Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications
Abstract
1. Introduction
2. Theoretical Models
2.1. Maxwell–Eucken Model
2.2. Hasselman–Johnson Model
2.3. Differential Effective Medium Model
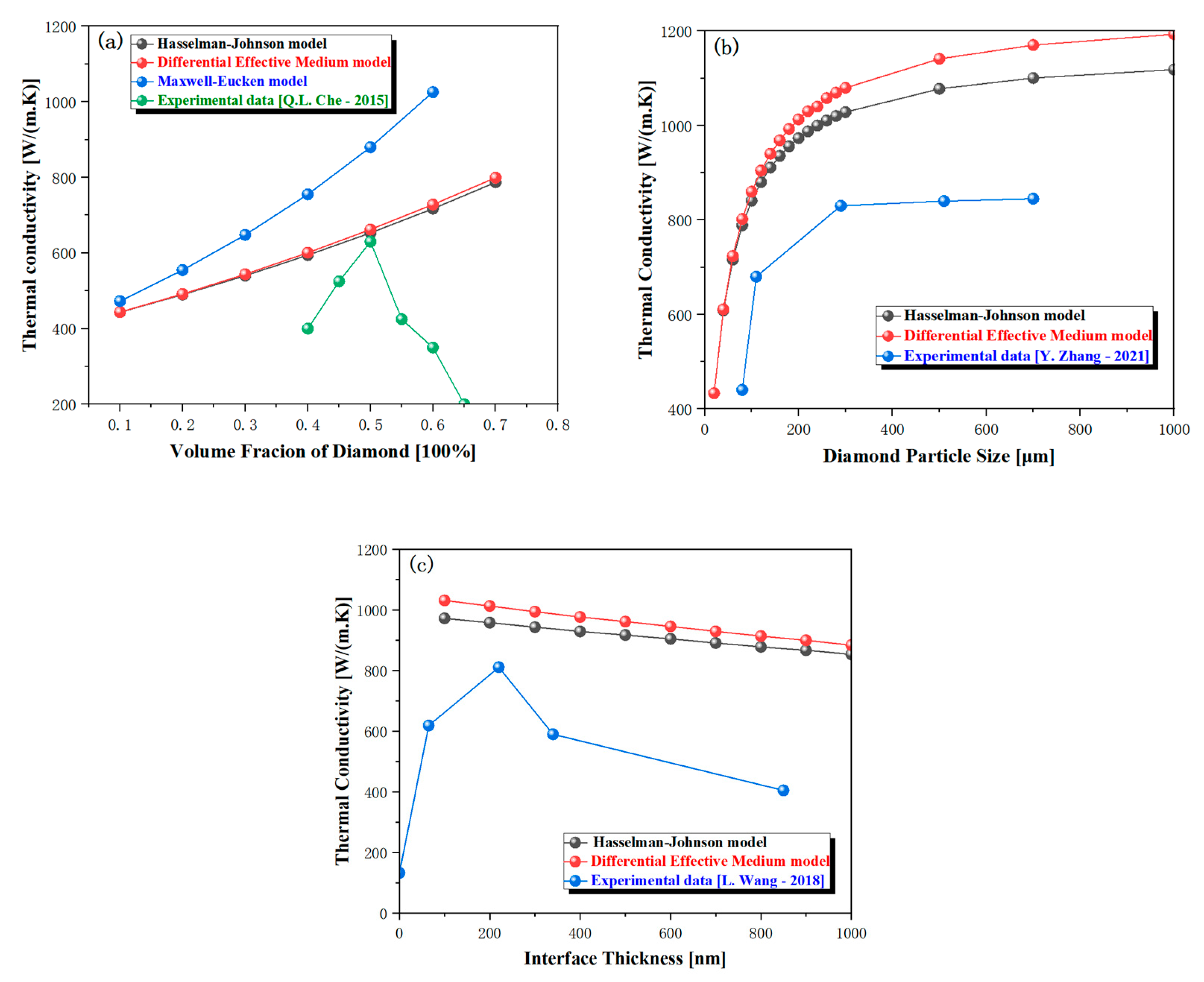
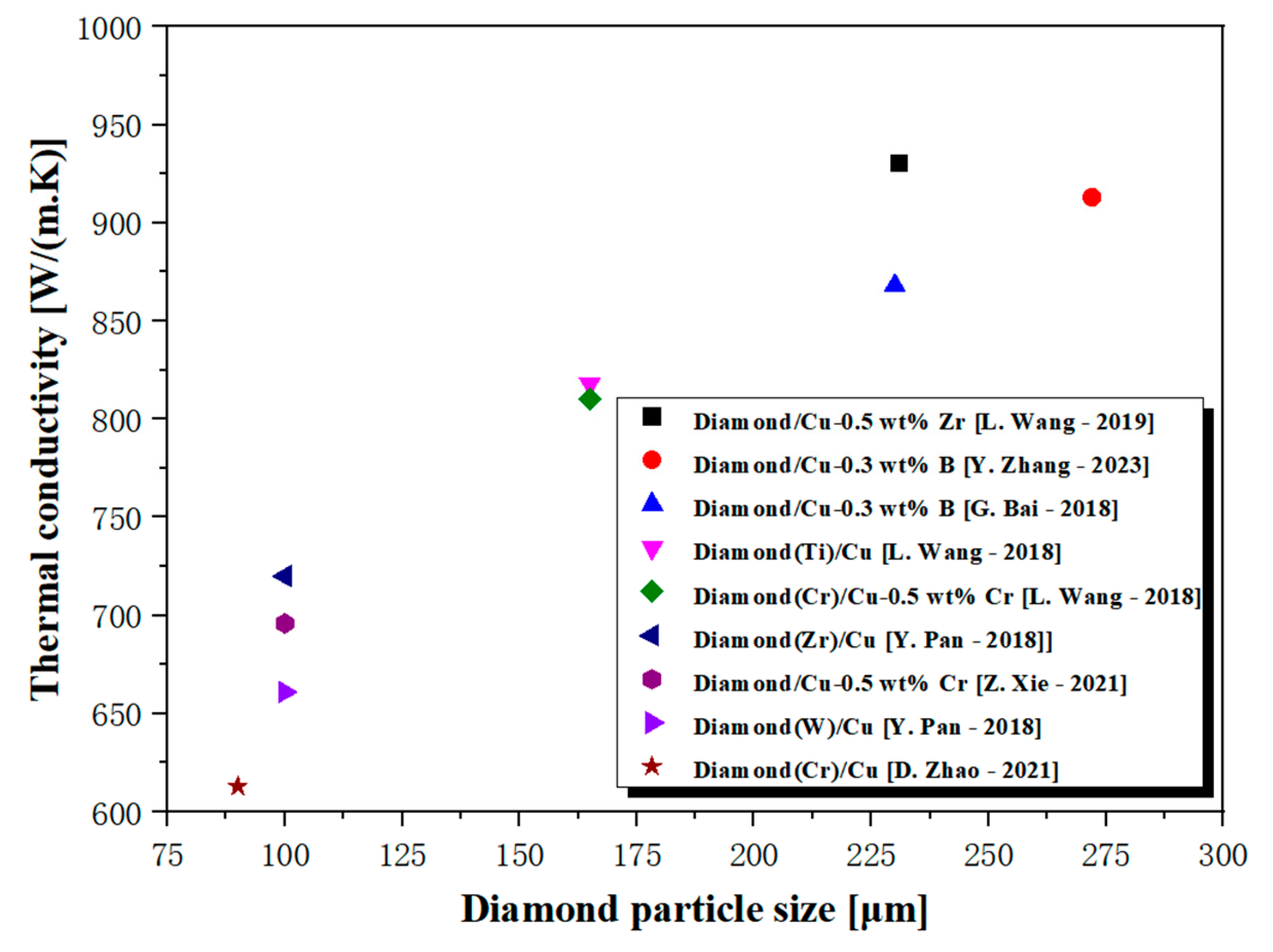
3. The Effect of Diamond Particle Size
4. Effect of Diamond Volume Fraction
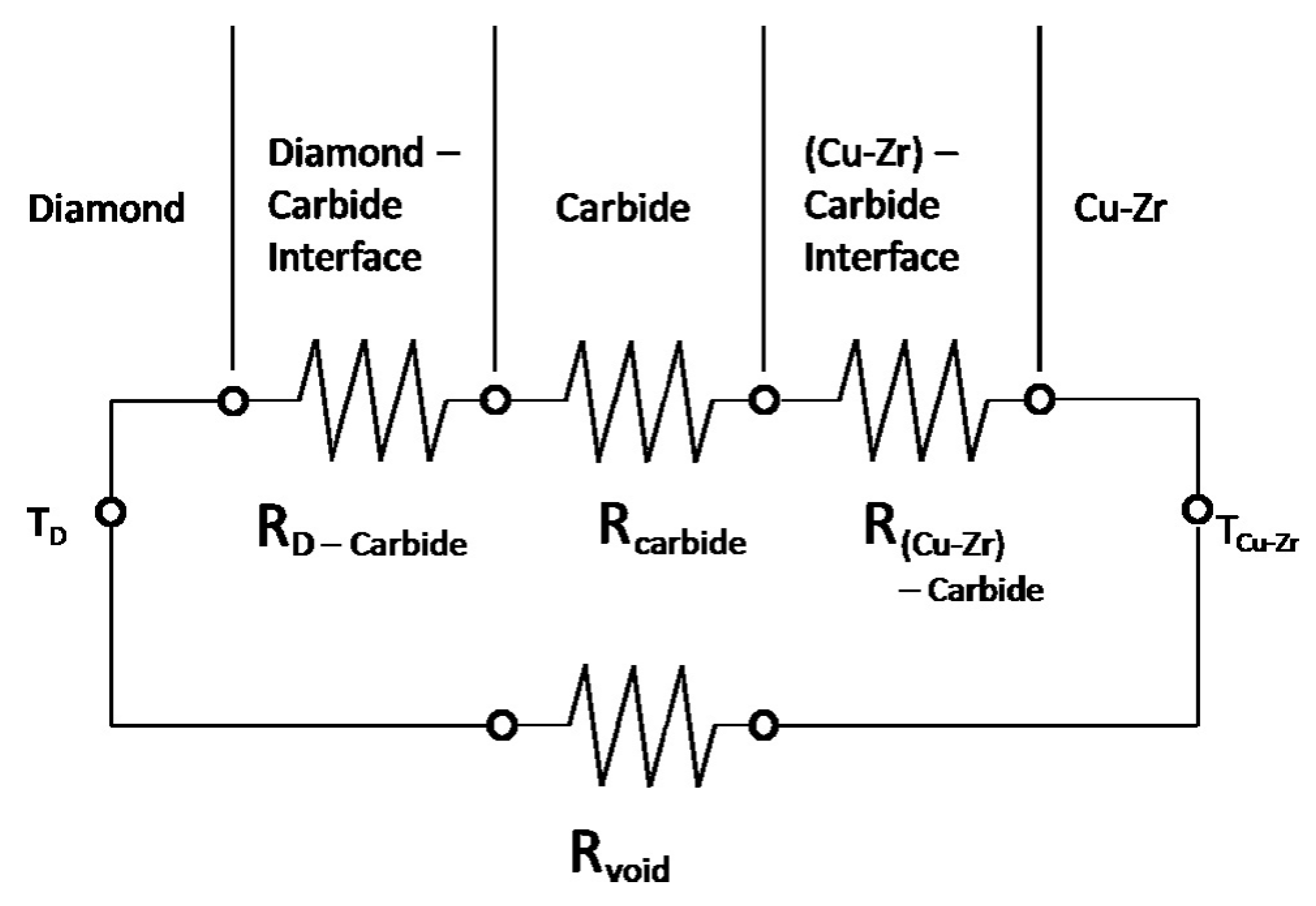
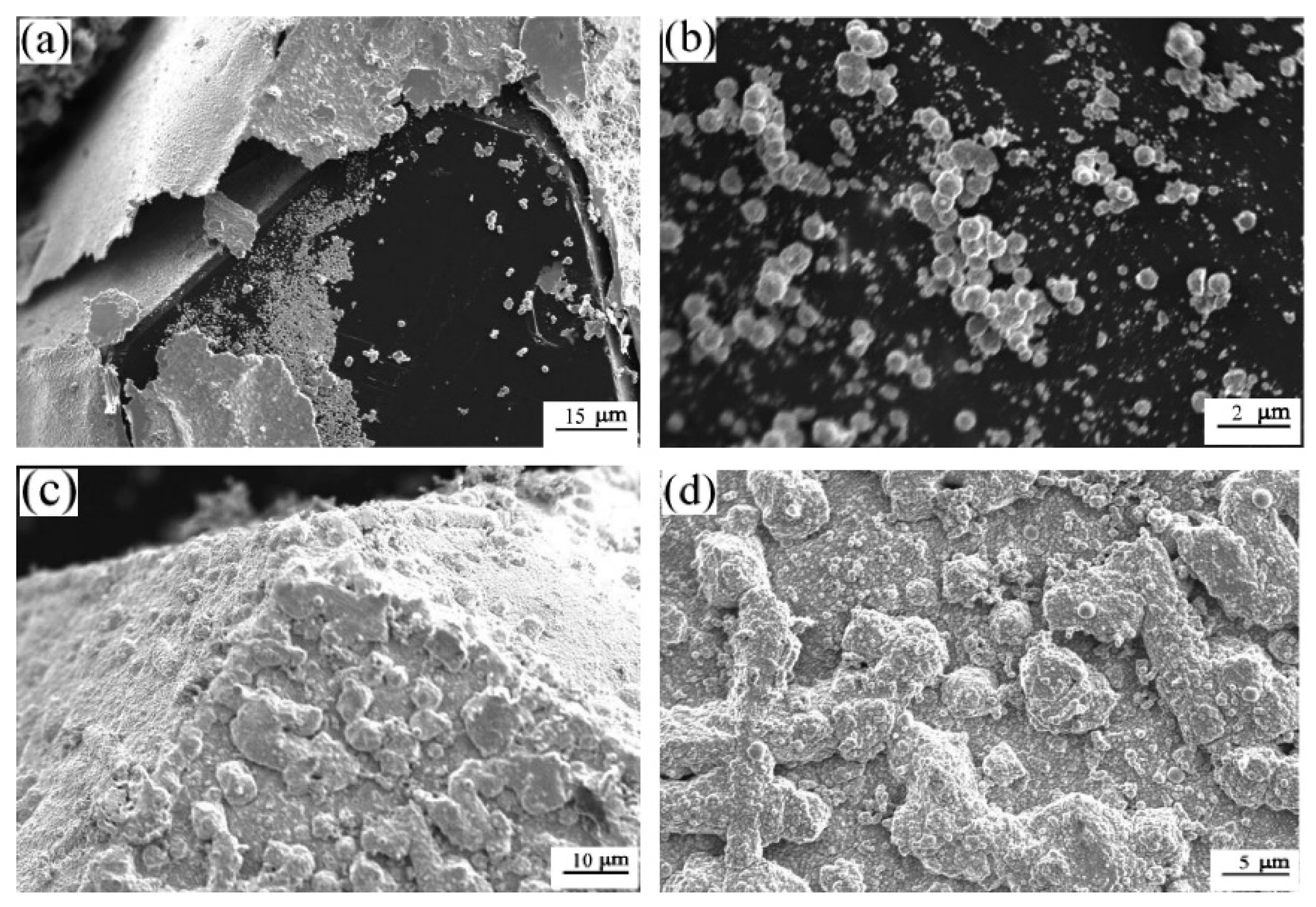
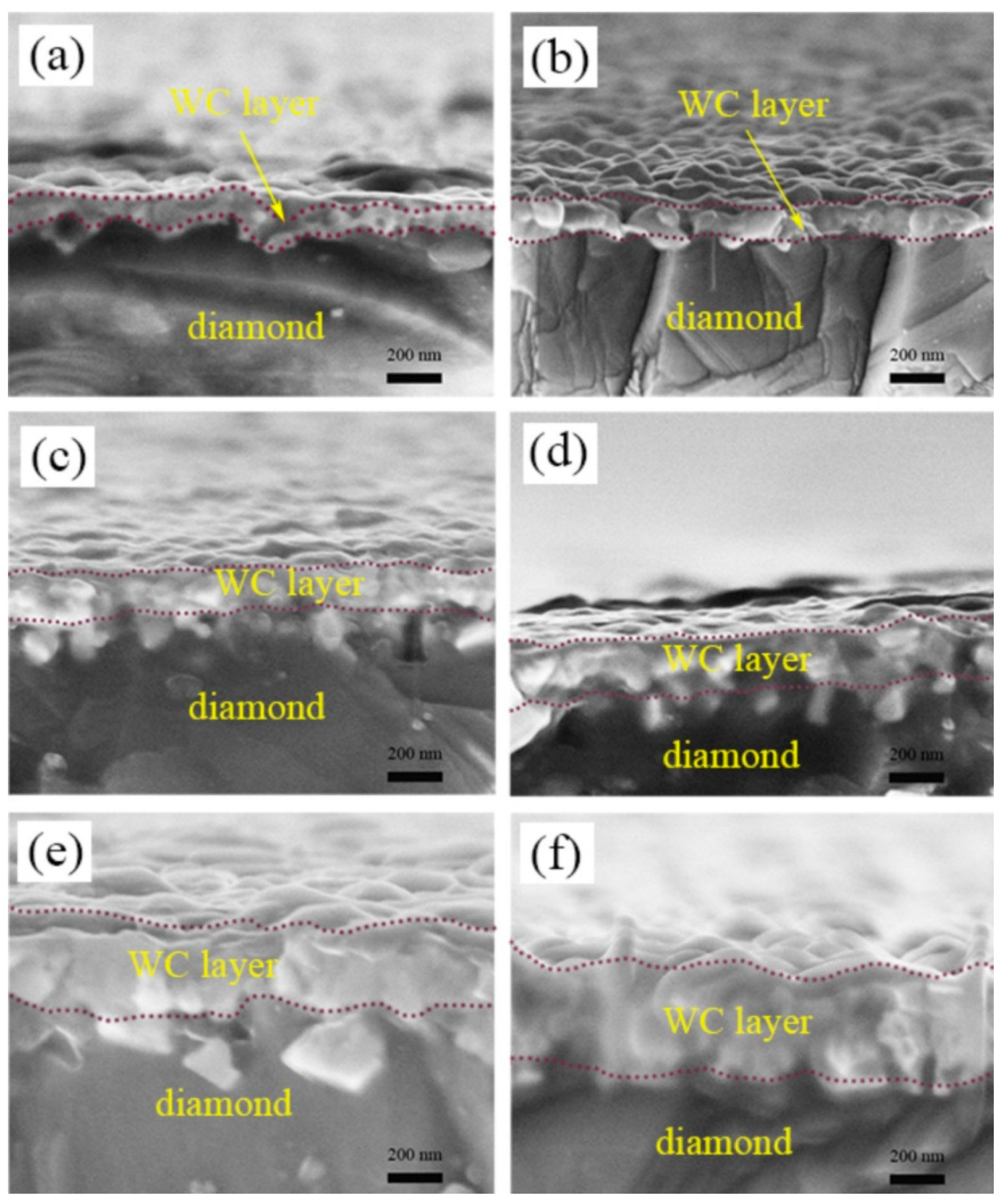
5. Factors Affecting Interfacial Thermal Conductivity
5.1. Methods for Improving Wettability
5.1.1. Diamond Surface Treatment
5.1.2. Matrix Alloying
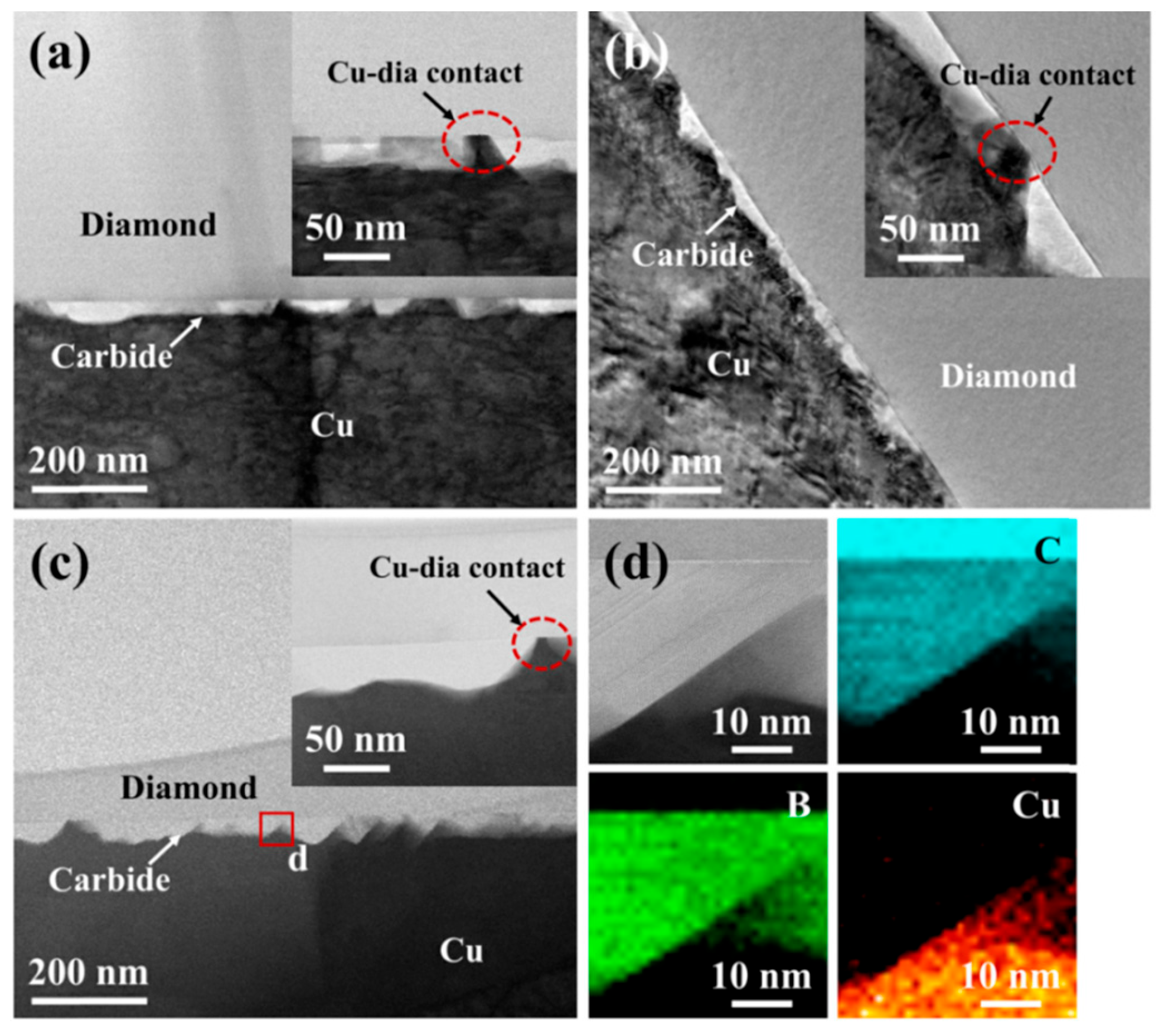
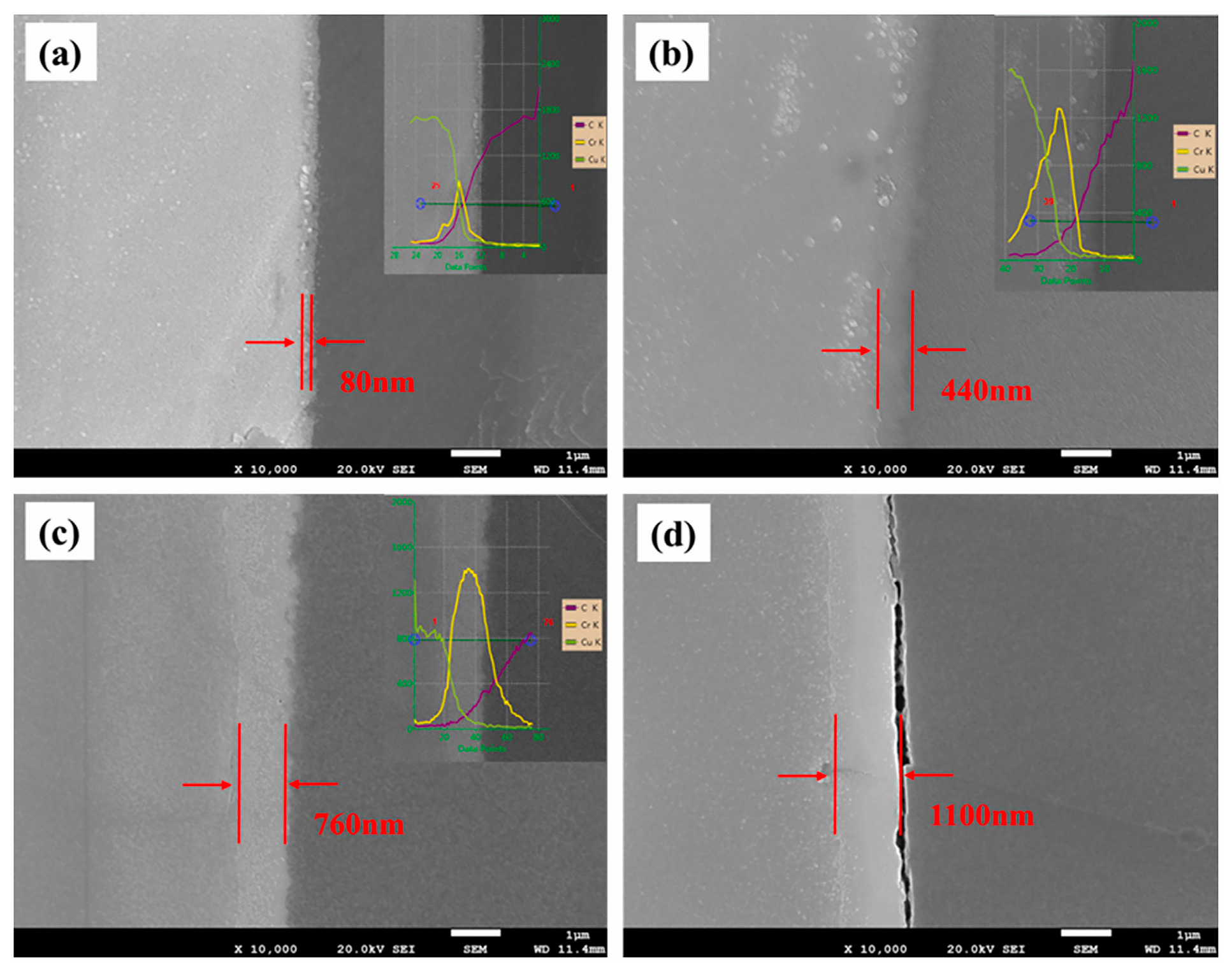
5.2. Effect of Interface
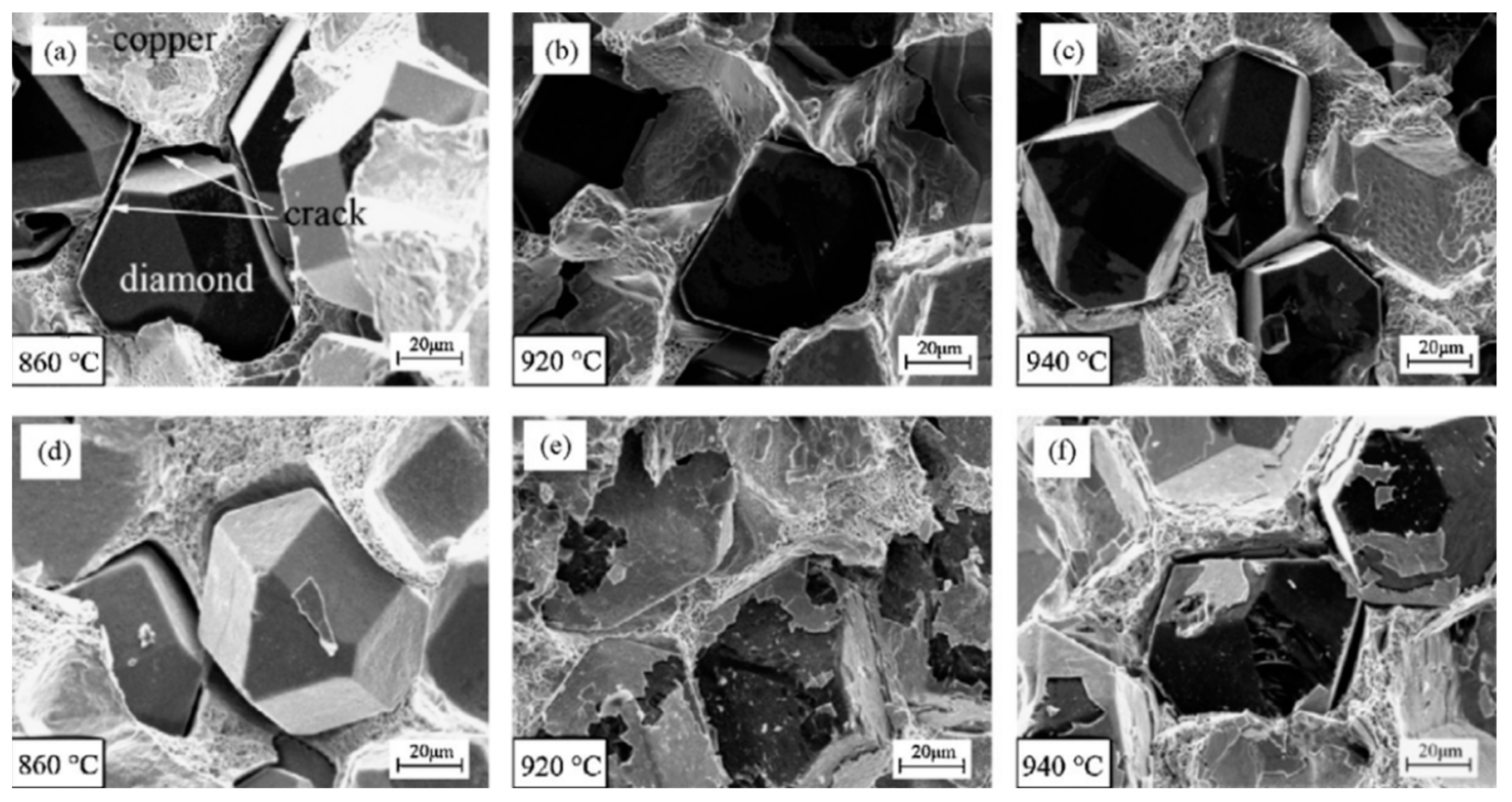
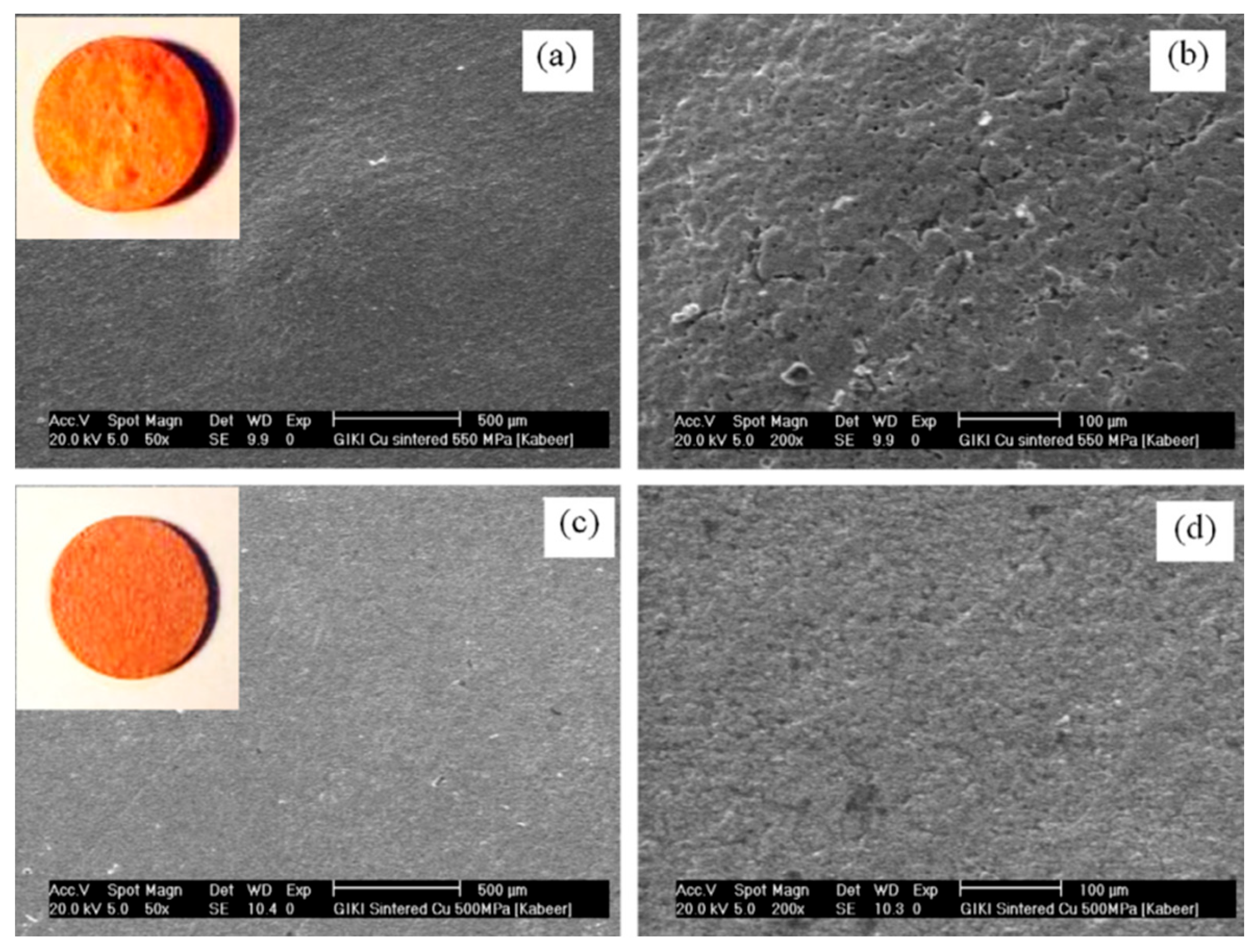
5.3. Effect of Temperature, Pressure and Holding Time
5.4. Effects of Heat Treatment
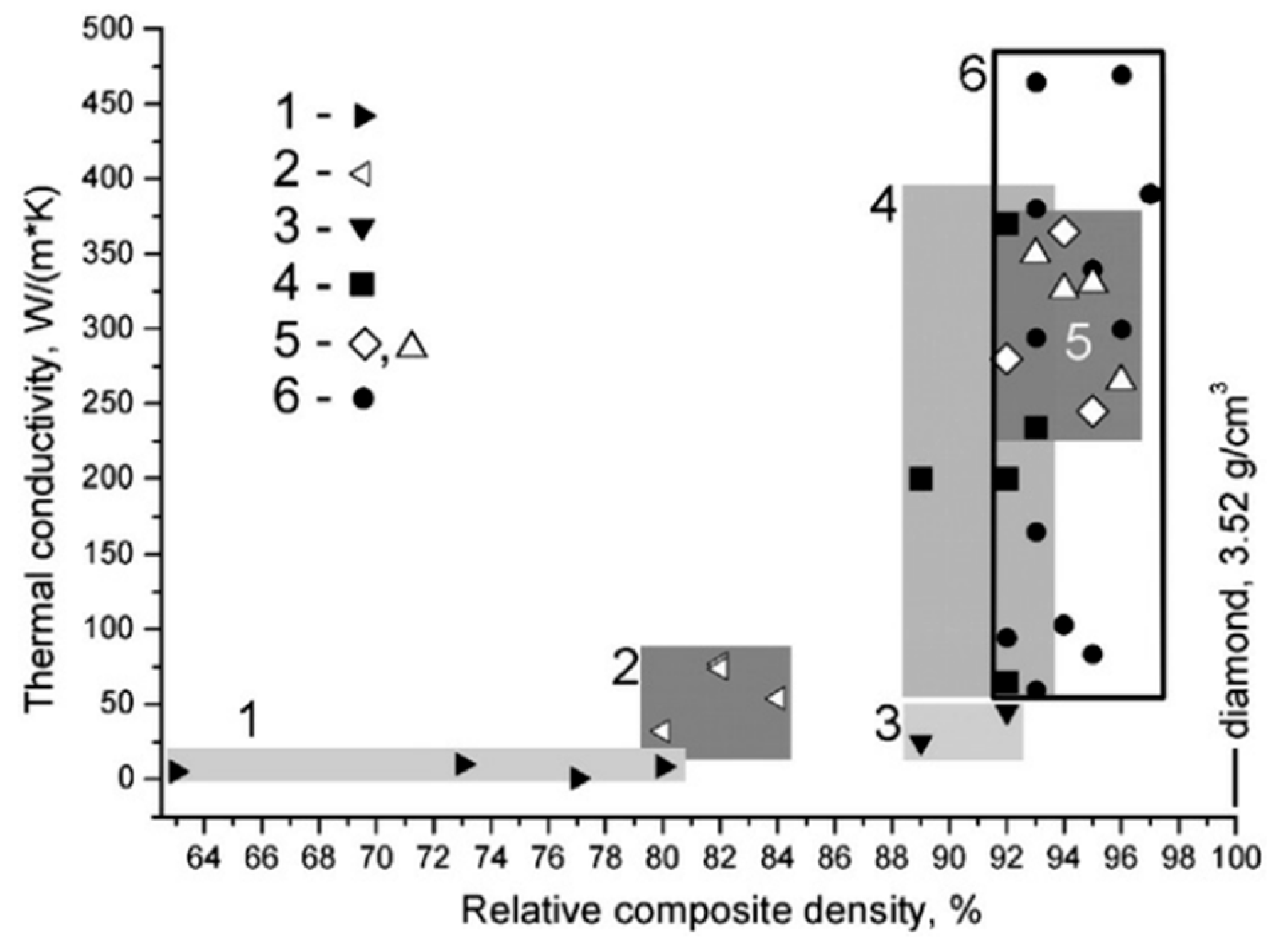
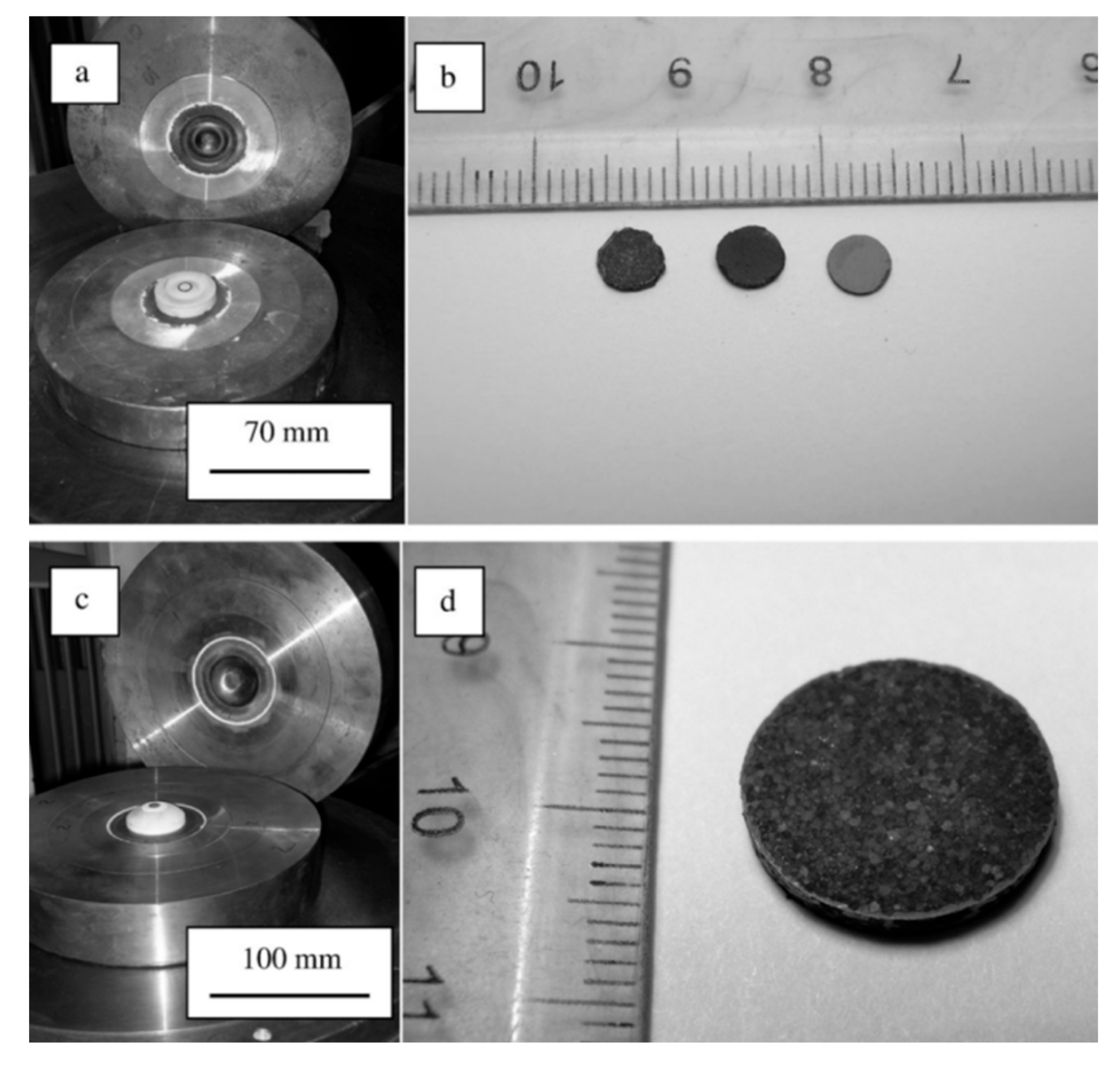
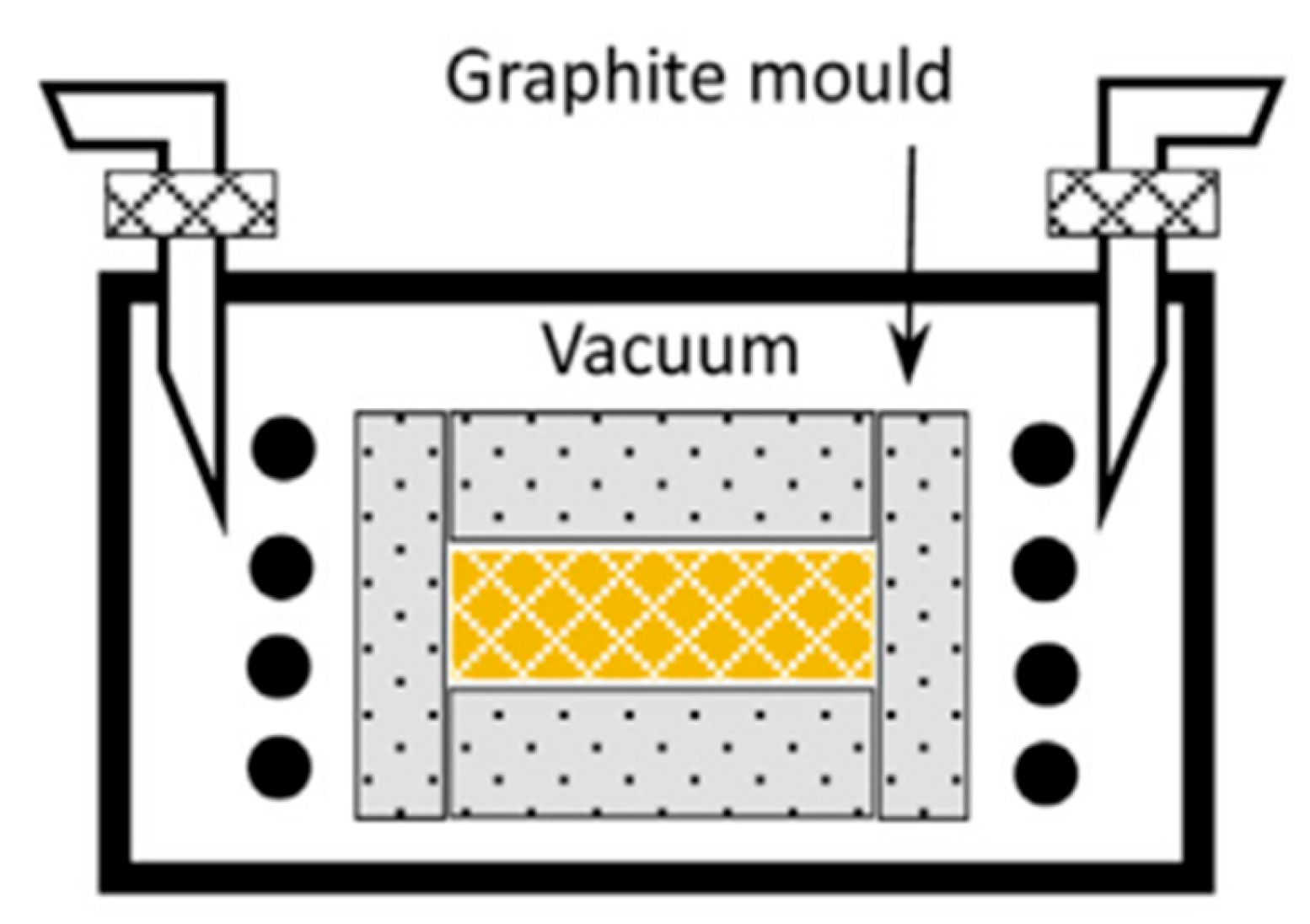
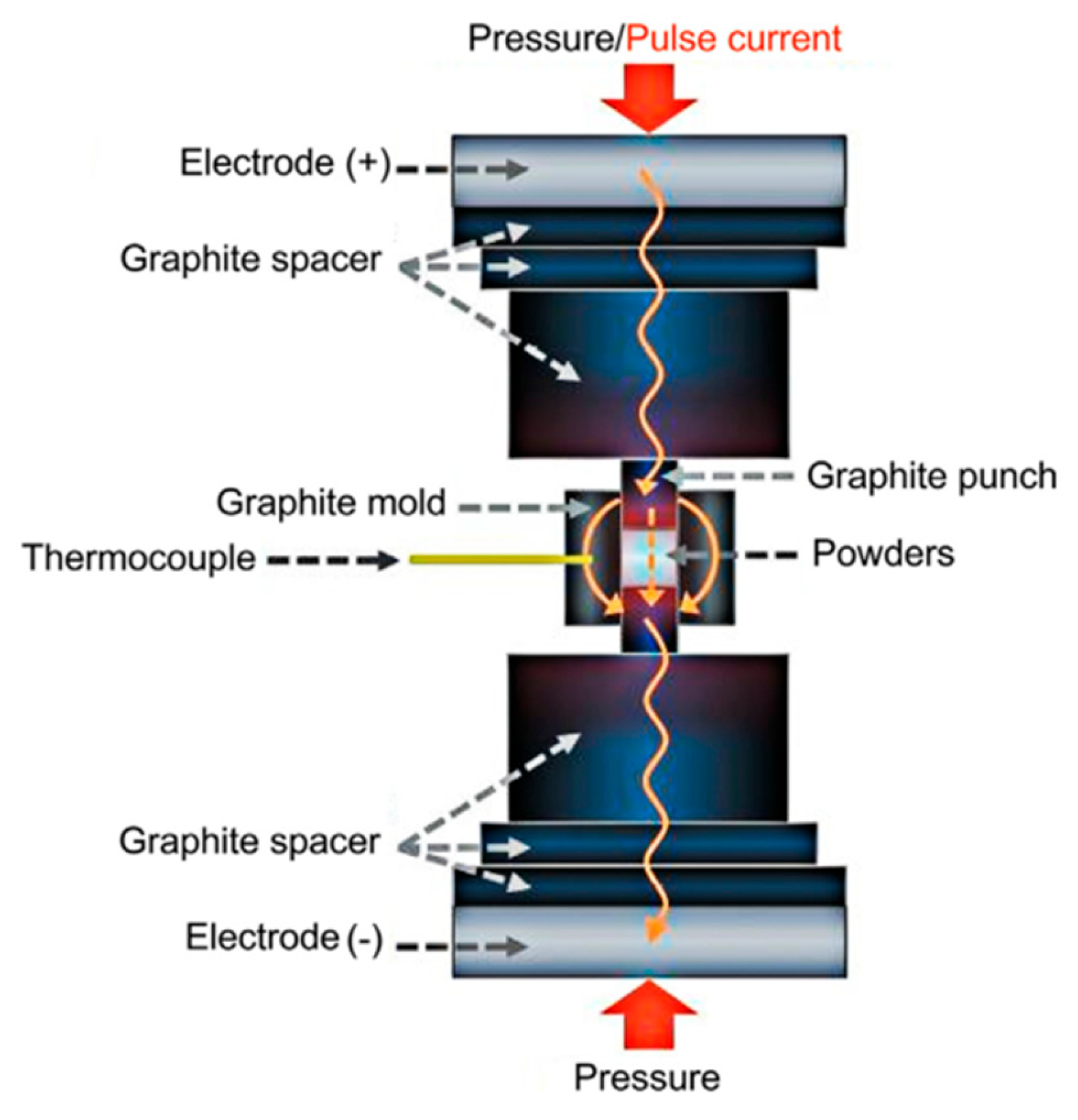
6. Forming Techniques
7. Conclusions and Outlook
- (1)
- There is a need to improve the existing models and to improve the preparation process of Cu/diamond composite materials. Based on our findings, there is still a significant gap between the theoretical models and the actual data. One method is to consider more factors to make the theoretical thermal conductivity closer to the actual thermal conductivity; however, this method would make the predictive model more complicated. Researchers can further improve the preparation process of Cu/diamond composite materials so that the thermal conductivity of the prepared materials is closer to the theoretical value. This would make the theoretical model more practical in guiding actual production.
- (2)
- Studies on forming techniques that focus on reducing power consumption, improving efficiency, and increasing thermal conductivity lay a foundation for better practical applications in production. The existing shaping methods are not very efficient. High-power equipment is required to achieve ultra-high thermal conductivity. In recent years, although shaping methods have been working on solving power consumption and efficiency issues, the obtained composite materials do not have outstanding thermal conductivity. To make copper-based diamond composite materials more widely used in daily life, we need to pay more attention to the development of new forming techniques.
- (3)
- Future studies on the copper-based diamond composites should focus on both the thermal and the mechanical properties. The existing studies have focused primarily on how to improve thermal conductivity, and many composite materials with high thermal conductivity have been developed. However, there are still many issues to be addressed before high thermal conductivity composite materials can be applied. We need to consider whether the strength of the composite material is sufficient. Similarly, we need to consider the processability of the composite material. Only when high thermal conductivity and high mechanical performance are simultaneously achieved, can copper-based diamond composite materials be more effectively applied in the field of electronic packaging.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarvar, F.; Whalley, D.C.; Conway, P.P. Thermal interface materials-A review of the state of the art. In Proceedings of the 2006 1st Electronic System Integration Technology Conference, Dresden, Germany, 5–7 September 2006; IEEE: New York, NY, USA, 2006; Volume 2, pp. 1292–1302. [Google Scholar]
- Moore, A.L.; Shi, L. Emerging challenges and materials for thermal management of electronics. Mater. Today 2014, 17, 163–174. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Imai, T.; Tanabe, K.; Tsuno, T.; Kumazawa, Y.; Fujimori, N. The measurement of thermal properties of diamond. Diam. Relat. Mater. 1997, 6, 1057–1061. [Google Scholar] [CrossRef]
- Ma, S.; Zhao, N.; Shi, C.; Liu, E.; He, C.; He, F.; Ma, L. Mo2C coating on diamond: Different effects on thermal conductivity of diamond/Al and diamond/Cu composites. Appl. Surf. Sci. 2017, 402, 372–383. [Google Scholar] [CrossRef]
- Xue, C.; Yu, J.K.; Zhu, X.M. Thermal properties of diamond/SiC/Al composites with high volume fractions. Mater. Des. 2011, 32, 4225–4229. [Google Scholar] [CrossRef]
- Liang, X.; Jia, C.; Chu, K.; Chen, H.; Nie, J.; Gao, W. Thermal conductivity and microstructure of Al/diamond composites with Ti-coated diamond particles consolidated by spark plasma sintering. J. Compos. Mater. 2012, 46, 1127–1136. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, H.; Zhang, Y.; Li, J.; Wang, X. Effect of copper content on the thermal conductivity and thermal expansion of Al–Cu/diamond composites. Mater. Des. 2012, 39, 87–92. [Google Scholar] [CrossRef]
- Ren, S.; Shen, X.; Guo, C.; Liu, N.; Zang, J.; He, X.; Qu, X. Effect of coating on the microstructure and thermal conductivities of diamond–Cu composites prepared by powder metallurgy. Compos. Sci. Technol. 2011, 71, 1550–1555. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Dong, Y.H.; Zhang, R.Q.; Ren, X.T.; Chu, A.M.; Chen, D.Z.; Chen, Q.J.; Ye, Z.G. Numerical simulation of thermal conductivity of diamond/copper composites. Trans. Mater. Heat Treat. 2018, 39, 110. [Google Scholar]
- Schubert, T.; Trindade, B.; Weißgärber, T.; Kieback, B. Interfacial design of Cu-based composites prepared by powder metallurgy for heat sink applications. Mater. Sci. Eng. A 2008, 475, 39–44. [Google Scholar] [CrossRef]
- Ekimov, E.A.; Suetin, N.V.; Popovich, A.F.; Ralchenko, V.G. Thermal conductivity of diamond composites sintered under high pressures. Diam. Relat. Mater. 2008, 17, 838–843. [Google Scholar] [CrossRef]
- Zhang, C.; Cai, Z.; Tang, Y.; Wang, R.; Peng, C.; Feng, Y. Microstructure and thermal behavior of diamond/Cu composites: Effects of surface modification. Diam. Relat. Mater. 2018, 86, 98–108. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xu, M.; Cao, S.Z.; Chen, W.B.; Yang, W.Y.; Yang, Q.Y. Enhanced thermal conductivity of diamond/copper composite fabricated through doping with rare-earth oxide Sc2O3. Diam. Relat. Mater. 2020, 104, 107755. [Google Scholar] [CrossRef]
- Chao, Z.; Wang, J. Enhanced mechanical properties in diamond/Cu composites with chromium carbide coating for structural applications. Mater. Sci. Eng. A 2013, 588, 221–227. [Google Scholar]
- Eucken, A. Heat transfer in ceramic refractory materials: Calculation from thermal conductivities of constituents. Fortchg. Gebiete Ingenieurw. B3 Forschungsheft 1932, 16, 353–360. [Google Scholar]
- Hasselman, D.P.H.; Donaldson, K.Y.; Liu, J.; Gauckler, L.J.; Ownby, P.D. Thermal Conductivity of a Particulate-Diamond-Reinforced Cordierite Matrix Composite. J. Am. Ceram. Soc. 1994, 77, 1757–1760. [Google Scholar] [CrossRef]
- Tavangar, R.; Molina, J.M.; Weber, L. Assessing predictive schemes for thermal conductivity against diamond-reinforced silver matrix composites at intermediate phase contrast. Scr. Mater. 2007, 56, 357–360. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.L.; Wu, J.H.; Wang, X.T. Enhanced thermal conductivity in copper matrix composites reinforced with titanium-coated diamond particles. Scr. Mater. 2011, 65, 1097–1100. [Google Scholar] [CrossRef]
- Che, Q.L.; Zhang, J.J.; Chen, X.K.; Ji, Y.Q.; Li, Y.W.; Wang, L.X.; Cao, S.Z.; Guo, L.; Wang, Z.; Wang, S.W.; et al. Spark plasma sintering of titanium-coated diamond and copper–titanium powder to enhance thermal conductivity of diamond/copper composites. Mater. Sci. Semicond. Process. 2015, 33, 67–75. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Liu, X.; Dai, J.; Wang, X.; Zhang, H. Reinforcement size effect on thermal conductivity in Cu-B/diamond composite. J. Mater. Sci. Technol. 2021, 91, 1–4. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Catalano, M.; Bai, G.; Li, N.; Dai, J.; Wanga, X.; Zhanga, H.; Kim, M.J. Enhanced thermal conductivity in Cu/diamond composites by tailoring the thickness of interfacial TiC layer. Compos. Part A Appl. Sci. Manuf. 2018, 113, 76–82. [Google Scholar] [CrossRef]
- Rape, A.; Gott, K.; Kulkarni, A.; Singh, J. Simulation of matrix conductivity in copper–diamond composites sintered by field assisted sintering technology. Comput. Mater. Sci. 2015, 110, 29–33. [Google Scholar] [CrossRef]
- Every, A.G.; Tzou, Y.; Hasselman, D.P.H.; Raj, R. The Effect of Microstructure on the Thermal Conductivity of Particulate ZnS/Diamond Composites. Acta Metall. Mater. 1992, 40, 123–129. [Google Scholar] [CrossRef]
- Swartz, E.T.; Pohl, R.O. Thermal boundary resistance. Rev. Mod. Phys. 1989, 61, 605. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Li, N.; Che, Z.; Liu, X.; Chang, G.; Hao, J.; Dai, J.; Wang, X.; Sun, F.; et al. Interfacial Thermal Conductance between Cu and Diamond with Interconnected W− W2C Interlayer. ACS Appl. Mater. Interfaces 2022, 14, 35215–35228. [Google Scholar] [CrossRef] [PubMed]
- Kidalov, S.V.; Shakhov, F.M.; Vul, A.Y. Thermal conductivity of nanocomposites based on diamonds and nanodiamonds. Diam. Relat. Mater. 2007, 16, 2063–2066. [Google Scholar] [CrossRef]
- Hanada, K.; Matsuzaki, K.; Sano, T. Thermal properties of diamond particle-dispersed Cu composites. J. Mater. Process. Technol. 2004, 153, 514–518. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, J.; Wang, Y.; Wang, G.; Wang, H.; Yang, Z.; Ding, G. Critical effect and enhanced thermal conductivity of Cu-diamond composites reinforced with various diamond prepared by composite electroplating. Ceram. Int. 2019, 45, 13225–13234. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Bai, G.; Li, N.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Interfacial structure evolution and thermal conductivity of Cu-Zr/diamond composites prepared by gas pressure infiltration. J. Alloy. Compd. 2019, 781, 800–809. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, G.; Zhu, X.; Dai, J.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Manipulating in-situ discrete carbide interlayer to achieve high thermal conductivity in Cu-B/diamond composite. Mater. Today Commun. 2023, 34, 105357. [Google Scholar] [CrossRef]
- Bai, G.; Li, N.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. High thermal conductivity of Cu-B/diamond composites prepared by gas pressure infiltration. J. Alloy. Compd. 2018, 735, 1648–1653. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Che, Z.; Wang, X.; Zhang, H.; Wang, J.; Kim, M.J. Combining Cr pre-coating and Cr alloying to improve the thermal conductivity of diamond particles reinforced Cu matrix composites. J. Alloy. Compd. 2018, 749, 1098–1105. [Google Scholar] [CrossRef]
- Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. High thermal conductivity of diamond/copper composites produced with Cu–ZrC double-layer coated diamond particles. J. Mater. Sci. 2018, 53, 8978–8988. [Google Scholar] [CrossRef]
- Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. Optimized thermal conductivity of diamond/Cu composite prepared with tungsten-copper-coated diamond particles by vacuum sintering technique. Vacuum 2018, 153, 74–81. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, X.; Huang, S.; Xie, H.; Mi, X. Tailoring the thermal and mechanical properties of diamond/Cu composites by interface regulation of Cr alloying. Diam. Relat. Mater. 2021, 114, 108309. [Google Scholar] [CrossRef]
- Zhao, D.; Zha, S.; Liu, D. Influence of sputtering and electroless plating of Cr/Cu dual-layer structure on thermal conductivity of diamond/copper composites. Diam. Relat. Mater. 2021, 115, 108296. [Google Scholar] [CrossRef]
- Tao, J.M.; Zhu, X.K.; Tian, W.W.; Peng, Y.A.; Hao, Y.A. Properties and microstructure of Cu/diamond composites prepared by spark plasma sintering method. Trans. Nonferrous Met. Soc. China 2014, 24, 3210–3214. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Zhang, Y.; Zhao, Y.; Zhang, H. Thermal conductivity of Cu–Zr/diamond composites produced by high temperature–high pressure method. Compos. Part B Eng. 2015, 68, 22–26. [Google Scholar] [CrossRef]
- Xie, Z.; Guo, H.; Zhang, Z.; Zhang, X. Thermal expansion behavior and dimensional stability of Diamond/Cu composites with different diamond content. J. Alloy. Compd. 2019, 797, 122–130. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Wang, J.; Chang, J. Effect of tungsten based coating characteristics on microstructure and thermal conductivity of diamond/Cu composites prepared by pressueless infiltration. Ceram. Int. 2019, 45, 10810–10818. [Google Scholar] [CrossRef]
- Chang, G.; Sun, F.; Duan, J.; Che, Z.; Wang, X.; Wang, J.; Kim, M.J.; Zhang, H. Effect of Ti interlayer on interfacial thermal conductance between Cu and diamond. Acta Mater. 2018, 160, 235–246. [Google Scholar] [CrossRef]
- Yang, L.; Sun, L.; Bai, W.; Li, L. Thermal conductivity of Cu-Ti/diamond composites via spark plasma sintering. Diam. Relat. Mater. 2019, 94, 37–42. [Google Scholar] [CrossRef]
- Chu, K.; Jia, C.; Guo, H.; Li, W. On the thermal conductivity of Cu–Zr/diamond composites. Mater. Des. 2013, 45, 36–42. [Google Scholar] [CrossRef]
- Sang, J.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Regulating interface adhesion and enhancing thermal conductivity of diamond/copper composites by ion beam bombardment and following surface metallization pretreatment. J. Alloy. Compd. 2018, 740, 1060–1066. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Zhang, W.; Song, M.; Yang, W.; Peng, K. Effect of surface roughening on the interfacial thermal conductance of diamond/copper composites. Diam. Relat. Mater. 2019, 98, 107467. [Google Scholar] [CrossRef]
- Wu, X.; Wan, D.; Zhang, W.; Song, M.; Peng, K. Constructing efficient heat transfer channels at the interface of Diamond/Cu composites. Compos. Interfaces 2021, 28, 625–635. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, R.; Cai, Z.; Peng, C.; Feng, Y.; Zhang, L. Effects of dual-layer coatings on microstructure and thermal conductivity of diamond/Cu composites prepared by vacuum hot pressing. Surf. Coat. Technol. 2015, 277, 299–307. [Google Scholar] [CrossRef]
- Li, H.; Xie, Y.; Zhang, L.; Wang, H. Thermal properties of diamond/Cu composites enhanced by TiC plating with molten salts containing fluoride and electroless-plated Cu on diamond particles. Diam. Relat. Mater. 2022, 129, 109337. [Google Scholar] [CrossRef]
- Sang, J.; Yuan, Y.; Yang, W.; Zhu, J.; Fu, L.; Li, D.; Zhou, L. Exploring the underlying causes of optimizing thermal conductivity of copper/diamond composites by interface thickness. J. Alloy. Compd. 2022, 891, 161777. [Google Scholar] [CrossRef]
- Jia, J.; Bai, S.; Xiong, D.; Xiao, J.; Yan, T. Enhanced thermal conductivity in diamond/copper composites with tungsten coatings on diamond particles prepared by magnetron sputtering method. Mater. Chem. Phys. 2020, 252, 123422. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Liu, T.; Liu, Q.; Wu, M.; Qu, X. Microstructure and thermal properties of copper–diamond composites with tungsten carbide coating on diamond particles. Mater. Charact. 2015, 105, 18–23. [Google Scholar] [CrossRef]
- Kang, Q.; He, X.; Ren, S.; Zhang, L.; Wu, M.; Guo, C.; Cui, W.; Qu, X. Preparation of copper–diamond composites with chromium carbide coatings on diamond particles for heat sink applications. Appl. Therm. Eng. 2013, 60, 423–429. [Google Scholar] [CrossRef]
- Fu, X.; Jiang, J.; Jiang, X. Research Progress in Interfacial Characteristics and Strengthening Mechanisms of Rare Earth Metal Oxide-Reinforced Copper Matrix Composites. Materials 2022, 15, 5350. [Google Scholar] [CrossRef]
- Kang, Q.; Wu, X. Preparation, mechanical properties and thermo physical properties of copper/diamond composites with WC coating diamond particles by vacuum-pressure infiltration. J. Alloy. Compd. 2019, 789, 310–316. [Google Scholar]
- Chu, K.; Liu, Z.; Jia, C.; Chen, H.; Liang, X.; Gao, W.; Tian, W.; Guo, H. Thermal conductivity of SPS consolidated Cu/diamond composites with Cr-coated diamond particles. J. Alloy. Compd. 2010, 490, 453–458. [Google Scholar] [CrossRef]
- Raza, K.; Khalid, F.A. Optimization of sintering parameters for diamond–copper composites in conventional sintering and their thermal conductivity. J. Alloy. Compd. 2014, 615, 111–118. [Google Scholar] [CrossRef]
- Li, H.; Wang, C.; Wu, L.; Chen, M.; Wu, C.; Wang, N.; Li, Z.; Tang, L.; Pang, Q. Optimization of process parameters, microstructure, and thermal conductivity properties of Ti-coated diamond/copper composites prepared by spark plasma sintering. J. Mater. Sci. Mater. Electron. 2021, 32, 9115–9125. [Google Scholar] [CrossRef]
- Sang, J.; Zhou, L.; Yang, W.; Zhu, J.; Fu, L.; Li, D. Enhanced thermal conductivity of copper/diamond composites by fine-regulating microstructure of interfacial tungsten buffer layer. J. Alloy. Compd. 2021, 856, 157440. [Google Scholar] [CrossRef]
- Lei, L.; Su, Y.; Bolzoni, L.; Yang, F. Evaluation on the interface characteristics, thermal conductivity, and annealing effect of a hot-forged Cu-Ti/diamond composite. J. Mater. Sci. Technol. 2020, 49, 7–14. [Google Scholar] [CrossRef]
- Sun, H.; Guo, L.; Deng, N.; Li, X.; Li, J.; He, G.; Li, J. Elaborating highly thermal-conductive diamond/Cu composites by sintering intermittently electroplated core-shell powders. J. Alloy. Compd. 2019, 810, 151907. [Google Scholar] [CrossRef]
- Pan, Y.; He, X.; Ren, S.; Wu, M.; Qu, X. Improvement of ZrC/Zr coating on the interface combination and physical properties of diamond-copper composites fabricated by spark plasma sintering. Materials 2019, 12, 475. [Google Scholar] [CrossRef]
- Azarniya, A.; Azarniya, A.; Sovizi, S.; Hosseini, H.R.M.; Varol, T.; Kawasaki, A.; Ramakrishna, S. Physicomechanical properties of spark plasma sintered carbon nanotube-reinforced metal matrix nanocomposites. Prog. Mater. Sci. 2017, 90, 276–324. [Google Scholar] [CrossRef]
- Dai, S.G.; LI, J.W.; Wang, C.J. Preparation and thermal conductivity of tungsten coated diamond/copper composites. Trans. Nonferrous Met. Soc. China 2022, 32, 2979–2992. [Google Scholar] [CrossRef]
- Constantin, L.; Fan, L.; Pontoreau, M.; Wang, F.; Cui, B.; Battaglia, J.L.; Silvain, J.F.; Lu, Y.F. Additive manufacturing of copper/diamond composites for thermal management applications. Manuf. Lett. 2020, 24, 61–66. [Google Scholar] [CrossRef]
- Jia, S.Q.; Bolzoni, L.; Li, T.; Yang, F. Unveiling the interface characteristics and their influence on the heat transfer behavior of hot-forged Cu–Cr/Diamond composites. Carbon 2021, 172, 390–401. [Google Scholar] [CrossRef]
- Lei, L.; Bolzoni, L.; Yang, F. High thermal conductivity and strong interface bonding of a hot-forged Cu/Ti-coated-diamond composite. Carbon 2020, 168, 553–563. [Google Scholar] [CrossRef]
- Lei, L.; Bolzoni, L.; Yang, F. The Impact of Interface Characteristics on Mechanical Performance of a Hot-Forged Cu/Ti-Coated-Diamond Composite. Mater. Sci. Forum 2021, 1016, 1682–1689. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Wu, C.; Yin, Z.; Liu, C.; Liu, J.; Shi, Z. Interfacial Characterization and Thermal Conductivity of Diamond/Cu Composites Prepared by Liquid-Solid Separation Technique. Nanomaterials 2023, 13, 878. [Google Scholar] [CrossRef]
- Zhou, H.; Ran, M.; Li, Y.; Yin, Z.; Tang, Y.; Zhang, W.; Zheng, W.; Liu, J. Improvement of thermal conductivity of diamond/Al composites by optimization of liquid-solid separation process. J. Mater. Process. Technol. 2021, 297, 117267. [Google Scholar] [CrossRef]
- Huda, N.; Bisht, A.; Moreau, E.; Corbin, S.; Rabkin, E.; Gerlich, A.P. Fabrication of copper–diamond composite by friction stir processing. J. Mater. Sci. 2023, 58, 4184–4198. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, H.; Yu, G.; Du, R.; He, P. A novel ultrasonic consolidation method for rapid preparation of diamond/Cu composites. Mater. Lett. 2022, 323, 132498. [Google Scholar] [CrossRef]
- Dai, S.; Li, J.; Lu, N. Research progress of diamond/copper composites with high thermal conductivity. Diam. Relat. Mater. 2020, 108, 107993. [Google Scholar] [CrossRef]
- Jia, S.Q.; Yang, F. High thermal conductive copper/diamond composites: State of the art. J. Mater. Sci. 2021, 56, 2241–2274. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Leng, X.; Zhao, R.; Kang, Y.; Chen, H. Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications. Crystals 2023, 13, 906. https://doi.org/10.3390/cryst13060906
Chen K, Leng X, Zhao R, Kang Y, Chen H. Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications. Crystals. 2023; 13(6):906. https://doi.org/10.3390/cryst13060906
Chicago/Turabian StyleChen, Kang, Xuesong Leng, Rui Zhao, Yiyao Kang, and Hongsheng Chen. 2023. "Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications" Crystals 13, no. 6: 906. https://doi.org/10.3390/cryst13060906
APA StyleChen, K., Leng, X., Zhao, R., Kang, Y., & Chen, H. (2023). Progress in the Copper-Based Diamond Composites for Thermal Conductivity Applications. Crystals, 13(6), 906. https://doi.org/10.3390/cryst13060906





