Synthesis and Spectroscopic Characterizations of Some Essential Metal Ion (MgII, CaII, ZnII, and FeIII) Pipemidic Acid Chemotherapeutic Agent Complexes
Abstract
1. Introduction
2. Experimental
2.1. Chemicals
2.2. Pipemidic Acid Complexes’ Synthesis
2.3. Instrumentals
3. Results and Discussion
3.1. Microanalytical and Conductance Measurements
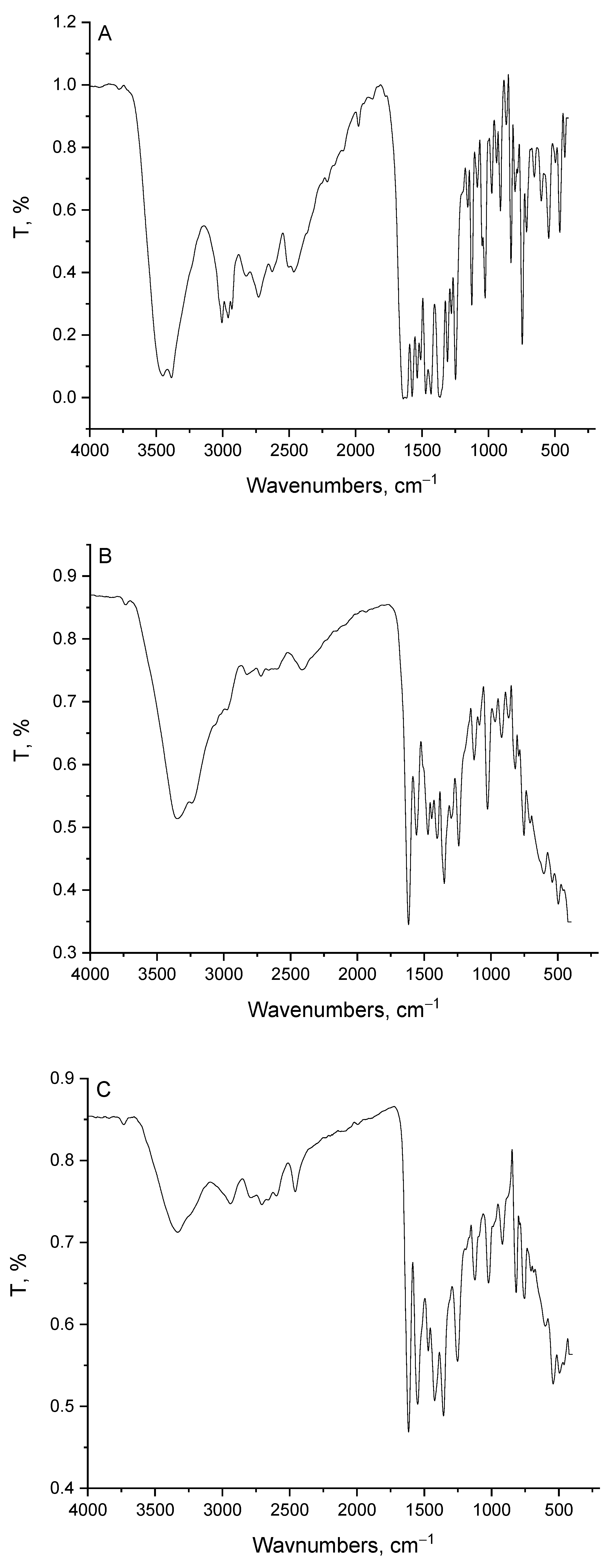
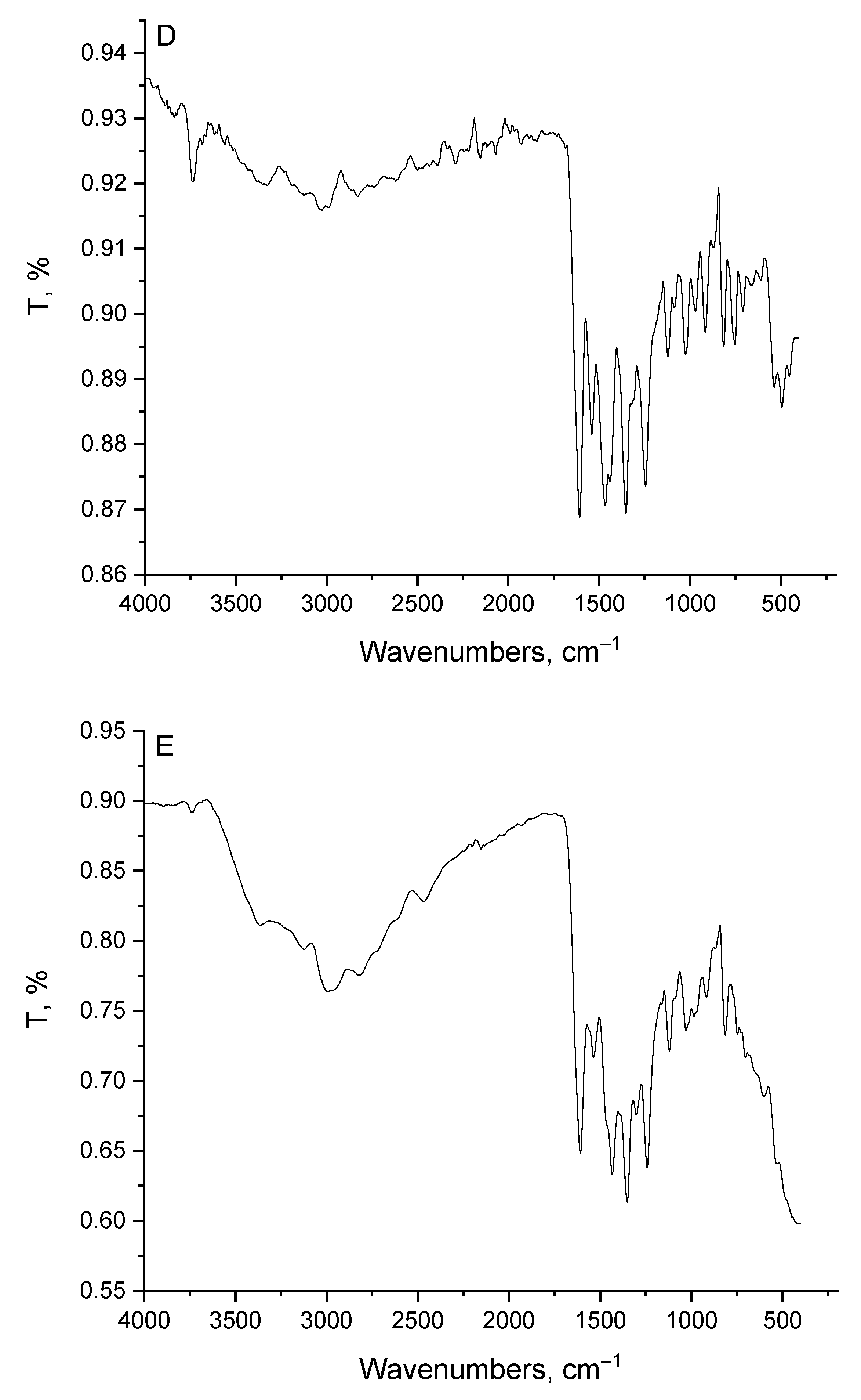
3.2. Infrared Assignments
3.3. Electronic Spectral Data of [Fe(pip)(H2O)2(Cl)2].6H2O Complex
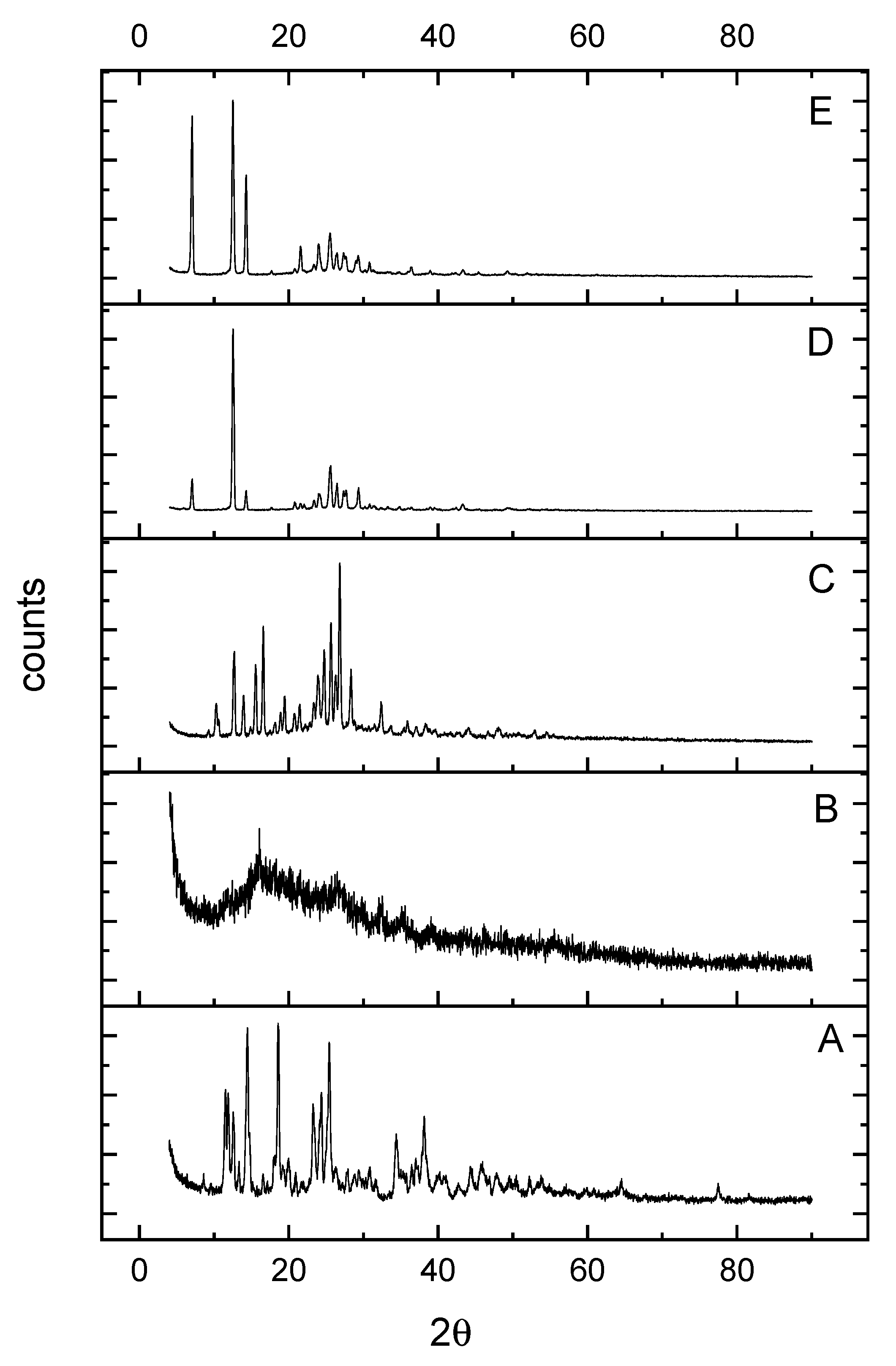
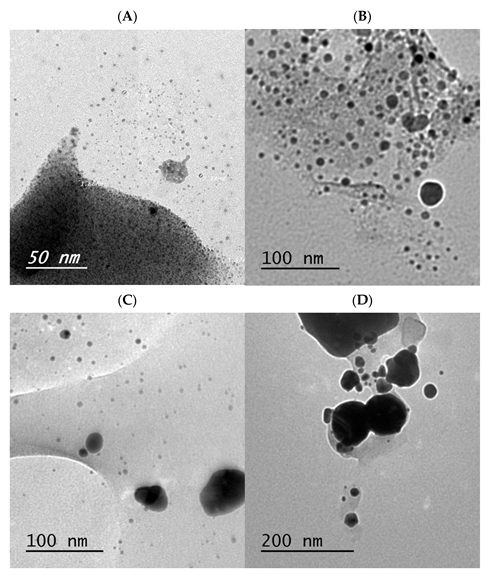
Powder X-ray Diffraction and TEM Morphology
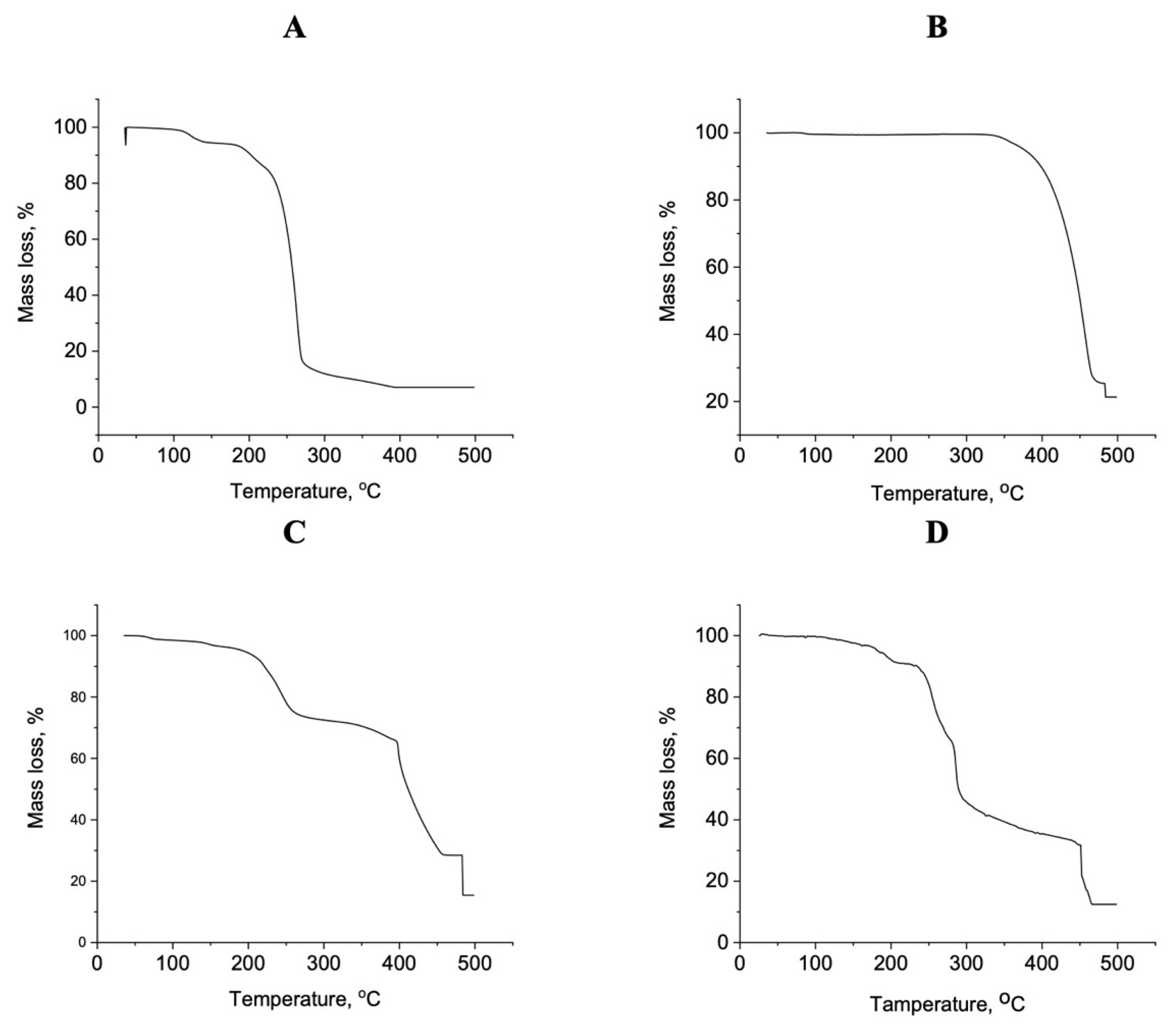
3.4. Thermal Studies
3.5. Kinetics Data
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EMA. Disabling and potentially permanent side effects lead to suspension or restrictions of quinolone and fluoroquinolone antibiotics. Eur. Med. Agency 2018, 31, 1–3. [Google Scholar]
- Zaki, A.; Schreiber, E.C.; Weliky, I.; Knill, J.R.; Hubsher, H.J. Clinical pharmacology of oral cephradine. J. Clin. Pharmacol. 1974, 14, 1180. [Google Scholar] [CrossRef] [PubMed]
- Anacona, J.R. Synthesis and antibacterial activity of some metal complexes of beta-lactamic antibiotics. J. Coord. Chem. 2001, 54, 355. [Google Scholar] [CrossRef]
- Lozano, J.; Borras, J. Antibiotic as ligand. Coordinating behavior of the cephalexin towards Zn (II) and Cd (II) ions. J. Inorg. BioChem. 1987, 31, 187. [Google Scholar] [CrossRef]
- Abdel-Gawad, F.M.; El-Guindi, N.M.; Ibrahim, M.N. Cephalexin complexes with some 3d transition-metal ions. J. Drug Res. 1987, 17, 197. [Google Scholar]
- Helaleh, M.I.H.; Nameh, E.S.M. Selective kinetic study for the degradation of cephalexin in alkaline aqueous media. An. Quim. Int. Ed. 1998, 94, 160. [Google Scholar]
- Sorenson, J.R.J. Copper chelates as possible active forms of the antiarthritic agents. J. Med. Chem. 1976, 19, 135. [Google Scholar] [CrossRef]
- Brown, D.H.; Lewis, A.E.; Smith, W.E.; Teape, J.W. Antiinflammatory effects of some copper complexes. J. Med. Chem. 1980, 23, 729. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.R. The Metals of Life; Van Nostrand Reinhold: London, UK, 1971. [Google Scholar]
- Ruiz, M.; Perello, L.; Ortiz, R.; Castineiras, A.; Maichlemossmer, C.; Canton, E. Synthesis, characterization, and crystal structure of [Cu (cinoxacinate) 2]· 2H2O complex: A square-planar CuO4 chromophore. Antibacterial studies. J. Inorg. Biochem. 1995, 59, 801. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Blum, S.E.; Barba-Behrens, N. Coordination chemistry of some biologically active ligands. Coord. Chem. Rev. 2000, 196, 3. [Google Scholar] [CrossRef]
- Naglah, A.M.; Al-Omar, M.A.; Almehizia, A.A.; AlKahtani, H.M.; Bhat, M.A.; Al-Shakliah, N.S.; Belgacem, K.; Majrashi, B.M.; Refat, M.; Adam, A.M.A. Synthesis, thermogravimetric, and spectroscopic characterizations of three palladium metal(II) ofloxacin drug and amino acids mixed ligand complexes as advanced antimicrobial materials. J. Mol. Struct. 2021, 1225, 129102. [Google Scholar] [CrossRef]
- Refat, M.S.; Saad, H.A.; Gobouri, A.A.; Alsawat, M.; Adam, A.M.A.; Shakya, S.; Gaber, A.; Alsuhaibani, A.M.; El-Megharbel, S.M. Synthesis and spectroscopic characterizations of nanostructured charge transfer complexes associated between moxifloxacin drug donor and metal chloride acceptors as a catalytic agent in a recycling of wastewater. J. Mol. Liq. 2021, 349, 118121. [Google Scholar] [CrossRef]
- Alibrahim, K.A.; Al-Saif, F.A.; Alghamdi, M.; El-Shahawi, M.; Althubeiti, K.; Aljuhani, E.; Refat, M. Spectroscopic, molecular structural, thermal, biological and voltammetric characterization of Ru3+, Pt4+and Ir3+complexes of lomefloxacin drug. Lat. Am. J. Pharm. 2019, 38, 1077–1090. [Google Scholar]
- Naglah, A.M.; Al-Omar, M.A.; Almehizia, A.A.; Obaidullah, A.J.; Bhat, M.A.; Al-Shakliah, N.S.; Belgacem, K.; Majrashi, B.M.; Refat, M.; Adam, A.M.A. Synthesis, spectroscopic, and antimicrobial study of binary and ternary ruthenium(III) complexes of ofloxacin drug and amino acids as secondary ligands. Crystals 2020, 10, 225. [Google Scholar] [CrossRef]
- Refat, M.; El-Sayed, M.Y.; Hassan, R.F. Study of the chemical structure and the microbial effect of the iron(III) metal ions with four consecutive generations of quinolones in a nanometric form for the purpose of raising the efficacy of anti-bacterial and fungal drugs. Appl. Organomet. Chem. 2018, 32, e4195. [Google Scholar] [CrossRef]
- Al-Saif, F.A.; Alibrahim, K.A.; Alfurhood, J.A.; Refat, M. Synthesis, spectroscopic, thermal, biological, morphological and molecular docking studies of the different quinolone drugs and their cobalt(II) complexes. J. Mol. Liq. 2018, 249, 438–453. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Hegab, M.S.; Manaaa, E.-S.A.; Al-Humaidi, J.Y.; Refat, M.S. Synthesis and physicochemical characterizations of coordination between palladium(II) metal ions with floroquinolone drugs as medicinal model against anticancer cells: Novel metallopharmaceuticals. New J. Chem. 2018, 42, 9709–9719. [Google Scholar] [CrossRef]
- Alibrahim, K.A.; Al-Saif, F.A.; Alghamdi, M.; El-Shahawi, M.S.; Moustafa, Y.M.; Refat, M.S. Synthesis, spectroscopic, thermal, antimicrobial and electrochemical characterization of some novel Ru(III), Pt(IV) and Ir(III) complexes of pipemidic acid. RSC Adv. 2018, 8, 22515–22529. [Google Scholar] [CrossRef]
- Alghamdi, M.T.; Alsibaai, A.A.; Shahawi, M.S.; Refat, M.S. Structural and chelation behaviors of new Ru(III), Pt(IV) and Ir(III) gatifloxacin drug complexes: Spectroscopic characterizations. J. Mol. Struct. 2017, 1130, 264–275. [Google Scholar] [CrossRef]
- Hussien, M.A.; El-Megharbel, S.M.; Refat, M.S. In-situ copper(II) complexes of some quinolone drug ligands were discussed for their molecular structures: Synthesis in binary solvent. J. Comput. Theor. Nanosci. 2017, 14, 561–576. [Google Scholar]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Li, B.-Q.; Xie, Y.-R.; Xiong, R.-G.; You, X.-Z.; Feng, X.-L. Synthesis, crystal structure, and characterization of mixed-ligand complex of copper (I) with drug of norfloxacin and triphenyl phosphine:[Cu (PPh3) 2 (H-Norf)] ClO4. Inorg. Chem. Commun. 2001, 4, 346. [Google Scholar] [CrossRef]
- Wang, L.-Z.; Chen, Z.-F.; Wang, X.-S.; Li, Y.-H.; Xiong, R.-G.; You, X.-Z. 2D nanoporous molecular square grid: Manganese (II) norfloxacin complex. Chin. J. Inorg. Chem. 2002, 18, 1185. [Google Scholar]
- Chen, Z.-F.; Liang, H.; Hu, H.-M.; Li, Y.; Xiong, R.-G.; You, X.-Z. A neutral 2D nanosized molecular square grid: The first vanadium(II)coordination polymer of norfloxacin. Inorg. Chem. Commun. 2003, 6, 241. [Google Scholar] [CrossRef]
- Li, Y.-X.; Chen, Z.-F.; Xiong, R.-G.; Xue, Z.; Ju, H.-X.; You, X.-Z. A mononuclear complex of norfloxacin with silver (I) and its properties. Inorg. Chem. Commun. 2003, 6, 819. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiely: New York, NY, USA, 1978. [Google Scholar]
- Ross, S.D. Inorganic Infrared and Raman Spectra; McGraw Hill: London, UK, 1972. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morril, T.C. Spectroscopic Identification of Organic Compounds, 5th ed.; Wiley: New York, NY, USA, 1991. [Google Scholar]
- Anuradha, S.; Pramila, S. Synthesis, characterization, in-vitro anti-inflammatory and antimicrobial screening of metal (II) mixed diclofenac and acetaminophen complexes. Indian J. Chem. 2000, 39, 874–876. [Google Scholar]
- Cullity, B.D. Elements of X-ray Diffraction; Addison-Wesley, Reading: Boston, MA, USA, 1972; p. 102. [Google Scholar]
- Salavati-Niasari, M.; Mohandes, F.; Davar, F.; Mazaheri, M.; Monemzadeh, M.; Yavarinia, N. Preparation of NiO nanoparticles from metal-organic frameworks via a solid-state decomposition route. Inorg. Chim. Acta 2009, 362, 3691. [Google Scholar] [CrossRef]
- Velumani, S.; Mathew, X.; Sebastian, P.J. Structural and optical characterization of hot wall deposited CdSexTe1− x films. Solar Energy Mater. Solar Cells 2003, 76, 359. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic parameters from thermogravimetric data. Nature 1964, 201, 68. [Google Scholar] [CrossRef]
- Horowitz, H.H.; Metzger, G. A new analysis of thermogravimetric traces. Anal. Chem. 1963, 35, 1464. [Google Scholar] [CrossRef]
- Wendlandt, W.W. Thermal Methods of Analysis; Interscience: New York, NY, USA, 1964. [Google Scholar]
- Avsar, G.; Kulcu, N.; Arslan, H. Thermal behaviour of copper (II), nickel (II), cobalt (II) and palladium (II) complexes of N, N-dimethyl-N‣-benzoylthiourea. Tur. J. Chem. 2002, 26, 607. [Google Scholar]
- Sodhi, G.S. Correlation of thermal stability with structures for some metal complexes. Therm. Chim. Acta 1987, 120, 107. [Google Scholar] [CrossRef]
- Arslan, H.; Ozpozan, N.; Tarkan, N. Kinetic analysis of thermogravimetric data of p-toluidino-p-chlorophenylglyoxime and of some complexes. Therm. Chim. Acta 2002, 383, 69. [Google Scholar] [CrossRef]

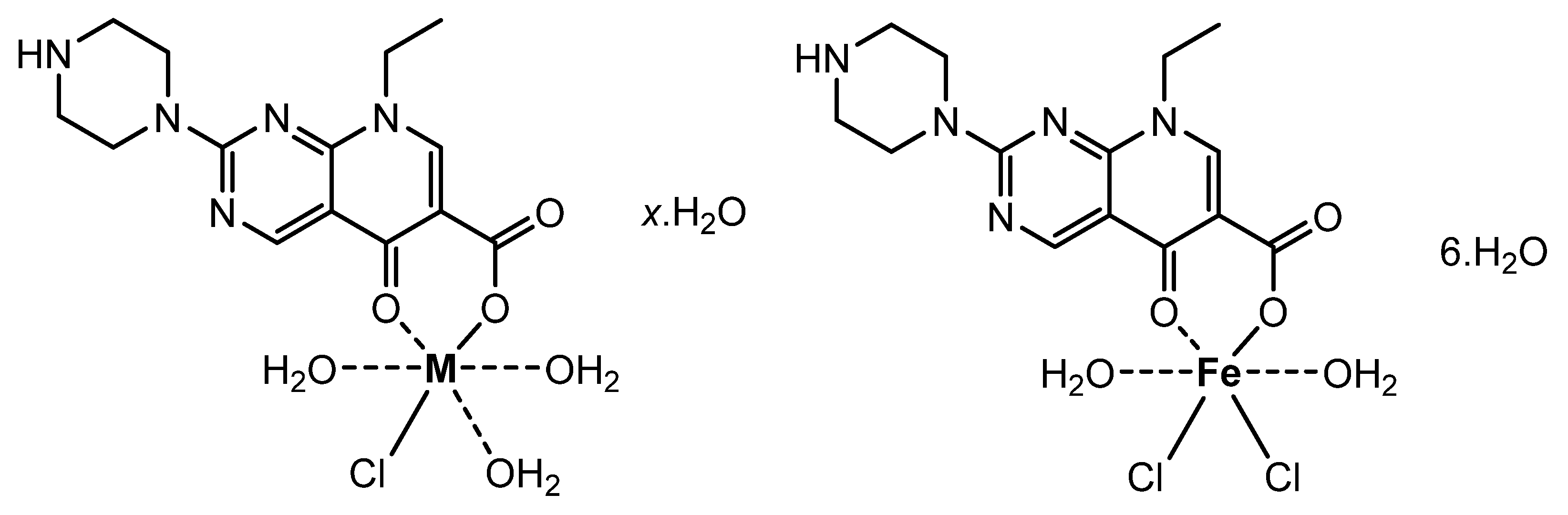
| Complexes/ F·Wt | Color | Yield | M.Wt/g/mol | Mp/°C | ΛM/ Ω−1·cm2·mol−1 | Elemental Analysis Found (Calcd.) | |||
|---|---|---|---|---|---|---|---|---|---|
| %C | %H | %N | %M | ||||||
| Complex 1 MgC14H34N5O12Cl | pale yellow | 77% | 524 | >300 | 18 | 31.98 (32.08) | 6.42 (6.54) | 13.34 (13.36) | 4.61 (4.64) |
| Complex 2 CaC14H26N5O8Cl | yellow | 74 | 468 | >300 | 21 | 35.77 (35.94) | 5.51 (5.60) | 14.92 (14.97) | 8.46 (8.57) |
| Complex 3 ZnC14H30N5O10Cl | yellow | 77 | 529 | >300 | 24 | 31.31 (31.77) | 5.59 (5.71) | 12.95 (13.23) | 12.18 (12.35) |
| Complex 4 FeC14H32N5O11Cl2 | brown | 72 | 573 | >300 | 28 | 29.30 (29.34) | 5.60 (5.63) | 12.10 (12.22) | 9.71 (9.74) |
| pipH | Mg(II) | Ca(II) | Zn(II) | Fe(III) | Assignments * |
|---|---|---|---|---|---|
| 3454, 3383 | 3348 | 3333 | 3327 | 3363 | ν(N-H) + νas(O-H); H2O |
| 3005, 2955 2929, 2818 2728, 2621 | 3237, 2969 2823 | 2939, 2788 | 3131, 3020 2823 | 3125, 2984 2818 | νs(O-H) + ν(C-H) |
| 1774 | -- | -- | -- | -- | ν(C=O): (COOH) |
| -- | 1618 | 1618 | 1607 | 1607 | νas(COO-) |
| 1628, 1573 | 1557 | 1548 | 1543 | 1537 | ν(C=O) + δb(H2O) Phenyl breathing modes |
| 1537, 1507 1471, 1432 | 1471, 1441 | 1466, 1421 | 1467 | 1432 | CH; deformation of –CH2– |
| -- | 1401 | 1355 | 1351 | 1355 | νs(COO-) |
| 1366 | 1346 | 1310 | 1330 | 1300 | δb(CH2) |
| 1280, 1250 | 1295, 1244 | 1250 | 1244 | 1239 | ν(C-C) |
| 1159 | 1128 | 1124 | 1124 | 1119 | ν(C-O) + ν(C-N) |
| 1128, 1083 1023, 972 | 1023 | 1017 | 1023 | 1028 | δr(CH2) |
| 942, 906 867, 826 801 | 922, 861 | 917, 816 | 912, 817 | 912, 816 | CH- bend; phenyl |
| 745 | 784 | 756 | 751, 705 | 751 | δb(COO-) |
| 654, 604 543, 423 | 604, 543 493 | 599, 543 493, 458 | 660, 610 539, 493, 453 | 599, 534 | ν(M-O) + ring deformation |
| Complexes | D (nm) | δ (1012·lin·m−2) | 2Theta | Intensity | d-Value (nm) |
|---|---|---|---|---|---|
| Complex 1 | 2 | 0.2500 | 16 | 100 | 0.55 |
| Complex 2 | 18 | 0.0031 | 26 | 100 | 0.34 |
| Complex 3 | 17 | 0.0035 | 12 | 100 | 0.74 |
| Complex 4 | 37 | 0.0007 | 12 | 100 | 0.74 |
| Samples | Stage | TGA Range (°C) | DTG Peak (°C) | Weight Loss (%) | Evolved Moiety | |
|---|---|---|---|---|---|---|
| Found | Calc | |||||
| Mg(II) complex | I | 30–180 | 150 | 20.55 | 20.61 | 6H2O |
| II | 180–270 | 250 | 17.10 | 17.08 | 3H2O + ½Cl2 | |
| III | 270–500 | 330 | 55.31 | 54.58 | C14H16N5O2 | |
| Residue | 7.04 | 7.73 | MgO | |||
| Ca(II) complex | I | 30–300 | 260 | 26.91 | 26.82 | 5H2O + ½Cl2 |
| II | 300–500 | 430 | 51.76 | 51.71 | C13H16N5 | |
| Residue | 21.33 | 21.47 | CaCO3 | |||
| Zn(II) complex | I | 30–250 | 200 | 13.65 | 13.61 | 4H2O |
| II | 250–400 | 320 | 17.10 | 16.92 | 3H2O + ½Cl2 | |
| III | 400–500 | 420 | 53.80 | 54.06 | C14H16N5O2 | |
| Residue | 15.45 | 15.41 | ZnO | |||
| Fe(III) complex | I | 30–230 | 150 | 18.90 | 18.85 | 6H2O |
| II | 230–300 | 270 | 18.82 | 18.67 | 2H2O + Cl2 | |
| III | 300–500 | 330, 450 | 49.83 | 49.91 | C14H16N5O2 | |
| Residue | 12.45 | 12.57 | FeO | |||
| Complexes | Ionic Radius/pm | Parameters * | Coats-Redfern eq. | Horowitz-Metzger eq. |
|---|---|---|---|---|
| Mg(II) complex | 0.72Å | E | 2.44E+05 | 2.54E+05 |
| A | 2.27E+22 | 5.22E+23 | ||
| ΔS | 1.78E+02 | 2.04E+02 | ||
| ΔH | 2.40E+05 | 2.50E+05 | ||
| ΔG | 1.46E+05 | 1.42E+05 | ||
| Ca(II) complex | 0.99Å | E | 1.54E+05 | 1.74E+05 |
| A | 1.54E+09 | 6.71E+10 | ||
| ΔS | −7.62E+01 | −4.49E+01 | ||
| ΔH | 1.48E+05 | 1.68E+05 | ||
| ΔG | 2.02E+05 | 2.00E+05 | ||
| Zn(II) complex | 0.74 Å | E | 1.93E+05 | 2.03E+05 |
| A | 2.92E+12 | 2.58E+13 | ||
| ΔS | −1.33E+01 | 4.83E+00 | ||
| ΔH | 1.87E+05 | 1.97E+05 | ||
| ΔG | 1.96E+05 | 1.94E+05 | ||
| Fe(III) complex | 0.645 Å | E | 3.10E+05 | 3.83E+05 |
| A | 5.87E+05 | 6.98E+00 | ||
| ΔS | −1.40E+02 | −2.35E+02 | ||
| ΔH | 2.61E+04 | 3.33E+04 | ||
| ΔG | 1.10E+05 | 1.74E+05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Wasidi, A.S. Synthesis and Spectroscopic Characterizations of Some Essential Metal Ion (MgII, CaII, ZnII, and FeIII) Pipemidic Acid Chemotherapeutic Agent Complexes. Crystals 2023, 13, 596. https://doi.org/10.3390/cryst13040596
Al-Wasidi AS. Synthesis and Spectroscopic Characterizations of Some Essential Metal Ion (MgII, CaII, ZnII, and FeIII) Pipemidic Acid Chemotherapeutic Agent Complexes. Crystals. 2023; 13(4):596. https://doi.org/10.3390/cryst13040596
Chicago/Turabian StyleAl-Wasidi, Asma S. 2023. "Synthesis and Spectroscopic Characterizations of Some Essential Metal Ion (MgII, CaII, ZnII, and FeIII) Pipemidic Acid Chemotherapeutic Agent Complexes" Crystals 13, no. 4: 596. https://doi.org/10.3390/cryst13040596
APA StyleAl-Wasidi, A. S. (2023). Synthesis and Spectroscopic Characterizations of Some Essential Metal Ion (MgII, CaII, ZnII, and FeIII) Pipemidic Acid Chemotherapeutic Agent Complexes. Crystals, 13(4), 596. https://doi.org/10.3390/cryst13040596





