Synthesis of Thiophene-Based Derivatives and the Effects of Their Molecular Structure on the Mesomorphic Behavior and Temperature Range of Liquid-Crystalline Blue Phases
Abstract
1. Introduction
2. Results and Discussion
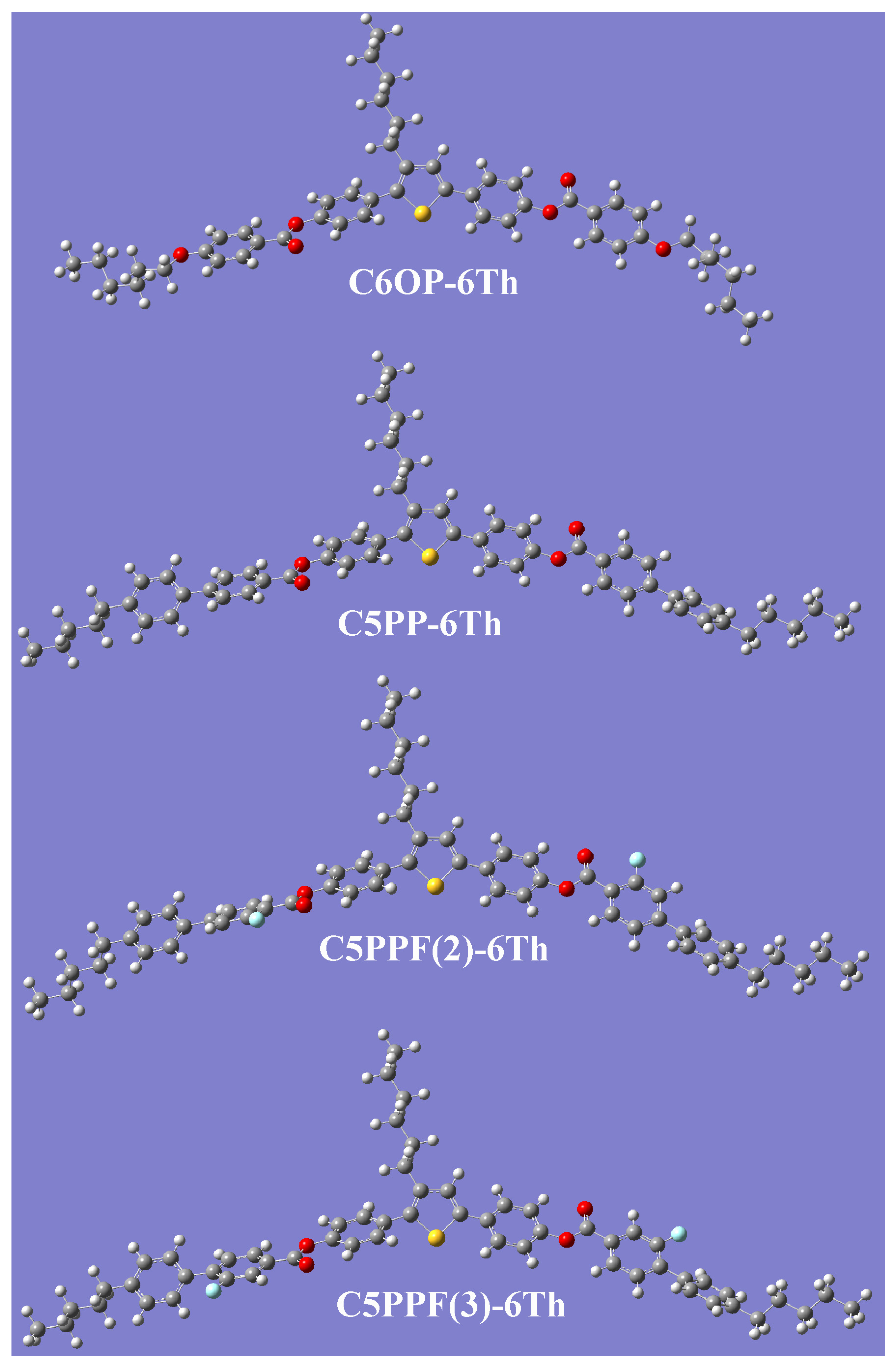
2.1. Mesomorphic Behavior
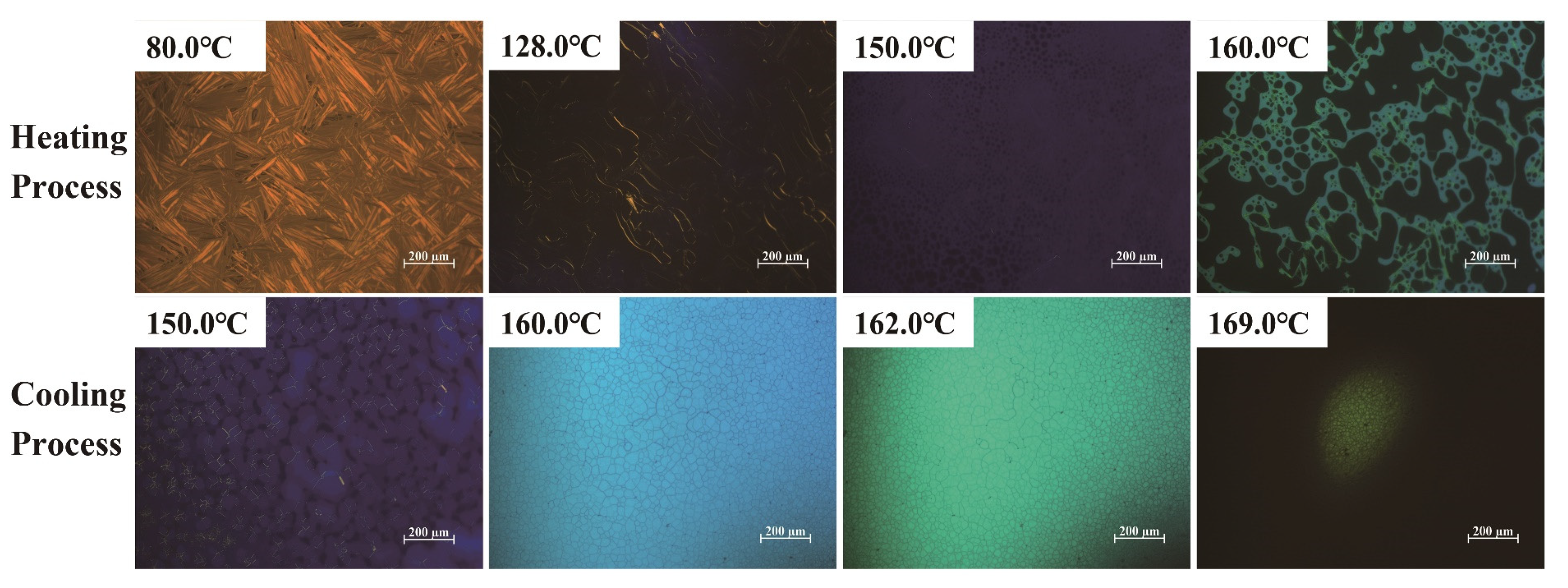
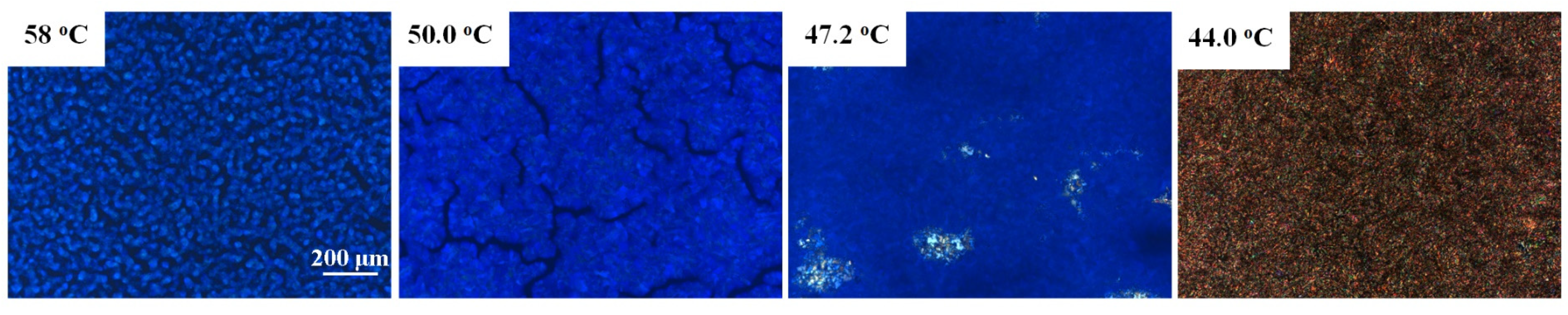
2.2. BP Behavior of Thiophene Bent-Shaped Molecules with Added Chiral Compounds
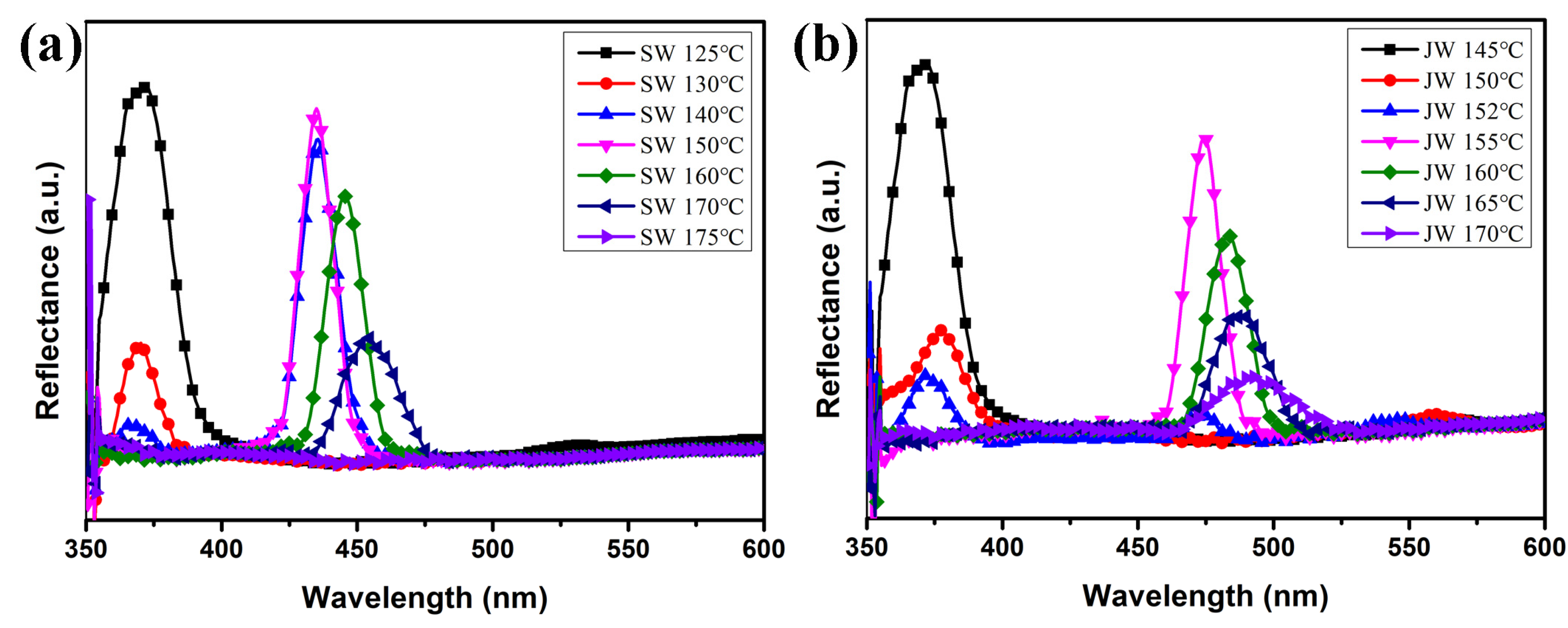
2.3. Effect of Thiophene Bent-Shaped Molecules on BP Temperature Range
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisoyi, H.K.; Li, Q. Liquid Crystals: Versatile Self-Organized Smart Soft Materials. Chem. Rev. 2022, 122, 4887–4926. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Urbas, A.M.; Li, Q. Nature-Inspired Emerging Chiral Liquid Crystal Nanostructures: From Molecular Self-Assembly to DNA Mesophase and Nanocolloids. Adv. Mater. 2020, 32, 1801335. [Google Scholar] [CrossRef]
- Zheng, Z.-G.; Yuan, C.-L.; Hu, W.; Bisoyi, H.K.; Tang, M.-J.; Liu, Z.; Sun, P.-Z.; Yang, W.-Q.; Wang, X.-Q.; Shen, D.; et al. Light-Patterned Crystallographic Direction of a Self-Organized 3D Soft Photonic Crystal. Adv. Mater. 2017, 29, 1703165. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, W.; Guan, B.; Wang, B.; Shi, L.; Jin, F.; Zheng, Z.; Wang, J.; Ikeda, T.; Jiang, L. Diffusionless transformation of soft cubic superstructure from amorphous to simple cubic and body-centered cubic phases. Nat. Commun. 2021, 12, 3477. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.S.; Chien, L.-C. Unraveling the Mystery of the Blue Fog: Structure, Properties, and Applications of Amorphous Blue Phase III. Adv. Mater. 2017, 29, 1704296. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, W.; He, W.; Yang, Z.; Wang, D.; Cao, H. Liquid crystalline blue phase materials with three-dimensional nanostructures. J. Mater. Chem. C 2019, 7, 13352–13366. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, H.; He, W.; Yang, Z.; Cao, H.; Wang, D.; Li, Y. Research Progress on Blue-Phase Liquid Crystals for Pattern Replication Applications. Materials 2023, 16, 194. [Google Scholar] [CrossRef]
- Rahman, M.D.A.; Said, S.M.; Balamurugan, S. Blue phase liquid crystal: Strategies for phase stabilization and device development. Sci. Technol. Adv. Mater. 2015, 16, 033501. [Google Scholar] [CrossRef]
- Yoshizawa, A. Material design for blue phase liquid crystals and their electro-optical effects. RSC Adv. 2013, 3, 25475–25497. [Google Scholar] [CrossRef]
- Coles, H.J.; Pivnenko, M.N. Liquid crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef]
- Hu, W.; Wang, L.; Wang, M.; Zhong, T.; Wang, Q.; Zhang, L.; Chen, F.; Li, K.; Miao, Z.; Yang, D.; et al. Ultrastable liquid crystalline blue phase from molecular synergistic self-assembly. Nat. Commun. 2021, 12, 1440. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Sato, M.; Rokunohe, J. A blue phase observed for a novel chiral compound possessing molecular biaxiality. J. Mater. Chem. 2005, 15, 3285–3290. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, X.W.; Wei, J.; Yang, H.; Guo, J.B. Stabilization of blue phases by hydrogen-bonded bent-shaped and T-shaped molecules featuring a branched terminal group. Soft Matter 2013, 9, 10186–10195. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Kogawa, Y.; Kobayashi, K.; Takanishi, Y.; Yamamoto, J. A binaphthyl derivative with a wide temperature range of a blue phase. J. Mater. Chem. 2009, 19, 5759–5764. [Google Scholar] [CrossRef]
- Etxebarria, J.; Ros, M.B. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926. [Google Scholar] [CrossRef]
- Jakli, A. Liquid crystals of the twenty-first century—Nematic phase of bent-core molecules. Liq. Cryst. Rev. 2013, 1, 65–82. [Google Scholar] [CrossRef]
- Abberley, J.P.; Killah, R.; Walker, R.; Storey, J.M.D.; Imrie, C.T.; Salamonczyk, M.; Zhu, C.; Gorecka, E.; Pociecha, D. Heliconical smectic phases formed by achiral molecules. Nat. Commun. 2018, 9, 228. [Google Scholar] [CrossRef]
- Jákli, A.; Nastishin, Y.; Lavrentovich, O.D. Defects in bent-core liquid crystals. Liq. Cryst. Rev. 2022. [Google Scholar] [CrossRef]
- Nakata, M.; Takanishi, Y.; Watanabe, J.; Takezoe, H. Blue phases induced by doping chiral nematic liquid crystals with nonchiral molecules. Phys. Rev. E 2003, 68, 041710. [Google Scholar] [CrossRef]
- Lee, M.; Hur, S.-T.; Higuchi, H.; Song, K.; Choi, S.-W.; Kikuchi, H. Liquid crystalline blue phase I observed for a bent-core molecule and its electro-optical performance. J. Mater. Chem. 2010, 20, 5813–5816. [Google Scholar] [CrossRef]
- Ocak, H.; Bilgin-Eran, B.; Prehm, M.; Schymura, S.; Lagerwall, J.P.F.; Tschierske, C. Effects of chain branching and chirality on liquid crystalline phases of bent-core molecules: Blue phases, de Vries transitions and switching of diastereomeric states. Soft Matter 2011, 7, 8266–8280. [Google Scholar] [CrossRef]
- Yoshizawa, A. Liquid crystal supermolecules stabilizing an optically isotropic phase with frustrated molecular organization. Polym. J. 2012, 44, 490–502. [Google Scholar] [CrossRef]
- Wang, L.; He, W.L.; Xiao, X.; Yang, Q.; Li, B.R.; Yang, P.Y.; Yang, H. Wide blue phase range and electro-optical performances of liquid crystalline composites doped with thiophene-based mesogens. J. Mater. Chem. 2012, 22, 2383–2386. [Google Scholar] [CrossRef]
- Wang, L.; Yu, L.; Xiao, X.; Wang, Z.; Yang, P.; He, W.; Yang, H. Effects of 1,3,4-oxadiazoles with different rigid cores on the thermal and electro-optical performances of liquid crystalline blue phases. Liq. Cryst. 2012, 39, 629–638. [Google Scholar] [CrossRef]
- Punjani, V.; Mohiuddin, G.; Kaur, S.; Choudhury, A.R.; Paladugu, S.; Dhara, S.; Ghosh, S.; Pal, S.K. Chiral Bent-Shaped Molecules Exhibiting Unusually Wide Range of Blue Liquid-Crystalline Phases and Multistimuli-Responsive Behavior. Chem.—Eur. J. 2020, 26, 5859–5871. [Google Scholar] [CrossRef]
- Khan, R.K.; Mohiuddin, G.; Begum, N.; Turlapati, S.; Nandiraju, R.V.S.; Debbarma, B.K.; Ghosh, S. Extremely High Kerr Constant and Low Operating Voltage in a Stable Room-Temperature Blue Phase III Derived from Three-Ring-Based Bent-Core Molecules. ACS Appl. Mater. Interfaces 2022, 14, 42628–42634. [Google Scholar] [CrossRef]
- Alexander, G.; Yeomans, J. Stabilizing the blue phases. Phys. Rev. E 2006, 74, 061706. [Google Scholar] [CrossRef]
- Hur, S.-T.; Gim, M.-J.; Yoo, H.-J.; Choi, S.-W.; Takezoe, H. Investigation for correlation between elastic constant and thermal stability of liquid crystalline blue phase I. Soft Matter 2011, 7, 8800–8803. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light -Driven Liquid Crystalline Materials: From Photo -Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Begum, N.; Kaur, S.; Mohiuddin, G.; Nandi, R.; Gupta, S.P.; Rao, N.V.S.; Pal, S.K. Structural Understanding, Photoswitchability, and Supergelation of a New Class of Four Ring-Based Bent-Shaped Liquid Crystal. J. Phys. Chem. B 2019, 123, 4443–4451. [Google Scholar] [CrossRef]
- Wang, H.F.; Zheng, Z.G.; Shen, D. Blue phase liquid crystals induced by bent-shaped molecules based on 1,3,4-oxadiazole derivatives. Liq. Cryst. 2012, 39, 99–103. [Google Scholar] [CrossRef]
- Kaur, S.; Punjani, V.; Yadav, N.; Barthakur, A.; Baghla, A.; Dhara, S.; Pal, S.K. Chemical and physical aspects of recent bent-shaped liquid crystals exhibiting chiral and achiral mesophases. Liq. Cryst. 2022, 49, 1078–1146. [Google Scholar] [CrossRef]
- Qi, R.-Z.; Shi, Q.-K.; Wang, Y.-F.; Yang, J.-Y.; Kang, J.-T.; Jia, Y.-G. Effect of terminal chain length on mesomorphic behavior and selective reflection of blue phase liquid crystals. Liq. Cryst. 2022, 49, 1689–1697. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Wang, M.; Wei, M.; Sun, J.; Chen, X.; Yang, H. Effects of symmetrically 2,5-disubstituted 1,3,4-oxadiazoles on the temperature range of liquid crystalline blue phases: A systematic study. Liq. Cryst. 2013, 40, 354–367. [Google Scholar] [CrossRef]
- Han, J. 1,3,4-Oxadiazole based liquid crystals. J. Mater. Chem. C 2013, 1, 7779–7797. [Google Scholar] [CrossRef]
- Reddy, Y.S.K.; Lobo, N.P.; Sampath, S.; Narasimhaswamy, T. Morphology, Mesophase, and Molecular Order of 3-Hexyl Thiophene-Based π-Conjugated Mesogens. J. Phys. Chem. C 2016, 120, 17960–17971. [Google Scholar] [CrossRef]
- Bajzíková, K.; Veselý, J.; Kozmík, V.; Svoboda, J.; Novotná, V.; Pociecha, D. Diphenylthiophenes as central part for the design of bent-core liquid crystalline compounds. J. Mol. Liq. 2018, 267, 496–503. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Kun, S.-Q.; Kang, J.-T.; Jia, Y.-G. New (-)-menthol-based blue phase liquid crystals with different polar substituents in the terminal group: Synthesis, mesophase behaviors, and DFT calculations. J. Mol. Struct. 2022, 1263, 133147. [Google Scholar] [CrossRef]
- Yoshida, H.; Anucha, K.; Ogawa, Y.; Kawata, Y.; Ozaki, M.; Fukuda, J.-i.; Kikuchi, H. Bragg reflection band width and optical rotatory dispersion of cubic blue-phase liquid crystals. Phys. Rev. E 2016, 94, 042703. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Tschierske, C. Fluorinated Liquid Crystals: Design of Soft Nanostructures and Increased Complexity of Self-Assembly by Perfluorinated Segments. In Liquid Crystals: Materials Design and Self-Assembly; Tschierske, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Volume 318, pp. 1–108. [Google Scholar]
- Cigl, M.; Jurok, R.; Hampl, F.; Svoboda, J.; Podoliak, N.; Novotna, V. Lateral substituted phenyl biphenylcarboxylates—Non-chiral analogues of ferroelectric liquid crystals. Liq. Cryst. 2020, 47, 768–776. [Google Scholar] [CrossRef]
- Harkins, R.; Tauscher, T.; Nguyen, J.; Lewis, S.; Adamo, F.C.; Pisani, M.; Hermida-Merino, D.; Samulski, E.T.; Vita, F.; Francescangeli, O.; et al. Biaxial ordering in the supercooled nematic phase of bent-core mesogens: Effects of molecular symmetry and outer wing lateral groups. Liq. Cryst. 2020, 47, 1986–1998. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Liu, H.-H.; Lai, J.-L.; Chiu, C.-H.; Chou, J.-Y. Relation between physical parameters and thermal stability of liquid-crystal blue phase. Appl. Phys. Lett. 2010, 97, 181919. [Google Scholar] [CrossRef]
- Cheng, X.H.; Dong, X.; Zheng, T.; Ye, H.; Wei, G.H. Synthesis and Mesomorphic Behavior of 3-Alkyl-2,5- bis[p-(hexa-2,4-dienoyloxy)phenyl]-Thiophene Derivatives. Chin. J. Chem. 2008, 26, 146–149. [Google Scholar] [CrossRef]
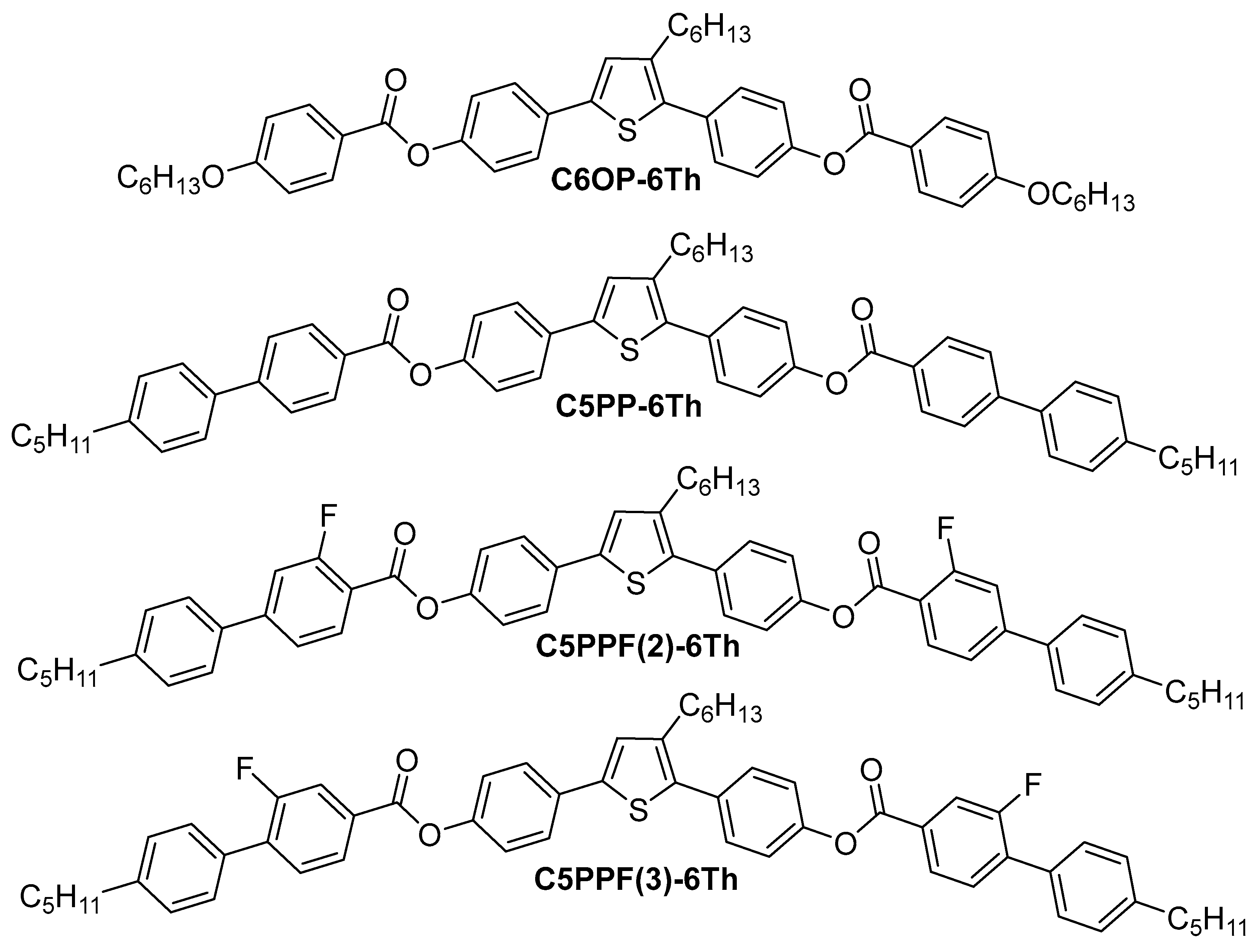


| Compound | Cooling Cycle/(°C, ΔH/J g−1) | Heating Cycle (°C, ΔH/J g−1) |
|---|---|---|
| C6OP-6Th | Iso 59.4 (48.1) Cr | Cr 84.4 (47.9) Iso |
| C5PP-6Th | Iso 262.2 (2.3) N 100.1 (34.3) Cr | Cr 126.4 (40.2) N 265.2 (2.8) Iso |
| C5PPF(2)-6Th | Iso 230.0 (0.9) N 82.5 (21.3) Cr | Cr 104.2 (22.8) N 232.7 (1.0) Iso |
| C5PPF(3)-6Th | Iso 262.0 (1.2) N 95.0 (23.2) Cr | Cr 107.0 (24.6) N 266.7 (1.0) Iso |
| Sample No. | R811 (wt%) | Heating (°C) | Cooling (°C) |
|---|---|---|---|
| A1 | 25 | Cr 105.0 Ch 205.0 Iso | Iso 193.0 Ch 90.0 Cr |
| A2 | 30 | Cr 105.0 Ch 190.0 Iso | Iso 178.0 Ch 85.0 Cr |
| A3 | 35 | Cr 102.0 Ch 128.0 BP 174.0 Iso | Iso 170.0 BP 150.0 Ch 83.0 Cr |
| A4 | 40 | Cr 100.0 Ch 130.0 BP 164.0 Iso | Iso 150.0 BP 130.0 Ch 100.0 Cr |
| Sample No. | Component (wt%) | Phase Transition Temperature | Temperature Range | ||
|---|---|---|---|---|---|
| LC-1 | C6OP-6Th | Iso-BP | BP-Ch | ΔT | |
| 0 | 100.0 | 0 | 64.5 | 61.0 | 3.5 |
| 1 | 99.5 | 0.5 | 63.2 | 54.5 | 8.7 |
| 2 | 99.0 | 1.0 | 61.3 | 49.7 | 11.6 |
| 3 | 98.0 | 2.0 | 58.9 | 45.7 | 13.2 |
| 4 | 97.0 | 3.0 | 57.4 | 45.0 | 12.4 |
| 5 | 96.0 | 4.0 | 56.2 | 44.6 | 11.6 |
| Sample. No | E(B3LYP) | Dipole Moment | Length | Width | L/W |
|---|---|---|---|---|---|
| C6OP-6Th | 2711.8 | 0.95 | 39.24 | 11.00 | 3.57 |
| C5PP-6Th | 2944.8 | 1.52 | 45.34 | 11.03 | 4.11 |
| C5PPF(2)-6Th | 3143.2 | 3.67 | 45.22 | 11.21 | 4.03 |
| C5PPF(3)-6Th | 3143.3 | 2.19 | 45.32 | 11.32 | 4.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Song, H.; Wu, C.; Liu, B.; Wang, Z.; Yang, H. Synthesis of Thiophene-Based Derivatives and the Effects of Their Molecular Structure on the Mesomorphic Behavior and Temperature Range of Liquid-Crystalline Blue Phases. Crystals 2023, 13, 916. https://doi.org/10.3390/cryst13060916
Wang M, Song H, Wu C, Liu B, Wang Z, Yang H. Synthesis of Thiophene-Based Derivatives and the Effects of Their Molecular Structure on the Mesomorphic Behavior and Temperature Range of Liquid-Crystalline Blue Phases. Crystals. 2023; 13(6):916. https://doi.org/10.3390/cryst13060916
Chicago/Turabian StyleWang, Meng, He Song, Chongye Wu, Beiqi Liu, Zichen Wang, and Huai Yang. 2023. "Synthesis of Thiophene-Based Derivatives and the Effects of Their Molecular Structure on the Mesomorphic Behavior and Temperature Range of Liquid-Crystalline Blue Phases" Crystals 13, no. 6: 916. https://doi.org/10.3390/cryst13060916
APA StyleWang, M., Song, H., Wu, C., Liu, B., Wang, Z., & Yang, H. (2023). Synthesis of Thiophene-Based Derivatives and the Effects of Their Molecular Structure on the Mesomorphic Behavior and Temperature Range of Liquid-Crystalline Blue Phases. Crystals, 13(6), 916. https://doi.org/10.3390/cryst13060916





