Abstract
The negative effects of refractory organic substances in water on the environment and life have aroused worldwide attention. The efficiency of using photoelectrocatalysis (PEC) to degrade refractory organic pollutants depends to a large extent on the properties of the photoanode semiconductor. Therefore, the selection of a satisfactory photoanode semiconductor material to promote the production of intermediate reactive species (hydroxyl radicals and superoxide radicals) has become a key issue in improving the efficiency of PEC. Among the available catalysts, transition metal oxides have received a lot of attention in recent years due to their low price and significant advantages. Due to its outstanding photoelectrocatalytic properties, Co3O4 has emerged as a candidate to serve as a photoelectrocatalyst specifically for the oxidation of water with oxygen in these materials. This paper summarizes in detail the recent advances in Co3O4 materials for PEC, both pure Co3O4 and Co3O4-based composites. In addition, this review discusses the impact of strategies on the performance of photoelectrocatalysts, such as synthesis methods, crystal surface structures, and composites. Finally, this review concludes with a presentation of the challenges and workable solutions for Co3O4-based materials in PEC, along with a discussion of their potential for future research.
1. Introduction
Wastewater is a global issue that has seriously harmed both human survival and ecology. Among various organic pollution sources in wastewater, the discharge of dye-containing effluents into water systems is a critical issue. Organic dyes and their intermediate products may be subjected to various chemical reactions, which may cause in these products becoming carcinogenic, mutated, or abnormal, and can have adverse effects on micro-organisms, aquatic life, soil, and water [1,2]. Research by type shows that since the beginning of the 20th century, the textile and pharmaceutical industries [3], which produce a large number of pollutants in the water environment, have experienced significant growth [4,5]. It is well known that wastewater containing intractable organic dyes has a high chemical oxygen demand (COD), high color, a high amount of total dissolved solids (TDS), uneven pH, and low biodegradability [6]. Undoubtedly, a clean and secure environment without the contamination of air, water, and soil is essential for people’s health and survival. Research shows that traditional water treatment technology cannot solve the problems of sludge and other secondary pollutants, resulting in the incomplete removal of pollutants and the transport of pollutants to other media [7]. In contrast, advanced oxidation processes (AOPs) are effective in degrading harmful and high-resistance pollutants by producing powerful oxidizing agents in situ, such as hydroxyl radicals (·OH), which have the capability of fully mineralizing toxic organic pollutants. In AOPs, there are four known methods [8,9,10], including biological oxidation [11,12], chemical oxidation [13,14], photochemical oxidation [15,16], and electrochemical oxidation processes [17,18]. Nevertheless, the single technical method is not entirely effective and successful in treating dyed wastewater due to its non-degradability and volatility. In practice, different methods are often combined to achieve the desired water quality in the most cost-effective way.
In recent years, photocatalysis (PC) has performed well in the treatment of pollution such as staining [19,20], drugs [21,22], and other endocrine disruptors [23,24,25,26]. Electrochemical (EC) catalysis is considered a highly efficient, sustainable, and low-cost technology for the degradation of wastewater [27]. AOPs are also a sub-class of photoelectrocatalysis (PEC). PEC aims to achieve a synergistic effect by combining photocatalysis with electrocatalysis. PEC has several advantages over PC and EC, including the ease of reuse of electrodes concerning catalyst power. For instance, in sewage, the rate of synthesis can be reduced and the degradation of organic compounds can be achieved by applying a bias voltage. PEC also provides an opportunity to generate reactive oxygen species at the cathode, which is beneficial for organic decomposition. To make full use of this free energy, it is possible to use the sun’s rays to power the PEC, which is called Solar PEC [28]. Fujishima and Honda established the cornerstone of PEC technology by using the n-type TiO2 and Pt electrodes in 1972 as the anode and cathode for water decomposition. This approach opened a vital door to the PEC field [29]. Then, Vinodgopal et al. first degraded organic pollutants in the PEC process using a particulate TiO2 film electrode in 1993 [30]. This success was extended to the degradation of dyes using many various semiconductor materials in PEC processes, such as TiO2 [31,32], WO3 [33], ZnO, α-Fe2O3 [34], Sn3O4 [35], and Co3O4.
Co3O4 is a typical spinel-type oxide, where Co(II) cations occupy tetrahedrally (Co(II)Td) [36,37]. On the other hand, Co(III) cations occupy octahedrally (Co(III)OH) [38,39]. This gives the oxidation–reduction pair Co(II)/Co(III). It has been reported that the bond of Co with other atoms is better suited to the process of water oxidation. This can provide a redox pair Co(II)/Co(III) that is well suited to several redox reactions and electron transfers. It has been reported that the binding force between Co atoms and other atoms is more suitable. Because of its superior catalytic activity, low cost (in comparison with Au, Ag, Pt), and high permanence, O2 has little capability to prevent “toxicity” (readily adsorbed into an intermediate, hard to desorb), which influences the catalysis [40,41]. One-dimensional (1D) Co3O4 is currently attracting a great deal of attention because of its high conductivity, large surface area, wide optical response range, chemical stability [42,43], and its expected synergistic effect with PC and EC [44,45]. Spinel Co3O4 may be a promising candidate to replace precious metals as anode materials. Similarly, in recent years, more articles have been published on Co3O4 than any other spinel oxide. Among them, Fe3O4, NiO, and CuO are less active and have smaller electrochemical active surface areas (ECSA) than Co3O4. To enhance the PEC performance of the Co3O4 photoanode, different strategies have been utilized, such as facet engineering [46,47], heteroatom doping, heterojunction construction, and the deposition of particles of noble metals [48]. They identified a number of active sites that benefit OER in catalysis [49,50]. For instance, Yang and his colleagues prepared hollow Co3O4 dodecahedrons, which were designed by the calcination of a ZIF-67 precursor under various conditions of argon and oxygen. It had a high percentage showing good photoelectrocatalytic properties for oxygen evolution reaction (OER) [51]. Yan and colleagues synthesized highly conductive, Ag-doped Co3O4 nanowires via electrodeposition. Ag-Co/FTO is a kind of OER material with good catalytic performance [52]. Recently, Li and colleagues reviewed the latest advances in cobalt-based materials as bi-functional photoelectrocatalysts for oxygen reduction reaction (ORR) [53]. There are a few types of research on the degradation of pollutants using different anode materials.
This paper presents a summary of the methods and ideas for the design and synthesis of materials and provides a perspective on methods to improve the performance of cobalt-based materials. These studies are more conducive to the development of photoelectrocatalytic hydrolysis.
2. Co3O4: Synthesis Routes
The physical and chemical properties of a substance are a reflection of its properties, and we usually learn about the properties of a substance through observation, experimentation, and analysis. However, we can also learn about a substance in terms of its physical, chemical, and biological properties and its patterns of change. We have investigated the properties of Co3O4 (Table 1) to gain a fuller understanding of its properties and to select a suitable synthesis method. Up to now, several physics and chemistry methods have been used to synthesize Co3O4. Table 2 summarizes the synthesis pathway and the advantages and disadvantages of Co3O4.

Table 1.
Characteristics of Co3O4.

Table 2.
Various synthesis methods and advantages and disadvantages of Co3O4.
2.1. Hydrothermal
Among the known methods for the synthesis of Co3O4, the hydrothermal process seems to be the most prominent because it produces a highly crystalline Co3O4. Furthermore, it is possible to control the shape of Co3O4 by simple optimization of the reaction pressure, temperature, time, and pH of the solution.
Controlled continuous hydrothermal synthesis is one method of obtaining Co3O4 nanoparticles. Stripper water containing 0.25% v/v H2O2 is fed into the reactor by a high-pressure pump through a preheating device. Experiments at the University of Nottingham have shown that diluted aqueous hydrogen peroxide solutions decompose into a mixture of oxygen and water [58,59]. Cobalt acetate tetrahydrate (II) is added to the reactor at room temperature. In a nozzle reactor, oxygen-enriched water is preheated through an inner tube, and an aqueous solution of cobalt acetate tetrahydrate (II) flows upward [60]. The Nottingham team has previously reported in detail on the design of the nozzle reactor and the mixing of fluids therein. At the outlet of the reactor, the mixture is pumped into the cooling device. A dry powder of Co3O4 nanoparticles was obtained by freeze-drying with liquid nitrogen and under low-temperature vacuum (−50 °C) for more than 36 h.
However, this method requires a longer reaction time and is not sufficiently cost-effective. Zhao et al. proposed a more energy-efficient solution with lower consumption. Initially, 5 mmol of Co(NO3)2·6H2O, 10.0 mmol of urea, and 5.0 mmol of NH4F were dissolved thoroughly into 40 mL of high-purity water at room temperature to form a homogeneous pink solution. The solution was transferred to a 60 mL, Teflon-lined stainless-steel autoclave, a Ti substrate was inserted as prepared, and the solution was held at 120 °C for 6 h. Subsequently, the Ti substrate having a pink precursor was naturally cooled to ambient temperature and cleaned with deionized water and vacuum dried, followed by annealing at 450 °C for 2 h at 2 °C/min to obtain a Co3O4 nanowire array on the Ti substrate. Nanorods of Co3O4 were obtained using this method [61] (see Figure 1).
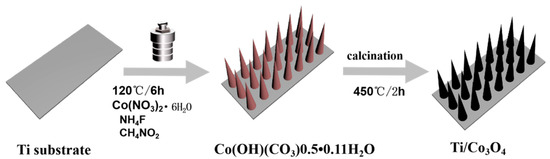
Figure 1.
Schematic diagram of the hydrothermal synthesis of Co3O4 nanorods.
2.2. Sol–Gel
Among the various conditions affecting material properties, the effect of temperature on the material structure is not negligible. Annealing temperature affects the structure, morphology, conductivity, and band gap of nanocrystalline Co3O4 films obtained via sol–gel spin-coating. Cobalt acetate tetrahydrate is added into 40 mL methanol and stirred vigorously for 1 h at 60 °C, resulting in a pale pink powder. The as-prepared powder is sintered at various temperatures ranging from 400 to 700 °C with a fixed annealing time of 1 h in ambient air to obtain Co3O4 with different crystallite sizes. Table 3 of the revised article illustrates the effect of calcination temperature on the size of the Co3O4 crystals. The nanocrystal Co3O4 powder is further dissolved in m-cresol, and the solution continues to be stirred at room temperature for 11 h, then filtered. The filtered solution is deposited on a glass substrate by a single-wafer spin processor [62].

Table 3.
Effect of annealing on Co3O4 thin film properties.
2.3. Vapor Deposition Method
Davide Barreca et al. deposited the film in a low-pressure chemical vapor deposition (CVD) reactor with a heated susceptor. O2 is used as a carrier and a reactive gas in the process of synthesizing the oxide and removing the organic ligands as oxidation by-products. The precursor is placed in a vaporizer attached to the tube of the reactor and kept at 90 °C for the entire duration of the film deposition. The gas pipe and the valve between the bubble and the reaction tube are heated to prevent the precursor from condensing. The pressure is measured with a capacitive pressure gauge, and a mass flow controller is used to control the gas flow. Before CVD, the substrates were desorbed in soapy water, washed with water and isopropanol, and then air-dried. In order to minimize carbon contamination, their surfaces were heated by O2 flow in the reaction chamber for 40 min [33]. Chen et al. show the schematic of the direct liquid injection chemical vapor deposition (DLI-CVD) apparatus. A novel spray atomizing and co-precipitating precursor delivery system was developed; this consisted of a liquid precursor tank, the extraction switch, an atomizer, an evaporator, and some necessary stainless-steel connection tubes. Solid Co(dpm)3 (DPM; dipivaloylmethanate; Wuhan CVD Science & Technology Co., Ltd., Wuhan, China) was used as the precursor into tetrahydrofuran [63] (see Figure 2).
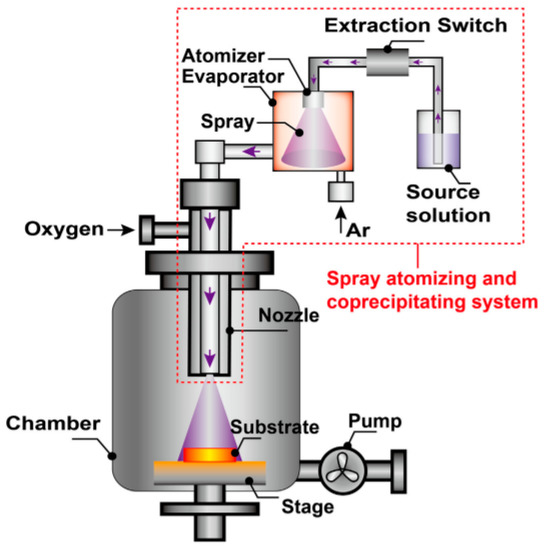
Figure 2.
Schematic of the DLI-CVD.
2.4. Green Synthesis
At present, the green synthesis of metal-oxide NPs is one of the most promising fields in green chemistry and nanotechnology. The method of preparing Co3O4 nanoparticles from latex at room temperature was studied. In addition, it is unnecessary to apply large quantities of heat, power [64], pressure, or poisonous substances [65,66]. Because of its environmental friendliness, simplicity, rapidity, toxicity, and economy, green synthesis offers a one-step method to synthesize Co3O4 NPs [67,68]. The Co3O4 NPs are stabilized by combining them with the biological material of amino acids, saponins, enzymes, proteins, steroids, phenol, tannin, vitamins, sugars, flavone, etc. [69,70] (see Figure 3).
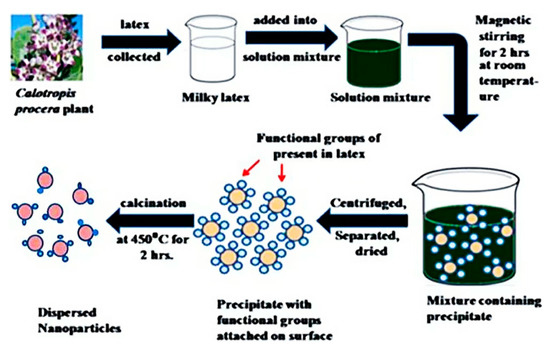
Figure 3.
A novel green synthesis of cobalt oxide (Co3O4) nanoparticles.
3. Crystal Structure Analysis of Co3O4 Samples
Co3O4 crystallizes in a cubic regular spinel structure, which consists of Co ions in Co2+, Co3+, and Co3+, respectively [71]. These are situated in the interstices in the tetrahedral (8a) and the octahedral (16d) positions of the closely packed, face-centered cubic (FCC) lattice formed by oxygen ions (Figure 4). In general, cobalt oxide consists of a spinel structure with an indirect band gap of ~1.5 eV and a direct band gap of 2.2 eV. The common spinel structure is expressed by the formula (A) [B2] C4, wherein A and B are cations in tetrahedral and octahedral coordination, while C represents anions. The spinal structure is significantly stable when A is divalent and B is trivalent, for instance, (A2+)[B3+]C4. Similarly, cobalt oxide (Co3O4) is known to follow a spinel structure, such as (Co2+)[Co23+]O4 [72,73]. The high-spin Co2+ occupies the interstitial sites of the tetrahedral (8a) interstices, while the low-spin Co3+ is known to occupy the octahedral (16d) interstices of the closely packed, face-centered, cubic lattice of CoO·Co2O3, as illustrated in Figure 4. It is known that the p-type conductivity of the material (CoO·Co2O3) is derived from a gap in the crystal lattice and an excess of oxygen at the interstitial site. However, the concentration of charge carriers is different from the operating temperature or the doping condition. A. Diallo et al. discussed that Co3O4 is generally active in the following order: {112} > {110} > {111} > {100} [74]. The understanding of Co3O4’s crystal structure is further deepened [75,76].
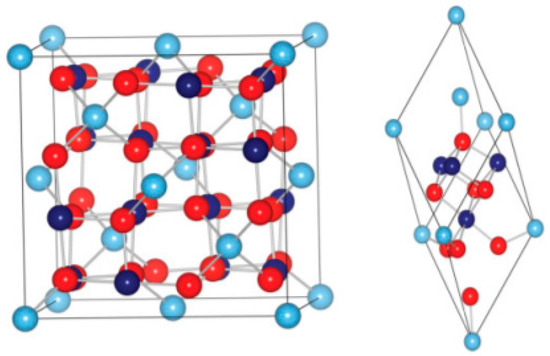
Figure 4.
Crystal structure of Co3O4. Unit cell (left) and primitive cell (right) of Co3O4. Light cyan and navy blue balls indicate Co2+ and Co3+ ions; red balls indicate O2−ions.
Zhou et al. studied the structure and the action mechanism of Co3O4 from the crystal structure and the atomic structure of the crystal surface. Figure 5a is a cell type (cell) structure with a pointed crystal. In Figure 5b–d, the topmost surface atoms and the first atoms, as well as the topmost suspended bonds, are exposed to the environment, and they can react oxidatively in water. Therefore, the main influential factor for the water-oxidizing activity of various well-defined Co3O4 crystals lies in the composition of the surface atoms and their respective catalytic active sites [77].
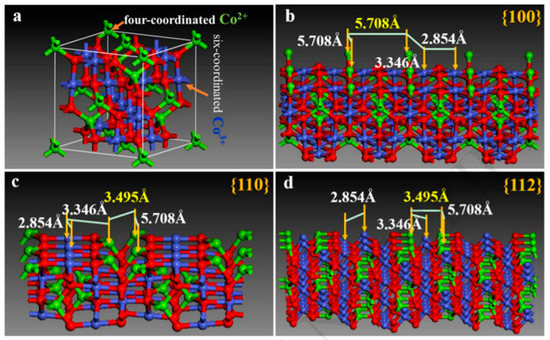
Figure 5.
(a) The unit cell structure of spinel Co3O4, green ball is Co2+, blue ball denotes Co3+, and red ball is O2−. The crystal structure model and arrangement of surface atoms of Co3O4 with different exposed facets for (b) {100}, (c) {110}, and (d) {112}. The Co3O4 structure model is obtained from inorganic crystal structure database (ICSD) with the corresponding JCPDS file.
4. Co3O4: Composites
Despite its unique properties, Co3O4 encounters a huge obstacle: the narrow band gap of photoelectrons can recombine easily with holes, resulting in a small quantum efficiency. Particle size and shape effects control the oxidation behavior of the nanostructured photoelectrocatalysis treatment of organic wastewater. The size of the nanoparticles can be altered by changing the cobalt concentration and reaction time. Spherical, cubic, octahedral, and platelike nanoparticles with narrow size distributions and size ranges were formed in high yields via thermal decomposition. These surfactant-free nanoparticles (around 10 nm) form an ideal substrate for the easy deposition of further elements, which in turn increases the efficiency of photoelectrocatalytic degradation (Figure 6). It has been suggested that the formation of composite materials, in which some are heterostructures, may be one of the approaches to improve the photoelectrocatalytic performance of Co3O4 (Table 4). The hybridization of Co3O4 with metals, non-metals, metal oxides, carbon-based materials, and plasmonic nano-metals such as gold and silver to form heterostructured composites has been extensively explored. The formation of heterogeneous composites by Co3O4 hybridization has been extensively studied [78,79]. Doping noble metals into simple metal oxides was shown to improve the photocatalytic activity by Chen et al. Among the noble metals, elemental Ag is widely used due to its lower cost. Its role in improving the catalytic activity mainly involves two aspects. First, Ag doping can separate the photogenerated carriers efficiently because of the formation of a Schottky barrier. Second, it can improve the response to visible light. A new electrochemically modified BiVO4-MoS2-Co3O4 thin film electrode for environmental applications has been successfully synthesized by Cong et al. [80]. The formation of composites has the potential to promote carrier migration, which leads to the formation of the internal electric field, thus improving carrier separation and finally improving the performance of Co3O4 as a photocatalyst. Yang et al. successfully used a simple method to blend Co3O4 and CoO on TiO2 NAs. A series of characterization methods was used to investigate the morphology, structure, and PEC water oxidation properties [81]. It was found that the photocurrent density of the obtained CoO-Co3O4/TiO2 photoelectrode was improved and could be maintained with good stability for more than 13 h. The improvement in the properties of CoO-Co3O4/TiO2 is due to the increase in light absorption and reduction in charge transfer resistance, thus improving charge separation.
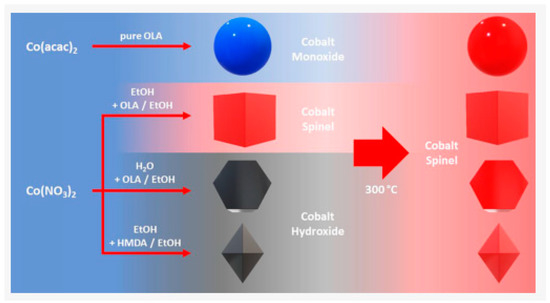
Figure 6.
Shape-selective synthesis of Co3O4 nanoparticles.

Table 4.
Modification approach and materials.
The photo-generated electrons of Co3O4 can be channeled into the dopant, thereby reducing the recombination rate [42]. This leads to the production of more oxidizing substances (h+ and OH−) that break down harmful organic substances. Although the process has some advantages, new electrons may be brought in by dopants, which may have adverse effects. On the other hand, the formation of heterojunction composite materials provides a more effective method for the separation of photogenerated carriers. This technology can increase the photocatalyst’s ability to capture light, enhance its charge separation ability, improve its charge utilization ratio, and prolong its service life.
5. Photoelectrocatalytic Application of Co3O4 Composites in Water Treatment
To date, Co3O4 and its composite materials are mainly used in capacitors and fuel cells. However, there are few studies on its application in dyes and photoelectrocatalysis. Applications in wastewater degradation are described in the following sections.
In the PC process, there is a problem with catalyst recycling. Nevertheless, in the photoelectrocatalysis process, the catalytic material is fixed on the surface of the carrier and used as an electrode, which is beneficial to the recycling of the material. In preparing the anodes, it is necessary to have a conductive substrate on which Co3O4 is deposited. Titanium plates, anodized TiO2, and fluoro-doped substrates have been used. Wang and co-workers doped F− into Co3O4 as the conducting substrate. A titanium plate was formed by etching, and cobalt nitrate was made from cobalt. Co3O4 nanowires have been prepared using the hydrothermal method and the calcining method on the titanium substrate. The best degradation conditions were obtained by varying the water temperature and electric current during the decomposition [89]. Co3O4 is unsuitable as the photoanode material to degrade organic pollutants because conventional Co3O4 is a p-type semiconductor. It is well-known that the introduction of F− can not only promote the morphology of the oxides but can also convert the intrinsic semiconductor to the n-type (e.g., SnO2) [90,91,92]. Moreover, the presence of F− in the crystal plays a key role in reducing the enucleation rate and activating the substrate, which leads to strong mechanical adhesion between the nano-architecture and the substrate. Based on the above results, the possible PEC processes were described as follows (see Figure 7): Firstly, the electrons (e−) in the valence band of Co3O4 under light irradiation could be excited to the conduction band, leaving the holes (h+) in the valence band of Co3O4. When applying an anodic bias potential to the semiconductor (i.e., the applied potential is greater than the flat band potential), there will be an increase in band bending. Thus, electrons in the conduction band are flown through the counter electrode via the external circuit, and the holes are transferred to the surface. Thus, the bands are bent downwards, producing an ohmic contact. The band-bending causes no impediment to the motion of the induced electrons from the conduction band of Co3O4 into the metal Ti. Then, the electrons are moved to the external circuit faster via an electric field, and the induced charge carriers were effectively separated.
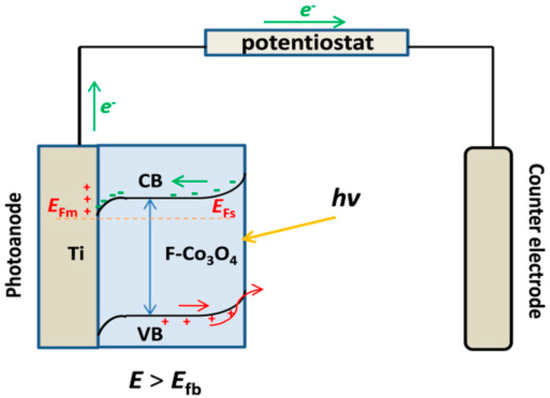
Figure 7.
Charge transfer mechanism in the Ti/Co3O4 electrode under PEC process.
As outlined in the introduction, many different semiconductor materials are used in the PEC process to degrade dyes, such as titanium dioxide, tungsten trioxide, zinc oxide, Sn3O4, and Co3O4. We have compared the photoelectric catalysis degradation capabilities of commonly used compounds. It was found that Co3O4 is a suitable material for photoelectric catalysis (Table 5). Coupling two or more different types of semiconductor materials into a single photoanode can improve the photo-carrier transmission efficiency and photo-conversion efficiency. Wang et al. reported one of the first studies on the application of Co3O4 in the formation of heterojunction for water treatment. In this paper, a new heterostructure of the PbO2-tipped Co3O4 nanowire array (NW) was prepared by the methods of hydrothermal synthesis and electrochemical deposition. The results show that the as-built PbO2/Co3O4 composite exhibits a large electro-active area, a low charge-transfer resistance, and a high efficiency in the production of hydroxyl radicals. The photoelectrochemical (PEC) performance of the as-constructed PbO2/Co3O4 composite has been assessed by the decoloration of dye (Reactive Brilliant Blue KN−R). The PEC test showed that the PbO2/Co3O4 composite prepared by this process had good repeatability and photoelectric properties. The enhancement of the PEC capability of composites may be attributed to the formation of heterostructures. This work provides a good prospect for using Co3O4 NWs doped with lead dioxide as photoanodes for treating refractory organic pollutants. This further reinforces the argument that the PEC is a more effective approach than EC and PC due to the synergy occurring in the PEC [89]. Co3O4 heterojunctions have been explored in the remediation of water contaminated with organic pollutants using photoelectric synergy, as outlined in Table 6.

Table 5.
Studies on photoelectrocatalytic degradation of toxic organics.

Table 6.
Recent studies on photoelectrocatalytic degradation of toxic organics involving Co3O4.
6. Conclusions and Future Perspectives
In recent years, Co3O4 has been used in photoelectrocatalysis for wastewater treatment. This material has good light-capturing performance in the visible light regions, so it has good application prospects. The shape and the crystal structure of Co3O4 have been reported to affect the photoelectrocatalytic properties of Co3O4, especially the crystal shape, which can change in the course of the application. Accordingly, it is possible to examine the crystalline form or structure of Co3O4 before and following a degradation cycle.
In the field of photoelectrocatalysis, further work should focus on the degradation of various types of pollutants. In this paper, the application of Co3O4 in PEC for the treatment of organic pollutants in water was shown to be in its initial stages. Indeed, a section of this paper highlights reports that can be compared to other types of semiconductors found in PEC applications. Therefore, it is expected that Co3O4 will be widely used as a photoelectrocatalyst. In addition, it would be worthwhile to find basic research on how some pollutants are degraded and how they are treated. Through the discussion of the above problems, we can better reveal its favorable structural form.
Although Co3O4-based catalysts, as frequently used photoelectrocatalysts, have made significant progress in degradation through various effective strategies in recent years, there are still some problems and challenges that cannot be ignored in its application:
(1) Morphology engineering can effectively improve the surface area. Increased porosity is beneficial to the photoelectrocatalytic performance, and an ordered nano-structure is beneficial to catalysis. Therefore, Co3O4-based catalysts with an array structure will be widely studied in the degradation field.
(2) The material lends itself to various synthesis methods, and thus one can expect more novel synthesis routes shortly.
(3) The Co3O4 composite exhibits high activity, but its catalytic performance is improved, and its preparation process is also complicated, which is not conducive to industrial applications. Therefore, the preparation technology suitable for practical applications still needs to be studied.
(4) Stability is an important part of the catalyst performance, and corrosion-resistant supports such as Cu, Pt and Ti should be considered as substrates, and cost should also be considered. At the same time, in situ growth technology with binding characteristics can avoid catalyst shedding, so in situ growth will be widely used by researchers in the process of catalyst preparation.
(5) The p–n variability can be exploited in preparing a myriad of heterojunctions with other semiconductors.
Author Contributions
F.Z.: Conceptualization, Investigation, Methodology, Writing—review and editing, and Writing—original draft. H.M.: Conceptualization, Funding acquisition, Project administration, and Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21875026, 21878031) and the Liaoning Revitalization Talents Program (XLYC1802124). This research was funded by Liaoning BaiQianWan Talents Program and the scientific research fund of the educational department of Liaoning province (J2019013). This research was funded by the Joint Research Fund of the Liaoning-Shenyang National Laboratory for Materials Science (Project number: 2019JH3/30100034; Contract number: 2019010278-JH3/301).
Data Availability Statement
This paper consists of review material; thus, there were no new data used for its preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wazir, M.B.; Daud, M.; Ali, F.; Al-Harthi, M. Dendrimer assisted dye-removal: A critical review of adsorption and catalytic degradation for wastewater treatment. J. Mol. Liq. 2020, 315, 113775. [Google Scholar] [CrossRef]
- Crini, G.L.E.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in german sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Gani, K.M.; Kazmi, A.A. Evaluation of three full scale sewage treatment plants for occurrence and removal efficacy of priority phthalates. J. Environ. Chem. Eng. 2016, 4, 2628–2636. [Google Scholar] [CrossRef]
- Ignatev, A.; Ngumba, E.; Kosunen, P.; Tuhkanen, T. Removal of selected antibiotics and antiretroviral drugs from water by ozonation and ozone-based AOPs. In Proceedings of the 15th EuCheMS International Conference on Chemistry and the Environment, Leipzig, Germany, 20–24 September 2015. [Google Scholar]
- Körbahti, B.; Aktaş, N.; Tanyolaç, A. Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J. Hazard. Mater. 2007, 148, 83–90. [Google Scholar] [CrossRef]
- Hameed, B.H.; Akpan, U.G.; Wee, K.P. Photocatalytic degradation of acid red 1 dye using zno catalyst in the presence and absence of silver. Desalin. Water Treat. 2011, 27, 204–209. [Google Scholar] [CrossRef]
- Arslan-Alaton, I. Degradation of a commercial textile biocide with advanced oxidation processes and ozone. J. Environ. Manag. 2007, 82, 145–154. [Google Scholar] [CrossRef]
- Rivas, F.J.; Beltran, F.J.; Encinas, A. Removal of emergent contaminants: Integration of ozone and photocatalysis. J. Environ. Manag. 2012, 100, 10–15. [Google Scholar] [CrossRef]
- Reddy, P.; Kim, K.H. A review of photochemical approaches for the treatment of a wide range of pesticides. J. Hazard. Mater. 2015, 285, 325–335. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef]
- Korotta-Gamage, S.M.; Sathasivan, A. A review: Potential and challenges of biologically activated carbon to remove natural organic matter in drinking water purification process. Chemosphere 2017, 167, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Wacławek, S.; Silvestri, D.; Hrabák, P.; Padil, V.V.; Torres-Mendieta, R.; Wacławek, M.; Dionysiou, D.D. Chemical oxidation and reduction of hexachlorocyclohexanes: A review. Water Res. 2019, 162, 302–319. [Google Scholar] [CrossRef]
- Talaiekhozani, A.; Talaei, M.R.; Rezania, S. An overview on production and application of ferrate (VI) for chemical oxidation, coagulation and disinfection of water and wastewater. J. Environ. Chem. Eng. 2017, 5, 1828–1842. [Google Scholar] [CrossRef]
- Chan, S.; Yeong, W.; Juan, J. Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J. Chem. Technol. Biotechnol. 2011, 86, 1130–1158. [Google Scholar] [CrossRef]
- Orge, C.A.; Faria, J.L.; Pereira, M.F.R. Photocatalytic ozonation of aniline with tio2-carbon composite materials. J. Environ. Manag. 2016, 195, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Peng, C. Treatment of textile wastewater by electrochemical method. Water Res. 1994, 28, 277–282. [Google Scholar] [CrossRef]
- Changotra, R.; Rajput, H.; Dhir, A. Natural soil mediated photo fenton-like processes in treatment of pharmaceuticals: Batch and continuous approach. Chemosphere 2017, 188, 345–353. [Google Scholar] [CrossRef]
- Babu, V.J.; Sireesha, M.; Bhavatharini, R.S.R.; Ramakrishna, S. Electrospun biobr lamellae for efficient photocatalysis on ars dye degradation. Mater. Lett. 2016, 169, 50–53. [Google Scholar] [CrossRef]
- Mahmoud, S.A.; Fouad, O.A. Synthesis and application of ZiNC/TiN oxide nanostructures in photocatalysis and dye sensitized solar cells. Sol. Energy Mater. Sol. Cells 2015, 136, 38–43. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, R.; Fu, Y.; Li, R.; Yao, J.; Jiang, S. Novel multifunctional NiFe2O4/ZnO hybrids for dye removal by adsorption, photocatalysis and magnetic separation. Appl. Surf. Sci. 2016, 369, 1–10. [Google Scholar] [CrossRef]
- Ahern, J.C.; Fairchild, R.; Thomas, J.S.; Carr, J.; Patterson, H.H. Characterization of biox compounds as photocatalysts for the degradation of pharmaceuticals in water. Appl. Catal. B 2015, 179, 229–238. [Google Scholar] [CrossRef]
- Fathinia, M.; Khataee, A.; Naseri, A.; Aber, S. Monitoring simultaneous photocatalytic-ozonation of mixture of pharmaceuticals in the presence of immobilized TiO2 nanoparticles using mcr-als: Identification of intermediates and multi-response optimization approach. Spectrochim. Acta Part A 2015, 136, 1275–1290. [Google Scholar] [CrossRef]
- He, Y.; Sutton, N.B.; Rijnaarts, H.; Langenhoff, A. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Maeng, S.K.; Cho, K.; Jeong, B.; Lee, J.; Lee, Y.; Lee, C.; Choi, K.J.; Hong, S.W. Substrate-immobilized electrospun TiO2 nanofibers for photocatalytic degradation of pharmaceuticals: The effects of ph and dissolved organic matter characteristics. Water Res. 2015, 86, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Li, Y.; Wu, Y.; Zhang, C.; Upreti, A.R. Mechanistic study of visible light driven photocatalytic degradation of EDC 17α-ethinyl estradiol and azo dye Acid Black-52: Phytotoxicity assessment of intermediates. RSC Adv. 2016, 6, 87246–87257. [Google Scholar] [CrossRef]
- Hu, Z.; Hao, L.; Quan, F.; Guo, R. Recent developments of Co3O4-based materials as catalysts for the oxygen evolution reaction. Catal. Sci. Technol. 2022, 12, 436–461. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Brillas, E. Applied photoelectrocatalysis on the degradation of organic pollutants in wastewaters. J. Photochem. Photobiol. C 2017, 31, 1–35. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Hotchandani, S.; Kamat, P.V. Electrochemically assisted photocatalysis: Titania particulate film electrodes for photocatalytic degradation of 4-chlorophenol. J. Phys. Chem. 1993, 97, 9040–9044. [Google Scholar] [CrossRef]
- Kondalkar, V.V.; Mali, S.S.; Mane, R.M.; Dandge, P.B.; Choudhury, S.; Hong, C.K.; Bhosale, P.N. Photoelectrocatalysis of cefotaxime using nanostructured TiO2 photoanode: Identification of the degradation products and determination of the toxicity level. Ind. Eng. Chem. Res. 2014, 53, 18152–18162. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, K.; Han, J.; Li, Y.; Cui, T.; Wang, B.; Zhou, C. Dendritic TiO2/ln2S3/AgInS2 trilaminar core–shell branched nanoarrays and the enhanced activity for photoelectrochemical water splitting. Small Mol. 2014, 10, 3153–3161. [Google Scholar] [CrossRef]
- Li, L.; Xiao, S.; Li, R.; Cao, Y.; Chen, Y.; Li, Z.; Li, H. Nanotube array-like WO3 photoanode with dual-layer oxygen-evolution cocatalysts for photoelectrocatalytic overall water splitting. ACS Appl. Energy Mater. 2018, 1, 6871–6880. [Google Scholar] [CrossRef]
- Cong, Y.; Ge, Y.; Zhang, T.; Wang, Q.; Shao, M.; Zhang, Y. Fabrication of Z-scheme Fe2O3–MoS2–Cu2O ternary nanofilm with significantly enhanced photoelectrocatalytic performance. Ind. Eng. Chem. Res. 2018, 57, 881–890. [Google Scholar] [CrossRef]
- Yang, R.; Ji, Y.; Li, Q.; Zhao, Z.; Zhang, R.; Liang, L.; Liu, H. Ultrafine Si nanowires/Sn3O4 nanosheets 3D hierarchical heterostructured array as a photoanode with high-efficient photoelectrocatalytic performance. Appl. Catal. B 2019, 256, 117798. [Google Scholar] [CrossRef]
- Pan, Y.; Ren, H.; Du, H.; Cao, F.; Jiang, Y.; Du, H.; Chu, D. Active site engineering by surface sulfurization for a highly efficient oxygen evolution reaction: A case study of Co3O4 electrocatalysts. J. Mater. Chem. A 2018, 6, 22497–22502. [Google Scholar] [CrossRef]
- Khalily, M.A.; Patil, B.; Yilmaz, E.; Uyar, T. Atomic layer deposition of Co3O4 nanocrystals on N-doped electrospun carbon nanofibers for oxygen reduction and oxygen evolution reactions. Nanoscale Adv. 2019, 1, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Shepit, M.; Paidi, V.K.; Roberts, C.A.; Van Lierop, J. Competing ferro-and antiferromagnetic exchange drives shape-selective Co3O4 nanomagnetism. Sci. Rep. 2020, 10, 20990. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Cui, X.; Dastafkan, K.; Wang, H.F.; Tang, C.; Zhao, C.; Zhang, Q. Recent advances in spinel-type electrocatalysts for bifunctional oxygen reduction and oxygen evolution reactions. J. Energy Chem. 2021, 53, 290–302. [Google Scholar] [CrossRef]
- Mathankumar, M.; Anantharaj, S.; Nandakumar, A.K.; Kundu, S.; Subramanian, B. Potentiostatic phase formation of β-CoOOH on pulsed laser deposited biphasic cobalt oxide thin film for enhanced oxygen evolution. J. Mater. Chem. A 2017, 5, 23053–23066. [Google Scholar] [CrossRef]
- Banerjee, S.; Debata, S.; Madhuri, R.; Sharma, P.K. Electrocatalytic behavior of transition metal (Ni, Fe, Cr) doped metal oxide nanocomposites for oxygen evolution reaction. Appl. Surf. Sci. 2018, 449, 660–668. [Google Scholar] [CrossRef]
- Hong, T.; Liu, Z.; Zheng, X.; Zhang, J.; Yan, L. Efficient photoelectrochemical water splitting over Co3O4 and Co3O4/Ag composite structure. Appl. Catal. B 2017, 202, 454–459. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Co3O4 nanoparticles synthesized from waste li-ion batteries as photocatalyst for degradation of methyl blue dye. Environ. Technol. Innov. 2021, 1222, 101765. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Ge, R.; Ren, X.; Ren, J.; Yang, D.; Sun, X. Phosphorus-doped Co3O4 nanowire array: A highly efficient bifunctional electrocatalyst for overall water splitting. ACS Catal. 2018, 8, 2236–2241. [Google Scholar] [CrossRef]
- Li, H.; Jian, L.; Chen, Y.; Wang, G.; Lyu, J.; Dong, X.; Ma, H. Fabricating Bi2MoO6@ Co3O4 core-shell heterogeneous architectures with Z-scheme for superior photoelectrocatalytic water purification. Chem. Eng. J. 2022, 427, 131716. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Z.; Fang, L.; Xu, H.; Zhang, H.; Gu, X.; Wang, Y. Probing the crystal plane effect of Co3O4 for enhanced electrocatalytic performance toward efficient overall water splitting. ACS Appl. Mater. Interfaces 2017, 9, 27736–27744. [Google Scholar] [CrossRef]
- Gao, C.; Meng, Q.; Zhao, K.; Yin, H.; Wang, D.; Guo, J.; Tang, Z. Co3O4 Hexagonal Platelets with Controllable Facets Enabling Highly Efficient Visible-Light Photocatalytic Reduction of CO2. Adv. Mater. 2016, 28, 6485–6490. [Google Scholar] [CrossRef]
- Faisal, F.; Stumm, C.; Bertram, M.; Waidhas, F.; Lykhach, Y.; Cherevko, S.; Libuda, J. Electrifying model catalysts for understanding electrocatalytic reactions in liquid electrolytes. Nat. Mater. 2018, 17, 592–598. [Google Scholar] [CrossRef]
- Liao, P.; Keith, J.A.; Carter, E.A. Water oxidation on pure and doped hematite (0001) surfaces: Prediction of Co and Ni as effective dopants for electrocatalysis. J. Am. Chem. Soc. 2012, 134, 13296–13309. [Google Scholar] [CrossRef]
- Elakkiya, R.; Maduraiveeran, G. Two-dimensional earth-abundant transition metal oxides nanomaterials: Synthesis and application in electrochemical oxygen evolution reaction. Langmuir 2020, 36, 4728–4736. [Google Scholar] [CrossRef]
- Yang, H.; Sun, H.; Fan, X.; Wang, X.; Yang, Q.; Lai, X. Hollow Co3O4 dodecahedrons with controlled crystal orientation and oxygen vacancies for the high performance oxygen evolution reaction. Mater. Chem. Front. 2021, 5, 259–267. [Google Scholar] [CrossRef]
- Yan, K.; Chi, J.; Xie, J.; Dong, B.; Liu, Z.; Gao, W.K.; Lin, J.H.; Chai, Y.M.; Liu, C.G. Mesoporous ag-doped Co3O4 nanowire arrays supported on fto as efficient electrocatalysts for oxygen evolution reaction in acidic media. Renew. Energy 2018, 119, 54–61. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Barreca, D.; Massignan, C.; Daolio, S.; Fabrizio, M.; Piccirillo, C.; Armelao, L.; Tondello, E. Composition and microstructure of cobalt oxide thin films obtained from a novel cobalt(ii) precursor by chemical vapor deposition. Chem. Mater. 2001, 13, 588–593. [Google Scholar] [CrossRef]
- Priyadharshini, T.; Saravanakumar, B.; Ravi, G.; Sakunthala, A.; Yuvakkumar, R. Hexamine role on pseudocapacitive behaviour of cobalt oxide (Co3O4) nanopowders. J. Nanosci. Nanotechnol. 2018, 18, 4093–4099. [Google Scholar] [CrossRef] [PubMed]
- Athar, T.; Hakeem, A.; Topnani, N.; Hashmi, A. Wet synthesis of monodisperse cobalt oxide nanoparticles. ISRN Mater. Sci. 2012, 2012, 691032. [Google Scholar] [CrossRef]
- Rim, H.; Park, H.R.; Song, M.Y. Synthesis of LiNi0.9Co0.1O2 from Li2Co3, NiO or NiCo3, and CoCO3 or Co3O4 and their electrochemical properties. Ceram. Int. 2013, 39, 7297–7303. [Google Scholar] [CrossRef]
- Croiset, E.; Rice, S.F.; Hanush, R.G. Hydrogen peroxide decomposition in supercritical water. AlChE J. 1997, 43, 2343–2352. [Google Scholar] [CrossRef]
- Garcia-Verdugo, E.; Venardou, E.; Thomas, W.B.; Whiston, K.; Partenheimer, W.; Hamley, P.A.; Poliakoff, M. Is it possible to achieve highly selective oxidations in supercritical water? Aerobic oxidation of methylaromatic compounds. Adv. Synth. Catal. 2004, 346, 307–316. [Google Scholar] [CrossRef]
- Lester, E.; Blood, P.; Denyer, J.; Giddings, D.; Azzopardi, B.; Poliakoff, M. Reaction engineering: The supercritical water hydrothermal synthesis of nano-particles. J. Supercrit. Fluids 2006, 37, 209–214. [Google Scholar] [CrossRef]
- Zhao, F.; Song, Y.; Liu, X.; Zhang, X.; Ma, C.; Wang, G.; Fu, Y.; Ma, H. Unraveling electron-deficient setaria-viridis-like Co3O4@MnO2 heterostructure with superior photoelectrocatalytic efficiency for water remediation. Appl. Surf. Sci. 2022, 573, 151473. [Google Scholar] [CrossRef]
- Patil, V.; Joshi, P.; Chougule, M.; Sen, S. Synthesis and characterization of Co3O4 thin film. Soft Nanosci. Lett. 2012, 2, 1–7. [Google Scholar] [CrossRef]
- Chen, J.; Du, H.; Zhang, J.; Lei, X.; Wang, Y.; Su, S.; Zhao, P. Influence of deposition temperature on crystalline structure and morphologies of Co3O4 films prepared by a direct liquid injection chemical vapor deposition. Surf. Coat. Technol. 2017, 319, 110–116. [Google Scholar] [CrossRef]
- Nikam, A.; Pagar, T.; Ghotekar, S.; Pagar, K.; Pansambal, S. A review on plant extract mediated green synthesis of zirconia nanoparticles and their miscellaneous applications. J. Chem. Rev. 2019, 1, 154–163. [Google Scholar]
- Dubey, S.; Kumar, J.; Kumar, A.; Sharma, Y.C. Facile and green synthesis of highly dispersed cobalt oxide (Co3O4) nano powder: Characterization and screening of its eco-toxicity. Adv. Powder Technol. 2018, 29, 2583–2590. [Google Scholar] [CrossRef]
- Ghotekar, S. A review on plant extract mediated biogenic synthesis of CdO nanoparticles and their recent applications. Asian J. Green Chem. 2019, 3, 187–200. [Google Scholar]
- Al-Qirby, L.M.; Radiman, S. Ultrasound-assisted green synthesis of nanocrystalline Co3O4 in the ionic liquid. AIP Conf. Proc. 2014, 1614, 41. [Google Scholar]
- Sivachidambaram, M.; Vijaya, J.J.; Kaviyarasu, K.; Kennedy, L.J.; Al-Lohedan, H.A.; Ramalingam, R.J. A novel synthesis protocol for Co3O4 nanocatalysts and their catalytic applications. RSC Adv. 2017, 7, 38861–38870. [Google Scholar] [CrossRef]
- Dewi, N.; Yulizar, Y.; Apriandanu, D. Green synthesis of Co3O4 nanoparticles using euphorbia heterophylla l. leaves extract: Characterization and photocatalytic activity. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012105. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Rashad, M.; Moharram, A.H.; Abdel-Rahim, M.A. Promising methane gas sensor synthesized by microwave-assisted Co3O4 nanoparticles. Mater. Sci. Semicond. Process. 2016, 46, 1–5. [Google Scholar] [CrossRef]
- Kumarage, G.W.; Comini, E. Low-dimensional nanostructures based on cobalt oxide (Co3O4) in chemical-gas sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Selloni, A. Electronic structure and bonding properties of cobalt oxide in the spinel structure. Phys. Rev. B 2011, 83, 245204. [Google Scholar] [CrossRef]
- Diallo, A.; Beye, A.C.; Doyle, T.B.; Park, E.; Maaza, M. Green synthesis of Co3O4 nanoparticles via Aspalathus linearis: Physical properties. Green Chem. Lett. Rev. 2015, 8, 30–36. [Google Scholar] [CrossRef]
- Zhang, M.; De Respinis, M.; Frei, H. Time-resolved observations of water oxidation intermediates on a cobalt oxide nanoparticle catalyst. Nat. Chem. 2014, 6, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Rosen, J.; Hutchings, G.S.; Jiao, F. Ordered mesoporous cobalt oxide as highly efficient oxygen evolution catalyst. J. Am. Chem. Soc. 2013, 135, 4516–4521. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, C.P.; van Santen, R.A. Structure sensitivity of the oxygen evolution reaction catalyzed by cobalt (II, III) oxide. J. Am. Chem. Soc. 2015, 137, 14660–14672. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Z.; Wang, Y.; Ding, Y. Facet effect of Co3O4 nanocrystals on visible-light driven water oxidation. Appl. Catal. B 2018, 237, 74–84. [Google Scholar] [CrossRef]
- Mat, A.N.C.; Sairi, N.A.; Basirun, W.J.; Rezayi, M.; Teridi, M.A.M.; Mazhar, M. Photoelectrocatalytic oxidation of methanol over RuO2MnO2Co3O4 supported porous anatase under visible light irradiation. Mater. Chem. Phys. 2019, 224, 196–205. [Google Scholar] [CrossRef]
- He, T.; Chen, D.; Jiao, X.; Xu, Y.; Gu, Y. Surfactant-assisted solvothermal synthesis of Co3O4 hollow spheres with oriented-aggregation nanostructures and tunable particle size. Langmuir 2004, 20, 8404–8408. [Google Scholar] [CrossRef]
- Cong, Y.; Zhang, W.; Ding, W.; Zhang, T.; Zhang, Y.; Chi, N.; Wang, Q. Fabrication of electrochemically-modified BiVO4-MoS2-Co3O4 composite film for bisphenol A degradation. J. Environ. Sci. 2021, 102, 341–351. [Google Scholar] [CrossRef]
- Yang, P.; Li, W.; Lian, Y.; Yu, F.; Dai, B.; Guo, X.; Peng, B. A facile approach to synthesize CoO-Co3O4/TiO2 NAs for reinforced photoelectrocatalytic water oxidation. J. Solid State Electrochem. 2020, 24, 941–950. [Google Scholar] [CrossRef]
- Shen, Q.; Chen, Z.; Huang, X.; Liu, M.; Zhao, G. High-yield and selective photoelectrocatalytic reduction of CO2 to formate by metallic copper decorated Co3O4 nanotube arrays. Environ. Sci. Technol. 2015, 49, 5828–5835. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Jing, C.; Zhang, H.; Shao, Q.; Ge, R. A visible-light active p-n heterojunction ZnO/Co3O4 composites supported on ni foam as photoanode for enhanced photoelectrocatalytic removal of methylene blue. Adv. Compos. Hybrid Mater. 2022, 5, 2406–2420. [Google Scholar] [CrossRef]
- Nan, J.; Guo, S.; Alhashmialameer, D.; He, Q.; Meng, Y.; Ge, R.; Guo, Z. Hydrothermal microwave synthesis of Co3O4/In2O3 nanostructures for photoelectrocatalytic reduction of Cr (VI). ACS Appl. Nano Mater. 2022, 5, 8755–8766. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Jin, E.; Lan, W.; Han, C.; Wang, G.; Ma, H. Constructing Z-scheme NiMoO4@ Co3O4 core-shell heterogeneous architectures with prominent photoelectrocatalytic performance toward water purification. Chemosphere 2023, 312, 137261. [Google Scholar] [CrossRef]
- Dai, G.; Liu, S.; Liang, Y.; Luo, T. Synthesis and enhanced photoelectrocatalytic activity of p-n junction Co3O4/TiO2 nanotube arrays. Appl. Surf. Sci. 2013, 264, 157–161. [Google Scholar] [CrossRef]
- Wang, X.; Jin, S.; Cui, Y.; Zhang, J.; Lu, H.; Ren, Y.; Xiong, C. Photocatalytic activity of Co3O4@ C enhanced by induction of amorphous cobalt-based MOF. Colloids Surf. A 2023, 657, 130597. [Google Scholar] [CrossRef]
- Chen, H.; Xue, C.; Cui, D.; Liu, M.; Chen, Y.; Li, Y.; Zhang, W. Co3O4-Ag photocatalysts for the efficient degradation of methyl orange. RSC Adv. 2020, 10, 15245–15251. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Fu, Y.; Zhang, Y.; Ma, C.; Dong, X.; Yu, Z. Study on the fabrication and photoelectrochemical performance of the F−doped Ti/Co3O4 electrodes with n-type semiconductor characteristics. J. Solid State Electrochem. 2019, 23, 1767–1777. [Google Scholar] [CrossRef]
- Fantini, M.; Torrian, I. The compositional and structural properties of sprayed SnO2. Thin Solid Films 1986, 138, 255–265. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Lei, Y.; Baoying, L.V.; Gao, J.; Zhang, Y. Fabrication and electrochemical treatment application of a novel lead dioxide anode with superhydrophobic surfaces, high oxygen evolution potential, and oxidation capability. Environ. Sci. Technol. 2010, 44, 1754–1759. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Gupta, B.K.; Raza, A.; Sharma, A.K.; Agnihotri, O.P. Fluorine-doped SnO2 films for solar cell application. Sol. Energy Mater. Sol. Cells 1981, 5, 39–49. [Google Scholar] [CrossRef]
- Joan, B.; Rita, S.; Encarnación, B.; Maria, J.; Ramón, M.F.; García-Antón, J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials 2019, 9, 583. [Google Scholar]
- Sapkal, R.T.; Shinde, S.S.; Mahadik, M.A.; Mohite, V.S.; Waghmode, T.R.; Govindwar, S.P. Photoelectrocatalytic decolorization and degradation of textile effluent using zno thin films. J. Photochem. Photobiol. B 2012, 114, 102–107. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Zeng, H.; Zhang, J.; Zhao, X. Enhanced photoelectrocatalytic degradation of bisphenol a by BiVO4 photoanode coupling with peroxymonosulfate. J. Hazard. Mater. 2019, 394, 121105. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Domene, R.M.; Sánchez-Tovar, R. Customized WO3 nanoplatelets as visible-light photoelectrocatalyst for the degradation of a recalcitrant model organic compound (methyl orange). J. Photochem. Photobiol. A 2017, 356, 46–56. [Google Scholar] [CrossRef]
- Yang, R.; Ji, Y.; Zhang, J.; Zhang, R.; Liu, F.; Chen, Y. Efficiently degradation of polyacrylamide pollution using a full spectrum Sn3O4 nanosheet/ni foam heterostructure photoelectrocatalyst. Catal. Today 2019, 335, 520–526. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Lyu, J.; Moussa, M.; Liu, X.; Fu, Y.; Ma, H. Constructed Co3O4-Sn3O4 hierarchical nanoflower-tree heterostructure with boosting photoelectrocatalytic efficiency for water decontamination. Biochem. Eng. J. 2021, 423, 130252. [Google Scholar] [CrossRef]
- Fei, W.; Gao, J.; Li, N.; Chen, D.; Xu, Q.; Li, H.; Lu, J. A visible-light active p-n heterojunction NiFe-LDH/Co3O4 supported on Ni foam as photoanode for photoelectrocatalytic removal of contaminants. J. Hazard. Mater. 2021, 402, 123515. [Google Scholar] [CrossRef]
- Cao, H.; Lu, Y.; Ning, W.; Zhang, H.; Zheng, G. Co3O4 Nanoparticles Modified TiO2 Nanotube Arrays with Improved Photoelectrochemical Performance. Russ. J. Appl. Chem. 2019, 92, 64–70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).