Abstract
The S-scheme photocatalyst system has become increasingly popular in recent years for its ability to efficiently degrade various pollutants, including organic dyes, pesticides, and other harmful substances. This system uses two semiconductor photocatalysts with different bandgap energies, working together in a redox reaction to produce a highly reactive species capable of pollutant breakdown. Here, an S-scheme Ag2WO4/Ag6Si2O7 p-n heterojunction nanocomposite was successfully developed by a coprecipitation method. By decomposing Rhodamine B (RhB) under visible-light irradiation, the photocatalytic activities of Ag6Si2O7/Ag2WO4 showed enhanced photocatalytic degradation performance of organic dyes, especially at a 4% molar ratio of the Ag2WO4-modified Ag6Si2O7 sample, whose degradation rate was 23.7 and 4.65 times those of Ag2WO4 and Ag6Si2O7, respectively. The physical and chemical properties of the samples were determined by identifying the physical structure, chemical element composition, and optical responsiveness. The optimum composite amongst the prepared materials was AgSW-4, achieving the maximum RhB degradation efficiency of 97.5%, which was higher by 60% and 20% than its counterparts Ag6Si2O7 and Ag2WO4, respectively. These results showed that in the nanocomposite structure, Ag6Si2O7 was a p-type semiconductor and Ag2WO4 was an n-type semiconductor. Based on the analysis data, a comprehensive p-n heterojunction S-scheme process was proposed to demonstrate the enhanced photocatalytic performance of the Ag6Si2O7/Ag2WO4 nanocomposite.
1. Introduction
Environmental disaster and water pollution can be caused by various factors, including but not limited to fossil fuel burning and industrial wastewater. The staggering problems faced by the earth in the coming century include the depletion of fossil fuel energy and a shortage of clean water. Many efforts are in progress to address environmental disasters and water pollution, as well as to find alternative solutions to the root causes of these problems [1]. To prevent water pollution, water treatment technologies have become a persistent interest of the scientific and industrial community because without water, life is not possible on earth. Shifting to renewable and clean energy sources such as solar, wind, and hydro power is an important step in reducing our reliance on fossil fuels and addressing the issue of climate change. These technologies allow us to generate energy in a way that is more sustainable and has lower environmental impact. However, one of the challenges with renewable energy sources is that they are often intermittent; that is, they generate energy only when the sun is shining, the wind is blowing, or water is flowing. Thus, energy storage technologies are needed to ensure that renewable energy can be used when needed even when not being generated at that moment. Efficient and long-lasting batteries are some of the most promising energy storage technologies currently being studied. Advances in battery technology have enabled the storage of larger amounts of energy in smaller and more portable devices and at lower costs [2]. Advanced photooxidation processes (AOPs) are a group of water treatment technologies that involve the use of highly reactive hydroxyl radicals to degrade and remove pollutants from water. A common method used in AOPs is semiconductor photocatalysis, which can enable light-energy absorption and create electron–hole pairs that can generate hydroxyl radicals through reactions with water and oxygen. These hydroxyl radicals are powerful oxidising agents that can break down organic contaminants and convert them into harmless by-products, such as carbon dioxide and water [3,4,5]. Visible-light-driven semiconductor photocatalysts harvest freely available sunlight and convert the absorbed photons into electron–hole pairs, which induce reduction and oxidation reactions to treat the pollutant species [6]. This technology is cost effective, efficient, and convenient to operate compared with its counterparts. Traditional semiconductors such as ZnO and TiO2 possess a wide bandgap and absorb only ultraviolet (UV) radiation, accounting for around 4% of the solar spectrum [7]. Given that visible light makes up a massive fraction of the solar spectrum (approximately 43%), creating semiconductor photocatalysts that respond to visible light is a major environmental priority [8]. Recently, various modification methods have been developed to expand the light absorption range and improve the photocatalytic performance of photocatalysts, including ion doping, noble metal recombination, and heterostructure construction. For example, Niu et al. [9] doped TiO2 nanotubes with the transition metal ion Nb. The defect states present in TiO2 lead to the formation of local intermediate bandgap states (MS) in the bandgap, thereby adjusting the band structure of TiO2 and extending the light absorption to the visible region. Lu et al. [10] used a one-step hydrothermal method to synthesise CdS@ZnO nanocomposites with a wide visible-light absorption range, greatly improving the visible-light utilisation.
Silver-based semiconductor materials have received significant attention for photocatalysis applications owing to their excellent bandgap and structural stability [11,12,13]. For instance, AgX-based (Cl, Br, and I) [14,15,16,17,18,19,20] semiconductors exhibit notable visible-sunlight harvesting and could be used to remove organic contaminants from water systems. Silver tungstate (Ag2WO4) has also been extensively explored as a semiconductor photocatalyst for the decontamination of organic pollutants [21,22]. However, pure Ag2WO4 always shows a low adsorption and high recombination rate, inhibiting its ability to function as a photocatalyst. Ag6Si2O7, which has a suitable band structure and bandgap, is reportedly a possible visible-light-responsive photocatalyst [23]. A distinctive internal electric field exists in the Ag6Si2O7 crystal structure, and the photogenerated holes and electrons could be quickly unpaired [13,24], which is conducive to enhancing photocatalytic performance. Forming heterojunction structures could further enhance the charge separation and carrier life span and achieve better photocatalysis performance than single-component semiconductors [25,26].
Research on the degradation of Rhodamine B (RhB) contaminants by using a combination of Ag6Si2O7 and Ag2WO4 photocatalysts is limited. However, the use of various photocatalysts for RhB degradation in wastewater has been extensively explored, and investigations on the development of new and more effective photocatalysts are ongoing. Some recent studies have examined the use of bimetallic or hybrid photocatalysts for RhB degradation, such as Ag/AgBr/TiO2, BiVO4/TiO2, and Fe2O3/TiO2. In the future, researchers may also explore the use of a combination of Ag6Si2O7 and Ag2WO4 for RhB degradation. Notably, the effectiveness of a photocatalyst for the degradation of a particular contaminant can depend on many factors, including the properties of the contaminant, the properties of the photocatalyst, and the conditions under which the photocatalysis is performed. Therefore, researchers must continue exploring different photocatalytic systems and optimising the conditions for their use to develop more efficient and effective treatment methods for wastewater containing RhB contaminants. In the current work, we synthesised a Ag2WO4-modified Ag6Si2O7 nanocomposite structure by a coprecipitation method. Under visible-light irradiation, the Ag6Si2O7/Ag2WO4 nanocomposite samples showed enhanced photocatalytic activity compared with pure Ag2WO4 or Ag6Si2O7 samples, and the 4% (molar ratio) Ag2WO4-modified Ag6Si2O7 (AgSW-4) had the optimum performance. After carefully analysing the Ag6Si2O7/Ag2WO4 nanocomposite’s structure, morphology, and optical characteristics, a potential charge-transfer mechanism, i.e., a p-n heterojunction S-scheme process, was put forward to explain how the Ag6Si2O7/Ag2WO4 nanocomposite heterojunction assisted in the breakdown of organic contaminants.
2. Experimental Section
2.1. Materials
Silver nitrate (AgNO3), sodium tungstate dehydrate (Na2WO4·2H2O), sodium silicate (Na2SiO3·9H2O), RhB, tertiary butanol, benzoquinone, ethylene diamine tetra acetic acid disodium salt, and ethanol were acquired from Tian in Fuyu Fine Chemical Co., Ltd., Tianjin, China. Without any other addition, all chemical reagents were used in their original form.
2.2. Synthesis of Ag6Si2O7
Ion exchange synthesis was used to synthesise Ag6Si2O7. Initially, 2.038 g of AgNO3 was dissolved in DI water at room temperature to prepare a 0.2 mol/L solution placed in darkness. Then, a 0.2 mol/L solution of Na2SiO3·9H2O with a typical mass of 1.136 g was prepared under continuous stirring. Afterwards, both solutions were mixed slowly under stirring and the mixture was further stirred for 40 min whilst shielding from light. The mixture was finally centrifuged at 7000 r/min and then washed with water. After ethanol washing and drying at 60 °C for 20 h in an oven, the obtained Ag6Si2O7 powder was ground and collected.
2.3. Synthesis of Ag6Si2O7/Ag2WO4 Composites
We weighed a certain amount of AgNO3, Na2WO4, and Na2SiO3 solids, prepared them into a 0.1 mmol/L solution, and placed the solution in darkness. Magnetic stirring was performed for 15 min to ensure its complete dissolution, followed by slowly adding a certain amount of Na2WO4 solution to AgNO3 solution dropwise in darkness. White flocculent precipitate can be observed in the solution at this time. Stirring was continued for 30 min to ensure that the Na2WO4 had completely reacted. After slowly adding a specific quantity of Na2SiO3 solution to the suspension solution, the dosage ratio was determined according to the proportion of silver ions from Ag2WO4 in the Ag6Si2O7/Ag2WO4 composite sample, i.e., 2%, 3%, 4%, 5%, and 6% (denoted as AgSW-2, AgSW-3, AgSW-4, AgSW-5, and AgSW-6, respectively). Brown precipitate can be observed at this time. Continuous stirring was performed for 45 min to ensure that the solute reacted completely. Finally, the obtained mixed liquid was centrifuged at a speed of 7000 r/min, the supernatant was discarded, three water washes and one ethanol wash were performed, and the obtained Ag6Si2O7/Ag2WO4 heterojunction powder was ground and collected after drying at 60 °C for 20 h in an oven.
2.4. Characterisation
The morphology and microstructure of the prepared materials were characterised by scanning electron microscopy (SEM; Hitachi, SU8000, Tokyo, Japan) and transmission electron microscopy (TEM; FEI Talos F200X, Hillsboro, OR, USA). An XPERT-PRO diffractometer (Bruker D8 Advance, Bremen, Germany) was used for X-ray diffraction (XRD) analysis and Cu K-alpha radiation (1.5406) to reveal the structural properties for 2θ values from 10° to 90°. To compute the Brunauer–Emmett–Teller (BET) specific surface areas of the materials, N2 adsorption–desorption isotherms were used with an ASAP2460 (Micromeritics instrument Ltd., Norcross, GA, USA) aperture analyser. An electron spectrometer (model number 250Xi, Thermo Fisher, Waltham, MA, USA) was used to perform XPS measurements to ascertain the elemental composition of the surface. Using UV–visible (UV–vis) diffusion reflectance spectroscopy (DRS), PerkinElmer Lambda 35 UV/Vis (Waltham, MA, USA) spectrophotometer data of freshly produced powders were collected to analyse optical bandgaps. The investigation of electron spin resonance (ESR) was completed using a Bruker A300-10/12 equipment.
2.5. Photocatalytic Degradation Experiment
Photocatalytic activities were evaluated by observing the change in RhB solution concentration under visible light. A schematic of the photocatalytic experimental equipment is shown in Figure S1. Typically, 100 mL of RhB solution (20 mg/L) had 10 mg of catalyst added to it. To balance the adsorption–desorption, the solution was agitated for 30 min whilst placing it in complete darkness. As the light source with a 400 nm cutoff filter, a 300 W Xenon lamp was operated. To extract the photocatalyst, 6 mL of the solution was collected taken from each photocatalysis experiment at different irradiation durations (10, 20, 30, 60, and 90 min) and centrifuged. Finally, the concentration of residual organic dyes was determined by UV–vis absorption spectroscopy.
3. Results and Discussion
3.1. XRD Analysis
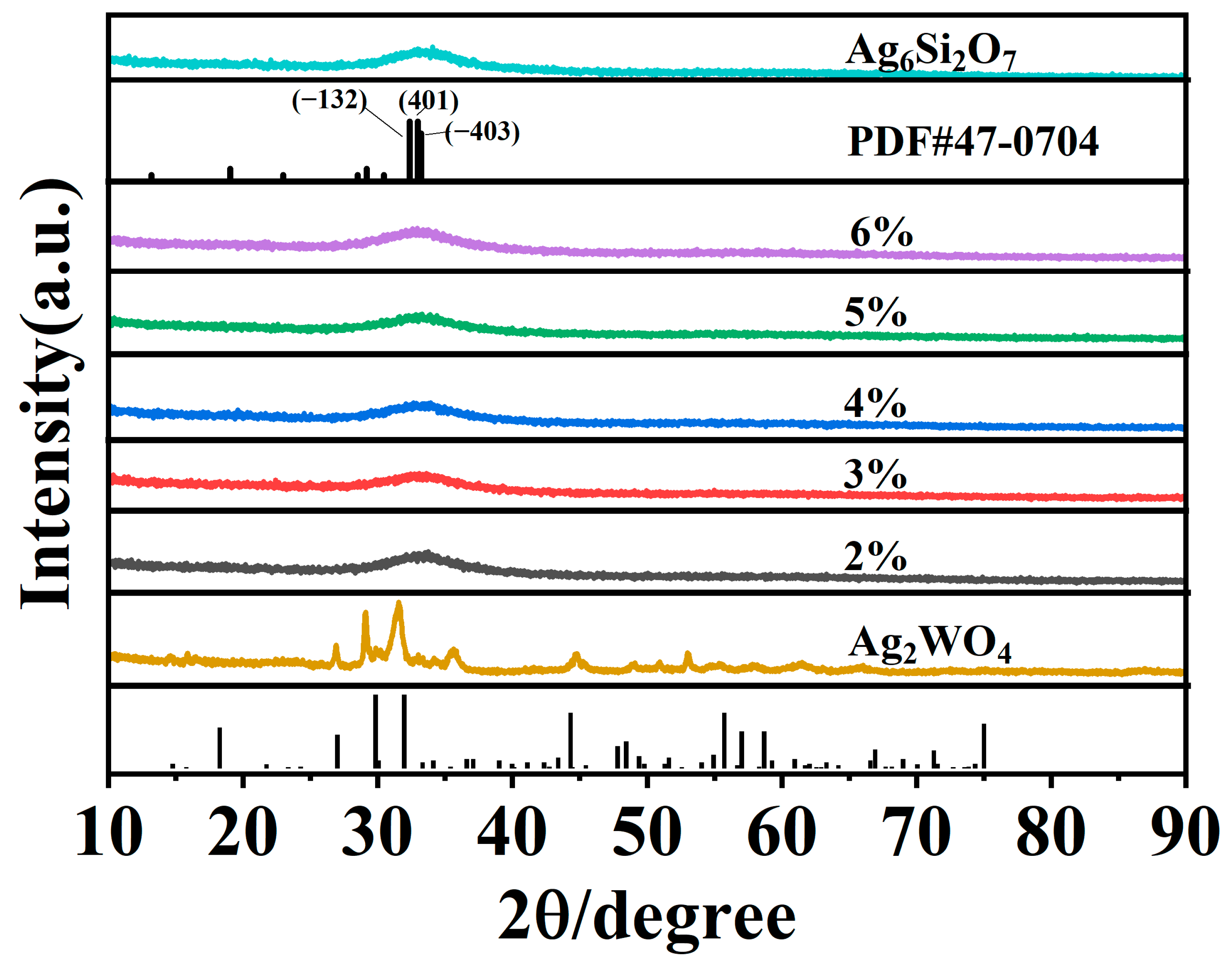
XRD analysis was used to examine the crystal phase and structure of the synthesised materials. The XRD patterns of Ag2WO4, Ag6Si2O7, and Ag2WO4-modified Ag6Si2O7 composite photocatalysts (AgSW-2, AgSW-3, AgSW-4, AgSW-5, and AgSW-6) are shown in Figure 1. Data revealed that Ag2WO4 exhibited six characteristic peaks at 2θ values of 27.3°, 30.1°, 32.3°, and 44.7°, respectively, which agreed with the crystal planes in the literature of hexagonal β-Ag2WO4 (JCPDS # 33-1195) [27,28,29]. Meanwhile, Ag6Si2O7 exhibited a broad peak at around 33° owing to the broadening of small particles and low crystallinity, corresponding with the XRD pattern of (JCPDS#47-0704) in the literature [24]. After composite Ag6Si2O7 with Ag2WO4, no obvious change was found in the XPS curves, which may be due to the very low ratio of Ag2WO4 to Ag6Si2O7.
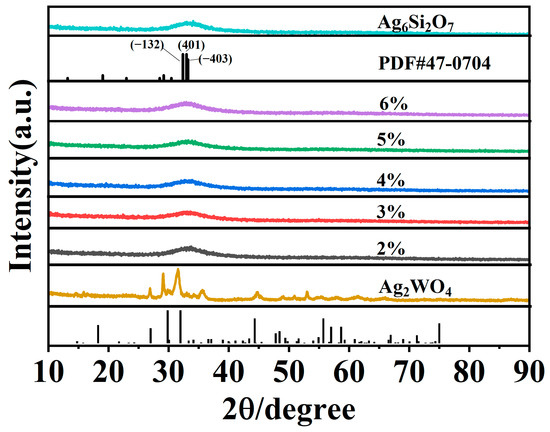
Figure 1.
XRD patterns of Ag2WO4, Ag6Si2O7, and Ag6Si2O7/Ag2WO4 (2%, 3%, 4%, 5%, and 6%).
3.2. Morphology and Composition Analyses
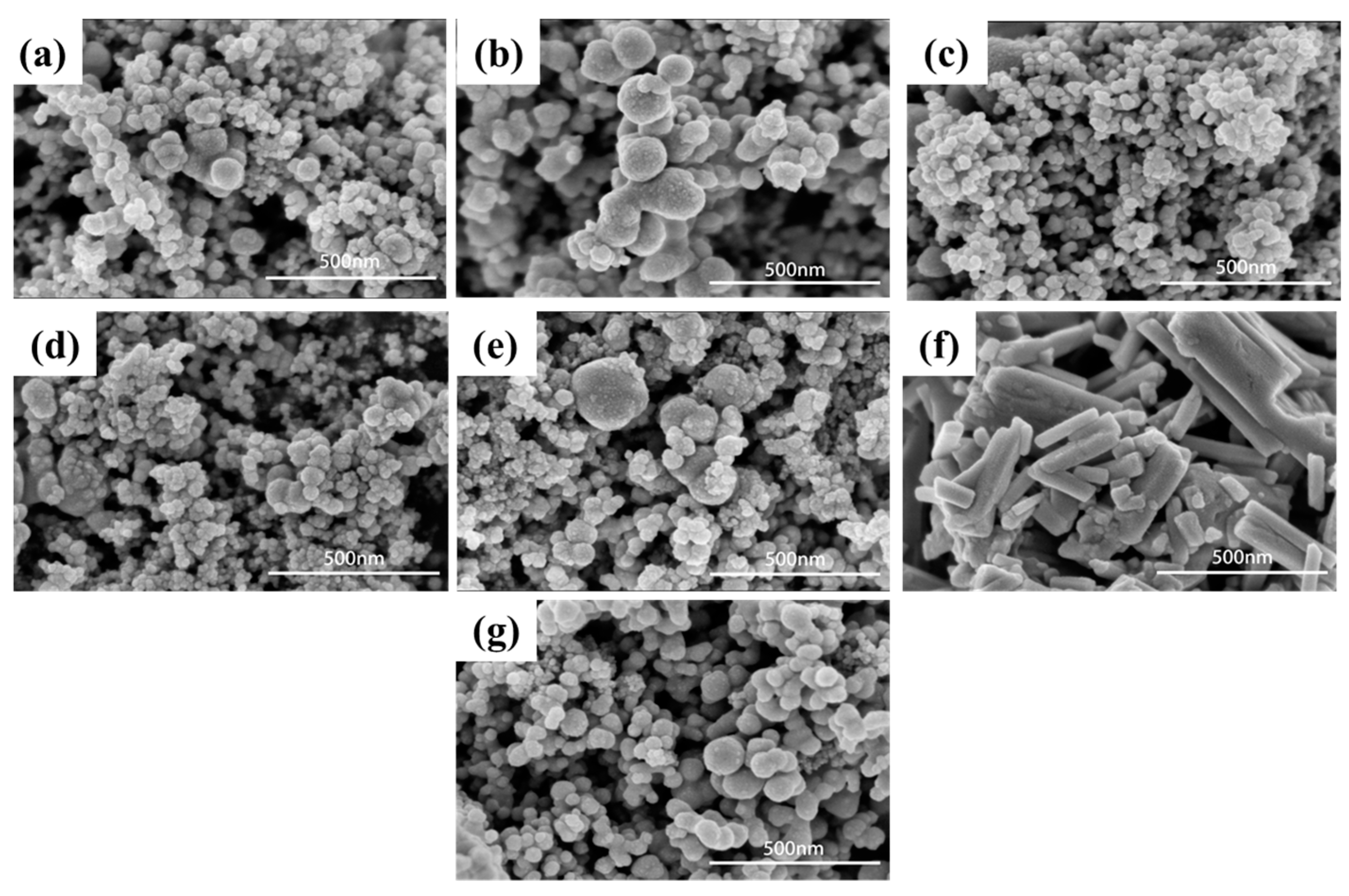
Scanning electron microscopy (SEM) techniques were used to reveal the surface morphology of the as-synthesised materials. The resulting patterns of the Ag2WO4-modified Ag6Si2O7 with different mole ratios and pure Ag2WO4 and Ag6Si2O7 are shown in Figure 2a–g. Figure 2f clearly shows that the Ag2WO4 possessed a rod-like morphology, indicating an inhomogeneous size having an average diameter of about 168.5 nm. Figure 2g is the SEM image of Ag6Si2O7, inferring a smooth spherical morphology with an average particle size of 75.6 nm. After compositing Ag2WO4 with Ag6Si2O7 at different ratios, the average sizes of the particles were smaller; for example, the 4% samples had the smallest particle sizes at 39.1 ± 6.5 nm.
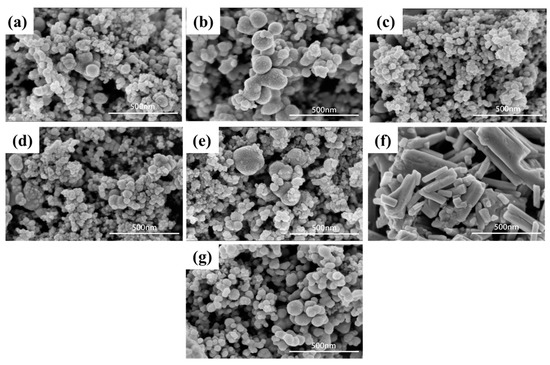
Figure 2.
SEM patterns of Ag2WO4-modified Ag6Si2O7 with different mole ratios: (a) 2%, (b) 3%, (c) 4%, (d) 5%, (e) 6%), and pure (f) Ag2WO4 and (g) Ag6Si2O7.
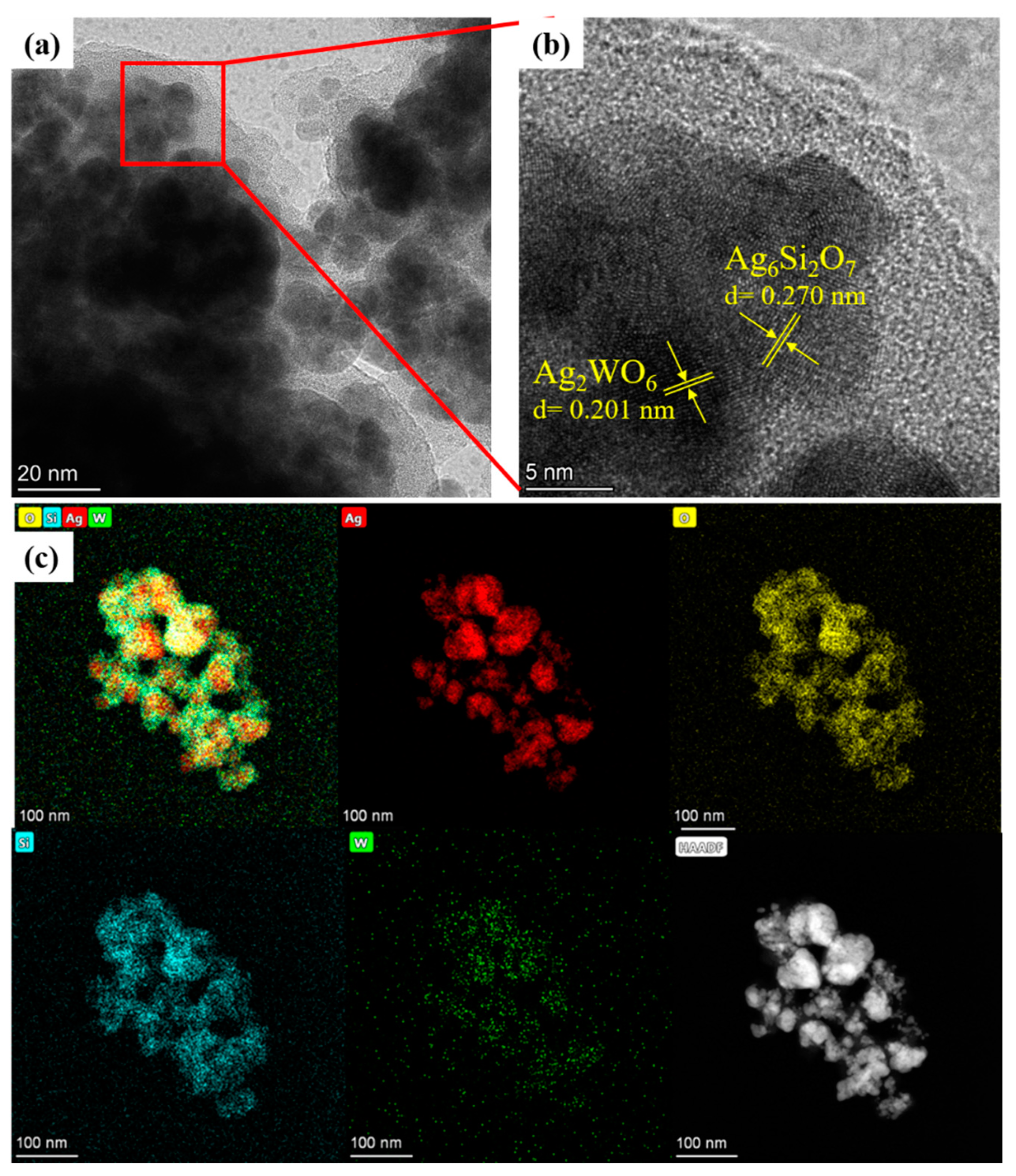
The microstructure of the 4% samples was further determined by high-resolution TEM (HRTEM). As shown in Figure 3a, small nanoparticles aggregated together to form larger irregular particles in Figure 2c. In Figure 3b, when enlarging some zones in high resolution, some small particles showed a lattice fringe spacing of 0.201 nm, corresponding with the peak of 2θ at 44.7° (JCPDS # 33-1195). This finding indicated that these particles were Ag2WO4 nanocrystals. Meanwhile, some small particles showed a lattice fringe spacing of 0.270 nm, which matched the 2θ peak at 33.0° (JCPDS # 47-0704) well, meaning that these particles were Ag6Si2O7 nanocrystals. To further illustrate the composite structure of the Ag2WO4-modified Ag6Si2O7, the element mapping of the composition elements were determined. As shown in Figure 3c, all tested elements, Ag, Si, W, and O, were detectable and uniformly distributed, suggesting that both the Ag2WO4 and Ag6Si2O7 structures existed in the particles, and the Ag2WO4-modified Ag6Si2O7 composite was developed. Meanwhile, the signal intensity of W was much weaker than that of the other elements, suggesting a much lower concentration of Ag2WO4 in the composite.
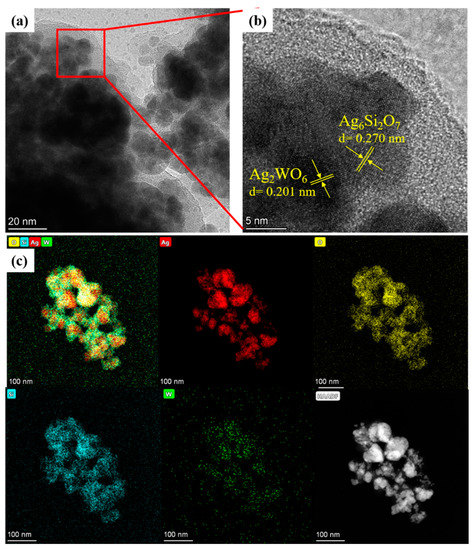
Figure 3.
(a,b) HRTEM images of the AgSW-4 sample; and (c) element mapping images of the AgSW-4 sample (red, Ag; yellow, O; blue, Si; green, W; white, TEM image of sample at the selected zone).
3.3. BET Analysis
To gain further insights into the as-prepared composite, BET was performed to reveal the specific surface area that anchored the chemical reaction ability of the materials. The enhanced surface area could provide more reaction sites on the particle surface, thereby improving the photocatalytic activity. Nitrogen adsorption and desorption coupled with BET was performed, and the results demonstrated specific surface areas of 0.9141 and 12.9647 m2/g for Ag2WO4 and Ag6Si2O7, respectively. Table 1 shows that for the samples of Ag2WO4-modified Ag6Si2O7 composite, the specific surface area data of the composite materials were larger than the Ag6Si2O7 sample (except for AgSW-5, whose specific surface area was smaller than that of Ag6Si2O7). AgSW-4 had the largest specific surface area of 15.0416 m2/g.

Table 1.
BET-specific surface area of the photocatalysts.
3.4. XPS Analysis
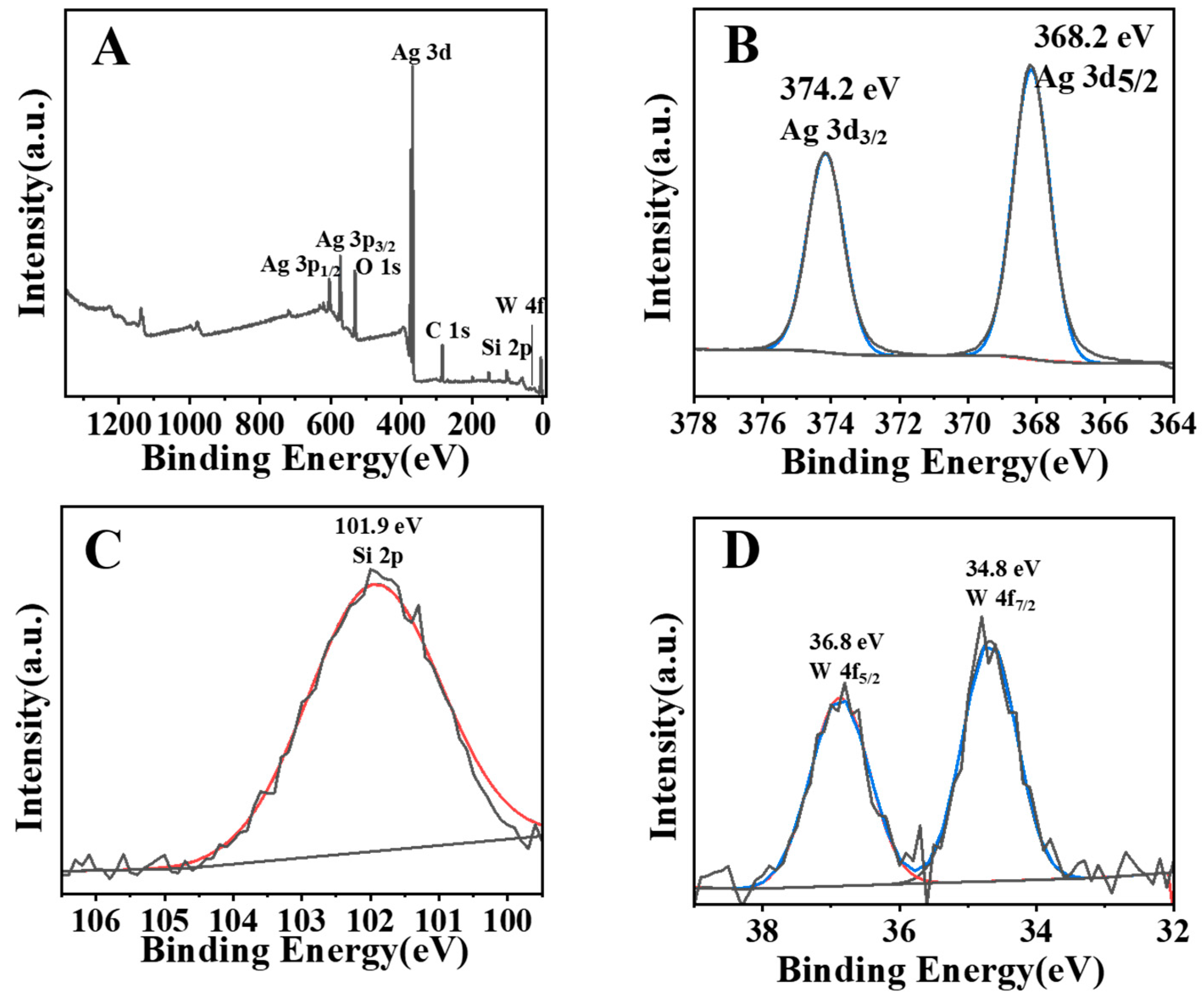
XPS involves a photoelectric effect-based technique to characterise the surface chemistry and density of electronic states residing on the photocatalyst surface. AgSW-4 was chosen as the representative composite to study the composition information. Figure 4A–D show the XPS spectra of AgSW-4, Ag 3d, Si 2p, and W 4f, respectively. We can infer the coexistence of Ag, Si, O, and W from Figure 4A. Figure 4B shows the Ag spectra of AgSW-4, and two characteristic peaks located at binding energies of 374.2 and 368 eV were found, corresponding with 3d3/2 and 3d5/2 of Ag+, respectively [30]. Similarly, Figure 4C depicts the existence of 2p orbitals of Si4+ associated with a binding energy of 101.9 eV, and Figure 4D exhibits two peaks at 34.8 and 36. 8 eV owing to an electronic states density of W6+ in 4f5/2 and 4f7/2, respectively [31,32]. Thus, the XPS spectra also confirmed the existence of Ag2WO4 and Ag6Si2O7 and the successful formation of the Ag2WO4-modified Ag6Si2O7 composite structure.
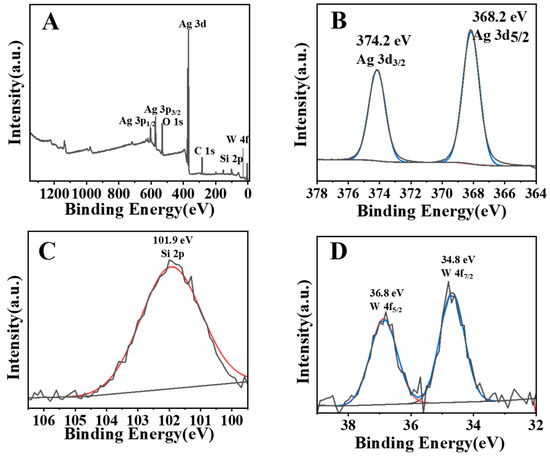
Figure 4.
XPS spectra of AgSW-4 (A), Ag 3 d (B), Si 2p (C), and W 4f (D). The red and blue lines are curves fitted by XPS data analysis software.
3.5. UV–vis Optical Properties and Valence Band (VB)/Conduction Band (CB) Potential Positions
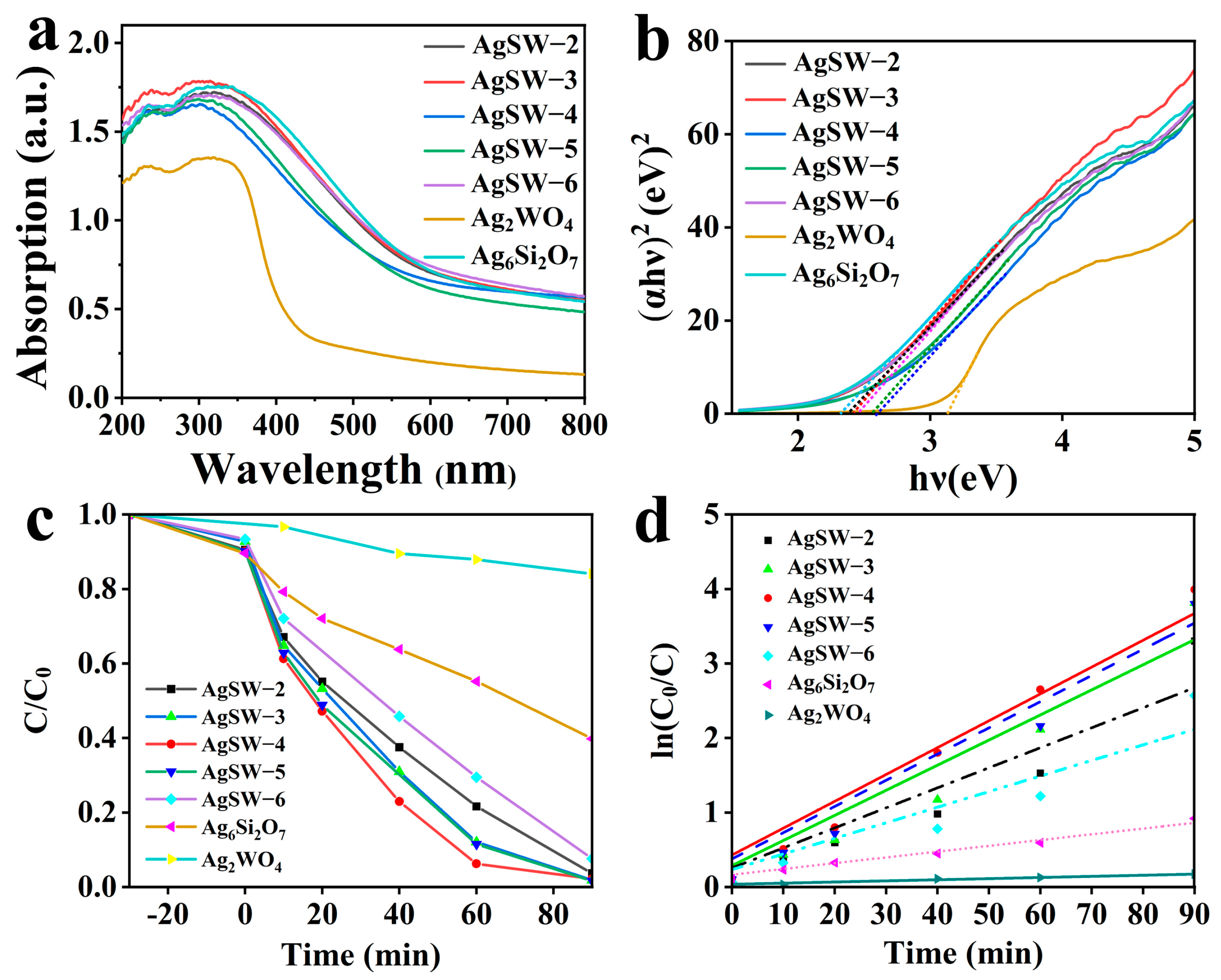
The optical properties of the synthesised materials were assessed through UV–vis DRS, and the absorbance spectra are shown in Figure 5a. Pure Ag2WO4 absorbed photons with wavelengths lower than 397 nm, whereas pure Ag6Si2O7 absorbed photons with wavelengths lower than 536 nm. All composite materials also exhibited similar curves in the visible region to Ag6Si2O7, with a little lower optical absorbance intensity, especially within the range of 380–510 nm. The DRS data were closely related to the bandgap of the materials. The optical bandgap was calculated utilising the wood Tauc equation for direct band evaluation as the studied materials were regarded as direct bandgap materials [33].
where α, h, ν, A, hν, and Eg are the absorption coefficient, Planck constant, photon frequency, constant of proportionality, photon energy, and energy bandgap of the material, respectively. The proportionality constant had no effect on optical absorbance, and n = 2 for the indirect bandgap and 1/2 for the direct bandgap. Ag6Si2O7 and Ag2WO4 are reportedly direct transition semiconductors, and their n value is ½ [34,35]. Bandgaps can be calculated by plotting (αhν)2 along the y-axis and along the y-axis, as shown in Figure 5b. By setting (αhν)2 to zero, the x-intercept provided the bandgaps of 3.12, 2.31, 2.38, 2.42, 2.61, 2.56, and 2.46 eV for Ag2WO4, Ag6Si2O7, AgSW-2, AgSW-3, AgSW-4, AgSW-5, and AgSW-6, respectively (Table 2).
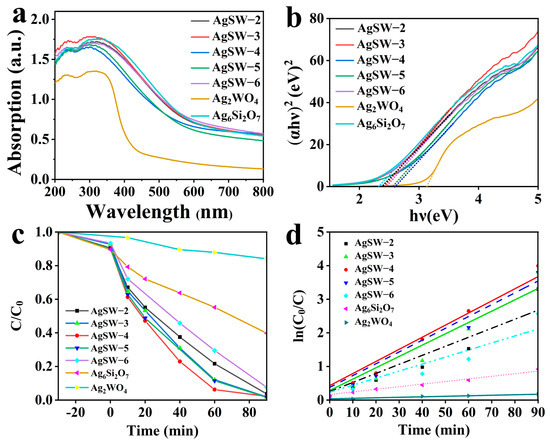
Figure 5.
(a) UV–vis DRS of Ag2WO4, Ag6Si2O7, and Ag6Si2O7/Ag2WO4 composites. (b) Plots of (αhν)2 versus (hν) for samples. (c) Rate of RhB photocatalytic degradation with different as-prepared photocatalysts. (d) Plots of the rate constants k for RhB degradation using the as-prepared samples.

Table 2.
The bandgap of the samples.
3.6. RhB Dye Degradation Analysis
After revealing the CB alignment of precursors in the Ag2WO4-modified Ag6Si2O7 composite, the suitable light absorption, and excellent specific area, the photocatalytic performance was evaluated through RhB dye photodegradation under visible-light irradiation. RhB dye exhibited a characteristic peak at 554 nm in the UV–vis spectrum in the absence of a photocatalyst [36], and the very moment the photocatalyst was introduced, the peak intensity declined. After certain time intervals, the UV–vis spectrum of the solution was obtained to reveal the degradation. As shown in Figure S2, an obvious absorption peak at 554 nm gradually weakened with prolonged illumination time and almost disappeared at 90 min, indicating that the skeleton structure of RhB was destroyed during photocatalysis and the content of RhB in the solution was continuously reduced. Figure 5c clearly shows that the Ag6Si2O7/Ag2WO4 composite photocatalysts exhibited much better degradation ability than the pure Ag2WO4 or Ag6Si2O7 samples. Notably, the AgSW-4 photocatalyst had the optimum photodegradation performance amongst all catalysts, with a degradation rate that reached 97.5% within 90 min. Moreover, the experiment was performed three times to ensure reproducibility, and the degradation efficiency was 97.5%, 94.9%, and 95.4%, respectively (mean = 95.9%; SD = 0.0138), indicating that the Ag6Si2O7/Ag2WO4 composite was reproducible (Figure S3).
Equation (2) was introduced to understand the reaction kinetics, where C0 C, t, and k are the initial concentration of RhB, concentration after time t, and reaction rate, respectively.
As shown in Figure 6d, the degradation rate value of this reaction was k = 0.03603 min–1 for the AgSW-4 photocatalyst, which was 23.7 times that of Ag2WO4 and 4.65 times that of Ag6Si2O7. The prepared Ag2WO4/Ag6Si2O7 Z-scheme heterostructure also exhibited better photocatalytic activity for RhB degradation compared with most Ag6Si2O7-based and Ag2WO4-based photocatalysts reported in recent years (Table S1).
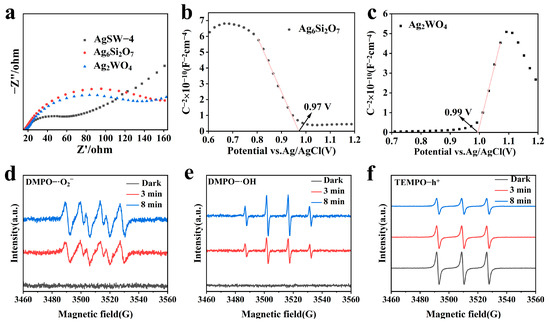
Figure 6.
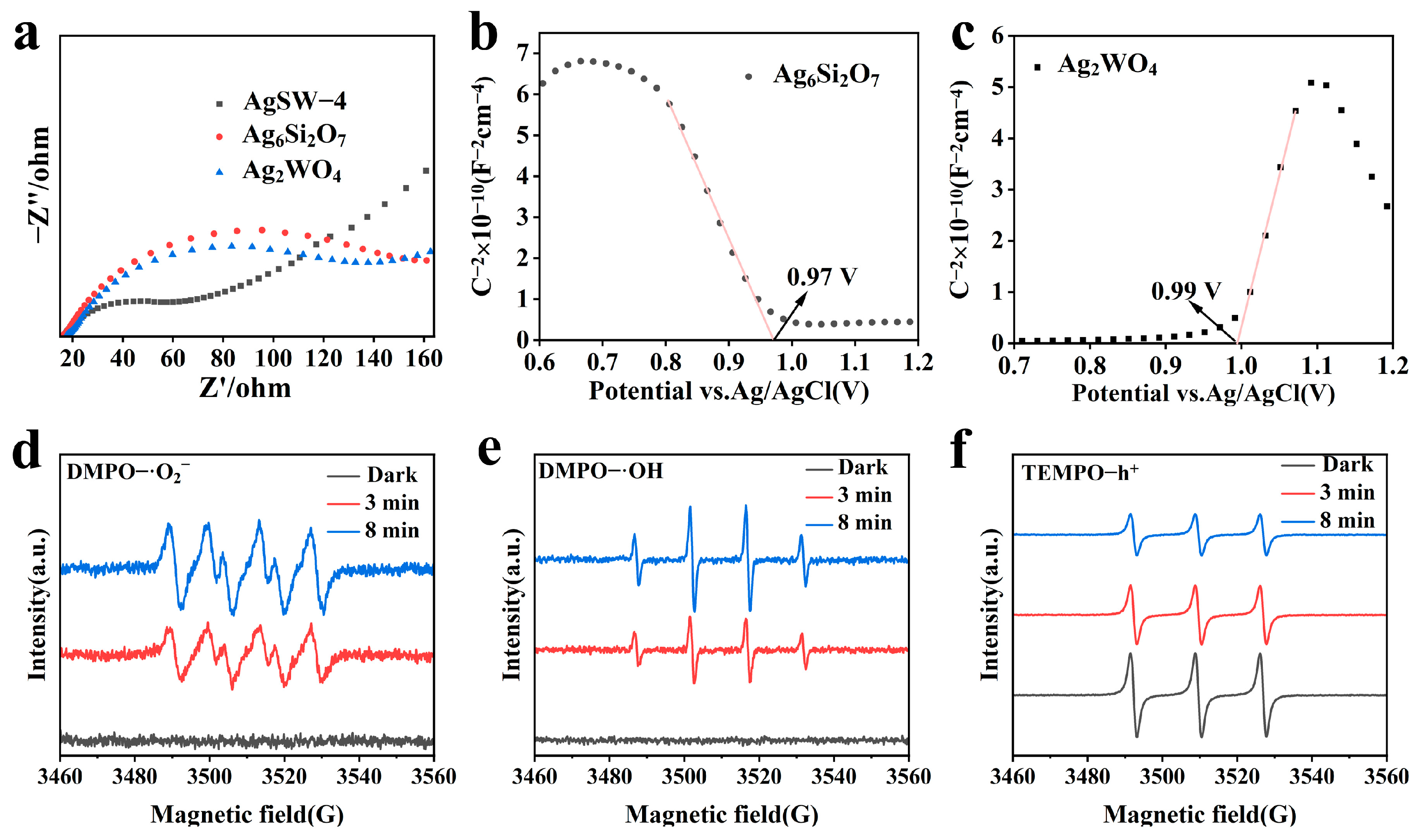
(a) Electrochemical impedance spectroscopy of AgSW-4, Ag2WO4, and Ag6Si2O7. MS plots of samples (b) Ag2WO4 and (c) Ag6Si2O7. ESR analysis of AgSW-4 sample: (d) superoxide anion radicals (·O2−), and (e) hydroxyl radicals (·OH) and (f) holes (h+).
Meanwhile, the cycle photocatalytic degradation performance of Ag6Si2O7/Ag2WO4 was studied. As shown in Figure S4, no significant decrease in the photodegradation efficiency of RhB with AgSW-4 was observed through six cycles. To further demonstrate the stability and reusability of Ag6Si2O7/Ag2WO4, we characterised the composition and structure of the used sample AgSW-4. The XRD spectra in Figure S5 showed no significant changes. As shown in Figure S6, the XPS spectrum of each element in AgSW-4 showed slight changes after use, but the valence states of each element were still the same as before the reaction. These results suggested that the structure of AgSW-4 was very stable during photocatalysis. Thus, AgSW-4 was selected to systematically analyse and reveal the probable mechanism of photocatalysis.
3.7. Electrochemical and Band Position Analysis
Photo-electrochemical properties could indicate the separation and transfer efficiency of the photogenerated electron–hole pairs in the semiconductor structures. The transient photocurrent analysis results of Ag6Si2O7, Ag2WO4, and AgSW-4 are shown in Figure S7. The photocurrent signal curves of the samples indicated that the AgSW-4 photocatalyst had much higher photocurrent intensity than Ag6Si2O7 and Ag2WO4, implying that the electron transfer and separation efficiency of photocharges were efficiently enhanced. This finding ultimately suggested that AgSW-4 had a higher capacity in the separation of photocharges, which was beneficial for the photocatalysis performance in accordance with the photodegradation results. EIS was performed to monitor the ability of photocatalysts to convert incident photons into charge carriers and transfer rate. The composite with the smaller arc radius in the EIS plots and smaller EIS reflection had lower movement resistance and thus are considered beneficial for redox processes [37]. Figure 6a clearly shows that AgSW-4 possessed much lower impedance than the pure Ag2WO4 or Ag6Si2O7 samples, with an improved ability to convert incident photons into electron–hole pairs. Moreover, the lower impedance indicated that AgSW-4 had a higher charge-transfer efficiency than its individual parts.
The band positions such as the VB and CB values of the photocatalysts were investigated to infer the photocatalysis mechanism of the composite material. The flat-band potential and naturally prepared semiconductors were investigated by Mott–Schottky (MS) plots. A negative MS slope represents an n-type semiconductor, whereas a positive slope represents a p-type one. Figure 6b,c show that Ag6Si2O7 is a p-type and Ag2WO4 is an n-type semiconductor [38]. By extrapolating the x-intercept of the linear region in the MS curve, the flat-band potential (Ef) of Ag2WO4 was calculated at 0.99 eV vs. Ag/AgCl, and was calculated to be 1.19 eV vs. NHE. For Ag6Si2O7, it was 0.97 eV vs. Ag/AgCl and 1.17 eV vs. NHE, respectively. Typically, the CB edge of the n-type semiconductors was 0.1 eV more negative than the Ef edge, whereas the VB edge of the p-type semiconductors was 0.1 eV more positive than the Ef edge [39]. On this basis, the CB potential (ECB) of Ag2WO4 was approximately 1.09 eV vs. NHE, and the VB potential (EVB) of Ag2WO4 was 1.27 eV vs. NHE.
Consequently, the following equation was used to calculate the VB or CB edge of Ag2WO4 or Ag6Si2O7:
From the UV–vis DRS analysis, the values of Ag2WO4 and Ag6Si2O7 were 3.12 and 2.31 eV, respectively, so the EVB of Ag2WO4 was calculated to be 4.21 and the ECB of Ag6Si2O7 was −1.04 eV.
3.8. Reactive Species Study
To gain insights into how dye degradation occurred during photocatalysis, ESR was performed to infer about the reactive species participating in the degradation and their corresponding roles. Once the photocatalyst was exposed to light, electron–hole pairs were generated and further produced superoxide (·O2−) or hydroxyl radicals (·OH) that interacted with dyes to degrade them [40,41,42]. Apart from these reactive species, holes were also considered to be the crucial candidates reacting directly with dyes to destroy and degrade them. Figure 6d shows the ESR graph demonstrating the production of (·O2−) radicals as a consequence of light irradiation. No peaks were observed when the photocatalyst was in darkness, and six peaks emerged after light irradiation, indicating the existence of reactive (·O2−) radicals in the presence of light. The intensity of peaks was further increased with prolonged irradiation exposure time, thereby confirming that they were the active species during the photocatalysis of dyes. Figure 6e depicts the subsequent hydroxyl radical (·OH) scenario when the photocatalyst was in darkness and light. They were also produced as a consequence of photogenerated carries. The increased peak intensity with increased irradiation time confirmed that they were also photo-induced reactive species. Finally, Figure 6f depicts the photogeneration of holes (h+). ESR results suggested that during the photocatalytic degradation of dyes, the ·O2−, ·OH, and holes all participated and played important roles.
3.9. Photocatalysis Mechanism
During advanced photocatalysis, which has been demonstrated to be more efficient for the degradation of organic contaminants, further studying the proposed process is necessary to better understand it. Generally, organic pollutants first adsorb onto the surface of photocatalytic material, and then they are broken down into nontoxic end-products by secondary reactive substances produced by photocatalysts during reaction.
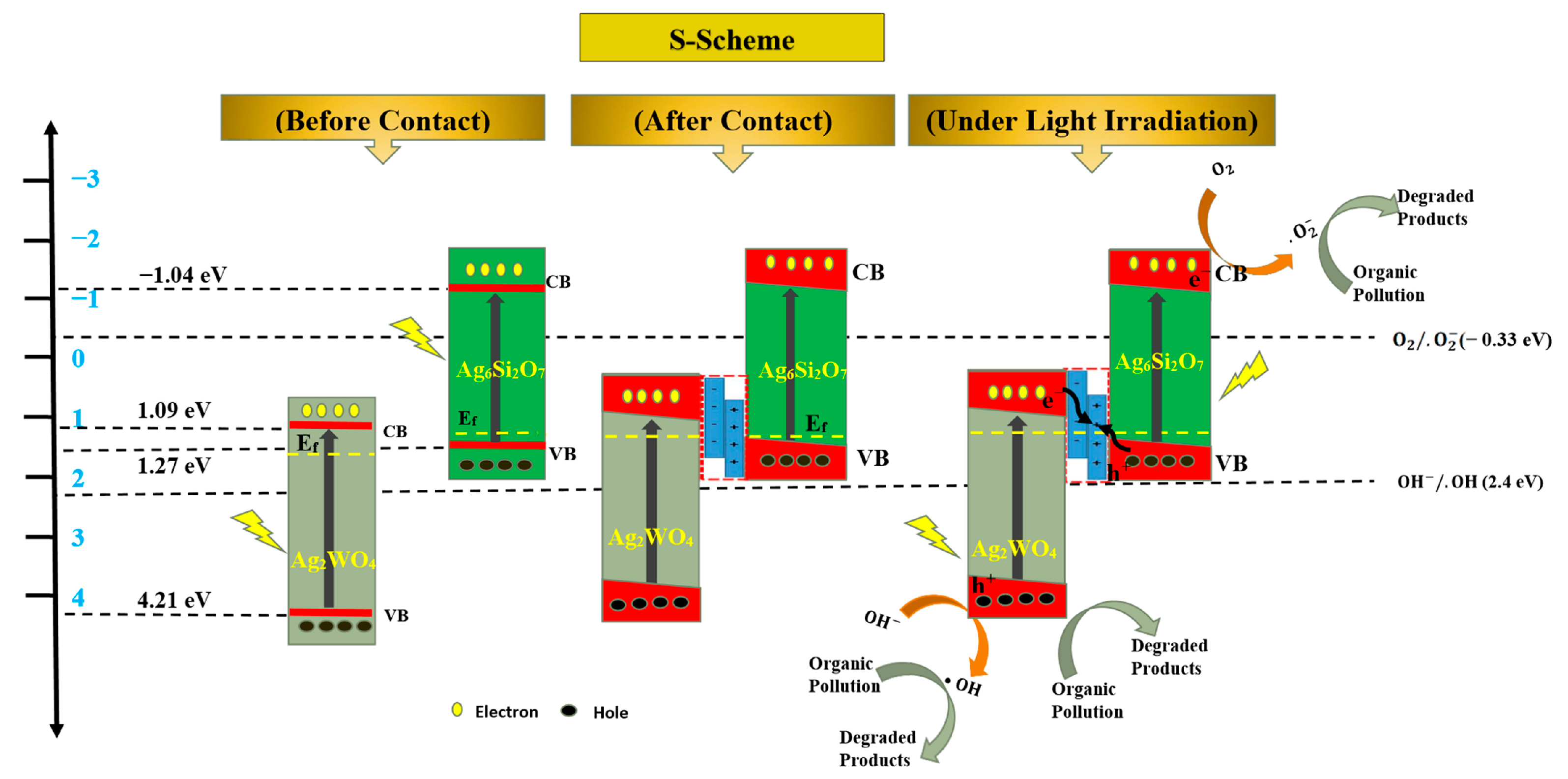
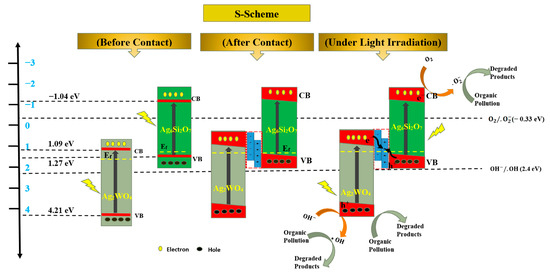
Scheme 1 shows that based on the previous data and analysis, before composition, the separated Ag2WO4 or Ag6Si2O7 nanocrystals absorbed the photons with suitable energy and generated photoelectrons and holes. However, the photocatalysis efficiency was low.
Scheme 1.
Proposed mechanism of AgSW-4 for the degradation of organic pollutions: before and after contact S-scheme of Ag2WO4 and Ag6Si2O7 nanostructure and the whole mechanism of organic pollutions’ photodegradation by using AgSW-4 photocatalyst.
When in contact with each other, the p-type Ag6Si2O7 and the n-type Ag2WO4 formed a p-n heterojunction. Along with this formation, an electric field was established between the interface of the two different nanostructures. With the effectiveness of the electric field at the interface, the Ef band positions of the two nanostructures Ag6Si2O7 and Ag2WO4 were unified at the same level. After exposure to visible light, the photogenerated electrons were transported to the CB levels of Ag2WO4 and Ag6Si2O7 and followed the traditional process of the heterojunction structure. The photogenerated electrons at a higher CB level of Ag6Si2O7 transferred to the lower CB level of Ag2WO4, whereas the photogenerated holes transferred from Ag2WO4 to Ag6Si2O7. However, data analysis revealed that the CB level of Ag2WO4 nanostructures (1.09 eV vs. NHE) was less negative than that of O2/·O2− (−0.33 eV vs. NHE) and the VB level of Ag6Si2O7 (1.27 eV vs. NHE) was less negative than that of ·OH (+2.4 eV vs. NHE) [43]. Given that ·O2− and ·OH were detected, other more rational mechanisms should be proposed.
When exposed to visible light, the photogenerated electrons’ Ag2WO4 nanostructures clearly aggregated in the CB level of Ag2WO4, whereas the holes of the Ag6Si2O7 nanostructures aggregated in the VB level of Ag6Si2O7, thereby enhancing the electric field at the p-n heterojunction.
The CB level of the Ag2WO4 nanostructures (1.09 eV vs. NHE) and the VB level of Ag6Si2O7 (1.27 eV vs. NHE) were very close. With the S-scheme photocatalysis mechanism [44,45,46,47,48], the photogenerated electrons in the CB level of the Ag2WO4 nanostructures and the holes in the VB level of Ag6Si2O7 very easily crossed the bandgap with the help of the electric field at the p-n heterojunction, and the electrons in the CB level of Ag6Si2O7 (−1.04 eV vs. NHE) and the holes in the VB level of Ag2WO4 (4.21 eV vs. NHE) were preserved and stable. They reacted with O2 and H2O to produce ·O2− and ·OH, consistent with the ESR results. The photogenerated h+, ·O2−, and ·OH further reacted with the adsorbed organic dyes and degraded the organic dyes to small molecules with much lower toxicity to the environment. This finding confirmed that the p-n heterojunction S-scheme photocatalytic degradation was valid and reliable.
4. Conclusions
A coprecipitation method was used to prepare Ag2WO4-modified Ag6Si2O7 nanocomposites. XRD, XPS, and TEM elemental mapping tests confirmed the composition of these two nanocrystals. Photodegradation performance tests suggested that the mole ratio of 4% of the Ag2WO4-modified Ag6Si2O7 (AgSW-4) photocatalyst had the optimum photocatalysis efficiency. Physical and chemical analyses confirmed that the AgSW-4 photocatalyst had appropriate light absorption, a large specific surface area, high efficiency to generate photocarriers, and enhanced the stability of the p-n heterojunction, which were the main factors for improving the photocatalysis performance. With the ESR data, all active species h+, ·O2−, and ·OH in the photodegradation were detected. Based on these data, an S-scheme photocatalysis degradation process was proposed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13040633/s1, Figure S1: Schematic diagram of the photocatalytic experimental equipment; Figure S2: UV–vis spectral changes of MO solution photocatalyzed by Ag2WO4/Ag6Si2O7 nanocomposites; Figure S3: Cycling experiments for RhB photodegradation with AgSW-4 nanocomposites; Figure S4: XRD patterns of AgSW-4 composites before and after photodegradation process; Figure S5: XPS spectra of AgSW-4 composites before and after photodegradation process: survey spectrum (b), Ag 3 d (b), Si 2p (c), and W 4f (d); Figure S6: Transient photocurrent response curves of samples Ag2WO4, Ag6Si2O7, and AgSW-4; Figure S7: Repeatability experiments for RhB photodegradation with AgSW-4 nanocomposites; Table S1: Degradation performance between the present work and previously reported Ag6Si2O7 and Ag2WO4 photocatalysts.
Author Contributions
Conceptualization, Y.C. and Y.W.; methodology, M.L., Y.C. and Y.W.; investigation, P.Z. and Y.C.; resources, M.L.; data curation, N.H.S., M.L., P.Z. and Y.W.; writing—original draft, N.H.S.; writing—review and editing, Y.C. and Y.W.; supervision, Y.C. and Y.W.; project administration, Y.W.; funding acquisition, Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFA1603700), the Program for International S&T Cooperation Projects of the Ministry of Science and Technology of China (2018YFE0117200), Beijing Natural Science Foundation (L212015), National Natural Science Foundation of China (22027810, 11621505). The National Basic Research Program of China (2021YFA1200900), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB36000000), CAMS Innovation Fund for Medical Sciences (CIFMS 2019-I2M-5-018), and the Research and Development Project in Key Areas of Guangdong Province (2019B090917011), Key-Area Research and Development Program of Guangdong Province for Guangdong High Level Innovation Research Institute (2020B0909010001).
Data Availability Statement
Data available on request from the authors.
Acknowledgments
We thank the Analysis and Testing Center at the Beijing Institute of Technology for the useful discussion and assistance in facility support.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Obaideen, K.; Shehata, N.; Sayed, E.T.; Abdelkareem, M.A.; Mahmoud, M.S.; Olabi, A.G. The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 2022, 7, 100112. [Google Scholar] [CrossRef]
- Nie, G.; Yao, Y.; Duan, X.; Xiao, L.; Wang, S. Advances of piezoelectric nanomaterials for applications in advanced oxidation technologies. Curr. Opin. Chem. Eng. 2021, 33, 100693. [Google Scholar] [CrossRef]
- Bethi, B.; Sonawane, S.H.; Bhanvase, B.A.; Gumfekar, S.P. Nanomaterials-based advanced oxidation processes for wastewater treatment: A review. Chem. Eng. Process. Process Intensif. 2016, 109, 178–189. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef] [PubMed]
- Skillen, N.; Daly, H.; Lan, L.; Aljohani, M.; Murnaghan, C.W.J.; Fan, X.; Hardacre, C.; Sheldrake, G.N.; Robertson, P.K.J. Photocatalytic reforming of biomass: What role will the technology play in future energy systems. Top. Curr. Chem. 2022, 380, 33. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.C. Solvothermal synthesis of facet-dependent BiVO4 photocatalyst with enhanced visible-light-driven photocatalytic degradation of organic pollutant: Assessment of toxicity by zebrafish embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 14100. [Google Scholar] [CrossRef]
- Niu, X.; Yan, W.; Zhao, H.; Yang, J. Synthesis of Nb doped TiO2 nanotube/reduced graphene oxide heterostructure photocatalyst with high visible light photocatalytic activity. Appl. Surf. Sci. 2018, 440, 804–813. [Google Scholar] [CrossRef]
- Lu, M.; Wang, X.; Xu, C.; Wang, Y. Synthesis of CdS@ZnO nanocomposites with wide visible light absorption range. J. Mater. Sci. Mater. Electron. 2020, 31, 17624–17632. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Liu, Y.; Xue, B.; Chen, X. Photocatalytic degradation of antibiotics using a novel Ag/Ag2S/Bi2MoO6 plasmonic p-n heterojunction photocatalyst: Mineralization activity, degradation pathways and boosted charge separation mechanism. Chem. Eng. J. 2021, 415, 128991. [Google Scholar] [CrossRef]
- Chen, P.; Chen, L.; Ge, S.; Zhang, W.; Wu, M.; Xing, P.; Rotamond, T.B.; Lin, H.; Wu, Y.; He, Y. Microwave heating preparation of phosphorus doped g-C3N4 and its enhanced performance for photocatalytic H2 evolution in the help of Ag3PO4 nanoparticles. Int. J. Hydrog. Energy 2020, 45, 14354–14367. [Google Scholar] [CrossRef]
- Chen, H.; Chen, N.; Feng, C.; Gao, Y. Synthesis of a novel narrow-band-gap iron (II,III) oxide/titania/silver silicate nanocomposite as a highly efficient and stable visible light-driven photocatalyst. J. Colloid Interface Sci. 2018, 515, 119–128. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, D.; Wang, F.; Chen, M. AgCl/Au/g-C3N4 ternary composites: Efficient photocatalysts for degradation of anionic dyes. J. Alloy. Compd. 2021, 868, 159266. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, C.; Zhou, L.; Yu, Z.; Liu, X.; Zhao, Z.; Yuan, H. Constructing a novel multi-hierarchical TiO2/g-C3N4/Ag-AgBr photocatalyst with dual Z-scheme heterojunction utilizing Ag as the charge transfer mediator. J. Alloys Compd. 2022, 900, 163514. [Google Scholar] [CrossRef]
- Jatav, N.; Kuntail, J.; Khan, D.; De, A.K.; Sinha, I. AgI/CuWO4 Z-scheme photocatalyst for the degradation of organic pollutants: Experimental and molecular dynamics studies. J. Colloid Interface Sci. 2021, 599, 717–729. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, M.; Zhou, Q.; Li, X.; Chen, T.; Wang, S. Preparation of Ag3PO4/TiO2(B) heterojunction nanobelt with extended light response and enhanced photocatalytic performance. Molecules 2021, 26, 6987. [Google Scholar] [CrossRef]
- Zhou, M.; Tian, X.; Yu, H.; Wang, Z.; Ren, C.; Zhou, L.; Lin, Y.-W.; Dou, L. WO3/Ag2CO3 mixed photocatalyst with enhanced photocatalytic activity for organic dye degradation. ACS Omega 2021, 6, 26439–26453. [Google Scholar] [CrossRef]
- Liu, L.; Hu, T.; Dai, K.; Zhang, J.; Liang, C. A novel step-scheme BiVO4/Ag3VO4 photocatalyst for enhanced photocatalytic degradation activity under visible light irradiation. Chin. J. Catal. 2021, 42, 46–55. [Google Scholar] [CrossRef]
- Rafiq, U.; Majid, K. Mitigating the charge recombination by the targeted synthesis of Ag2WO4/Bi2Fe4O9 composite: The facile union of orthorhombic semiconductors towards efficient photocatalysis. J. Alloys Compd. 2020, 842, 155876. [Google Scholar] [CrossRef]
- Ayappan, C.; Jayaraman, V.; Palanivel, B.; Pandikumar, A.; Mani, A. Facile preparation of novel Sb2S3 nanoparticles/rod-like α-Ag2WO4 heterojunction photocatalysts: Continuous modulation of band structure towards the efficient removal of organic contaminants. Sep. Purif. Technol. 2019, 236, 116302. [Google Scholar] [CrossRef]
- Shi, L.; Gou, J.; Liang, L.; Wang, F.; Sun, J. The crystal phase transformation of Ag2WO4 through loading onto g-C3N4 sheets with enhanced visible-light photocatalytic activity. RSC Adv. 2016, 6, 96861–96869. [Google Scholar] [CrossRef]
- Lou, Z.; Huang, B.; Wang, Z.; Ma, X.; Zhang, R.; Zhang, X.; Qin, X.; Dai, Y.; Whangbo, M.-H. Ag6Si2O7: A silicate photocatalyst for the visible region. Chem. Mater. 2014, 26, 3873–3875. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, H.; Xu, T.; Xu, N.; Ma, H. Highly efficient Ag6Si2O7/WO3 photocatalyst based on heterojunction with enhanced visible light photocatalytic activities. RSC Adv. 2016, 6, 103289–103295. [Google Scholar] [CrossRef]
- Wen, M.; Yang, N.; Wang, J.; Liu, D.; Zhang, W.; Bian, S.; Huang, H.; He, X.; Wang, X.; Ramakrishna, S.; et al. Activating carbon nitride by BP@Ni for the enhanced photocatalytic hydrogen evolution and selective benzyl alcohol oxidation. ACS Appl. Mater. Interfaces 2021, 13, 50988–50995. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. Hierarchical core-shell heterostructures of ZnIn2S4 nanosheets on electrospun In2O3 nanofibers with highly enhanced photocatalytic activity. J. Hazard. Mater. 2020, 398, 122889. [Google Scholar] [CrossRef]
- De Santana, Y.V.B.; Gomes, J.E.C.; Matos, L.; Cruvinel, G.H.; Perrin, A.; Perrin, C.; Andres, J.; Varela, J.A.; Longo, E. Silver molybdate and silver tungstate nanocomposites with enhanced photoluminescence. Nanomater. Nanotechnol. 2014, 4, 22. [Google Scholar] [CrossRef]
- Sreedevi, A.; Priyanka, K.P.; Babitha, K.K.; Sankararaman, S.I.; Thomas, V. Synthesis and characterization of silver tungstate/iron phthalocyanine nanocomposite for electronic applications synthesis and characterization of silver tungstate/iron phthalocyanine nanocomposite for electronic applications. Eur. Phys. J. B 2017, 90, 102. [Google Scholar] [CrossRef]
- Alvarez-Roca, R.; Gouveia, A.F.; De Foggi, C.C.; Lemos, P.S.; Gracia, L.; Da Silva, L.F.; Vergani, C.E.; San-Miguel, M.; Longo, E.; Andres, J. Selective synthesis of α-, β-, and γ-Ag2WO4 polymorphs: Promising platforms for photocatalytic and antibacterial materials. Inorg. Chem. 2021, 60, 1062–1079. [Google Scholar] [CrossRef]
- Ghobadifard, M.; Mohebbi, S. Novel nanomagnetic Ag/β-Ag2WO4/CoFe2O4 as a highly efficient photocatalyst under visible light irradiation. New J. Chem. 2018, 42, 9530–9542. [Google Scholar] [CrossRef]
- Hsu, M.H.; Chang, C.J. Ag-doped ZnO nanorods coated metal wire meshes as hierarchical photocatalysts with high visible-light driven photoactivity and photostability. J. Hazard. Mater. 2014, 278, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.; Gracia, L.; Gonzalez-Navarrete, P.; Longo, V.M.; Avansi, W., Jr.; Volanti, D.P.; Ferrer, M.M.; Lemos, P.S.; La Porta, F.A.; Hernandes, A.C.; et al. Structural and electronic analysis of the atomic scale nucleation of Ag on α-Ag2WO4 induced by electron irradiation. Sci. Rep. 2014, 4, 5391. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xing, P.; Chen, Z.; Hu, X.; Lin, H.; Zhao, L.; He, Y. In-situ synthesis of AgNbO3/g-C3N4 photocatalyst via microwave heating method for efficiently photocatalytic H2 generation. J. Colloid Interface Sci. 2019, 534, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Deng, Y.; Tang, L.; Feng, C.; Wang, J.; Yu, J.; Wang, J.; Liu, Z.; Zhou, H. Theoretical and experimental study of full spectrum response Z-scheme 0D/2D Ag6Si2O7/CN photocatalyst with enhanced photocatalytic activities. Appl. Surf. Sci. 2020, 514, 145963. [Google Scholar] [CrossRef]
- Liu, D.; Huang, W.; Li, L.; Liu, L.; Sun, X.; Liu, B.; Yang, B.; Guo, C. Experimental and theoretical investigation on photocatalytic activities of 1D Ag/Ag2WO4 nanostructures. Nanotechnology 2017, 28, 385702. [Google Scholar] [CrossRef]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Tang, M.; Jiang, S.; Gao, D. Incorporation of a Z-scheme AgI/Ag6Si2O7 heterojunction to PET fabric for efficient and repeatable photocatalytic dye degradation. New J. Chem. 2022, 46, 22550–22560. [Google Scholar] [CrossRef]
- Sabri, M.; Habibi-Yangjeh, A.; Chand, H.; Krishnan, V. Heterogeneous photocatalytic activation of persulfate ions with novel ZnO/AgFeO2 nanocomposite for contaminants degradation under visible light. J. Mater. Sci. Mater. Electron. 2021, 32, 4272–4289. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Xing, S. Insight into the catalytic mechanism of γ-Fe2O3/ZnFe2O4 for hydrogen peroxide activation under visible light. J. Colloid Interface Sci. 2018, 529, 247–254. [Google Scholar] [CrossRef]
- Cui, Y.; Li, M.; Zhu, N.; Cheng, Y.; Lam, S.S.; Chen, J.; Gao, Y.; Zhao, J. Bi-based visible light-driven nano-photocatalyst: The design, synthesis, and its application in pollutant governance and energy development. Nano Today 2022, 43, 101432. [Google Scholar] [CrossRef]
- Assis, M.; Robeldo, T.; Foggi, C.C.; Kubo, A.M.; Minguez-Vega, G.; Condoncillo, E.; Beltran-Mir, H.; Torres-Mendieta, R.; Andres, J.; Oliva, M.; et al. Ag Nanoparticles/α-Ag2WO4 composite formed by electron beam and femtosecond irradiation as potent antifungal and antitumor agents. Sci. Rep. 2019, 9, 9927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ma, Y.; Zhu, X.; Wang, L.; Ye, J.; Hou, X.; Liu, S.; Lu, M.; Tian, H.; Hu, X. In situ synthesis of α-Fe2O3/LaFeO3 modified with g-C3N4 and Ti3C2 for construction of multiple Z-scheme/Schottky heterojunctions as an efficient visible-light photocatalyst for Cr (VI) reduction and organic pollutants removal. J. Alloys Compd. 2022, 913, 165217. [Google Scholar] [CrossRef]
- Jo, W.-K.; Kumar, S.; Eslava, S.; Tonda, S. Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B Environ. 2018, 239, 586–598. [Google Scholar] [CrossRef]
- Fu, J.; Xu, Q.; Low, J.; Jiang, C.; Yu, J. Ultrathin 2D/2D WO3/g-C3N4 step-scheme H2-production photocatalyst. Appl. Catal. B Environ. 2019, 243, 556–565. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, J.; Du, Y.; Zhou, C.; Zhang, M.; Wang, Z.; Weng, Y.; Long, J.; Hofkens, J.; Steele, J.A.; et al. Direct Z-scheme heterojunction of semicoherent FAPbBr3/Bi2WO6 interface for photoredox reaction with large driving force. ACS Nano 2020, 14, 16689–16697. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, J.; Yu, H.; Yu, J. Emerging S-scheme photocatalyst. Adv. Mater. 2022, 34, 2107668. [Google Scholar] [CrossRef]
- Mei, F.; Li, Z.; Dai, K.; Zhang, J.; Liang, C. Step-scheme porous g-C3N4/Zn0.2Cd0.8S-DETA composites for efficient and stable photocatalytic H2 production. Chin. J. Catal. 2020, 41, 41–49. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, J.; Dai, K.; Fan, K.; Liang, C. Integrated S-Scheme Heterojunction of Amine-Functionalized 1D CdSe Nanorods Anchoring on Ultrathin 2D SnNb2O6 Nanosheets for Robust Solar-Driven CO2 Conversion. Sol. RRL 2021, 5, 2000805. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).