Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Iron Oxides
2.2. Fundamental Techniques Used in Characterizations
3. Result and Discussions
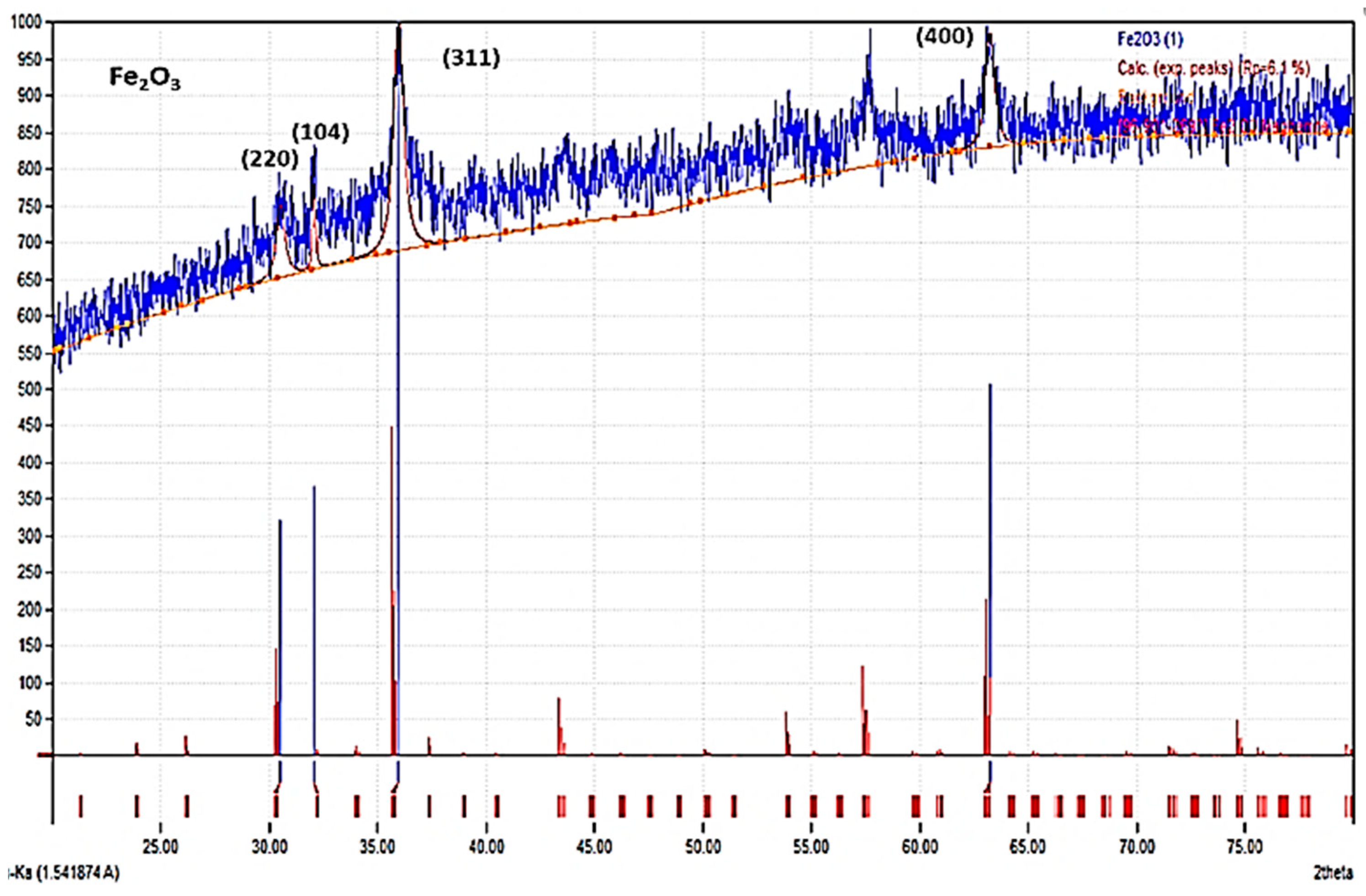
3.1. Crystalline Confirmations: XRD Study
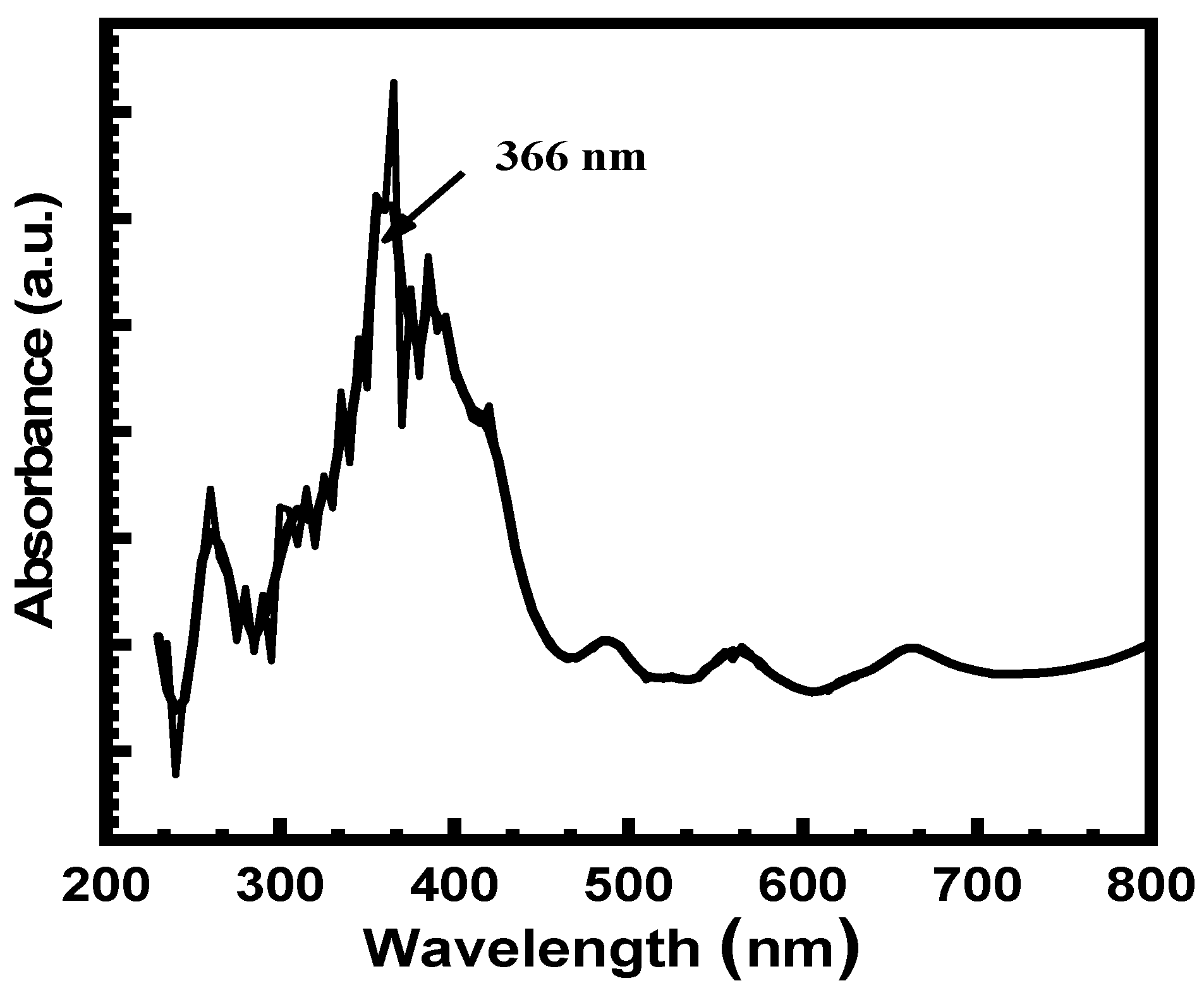
3.2. Optical Absorbance Study (UV-Vis Spectra)
3.3. Functional Group Confirmations: FTIR Analysis
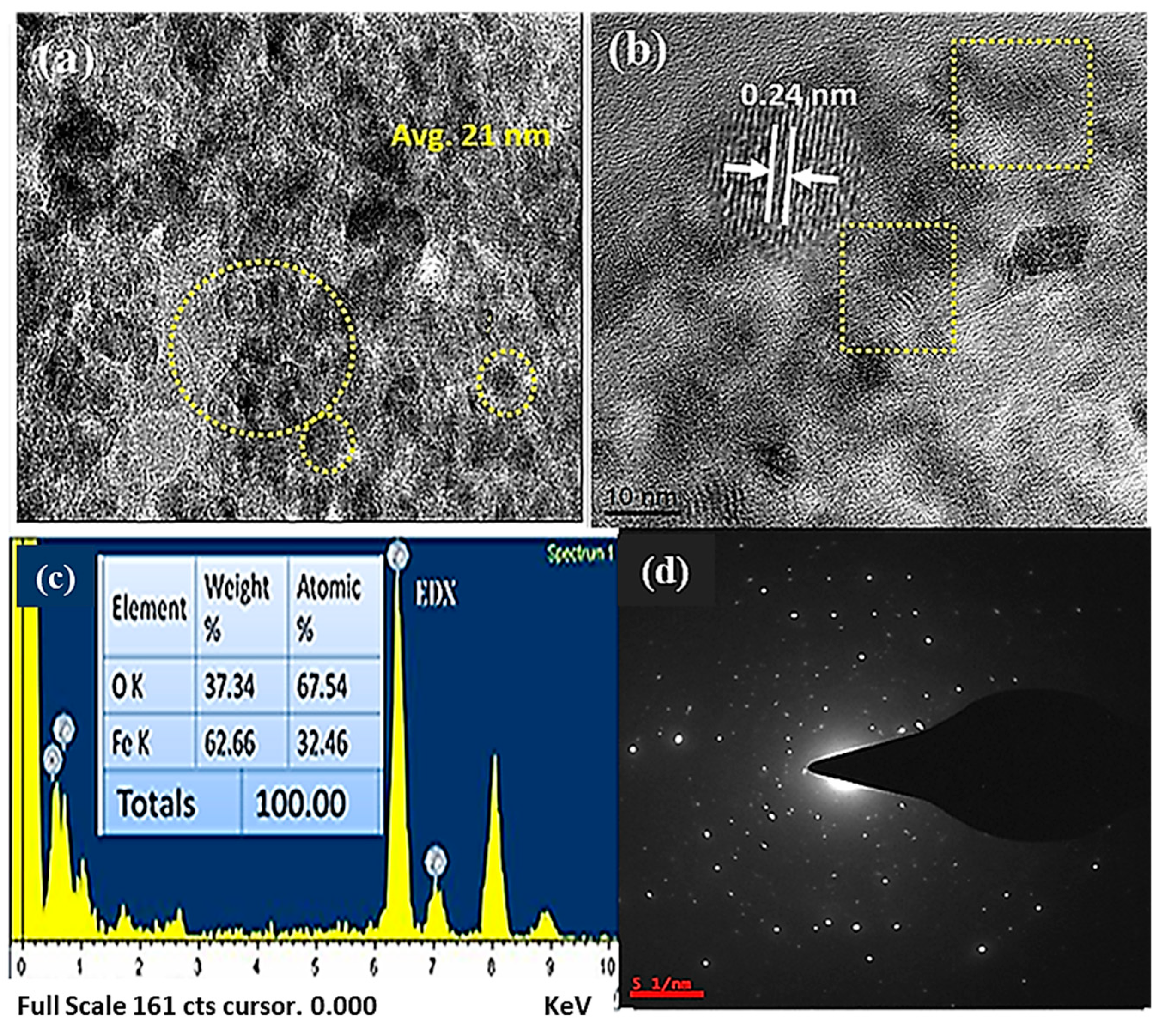
3.4. Morphological Studies: HRTEM Investigation
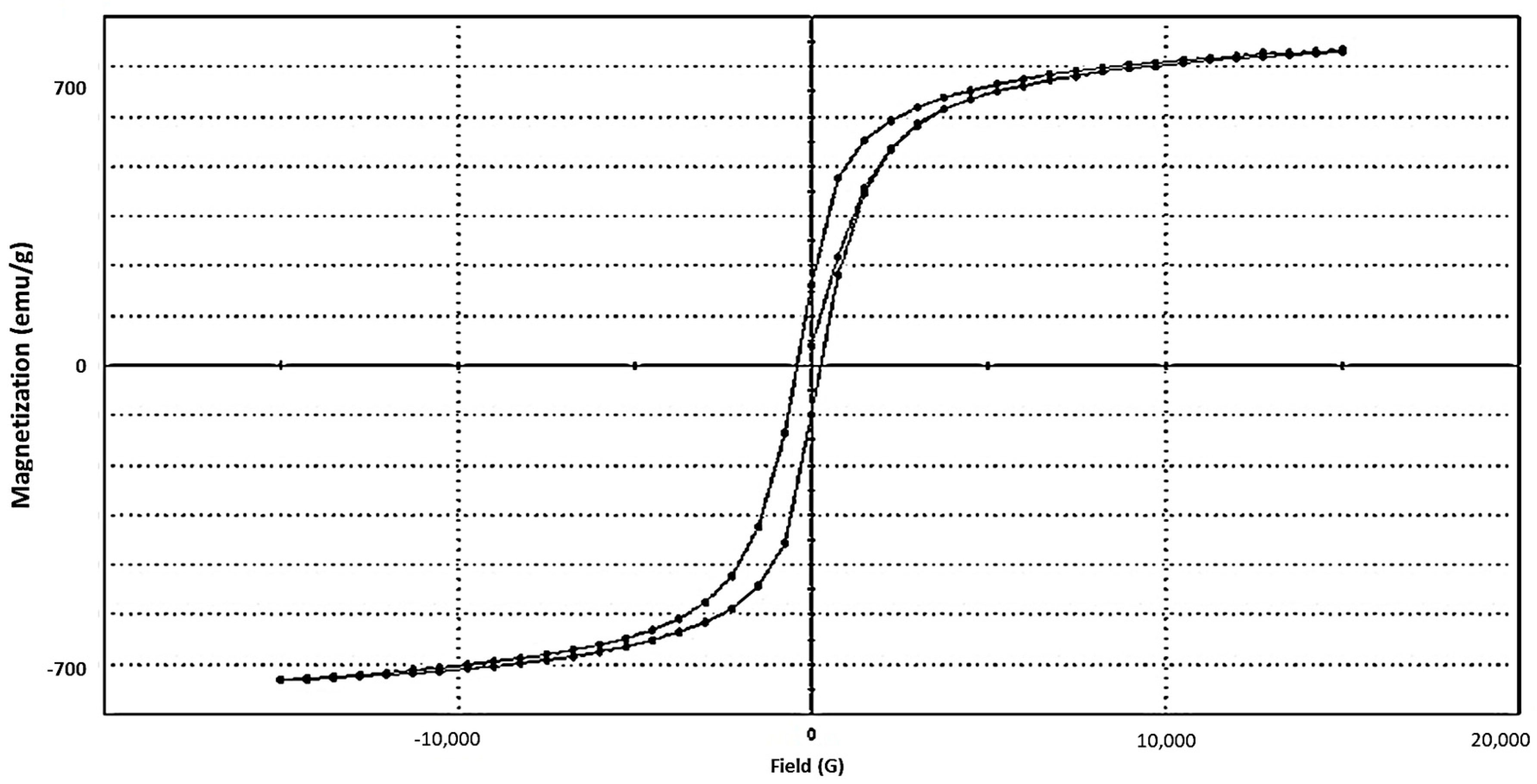
3.5. Vibrating Sample Magnetometer (VSM) Study of Iron Oxides NPs
4. Remediation of Industrial Effluents
4.1. Removal of Heavy Metals Using Iron Oxide Nanoparticles
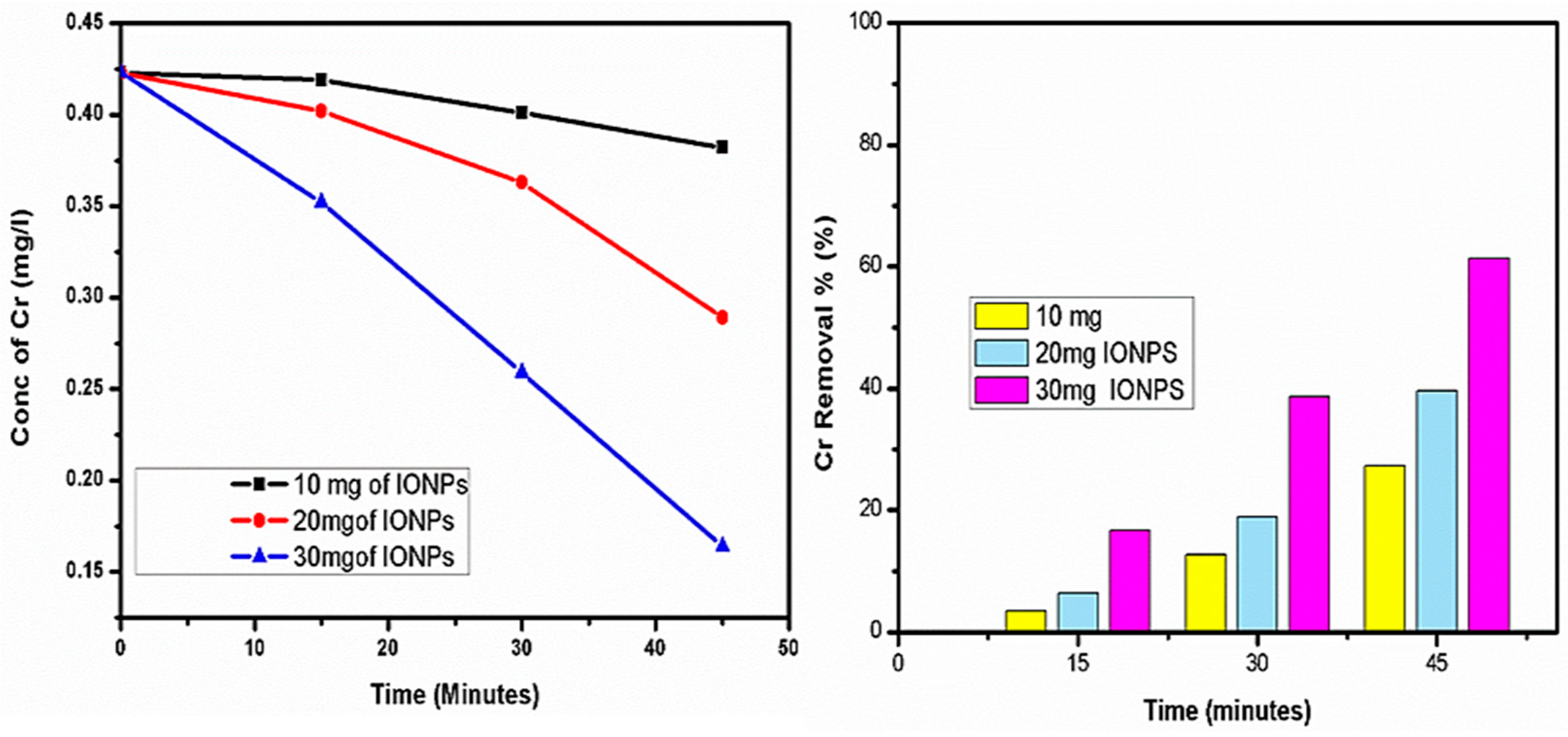
4.2. Chromium (Cr) Removal
4.3. Cadmium (Cd) Removal
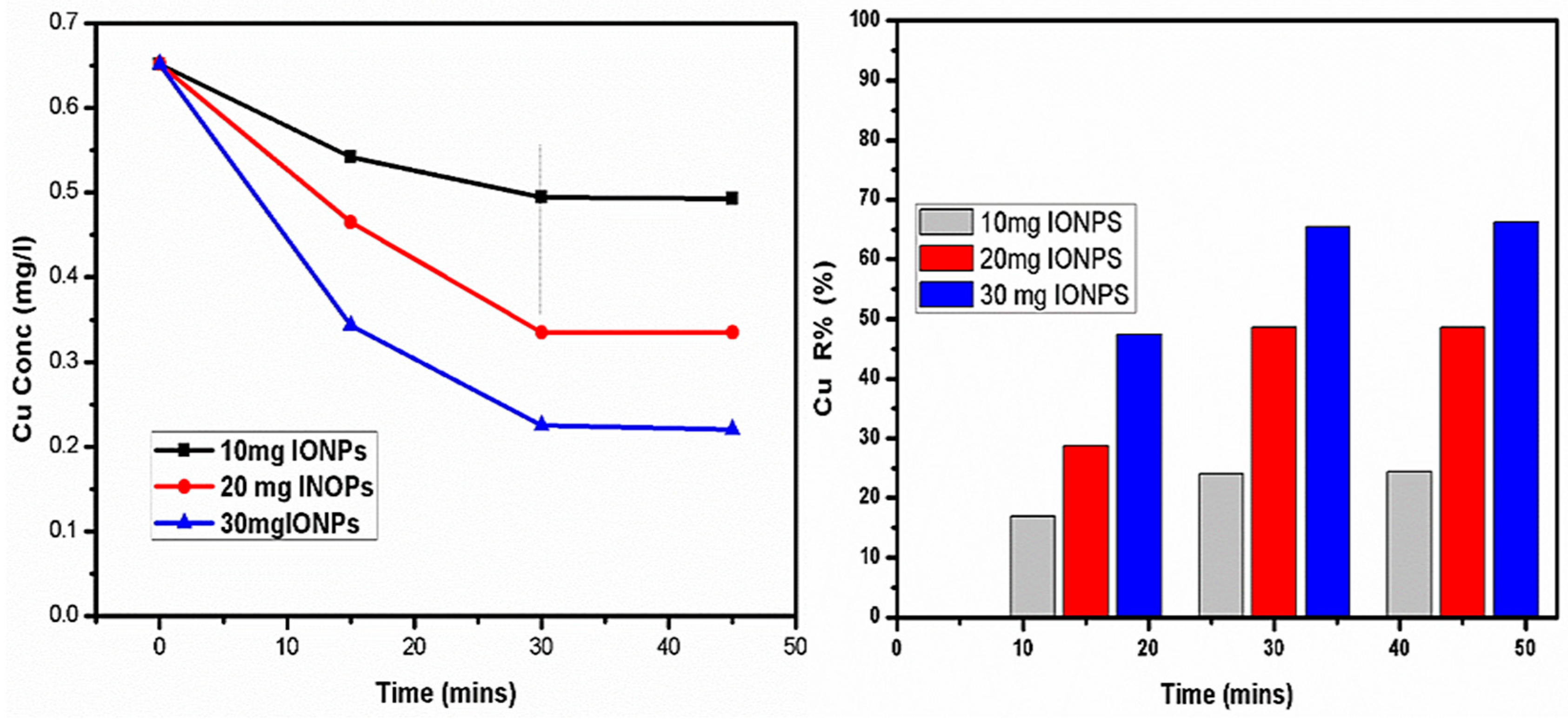
4.4. Elimination of Copper (Cu)
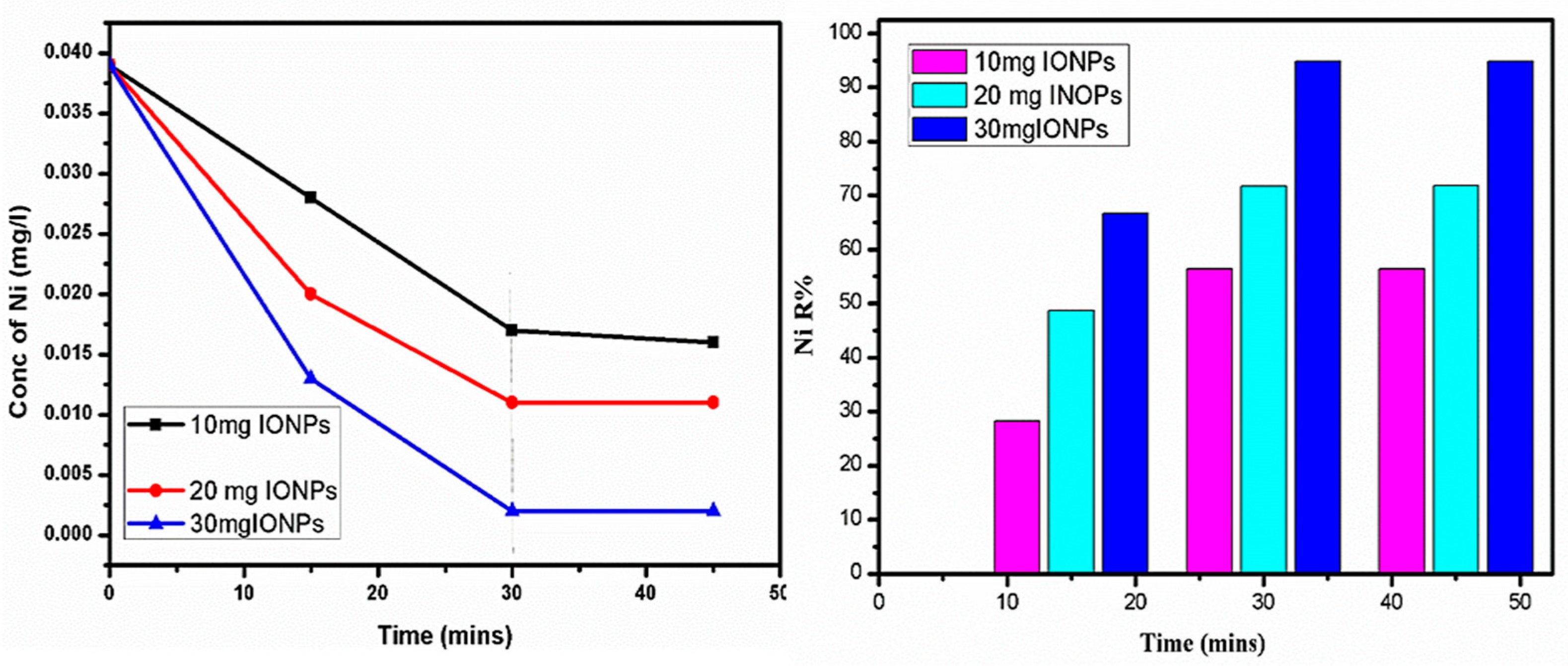
4.5. Nickel (Ni) Removal
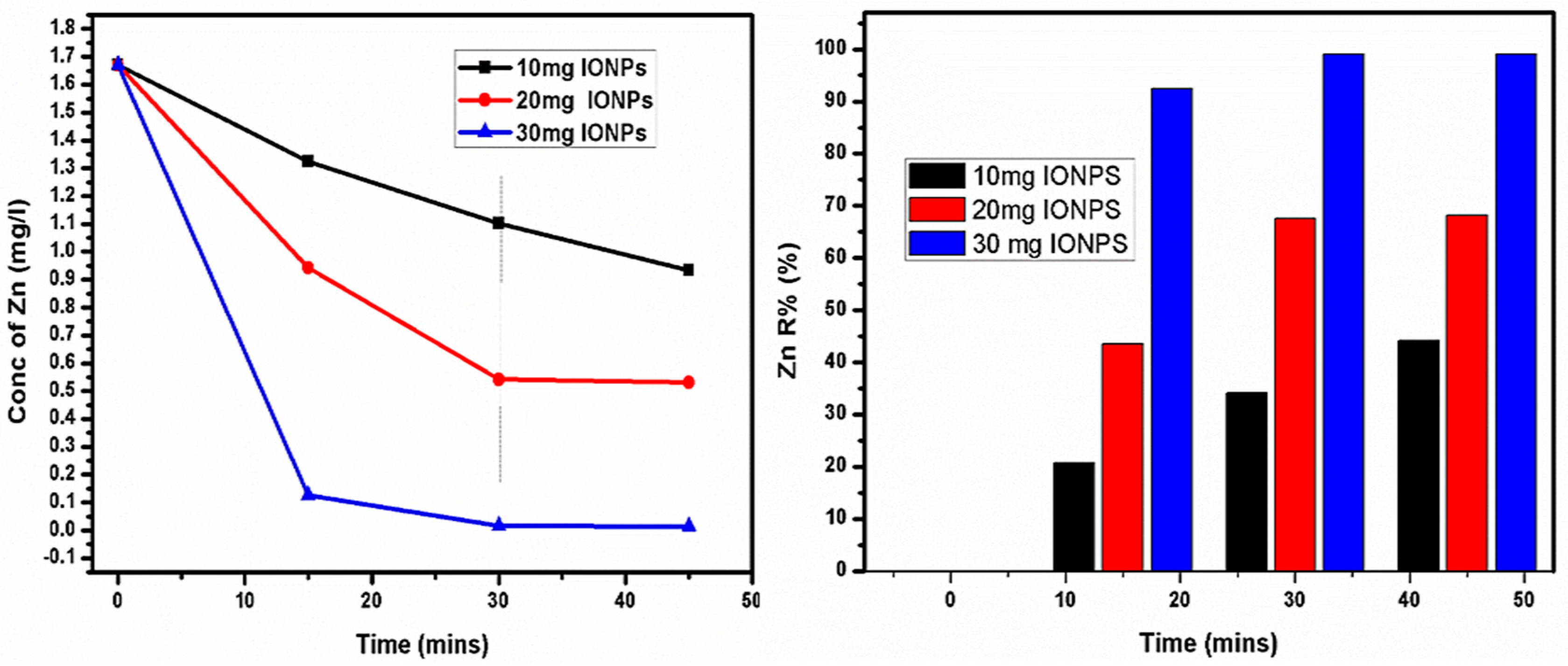
4.6. Zn Elimination Study
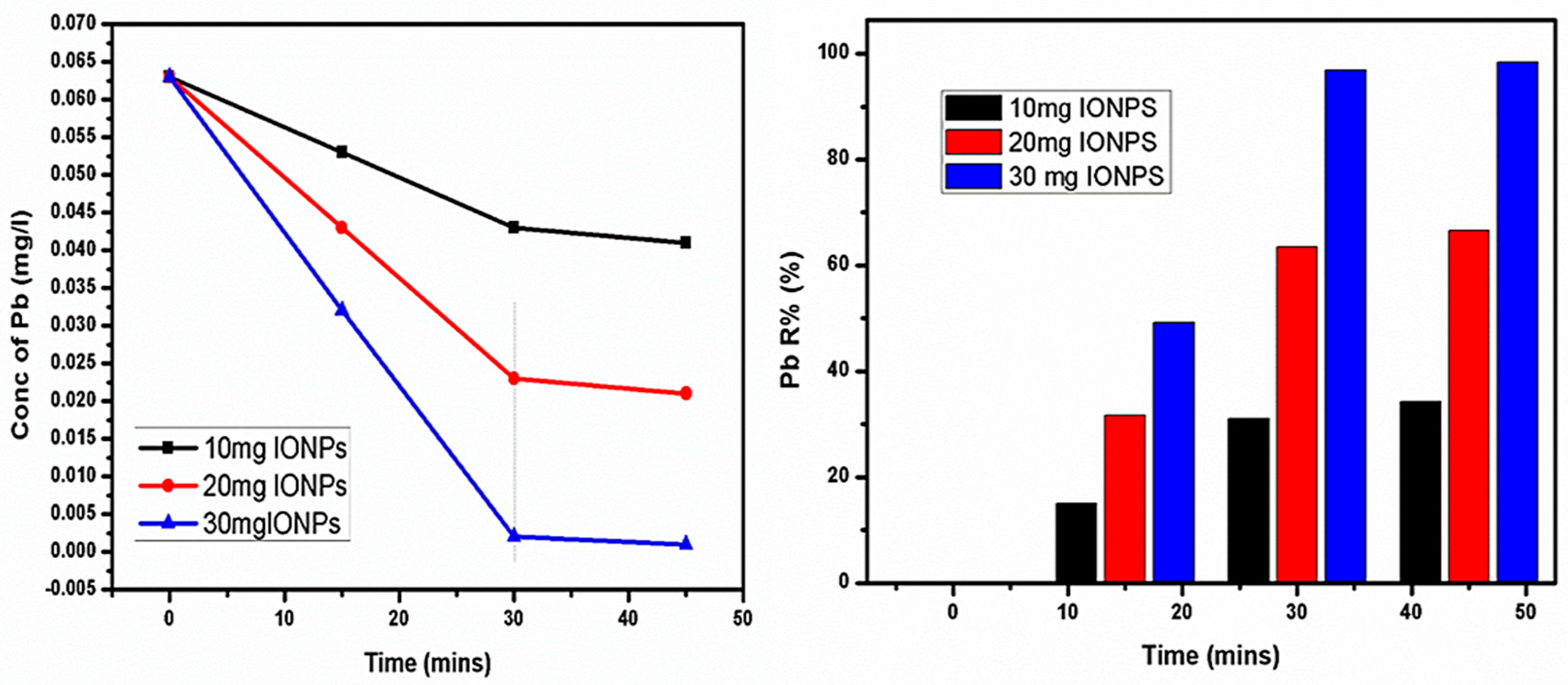
4.7. Lead (Pb) Removal
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yadav, V.K.; Yadav, K.K.; Gacem, A.; Gnanamoorthy, G.; Ali, I.H.; Khan, S.H.; Jeon, B.-H.; Kamyab, H.; Inwati, G.K.; Choudhary, N.; et al. A novel approach for the synthesis of vaterite and calcite from incense sticks ash waste and their potential for remediation of dyes from aqueous solution. Sustain. Chem. Pharm. 2022, 29, 100756. [Google Scholar] [CrossRef]
- Kumar, P.; Inwati, G.K.; Mathpal, M.C.; Maze, J.; Swart, H.C. Recent Advances on Ferrites Nanomaterial’s as Photocatalyst for Environment. In Advances in Nanostructured Materials. Materials Horizons: From Nature to Nanomaterials; Swain, B.P., Ed.; Springer: Singapore, 2022. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A.; Rizvi, M.A.; Moosvi, S.K.; Krishnan, V.; Duvenhage, M.; Roos, W.; Swart, H. Surface, optical and photocatalytic properties of Rb doped ZnO nanoparticles. Appl. Surf. Sci. 2020, 514, 145930. [Google Scholar] [CrossRef]
- Inwati, G.K.; Kumar, P.; Swart, H.C. Multifunctional properties of hybrid semiconducting nanomaterials and their applications. In Electronic and Optical Materials, Nanoscale Compound Semiconductors and Their Optoelectronics Applications; Elsevier: Amsterdam, The Netherlands, 2022; pp. 315–350. [Google Scholar] [CrossRef]
- Inwati, G.K.; Yadav, V.K.; Ali, I.H.; Vuggili, S.B.; Kakodiya, S.D.; Solanki, M.K.; Yadav, K.K.; Ahn, Y.; Yadav, S.; Islam, S.M.; et al. 2D Personality of Multifunctional Carbon Nitrides towards Enhanced Catalytic Performance in Energy Storage and Remediation. Appl. Sci. 2022, 12, 3753. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.; Islam, F.; Bin Emran, T.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10, 874742. [Google Scholar] [CrossRef]
- Rajendran, S.; Inwati, G.K.; Yadav, V.K.; Choudhary, N.; Solanki, M.B.; Abdellattif, M.H.; Yadav, K.K.; Gupta, N.; Islam, S.; Jeon, B.-H. Enriched Catalytic Activity of TiO2 Nanoparticles Supported by Activated Carbon for Noxious Pollutant Elimination. Nanomaterials 2021, 11, 2808. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, S.; Antonisamy, A.J.; Malayandi, S.; Rajendran, K.; Tsai, P.-C.; Pugazhendhi, A.; Ponnusamy, V.K. Silver nanoparticles in dye effluent treatment: A review on synthesis, treatment methods, mechanisms, photocatalytic degradation, toxic effects and mitigation of toxicity. J. Photochem. Photobiol. B Biol. 2020, 205, 111823. [Google Scholar] [CrossRef]
- Rahman, M.; Wadnerkar, N.; English, N.J.; MacElroy, J. The influence of Ti- and Si-doping on the structure, morphology and photo-response properties of α-Fe2O3 for efficient water-splitting: Insights from experiment and first-principles calculations. Chem. Phys. Lett. 2014, 592, 242–246. [Google Scholar] [CrossRef]
- Bibi, I.; Nazar, N.; Ata, S.; Sultan, M.; Ali, A.; Abbas, A.; Jilani, K.; Kamal, S.; Sarim, F.M.; Khan, M.I.; et al. Green synthesis of iron oxide nanoparticles using pomegranate seeds extract and photocatalytic activity evaluation for the degradation of textile dye. J. Mater. Res. Technol. 2019, 8, 6115–6124. [Google Scholar] [CrossRef]
- Inwati, G.K.; Mehetre, S.; Ameta, R.K.; Kumar, P.; Swart, H.C.; Yadav, V.K.; Makwana, B.A.; Kakodiya, S.D.; Gnanamoorthy, G. Smart Supercapacitors—A New Perspective; Elsevier: Amsterdam, The Netherlands, 2023; pp. 159–176. Available online: https://www.sciencedirect.com/science/article/pii/B9780323905305000277 (accessed on 27 January 2023). [CrossRef]
- Ebrahim, S.E.; Sulaymon, A.H.; Alhares, H.S. Competitive removal of Cu2+, Cd2+, Zn2+, and Ni2+ ions onto iron oxide nanoparticles from wastewater. Desalin. Water Treat. 2016, 57, 20915–20929. [Google Scholar] [CrossRef]
- Vaclavikova, M.; Misaelides, P.; Gallios, G.; Jakabsky, S.; Hredzak, S. Removal of cadmium, zinc, copper and lead by red mud, an iron oxides containing hydrometallurgical waste. Stud. Surf. Sci. Catal. 2005, 155, 517–525. [Google Scholar] [CrossRef]
- Julkapli, N.M.; Bagheri, S.; Bee, S.; Hamid, S.B.A. Recent Advances in Heterogeneous Photocatalytic Decolorization of Synthetic Dyes. Sci. World J. 2014, 2014, 692307. [Google Scholar] [CrossRef]
- Cavalu, S.; Simon, V. Microstructure and bioactivity of acrylic bone cements for prosthetic surgery. J. Optoelectron. Adv. Mater. 2006, 8, 1520–1523. [Google Scholar]
- Inwati, G.; Kumar, P.; Roos, W.; Swart, H.; Singh, M. UV-irradiation effects on tuning LSPR of Cu/Ag nanoclusters in ion exchanged glass matrix and its thermodynamic behaviour. J. Alloys Compd. 2020, 823, 153820. [Google Scholar] [CrossRef]
- Inwati, G.K.; Kumar, P.; Roos, W.; Swart, H. Thermally induced structural metamorphosis of ZnO: Rb nanostructures for antibacterial impacts. Colloids Surf. B Biointerfaces 2020, 188, 110821. [Google Scholar] [CrossRef] [PubMed]
- Darkwah, W.K.; Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Inwati, G.K.; Gacem, A.; Saquib Abullais, S.; Prajapati, R.; Yadav, V.K.; Syed, R.; Alqahtani, M.S.; Yadav, K.K.; Islam, S.; et al. Nanostructured Antibiotics and Their Emerging Medicinal Applications: An Overview of Nanoantibiotics. Antibiotics 2022, 11, 708. [Google Scholar] [CrossRef]
- Kong, L.; Lu, X.; Bian, X.; Zhang, W.; Wang, C. Constructing Carbon-Coated Fe3O4 Microspheres as Antiacid and Magnetic Support for Palladium Nanoparticles for Catalytic Applications. ACS Appl. Mater. Interfaces 2011, 3, 35–42. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.-M. α-Fe 2 O 3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Hassanjani-Roshan, A.; Vaezi, M.R.; Shokuhfar, A.; Rajabali, Z. Synthesis of iron oxide nanoparticles via sonochemical method and their characterization. Particuology 2011, 9, 95–99. [Google Scholar] [CrossRef]
- Shafi, K.V.P.M.; Ulman, A.; Yan, X.; Yang, N.-L.; Estournès, C.; White, H.; Rafailovich, M. Sonochemical Synthesis of Functionalized Amorphous Iron Oxide Nanoparticles. Langmuir 2001, 17, 5093–5097. [Google Scholar] [CrossRef]
- Inwati, G.K.; Rao, Y.; Singh, M. Thermodynamically induced in Situ and Tunable Cu Plasmonic Behaviour. Sci. Rep. 2018, 8, 3006. [Google Scholar] [CrossRef] [PubMed]
- Hei, S.; Jin, Y.; Zhang, F. Fabrication of γ-Fe2O3Nanoparticles by Solid-State Thermolysis of a Metal-Organic Framework, MIL-100(Fe), for Heavy Metal Ions Removal. J. Chem. 2014, 2014, 546956. [Google Scholar] [CrossRef]
- Kim, E.H.; Lee, H.S.; Kwak, B.K.; Kim, B.-K. Synthesis of ferrofluid with magnetic nanoparticles by sonochemical method for MRI contrast agent. J. Magn. Magn. Mater. 2005, 289, 328–330. [Google Scholar] [CrossRef]
- Chaki, S.H.; Malek, T.J.; Chaudhary, M.D.; Tailor, J.P.; Deshpande, M.P. Magnetite Fe3O4 nanoparticles synthesis by wet chemical reduction and their characterization. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 035009. [Google Scholar] [CrossRef]
- Mohammadikish, M. Hydrothermal synthesis, characterization and optical properties of ellipsoid shape α-Fe2O3 nanocrystals. Ceram. Int. 2014, 40, 1351–1358. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Deotale, A.J.; Nandedkar, R.V.; Sinha, A.K.; Upadhyay, A.; Mondal, P.; Srivastava, A.K.; Deb, S.K. Effect of isochronal annealing on phase transformation studies of iron oxide nanoparticles. Bull. Mater. Sci. 2015, 38, 599–606. [Google Scholar] [CrossRef]
- Abdo, N.I. Comparative Study between Magnetite Nanoparticles and Magnetite/Silver as a Core/Shell Nanostructure. Adv. Nanoparticles 2021, 10, 115–122. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.-J.; Tseng, W.-L. Effective adsorption of chromium(vi)/Cr(iii) from aqueous solution using ionic liquid functionalized multiwalled carbon nanotubes as a super sorbent. J. Mater. Chem. A 2015, 3, 7044–7057. [Google Scholar] [CrossRef]
- Mohamed, H.S.; Soliman, N.; Abdelrheem, D.A.; Ramadan, A.A.; Elghandour, A.H.; Ahmed, S.A. Adsorption of Cd2+ and Cr3+ ions from aqueous solutions by using residue of Padina gymnospora waste as promising low-cost adsorbent. Heliyon 2019, 5, e01287. [Google Scholar] [CrossRef]
- Boujelben, N.; Bouzid, J.; Elouear, Z.; Feki, M. Retention of nickel from aqueous solutions using iron oxide and manganese oxide coated sand: Kinetic and thermodynamic studies. Environ. Technol. 2010, 31, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Ayad, M.I.; Eledkawy, M.A.; Darweesh, M.; Elmelegy, E.M. Removal of iron, zinc, and nickel-ions using nano bentonite and its applications on power station wastewater. Heliyon 2021, 7, e06315. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.A.; Khan, T.A.; Ali, I. Adsorptive removal of Pb(II) and Zn(II) from water onto manganese oxide-coated sand: Isotherm, thermodynamic and kinetic studies. Egypt. J. Basic Appl. Sci. 2016, 3, 287–300. [Google Scholar] [CrossRef]
- Wang, B.; Yu, J.; Liao, H.; Zhu, W.; Ding, P.; Zhou, J. Adsorption of Lead (II) from Aqueous Solution with High Efficiency by Hydrothermal Biochar Derived from Honey. Int. J. Environ. Res. Public Health 2020, 17, 3441. [Google Scholar] [CrossRef]
- Yang, X.; Xu, G.; Yu, H. Removal of lead from aqueous solutions by ferric activated sludge-based adsorbent derived from biological sludge. Arab. J. Chem. 2019, 12, 4142–4149. [Google Scholar] [CrossRef]
- Cavalu, S.; Antoniac, I.V.; Fritea, L.; Mates, I.M.; Milea, C.; Laslo, V.; Vicas, S.; Mohan, A. Surface modifications of the titanium mesh for cranioplasty using selenium nanoparticles coating. J. Adhes. Sci. Technol. 2018, 32, 2509–2522. [Google Scholar] [CrossRef]
- Cavalu, S.; Simon, V.; Goller, G.; Akin, I. Bioactivity and antimicrobial properties of pmma/Ag2O acrylic bone cement collagen coated. Dig. J. Nanomater. Biostructures 2011, 6, 779–790. [Google Scholar]
- Cavalu, S.; Simon, V. Proteins adsorption to orthopaedic biomaterials: Vibrational spectroscopy evidence. J. Optoelectron. Adv. Mater. 2007, 9, 3297–3302. [Google Scholar]
- Simon, V.; Cavalu, S.; Simon, S.; Mocuta, H.; Vanea, E.; Prinz, M.; Neumann, M. Surface functionalisation of sol–gel derived aluminosilicates in simulated body fluids. Solid State Ion. 2009, 180, 764–769. [Google Scholar] [CrossRef]
- Ormanci, O.; Akin, I.; Sahin, F.; Yucel, O.; Simon, V.; Cavalu, S.; Goller, G. Spark plasma sintered Al2O3–YSZ–TiO2 composites: Processing, characterization and in vivo evaluation. Mater. Sci. Eng. C 2014, 40, 16–23. [Google Scholar] [CrossRef]


| Elements | S-1 | S-2 | S-3 | S-4 | S-5 |
|---|---|---|---|---|---|
| Conc. | Conc. | Conc. | Conc. | Conc. | |
| Cr | 0.423 | 0.311 | 0.287 | 0.316 | 0.117 |
| Ni | 0.013 | 0.020 | 0.017 | 0.008 | 0.039 |
| Cd | 0.001 | 0.003 | 0.006 | 0.004 | 0.001 |
| Cu | 0.05 | 0.032 | 0.652 | 0.083 | 0.054 |
| Pb | 0.013 | 0.01 | 0.015 | 0.063 | 0.005 |
| Zn | 1.670 | 1.34 | 0.096 | 0.87 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajendran, S.; Wanale, S.G.; Gacem, A.; Yadav, V.K.; Ahmed, I.A.; Algethami, J.S.; Kakodiya, S.D.; Modi, T.; Alsuhaibani, A.M.; Yadav, K.K.; et al. Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents. Crystals 2023, 13, 472. https://doi.org/10.3390/cryst13030472
Rajendran S, Wanale SG, Gacem A, Yadav VK, Ahmed IA, Algethami JS, Kakodiya SD, Modi T, Alsuhaibani AM, Yadav KK, et al. Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents. Crystals. 2023; 13(3):472. https://doi.org/10.3390/cryst13030472
Chicago/Turabian StyleRajendran, Suriyaprabha, Shivraj Gangadhar Wanale, Amel Gacem, Virendra Kumar Yadav, Inas A. Ahmed, Jari S. Algethami, Shakti Devi Kakodiya, Timsi Modi, Amnah Mohammed Alsuhaibani, Krishna Kumar Yadav, and et al. 2023. "Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents" Crystals 13, no. 3: 472. https://doi.org/10.3390/cryst13030472
APA StyleRajendran, S., Wanale, S. G., Gacem, A., Yadav, V. K., Ahmed, I. A., Algethami, J. S., Kakodiya, S. D., Modi, T., Alsuhaibani, A. M., Yadav, K. K., & Cavalu, S. (2023). Nanostructured Iron Oxides: Structural, Optical, Magnetic, and Adsorption Characteristics for Cleaning Industrial Effluents. Crystals, 13(3), 472. https://doi.org/10.3390/cryst13030472










