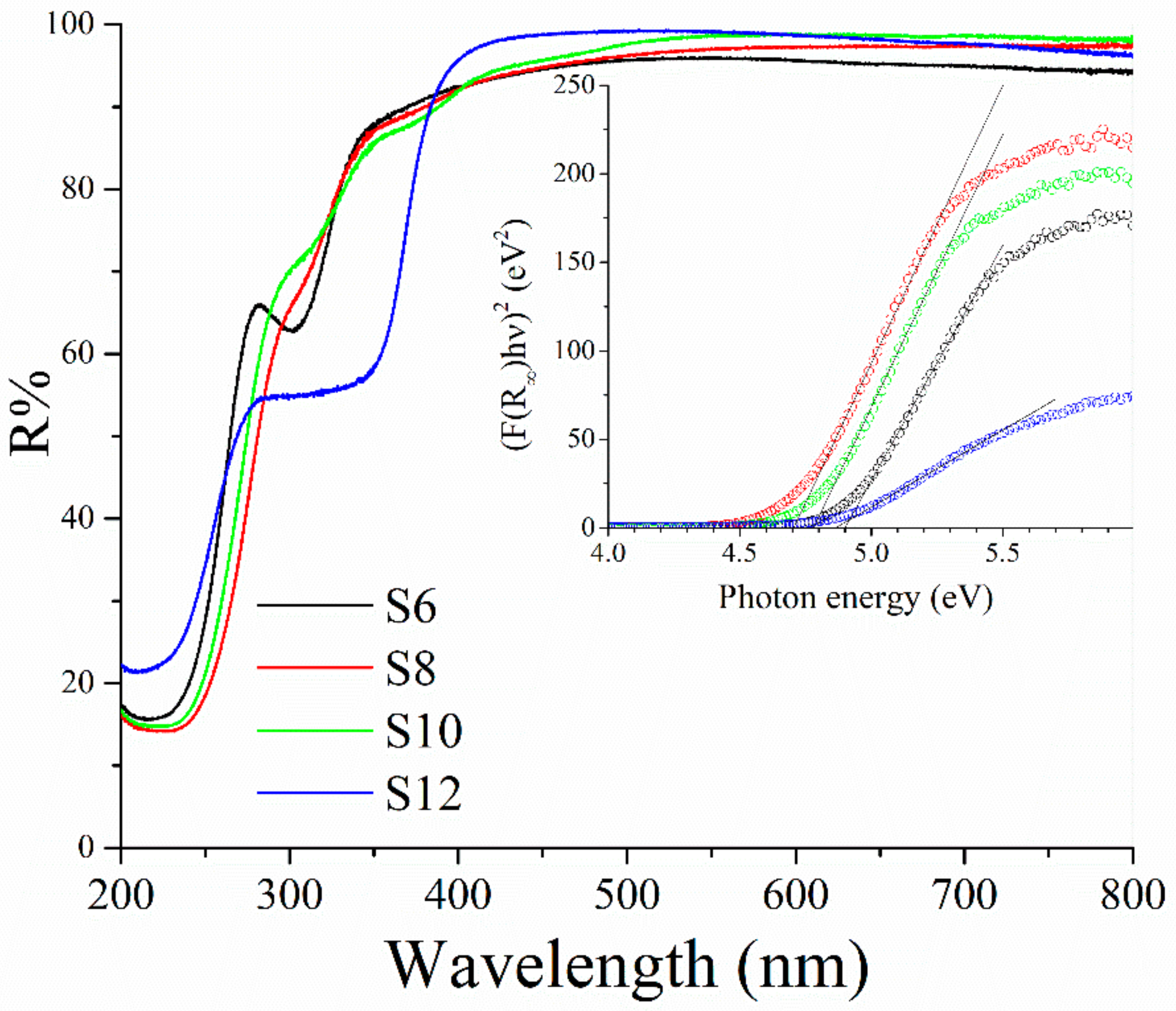
| Sample | ZnGa2O4 | Zn-Ga LDH | ZnO | ||||
|---|---|---|---|---|---|---|---|
| a, Å | L *, nm | Yield, % | a, Å | c, Å | a, Å | c, Å | |
| S6 | 8.34 | 4.5 | 50.8 ± 8.2 | - | - | - | - |
| S8 | 8.35 | 4.3 | 36.3 ± 6.1 | - | - | - | - |
| S10 | 8.38 | 5.5 | 32.0 ± 6.78 | - | - | - | - |
| S12 | 8.46 | 5.5 | 21.3 ± 7.8 | 3.11 | 22.86 | 3.26 | 5.18 |
| Sample | Eg (eV) | SSA (m2 g−1) | ||
|---|---|---|---|---|
| ZnGa2O4 | Zn2xGa2−2xO3+x | ZnO | ||
| S6 | 4.9(0) | 3.8(1) | - | 140 |
| S8 | 4.7(1) | 3.8(0) | - | 113 |
| S10 | 4.7(8) | 3.7(2) | - | 82 |
| S12 | 4.8(8) | - | 3.3(6) | * |
| Phenol Degradation (%) | |||||
|---|---|---|---|---|---|
| Time (min) | Photolysis | S6 | S8 | S10 | TiO2-Degussa P25 |
| 120 | 0 | 38.6(8) | 44.5(1) | 58.6(0) | 18.1(6) |
| 240 | 7.2(1) | 95.4(1) | 90.4(1) | 95.8(1) | 38.4(0) |
| 360 | 28.1(7) | 98.1(4) | 98.6(5) | 98.0(4) | 87.7(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Téllez-Flores, D.; Sánchez-Cantú, M.; Ruiz-Peralta, M.d.L.; López-Salinas, E.; Pérez-Centeno, A.; Tzompantzi, F.; Escobedo-Morales, A. Aqueous Chemical Synthesis of Nanosized ZnGa2O4 Using Mild Reaction Conditions: Effect of pH on the Structural, Morphological, Textural, Electronic, and Photocatalytic Properties. Crystals 2023, 13, 952. https://doi.org/10.3390/cryst13060952
Téllez-Flores D, Sánchez-Cantú M, Ruiz-Peralta MdL, López-Salinas E, Pérez-Centeno A, Tzompantzi F, Escobedo-Morales A. Aqueous Chemical Synthesis of Nanosized ZnGa2O4 Using Mild Reaction Conditions: Effect of pH on the Structural, Morphological, Textural, Electronic, and Photocatalytic Properties. Crystals. 2023; 13(6):952. https://doi.org/10.3390/cryst13060952
Chicago/Turabian StyleTéllez-Flores, Dalia, Manuel Sánchez-Cantú, María de Lourdes Ruiz-Peralta, Esteban López-Salinas, Armando Pérez-Centeno, Francisco Tzompantzi, and Alejandro Escobedo-Morales. 2023. "Aqueous Chemical Synthesis of Nanosized ZnGa2O4 Using Mild Reaction Conditions: Effect of pH on the Structural, Morphological, Textural, Electronic, and Photocatalytic Properties" Crystals 13, no. 6: 952. https://doi.org/10.3390/cryst13060952
APA StyleTéllez-Flores, D., Sánchez-Cantú, M., Ruiz-Peralta, M. d. L., López-Salinas, E., Pérez-Centeno, A., Tzompantzi, F., & Escobedo-Morales, A. (2023). Aqueous Chemical Synthesis of Nanosized ZnGa2O4 Using Mild Reaction Conditions: Effect of pH on the Structural, Morphological, Textural, Electronic, and Photocatalytic Properties. Crystals, 13(6), 952. https://doi.org/10.3390/cryst13060952





