Enhanced Photodegradation of Rhodamine B Using Visible-Light Sensitive N-TiO2/rGO Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
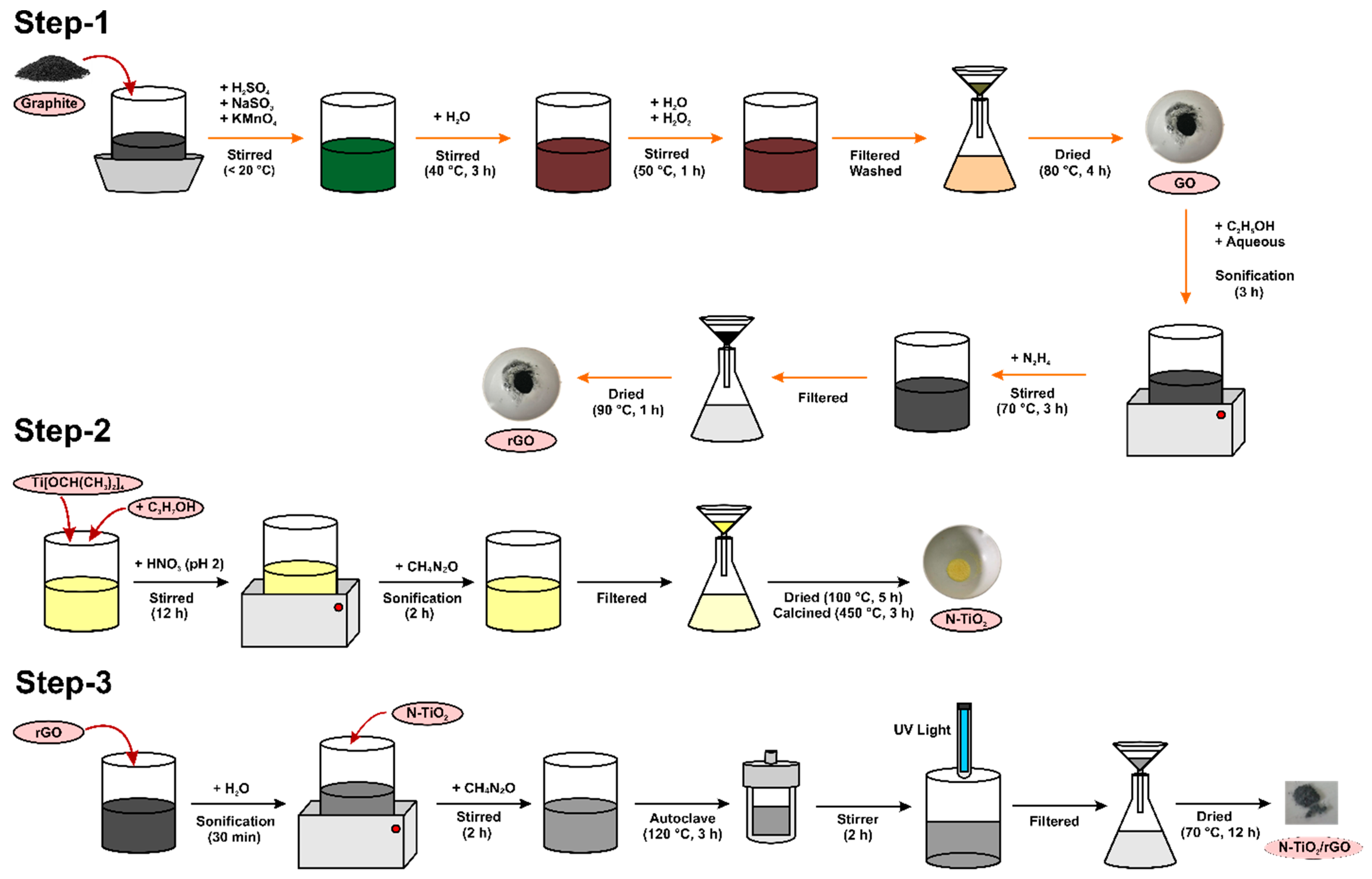
2.2. Material Synthesis
2.2.1. Synthesis of rGO
2.2.2. Synthesis of N-TiO2
2.2.3. Synthesis of N-TiO2-rGO
2.3. Material Characterization
2.4. Study of Photocatalytic Activity
3. Results and Discussion
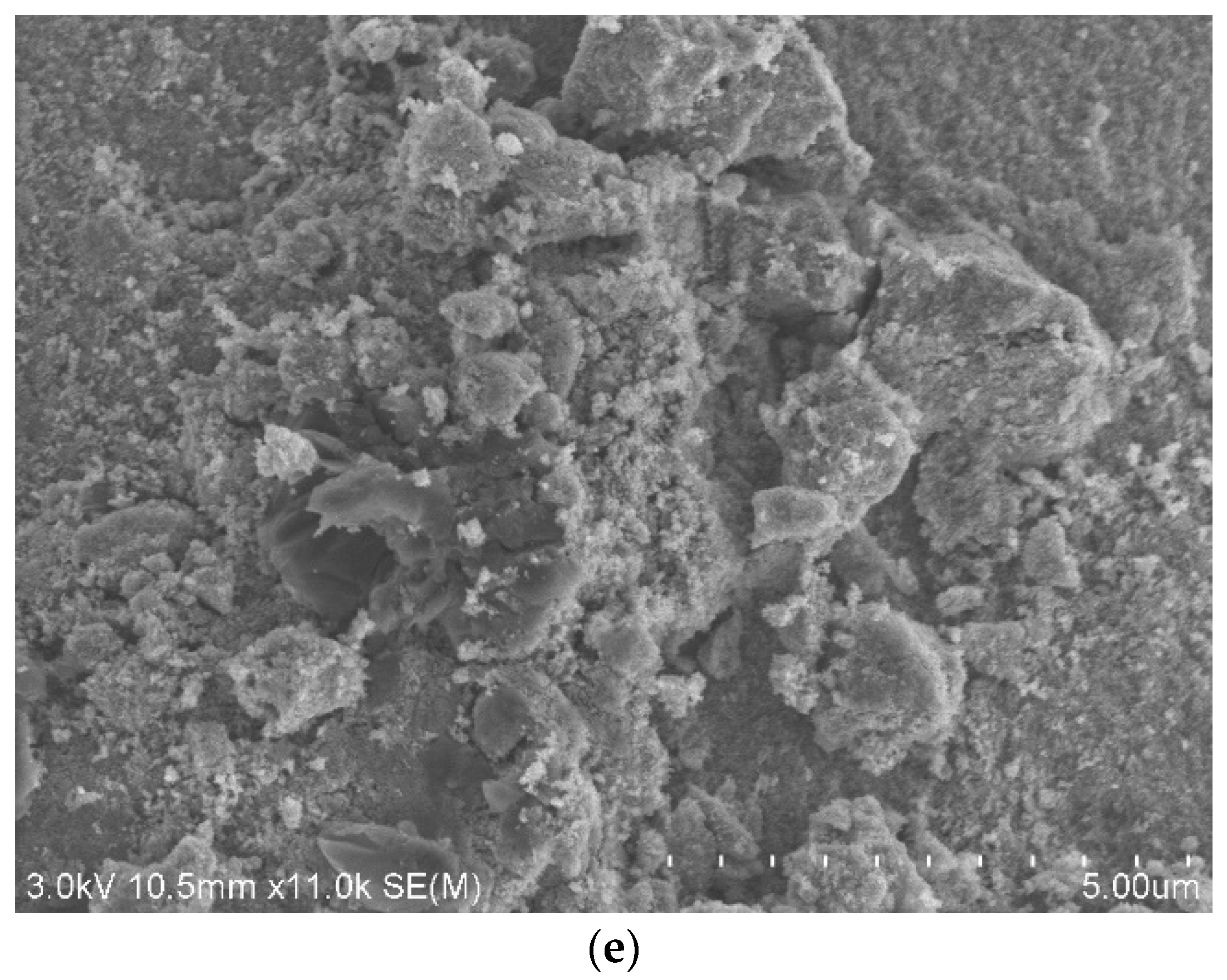
3.1. SEM-EDX Characterization
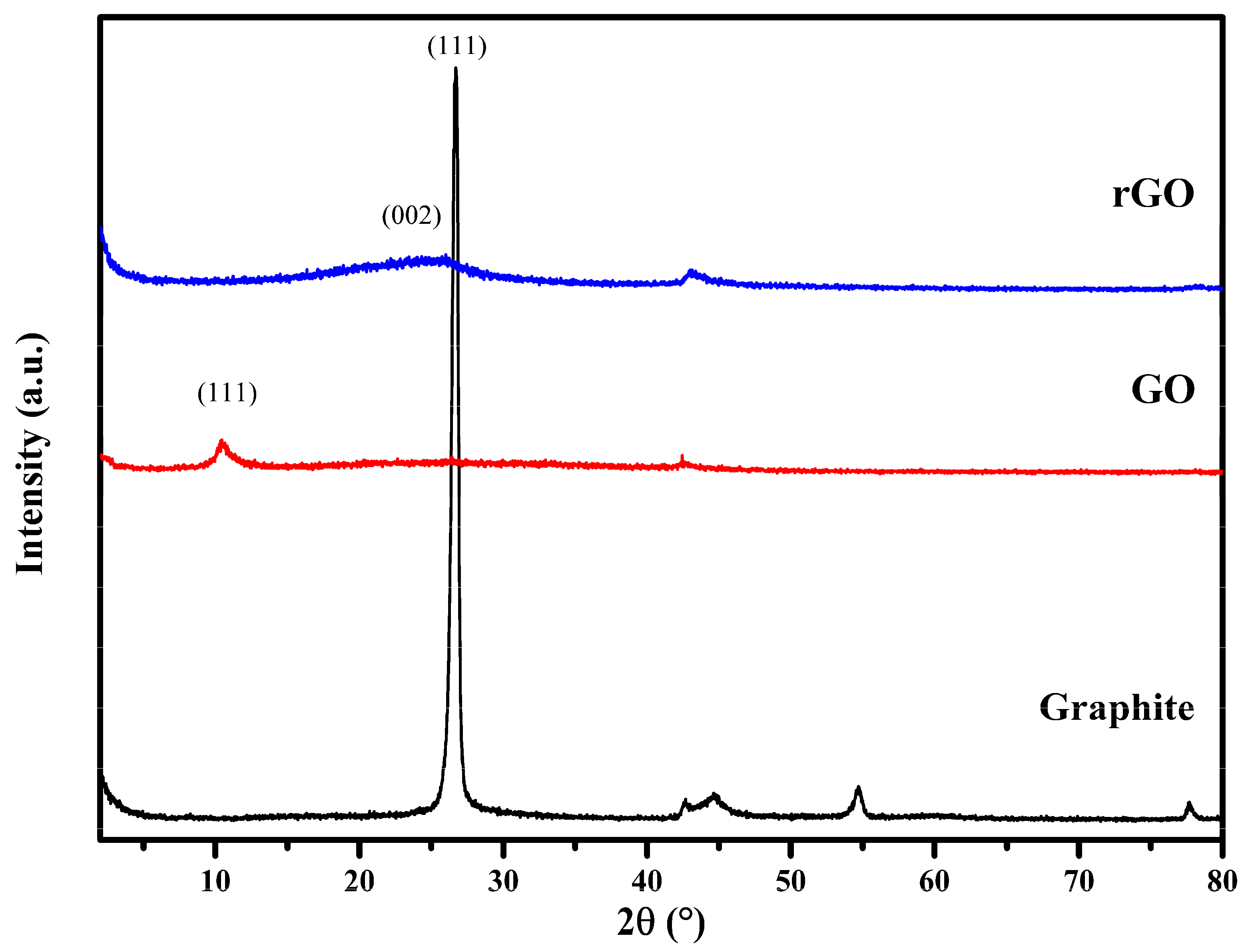
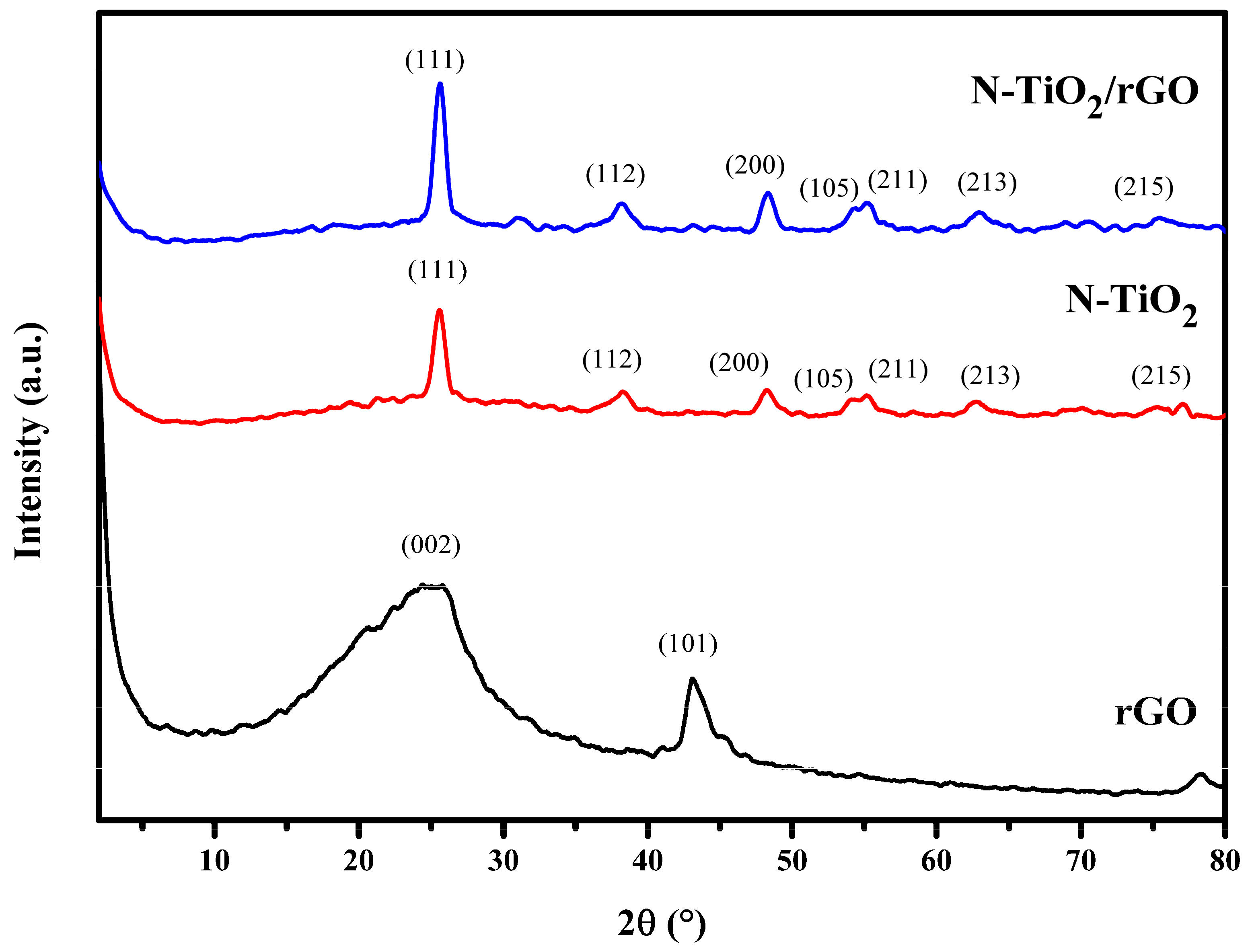
3.2. XRD Characterization
3.3. GSA Characterization
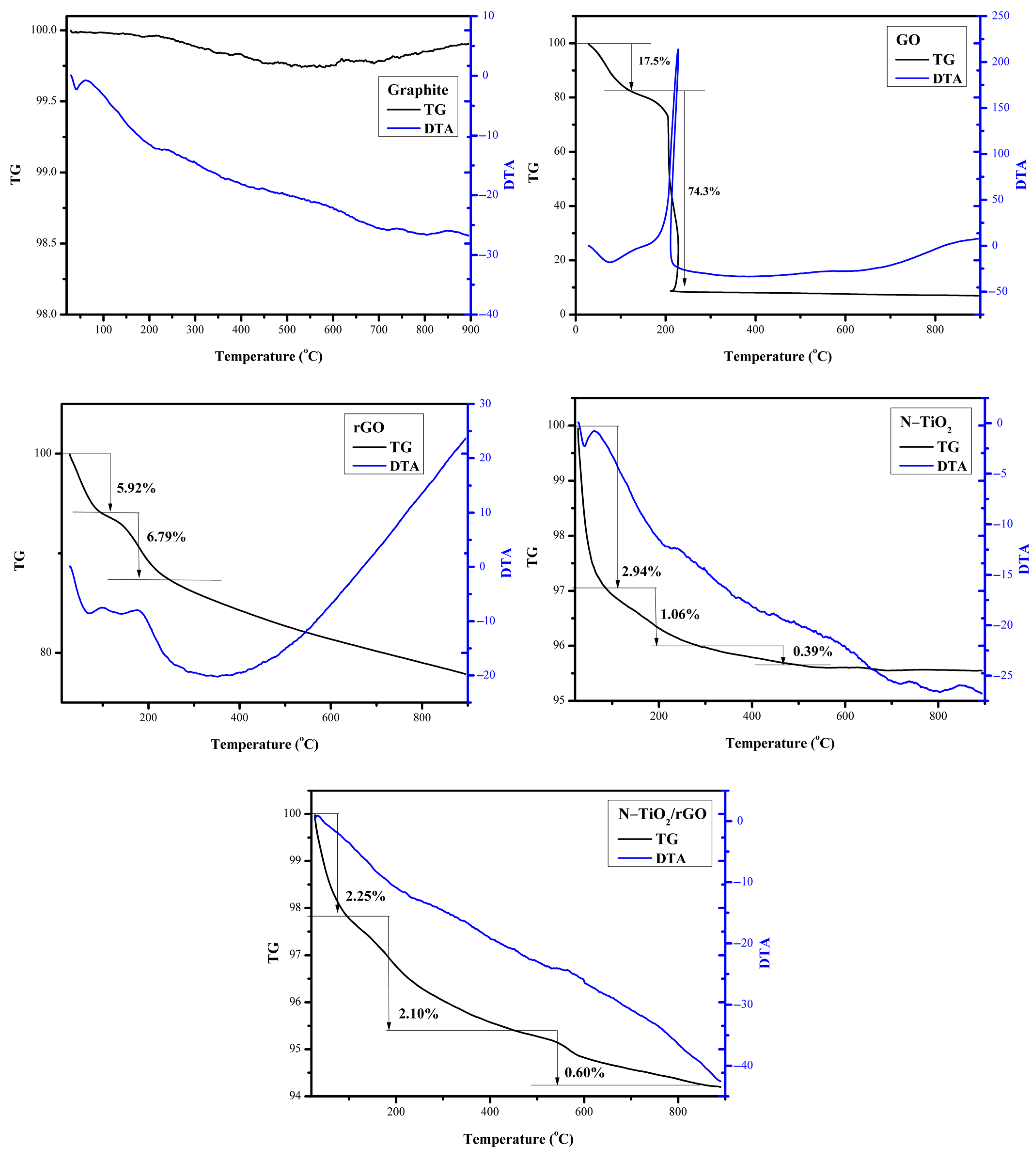
3.4. TG-DTA Characterization
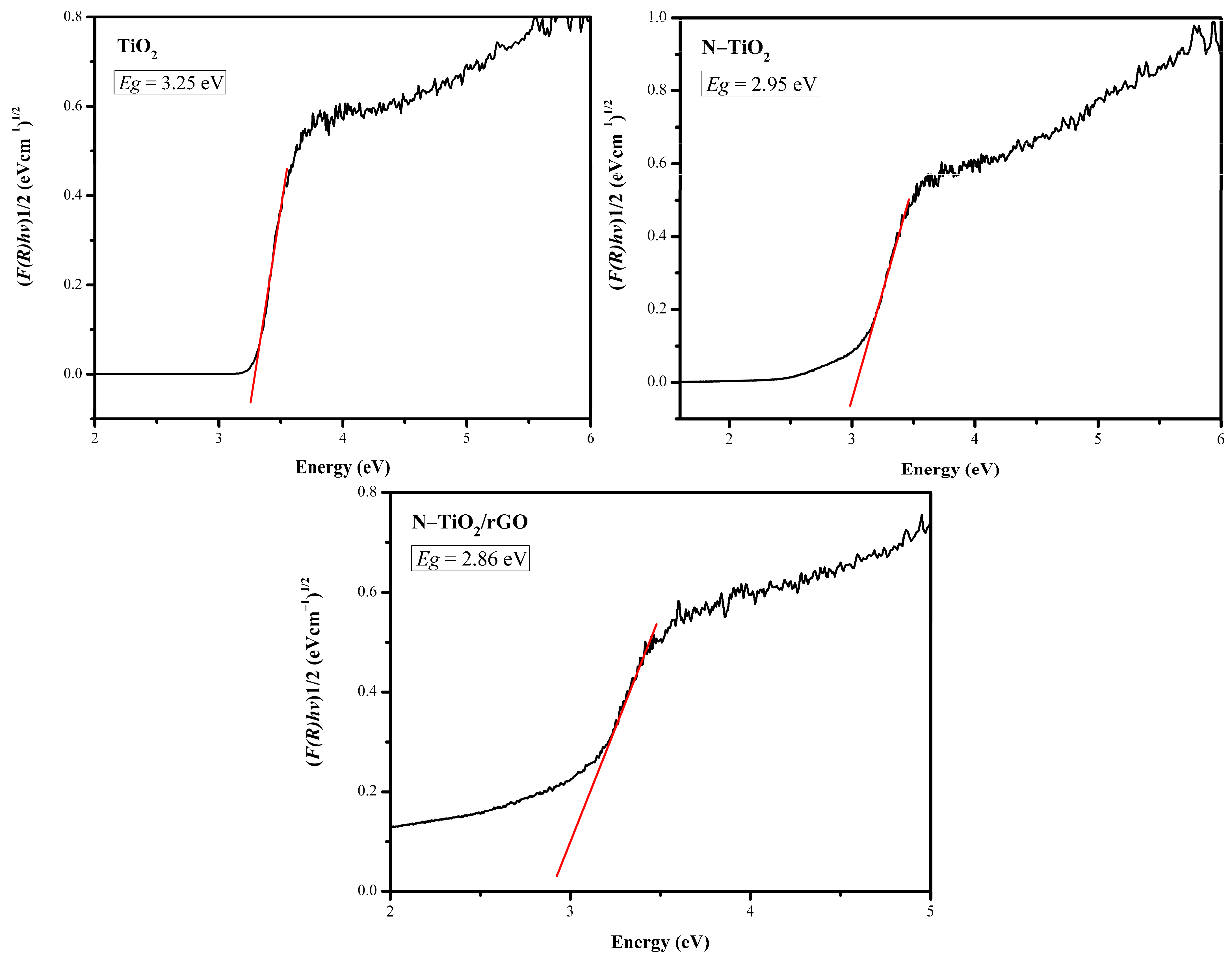
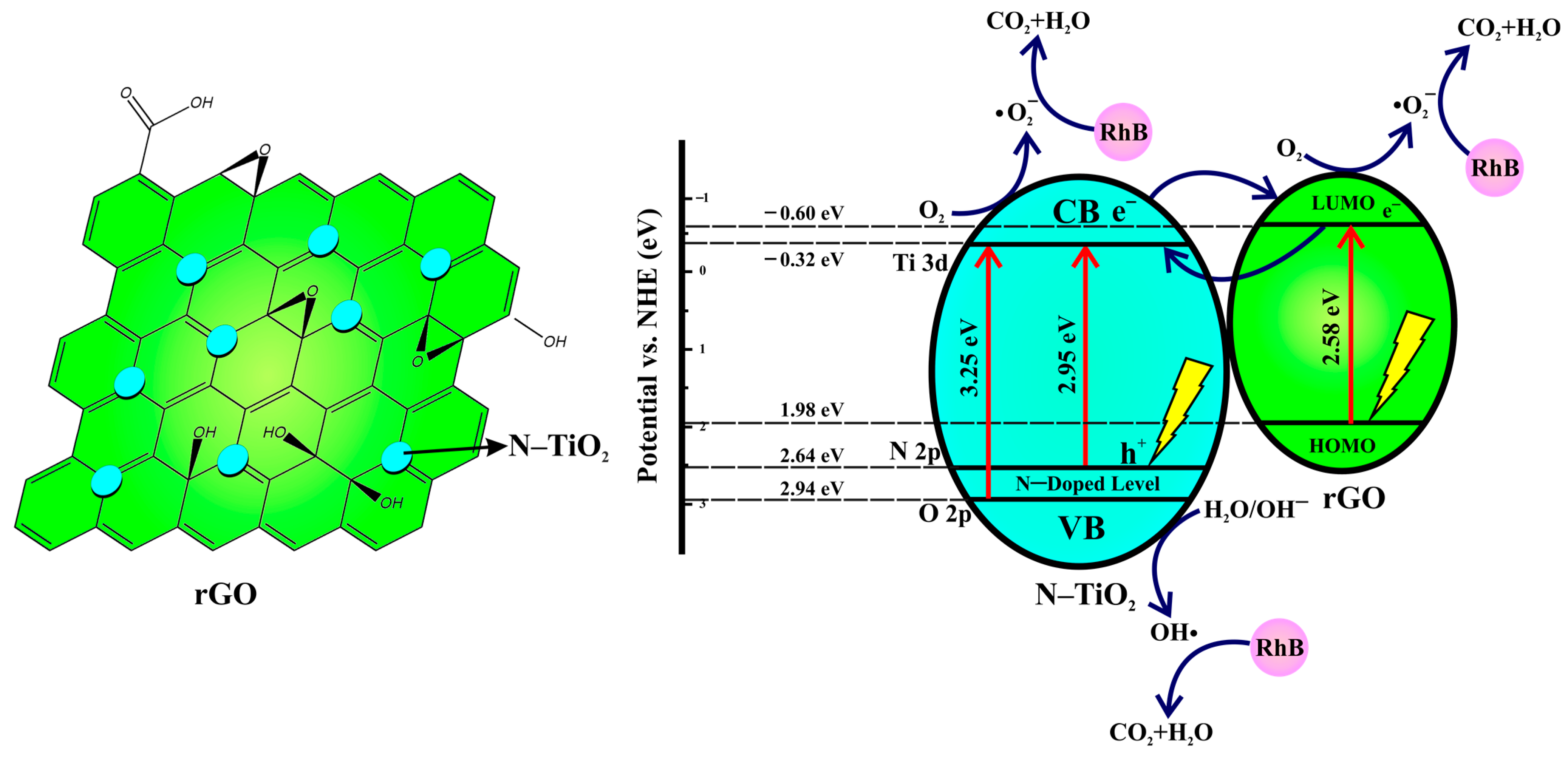
3.5. UV-Vis DRS Characterization
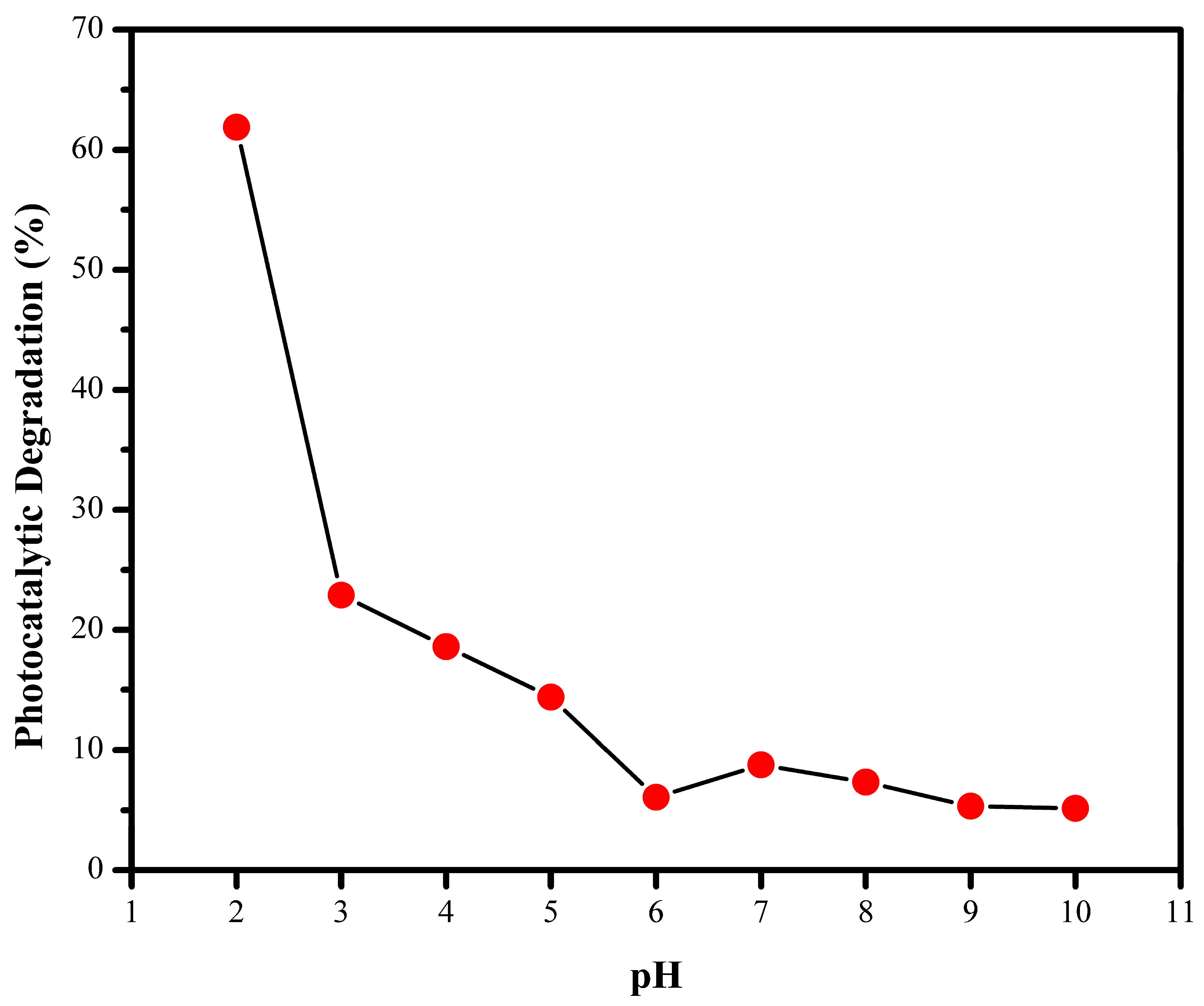
3.6. Effect of pH
3.7. Effect of Photocatalyst Mass
3.8. Effect of Irradiation Time
3.9. Effect of Light Irradiation
3.10. Kinetic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, P.S.; Saravanan, A. Sustainable wastewater treatments in textile sector. In Sustainable Fibres and Textiles; Muthu, S.S., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 323–346. [Google Scholar] [CrossRef]
- Adane, T.; Adugna, A.T.; Alemayehu, E. Textile industry effluent treatment techniques. J. Chem. 2021, 2021, 5314404. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Alsantali, R.I.; Raja, Q.A.; Alzahrani, A.Y.A.; Sadiq, A.; Naeem, N.; Mughal, E.U.; Al-Rooqi, M.M.; Guesmi, N.E.; Moussa, Z.; Ahmed, S.A. Miscellaneous azo dyes: A comprehensive review on recent advancements in biological and industrial applications. Dyes Pigments 2022, 199, 110050. [Google Scholar] [CrossRef]
- Saigl, Z.M. Various absorbents for removal Rhodamine B dye: A review. Indones. J. Chem. 2021, 21, 1039–1356. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-Shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.A.; Najam, T.; Jabeen, S.; Wattoo, M.A.; Bashir, M.S.; Shah, S.S.A.; Rehman, A. Facile synthesis of Tri-metallic layered double hydroxides (NiZnAl-LDHs): Adsorption of Rhodamine-B and methyl orange from water. Inorg. Chem. Commun. 2022, 145, 110008. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Natl. Libr. Med. 2021, 11, 1804. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Rangabhashiyam, S.; Singh, A.; Nguyen, V.H.; Le, Q.V.; Khan, A.A.P.; Hu, C.; Huang, C.W.; Ahamad, T.; et al. Copper sulfides based photocatalysts for degradation of environmental pollution hazards: A review on the recent catalyst design concepts and future perspectives. Surf. Interfaces 2022, 33, 102182. [Google Scholar] [CrossRef]
- Chen, X.; Selloni, A. Introduction: Titanium dioxide (TiO2) nanomaterials. Chem. Rev. 2014, 114, 9281–9282. [Google Scholar] [CrossRef]
- Li, R.; Li, T.; Zhou, Q. Impact of titanium dioxide (TiO2) modification on its application to pollution treatment: A review. Catalysts 2020, 10, 804. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of rGO and preparation method on the physicochemical and photocatalytic properties of TiO2/reduced graphene oxide photocatalysts. Catalysts 2021, 11, 1333. [Google Scholar] [CrossRef]
- Bell, N.J.; Ng, Y.H.; Du, A.; Coster, H.; Smith, S.; Amal, R. Understanding the enhancement in photoelectrochemical properties of photocatalytically prepared TiO2-reduced graphene oxide composite. J. Phys. Chem. C 2011, 115, 6004–6009. [Google Scholar] [CrossRef]
- Mishra, A.K.; Ramaprabhu, S. Functionalized graphene-based nanocomposites for supercapacitor application. J. Phys. Chem. C. 2011, 115, 14006–14013. [Google Scholar] [CrossRef]
- Anjusree, G.S.; Nair, A.S.; Nair, S.V.; Vadukumpully, S. One-pot hydrothermal synthesis of TiO2/graphene nanocomposites for enhanced visible light photocatalysis and photovoltaics. RSC Adv. 2013, 3, 12933–12938. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Monteagudo, J.; Durán, A.; Martínez, J.M.M.; Martín, I.S. Effect of reduced graphene oxide load into TiO2 P25 on the generationof reactive oxygen species in a solar photocatalytic reactor. Application to antipyrine degradation. Chem. Eng. J. 2019, 380, 122410. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M.; Polagani, R.K.; Vo, D.V.N. Removal of tetracycline from wastewater using g-C3N4 based photocatalysts: A review. Environ. Res. 2023, 216, 114660. [Google Scholar] [CrossRef]
- Wu, D.; Guo, J.; Wang, H.; Zhang, X.; Yang, Y.; Yang, C.; Gao, Z.; Wang, Z.; Jiang, K. Green synthesis of boron and nitrogen co-doped TiO2 with rich B-N motifs as lewis acid-base couples for the effective artificial CO2 photoreduction under simulated sunlight. J. Colloid Interface Sci. 2021, 585, 95–107. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Mushtaq, S.; Al Qahtani, H.S.; Sedky, A.; Alam, M.W. Investigation of TiO2 nanoparticles synthesized by sol-gel method for effectual photodegradation, Oxidation and Reduction Reaction. Crystals 2021, 11, 1456. [Google Scholar] [CrossRef]
- Divyasri, Y.V.; Reddy, N.L.; Lee, K.; Sakar, M.; Rao, V.; Rao, V.N.; Venkatramu, V.; Shankar, M.V.; Reddy, N.C.G. Optimization of N doping in TiO2 nanotubes for the enhanced solar light mediated photocatalytic H2 production and dye degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef]
- Andrade, Ó.R.; Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. Photocatalytic reduction of CO2 with N-doped TiO2-based photocatalysts obtained in one-pot supercritical synthesis. Nanomaterials 2022, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Afza, N.; Shivakumar, M.S.; Alam, M.W.; Kumar, A.N.; Bhatt, A.S.; Murthy, H.A.; Ravikumar, C.R.; Mylarappa, M.; Selvanandan, S. Facile hydrothermal synthesis of cerium oxide/rGO nanocomposite for photocatalytic and supercapacitor applications. Appl. Surf. Sci. Adv. 2022, 11, 100307. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, S.; Aqeel, M.; Ul-Hamid, A.; Imran, M.; Haider, J.; Haider, A.; Shahbaz, A.; Ali, S. Reduced graphene oxide nanosheets doped by Cu with highly efficient visible light photocatalytic behavior. J. Alloys Compd. 2020, 837, 155588. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, M.; Shi, J.; Shangguan, W. Preparations and photocatalytic hydrogen evolution of N-doped TiO2 from urea and titanium tetrachloride. Int. J. Hydrogen Energy 2006, 31, 1326–1331. [Google Scholar] [CrossRef]
- Jakimińska, A.; Pawlicki, M.; Macyk, W. Photocatalytic transformation of Rhodamine B to Rhodamine-110: The mechanism revisited. J. Photochem. Photobiol. A Chem. 2022, 433, 114176. [Google Scholar] [CrossRef]
- Sharon, M.; Sharon, M. Graphene: An Introduction to the Fundamentals and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 55, p. 5122. [Google Scholar] [CrossRef]
- Oliveira, A.E.F.; Braga, G.B.; Tarley, C.R.T.; Pereira, A.C. Thermally reduced graphene oxide: Synthesis, studies and characterization. J. Mater. Sci. 2018, 53, 12005–12015. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Synthesis and structural-mechanical property characteristics of graphene-polymer nanocomposites. In Nanocrystalline Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 335–375. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, S.; Fu, Z. Preparation of multicycle GO/TiO2 composite photocatalyst and study on degradation of methylene blue synthetic wastewater. Appl. Sci. 2019, 9, 3282. [Google Scholar] [CrossRef]
- Kumar, A.; Sadanandhan, A.M.; Jain, S.L. Silver doped reduced graphene oxide as a promising plasmonic photocatalyst for oxidative coupling of benzylamines under visible light irradiation. New J. Chem. 2019, 43, 9116–9122. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, D.S.; Ahn, B.T. Synthesis of mesoporous TiO2 and its application to photocatalytic activation of methylene blue and E. coli. Bull. Korean Chem. Soc. 2009, 30, 193–196. [Google Scholar]
- Yang, F.; Qu, J.; Zheng, Y.; Cai, Y.; Yang, X.; Li, C.M.; Hu, J. Recent advances of high-crystalline conjugated organic polymeric materials for photocatalytic CO2 conversion. Nanoscale 2022, 14, 15217–15241. [Google Scholar] [CrossRef] [PubMed]
- Rajkumari; Nandini, P.; Sangita, D.; Pallabi, G. Plant-Based natural dye-stimulated visible-light reduction of GO and physi-cochemical factors influencing the production of oxidizing species by a synthesized (RGO)/TiO2 nanocomposite for envi-ronmental remediation. ACS Omega 2021, 6, 2686. [Google Scholar] [CrossRef] [PubMed]
- Tobon, C.S.; Panžić, I.; Bafti, A.; Matijašić, G.; Ljubas, D.; Ćurković, L. Rapid microwave-assisted synthesis of n/tio2/rgo nanoparticles for the photocatalytic degradation of pharmaceuticals. Nanomaterial 2022, 12, 3975. [Google Scholar] [CrossRef] [PubMed]
- Kocijan, M.; Ćurković, L.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; Gonçalves, G.; Radošević, T.; Vengust, D.; Podlogar, M. Immobilised rGO/TiO2 nanocomposite for multi-cycle removal of methylene blue dye from an aqueous medium. Appl. Sci. 2022, 12, 385. [Google Scholar] [CrossRef]
- Tong, Z.W.; Yang, D.; Sun, Y.Y.; Jiang, Z.Y. Biomimetic synthesis of C3N4/TiO2/Ag nanosheet composites with high visible-light photocatalytic performance. RSC Adv. 2015, 5, 56913–56922. [Google Scholar] [CrossRef]
- Sovizi, M.R.; Eskandarpour, M.; Afshari, M. Synthesis, characterization, and application of magnetic-activated carbon nanocomposite (m-Fe3O4@ACCs) as a new low-cost magnetic adsorbent for removal of Pb(II) from industrial wastewaters. Desalin. Water Treat. 2016, 57, 28887–28899. [Google Scholar] [CrossRef]
- Ali, M.H.; Al-Afify, A.D.; Goher, M.E. Preparation and characterization of graphene–TiO2 nanocomposite for enhanced photodegradation of Rhodamine-B dye. Egypt. J. Aquat. Res. 2018, 44, 263–270. [Google Scholar] [CrossRef]
- Fang, H.; Cao, X.; Yu, J.; Lv, X.; Yang, N.; Wang, T.; Jiang, W. Preparation of the all-solid-state Z-scheme WO3/Ag/AgCl film on glass accelerating the photodegradation of pollutants under visible light. J. Mater. Sci. 2019, 54, 286–301. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Fu, M. Enhanced visible light photocatalytic activity of cluster modified N-doped for degradation of toluene in air. Int. J. Photoenergy 2012, 2012, 569716. [Google Scholar] [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Aragaw, B.A. Reduced graphene oxide-intercalated graphene oxide nano-hybrid for enhanced photoelectrochemical water reduction. J. Nanostruct. Chem. 2020, 10, 9–18. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Zhu, Y. Visible photocatalytic activity enhancement of ZnWO4 by graphene hybridization. ACS Catal. 2012, 2, 2769–2778. [Google Scholar] [CrossRef]
- Garrafa-Gálvez, H.E.; Alvarado-Beltrán, C.G.; Almaral-Sánchez, J.L.; Hurtado-Macías, A.; Garzon-Fontecha, A.M.; Luque, P.A.; Castro-Beltrán, A. Graphene role in improved solar photocatalytic performance of TiO2-RGO nanocomposite. Chem. Phys. 2019, 521, 35–43. [Google Scholar] [CrossRef]
- Lubis, S.; Sitompul, D.W. Photocatalytic degradation of indigo carmine dye using α-Fe2O3/bentonite nanocomposite prepared by mechanochemical synthesis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012142. [Google Scholar] [CrossRef]
- Rahimi, R.; Bathaee, H.; Rabbani, M. Degradation of Rhodamine B using Cr-doped TiO2 under visible light irradiation. In Proceedings of the 16th International Electronic Conference on Synthetic Organic Chemistry, Online, 1–30 November 2012; MDPI: Basel, Switzerland, 2012; Volume 16, pp. 1–9. [Google Scholar] [CrossRef]
- Abou-Gamra, Z.M.; Ahmed, M.A.; Hamza, M.A. Investigation of commercial PbCrO4/TiO2 for photodegradation of Rhodamine B in aqueous solution by visible light. Nanotechnol. Environ. Eng. 2017, 2, 12. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, D.; Ma, J.; Wu, D.; Yang, M.; Komarneni, S. Fabrication of AgBr/Ag2CrO4 composites for enhanced visible-light photocatalytic activity. Ceram. Int. 2015, 41, 12509–12513. [Google Scholar] [CrossRef]
- Leong, C.Y.; Teh, H.L.; Chen, M.C.; Lee, S.L. Effect of synthesis methods on properties of copper oxide doped titanium dioxide photocatalyst in dye photodegradation of Rhodamine B. Sci. Technol. Indones. 2022, 7, 91–97. [Google Scholar] [CrossRef]
- Fu, H.; Pan, C.; Yao, W.; Zhu, Y. Visible-light-induced degradation of Rhodamine B by nanosized Bi2WO6. J. Phys. Chem. B 2005, 109, 22432–22439. [Google Scholar] [CrossRef]
- Yai, H.; Nadu, T. Enhanced visible-light-driven photodegradation of Rhodamine B over Ag2C2O4/Bi2MoO6 nanocomposites. Iran. J. Chem. Chem. Eng. 2020, 39, 29–37. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, J.; Wang, N.; Xing, J.; Liu, L. In-situ synthesis of CNT/TiO2 heterojunction nanocomposite and its efficient photocatalytic degradation of Rhodamine B. Inorg. Chem. Commun. 2020, 119, 108071. [Google Scholar] [CrossRef]
- Isari, A.A.; Payan, A.; Fattahi, M.; Jorfi, S.; Kakavandi, B. Photocatalytic degradation of rhodamine B and real textile wastewater using Fe-doped TiO2 anchored on reduced graphene oxide (Fe-TiO2/rGO): Characterization and feasibility, mechanism and pathway studies. Appl. Surf. Sci. 2018, 462, 549–564. [Google Scholar] [CrossRef]
- Zhang, Y. High photocatalytic activity of hierarchical SiO2@C-doped TiO2 hollow spheres in UV and visible light towards degradation of Rhodamine B. J. Hazard. Mater. 2017, 340, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, V.; Ramesh, P.S.; Geetha, D. Synthesis of Cu loaded TiO2 nanoparticles for the improved photocatalytic degradation of Rhodamine B. Int. J. Nanosci. 2016, 15, 1660002. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Acemioğlu, B. Removal of a reactive dye using NaOH-activated biochar prepared from peanut shell by pyrolysis process. Int. J. Coal Prep. Util. 2022, 42, 671–693. [Google Scholar] [CrossRef]




| Sample | Element Percentage (wt%) | |||
|---|---|---|---|---|
| C | O | N | Ti | |
| Graphite | 100 | - | - | - |
| GO | 60.42 | 39.58 | - | - |
| rGO | 83.17 | 16.83 | - | - |
| N-TiO2 | 5.01 | 48.36 | 1.85 | 44.78 |
| N-TiO2/rGO | 27.88 | 37.94 | 0.61 | 33.57 |
| Sample | Distance between Layers (nm) | D (nm) | Average D (nm) |
|---|---|---|---|
| Graphite | 0.334 | 15.801 | 15.801 |
| 0.830 | 6.999 | ||
| GO | 0.339 | 7.416 | 7.208 |
| rGO | 0.342 | 4.160 | 6.438 |
| 0.348 | 9.641 | ||
| 0.235 | 9.261 | ||
| N-TiO2 | 0.188 | 9.296 | 13.215 |
| N-TiO2/rGO | 0.169 | 10.304 | 12.818 |
| 0.166 | 11.792 | ||
| 0.148 | 11.926 | ||
| 0.126 | 13.558 |
| Sample | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Size (nm) |
|---|---|---|---|
| Graphite | 3.951 | 0.014 | 7.144 |
| GO | 216.313 | 0.565 | 5.225 |
| rGO | 83.407 | 0.103 | 2.469 |
| N-TiO2 | 60.502 | 0.109 | 3.602 |
| N-TiO2/rGO | 53.393 | 0.096 | 3.588 |
| Photocatalyst Materials | Light | Dye Concentration | Photocatalyst Mass | Irradiation Time | Photodegradation | Ref. |
|---|---|---|---|---|---|---|
| Cr-doped TiO2 | Hg lamp 500 W | 10 mg/L | 0.02 g | 90 min | 98% | [48] |
| PbCrO4/TiO2 | Tungsten 200 W | 1 × 10−5 M | 0.5 g | 600 min | 70% | [49] |
| Ag2CrO4 | LED 5 × 24 W | 10 mg/L | 0.1 g | 35 min | 73% | [50] |
| Cu-TiO2 | LED 15 W | 15 mg/L | 0.05 g | 360 min | 65% | [51] |
| Bi2WO6 | Xenon 500 W | 1 × 10−5 M | 0.5 g | 60 min | 19% | [52] |
| Ag2C2O4/BiMoO6 | Xenon | 1 × 10−5 M | 0.2 g | 100 min | 78.84% | [53] |
| CNT-TiO2 | UV | 10 mg/L | 10 g | 20 min | 85% | [54] |
| Fe-TiO2/rGO | Solar simulator | 20 mg/L | 0.6 g | 120 min | 91% | [55] |
| SiO2/C-TiO2 | UV | 20 mg/L | 0.1 g | 60 min | 61.7% | [56] |
| Cu-TiO2 | Natural light | 10 mg/L | 0.02 g | 120 min | 97% | [57] |
| N-TiO2/rGO | Xenon | 10 mg/L | 0.02 g | 90 min | 78.29% | This Study |
| Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|
| qe1 | k1 | R2 | qe2 | k2 | R2 |
| 1.490992463 mg/g | 0.000047 min−1 | 0.4194 | 10.96417229 mg/g | 0.4287318 g/mg·min | 0.9999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utami, M.; Wang, S.; Fajarwati, F.I.; Salsabilla, S.N.; Dewi, T.A.; Fitri, M. Enhanced Photodegradation of Rhodamine B Using Visible-Light Sensitive N-TiO2/rGO Composite. Crystals 2023, 13, 588. https://doi.org/10.3390/cryst13040588
Utami M, Wang S, Fajarwati FI, Salsabilla SN, Dewi TA, Fitri M. Enhanced Photodegradation of Rhodamine B Using Visible-Light Sensitive N-TiO2/rGO Composite. Crystals. 2023; 13(4):588. https://doi.org/10.3390/cryst13040588
Chicago/Turabian StyleUtami, Maisari, Shaobin Wang, Febi Indah Fajarwati, Siva Nur Salsabilla, Tania Amara Dewi, and Melinda Fitri. 2023. "Enhanced Photodegradation of Rhodamine B Using Visible-Light Sensitive N-TiO2/rGO Composite" Crystals 13, no. 4: 588. https://doi.org/10.3390/cryst13040588
APA StyleUtami, M., Wang, S., Fajarwati, F. I., Salsabilla, S. N., Dewi, T. A., & Fitri, M. (2023). Enhanced Photodegradation of Rhodamine B Using Visible-Light Sensitive N-TiO2/rGO Composite. Crystals, 13(4), 588. https://doi.org/10.3390/cryst13040588






