Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities
(This article belongs to the Section Inorganic Crystalline Materials)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation and Identification of Fungal Isolate
2.3. Extracellular Biosynthesis of SeNPs
2.4. Characterization of SeNPs
2.5. Antimicrobial Activity
2.6. Photocatalytic Degradation of Malachite Green Dye Using SeNPs
2.7. Statistical Analysis
3. Results and Discussion
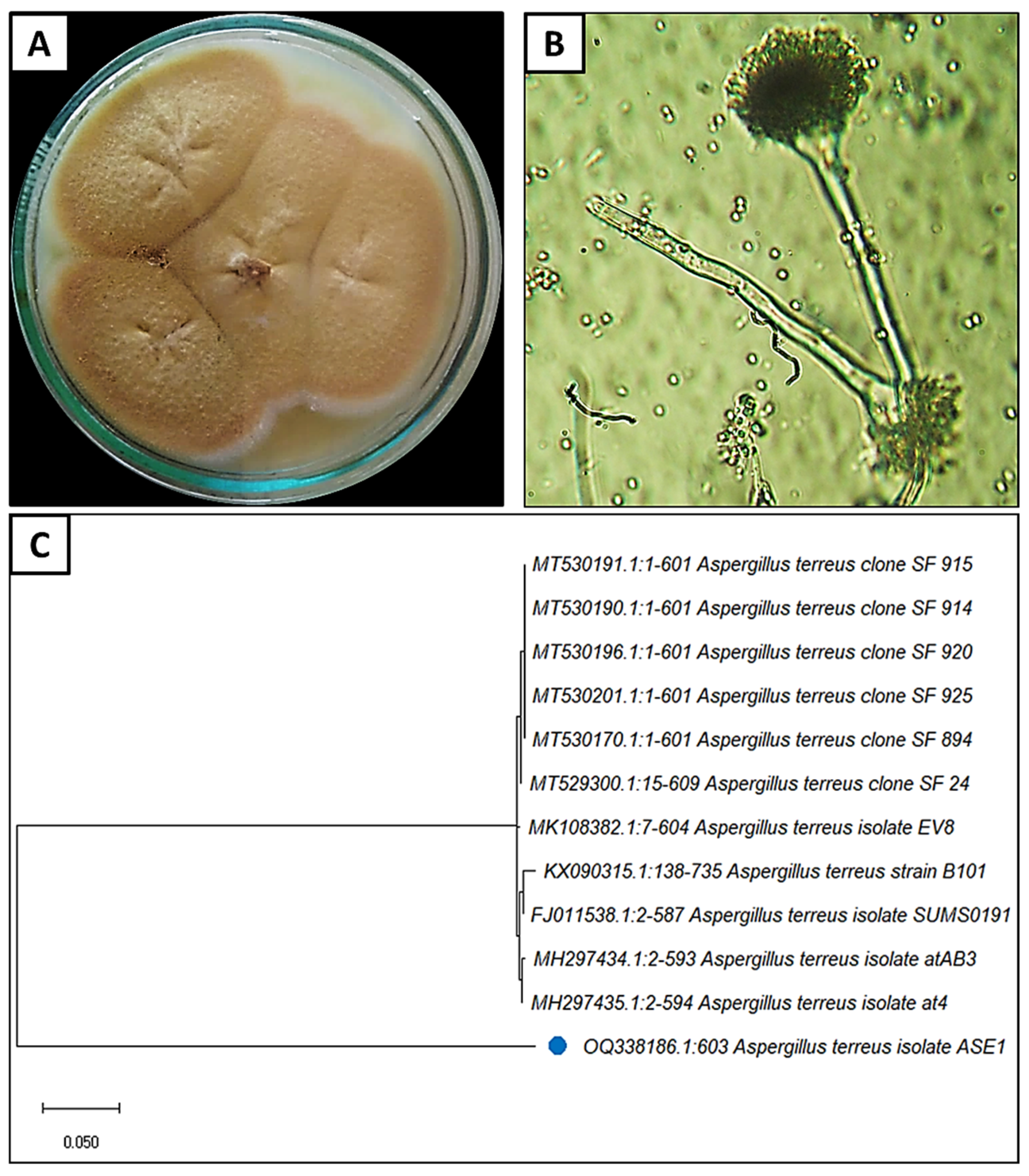
3.1. Isolation and Identification of the Fungal Isolate
3.2. Mycosynthesis of SeNPs
3.3. Characterization of SeNPs
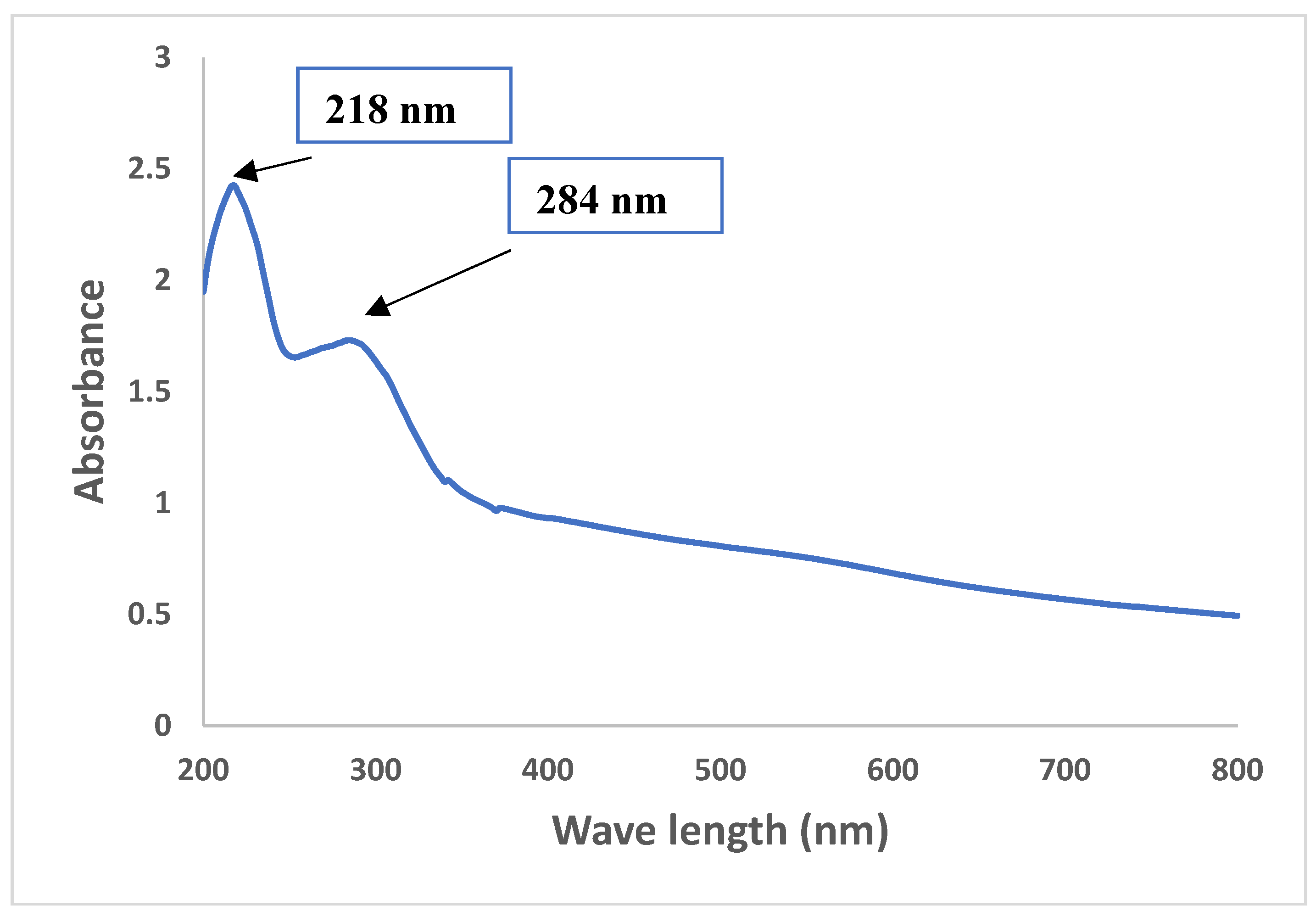
3.3.1. UV-Visible Spectroscopy
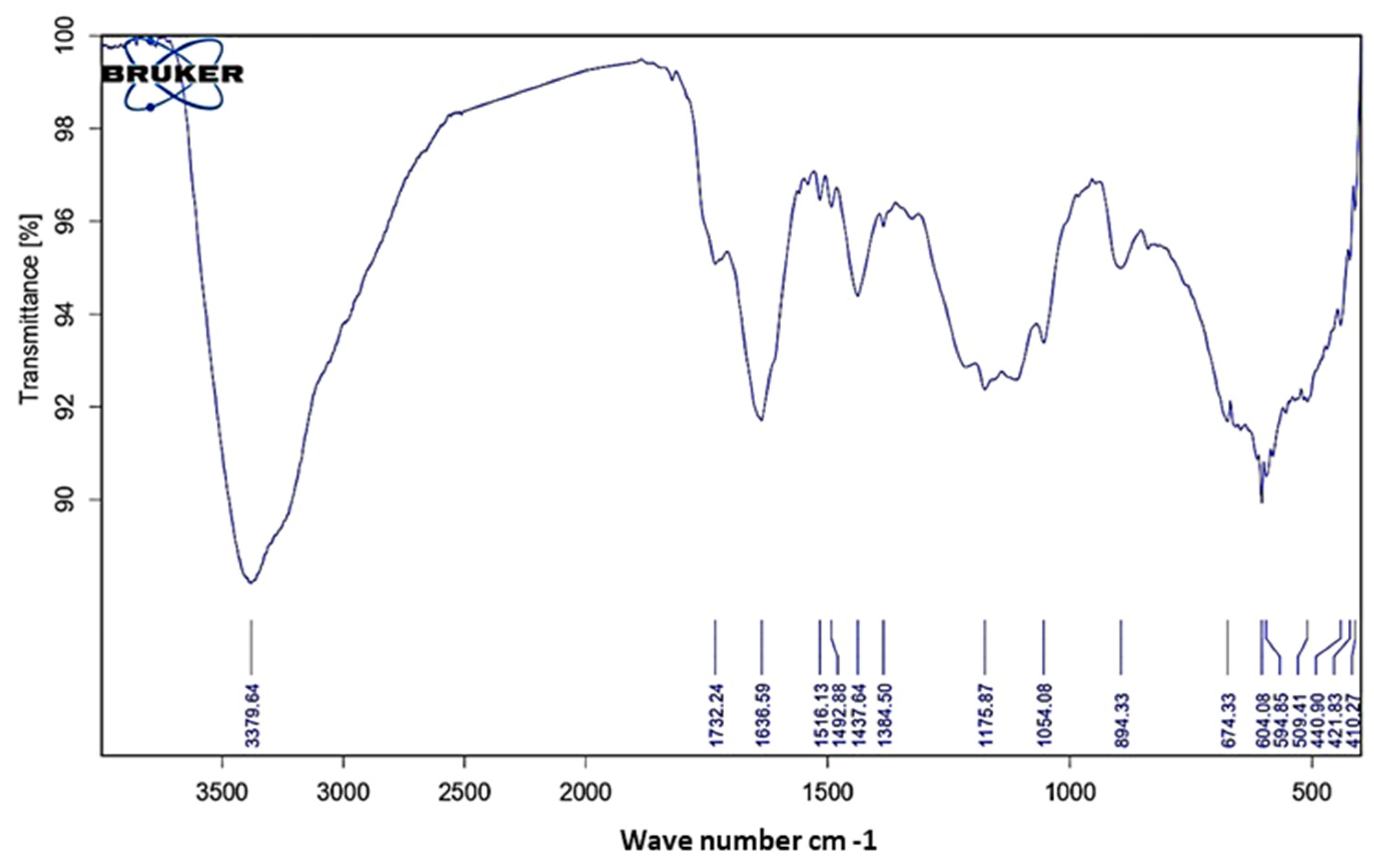
3.3.2. FT-IR Analysis
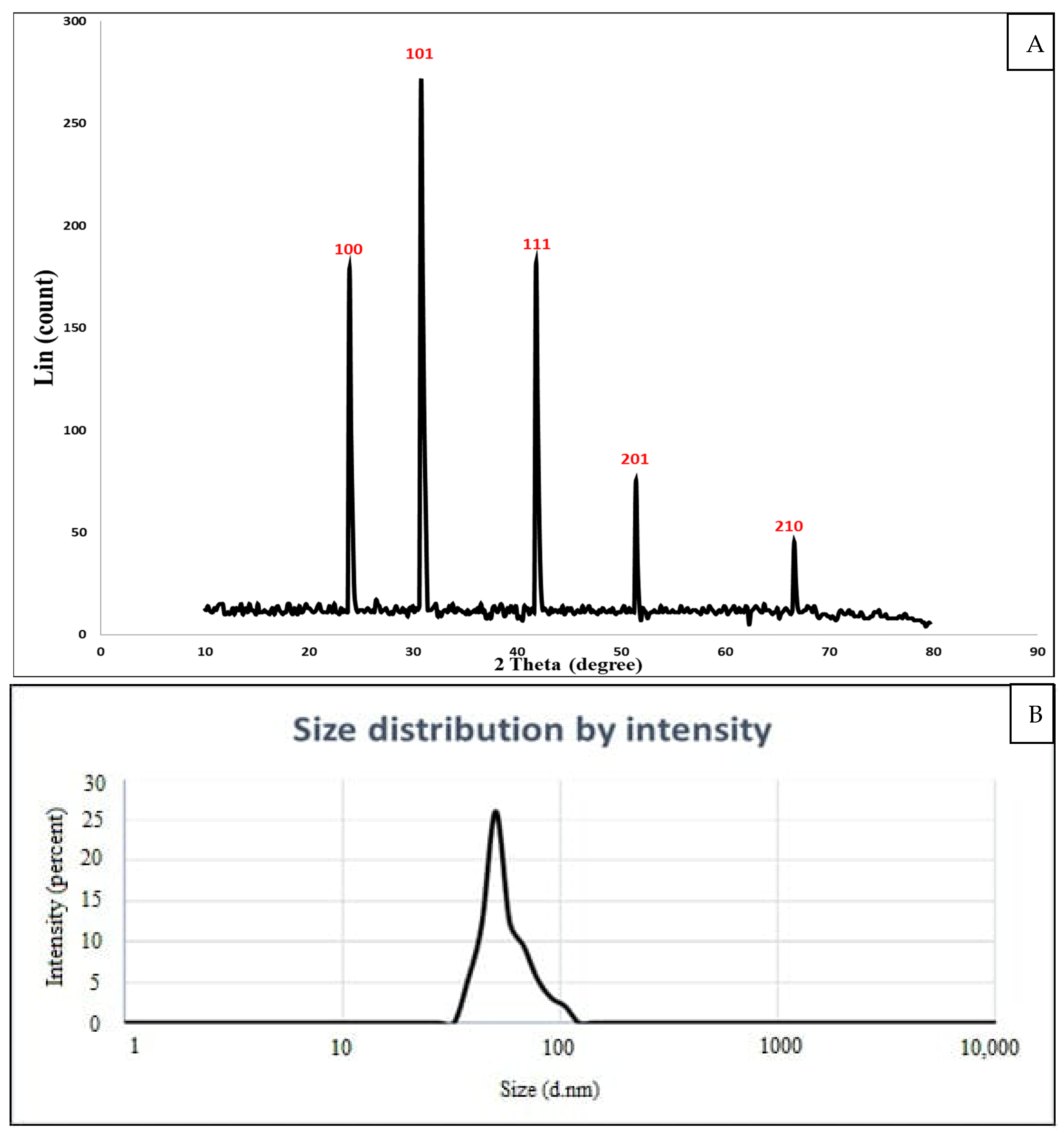
3.3.3. XRD Analysis
3.3.4. DLS Analysis
3.3.5. TEM Analysis
3.3.6. Scanning Electron Microscopic-Energy-Dispersive X-ray (SEM-EDX)
3.4. Antimicrobial Activity
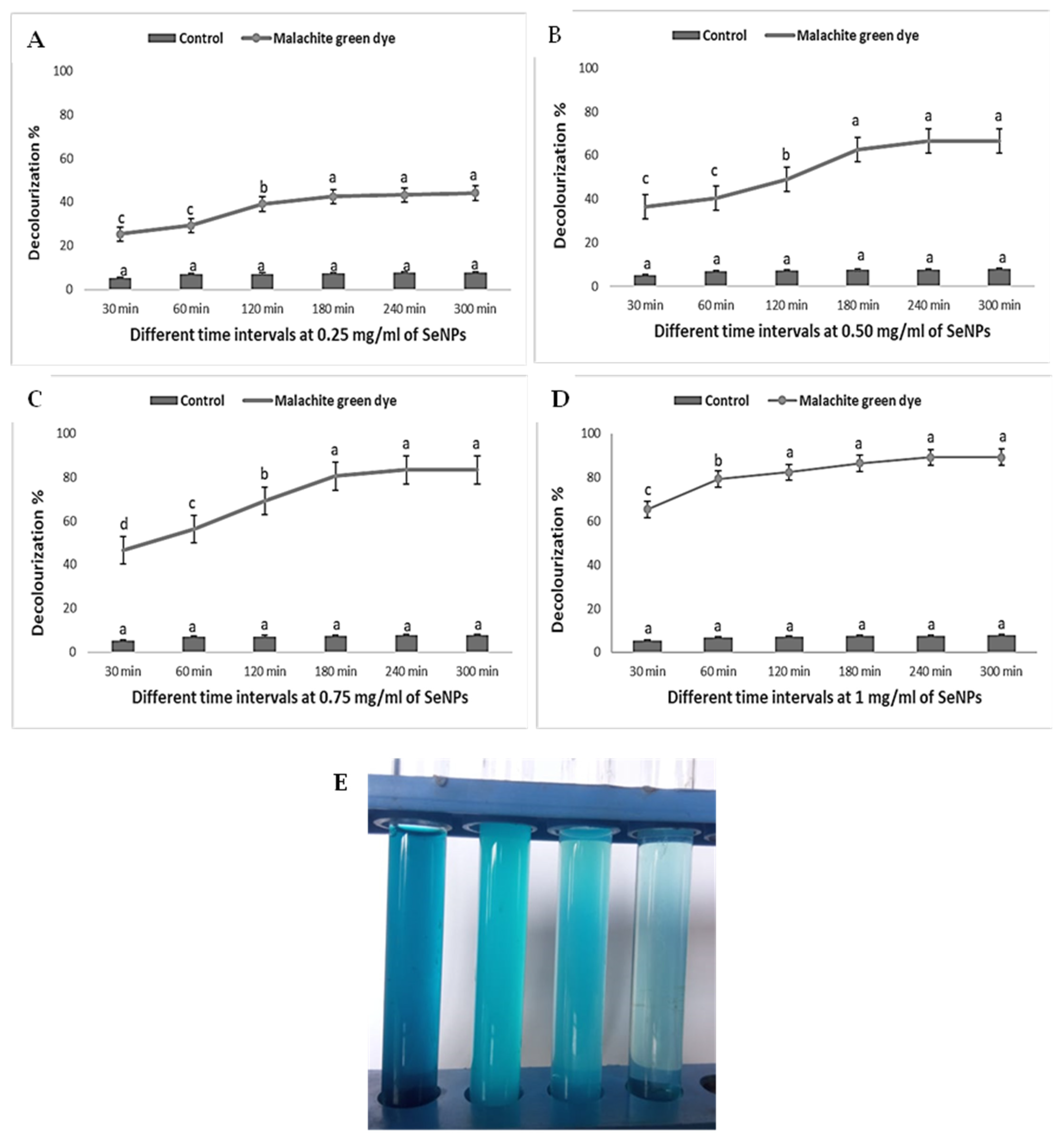
3.5. Photocatalytic Activity
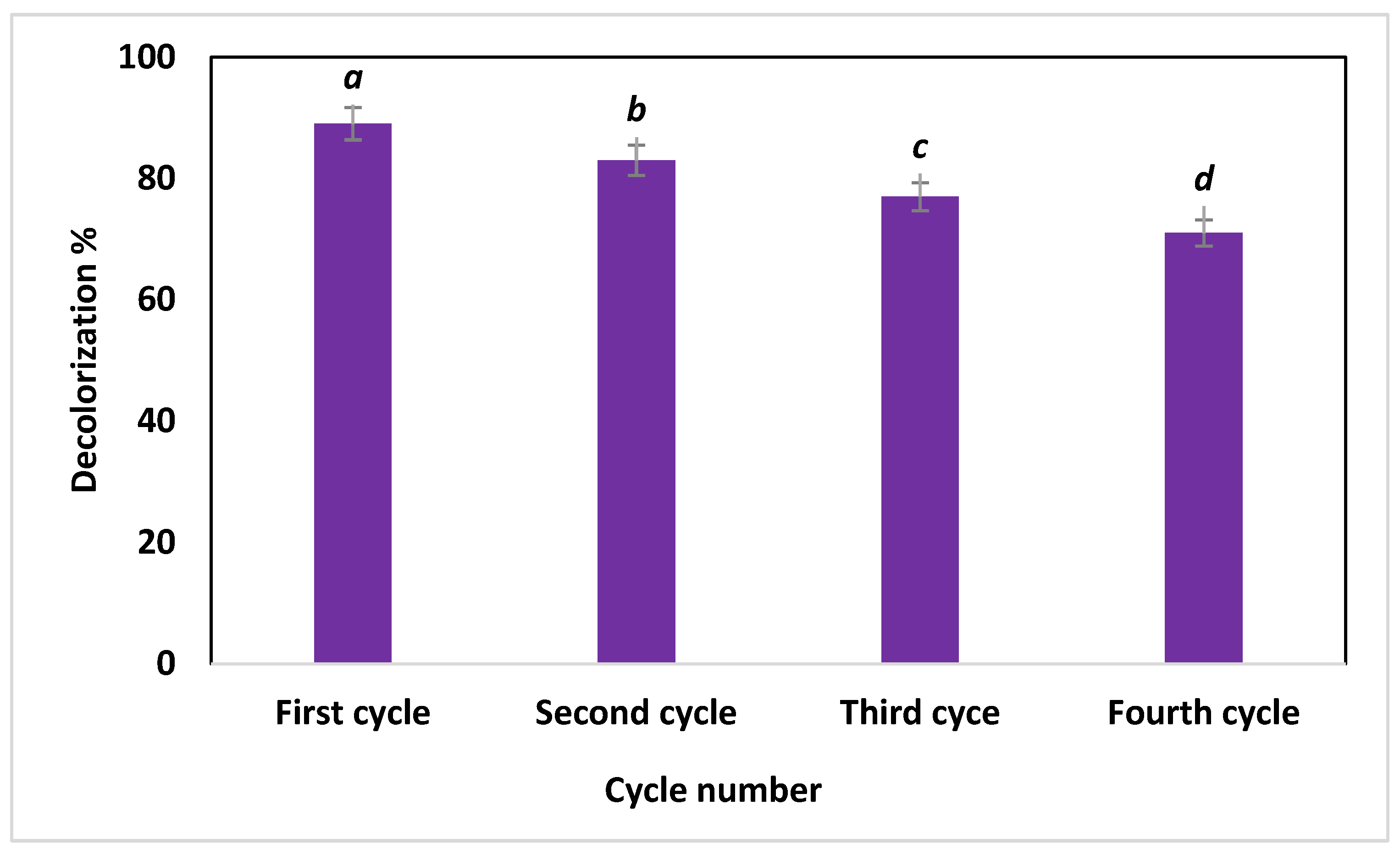
3.6. Recyclability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sathya, R.; Arasu, M.V.; Al-Dhabi, N.A.; Vijayaraghavan, P.; Ilavenil, S.; Rejiniemon, T. Towards sustainable wastewater treatment by biological methods—A challenges and advantages of recent technologies. Urban Clim. 2023, 47, 101378. [Google Scholar] [CrossRef]
- Nachana’a Timothy, E.T.W. Environmental pollution by heavy metal: An overview. Chemistry 2019, 3, 72–82. [Google Scholar]
- Tianzhi, W.; Weijie, W.; Hongying, H.; Khu, S.-T. Effect of coagulation on bio-treatment of textile wastewater: Quantitative evaluation and application. J. Clean. Prod. 2021, 312, 127798. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Z.; Zhang, J.; Shan, D.; Wu, Y.; Bai, L.; Wang, B. Treatment of industrial dye wastewater and pharmaceutical residue wastewater by advanced oxidation processes and its combination with nanocatalysts: A review. J. Water Process. Eng. 2021, 42, 102122. [Google Scholar] [CrossRef]
- Mehta, M.; Sharma, M.; Pathania, K.; Jena, P.K.; Bhushan, I. Degradation of synthetic dyes using nanoparticles: A mini-review. Environ. Sci. Pollut. Res. 2021, 28, 49434–49446. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Jaikumar, V.J.; Chemistry, E. Removal of colorants from wastewater: A review on sources and treatment strategies. J. Ind. Eng. Chem. 2019, 75, 1–19. [Google Scholar] [CrossRef]
- Ismail, M.; Akhtar, K.; Khan, M.I.; Kamal, T.; Khan, M.A.; Asiri, A.M.; Seo, J.; Khan, S.B. Pollution, Toxicity and Carcinogenicity of Organic Dyes and their Catalytic Bio-Remediation. Curr. Pharm. Des. 2019, 25, 3645–3663. [Google Scholar] [CrossRef]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2020, 12, 70–87. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater—A review. J. Water Process. Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Hamad, H.N.; Idrus, S. Recent Developments in the Application of Bio-Waste-Derived Adsorbents for the Removal of Methylene Blue from Wastewater: A Review. Polymers 2022, 14, 783. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Karishma, S.; Vo, D.-V.N.; Jeevanantham, S.; Yaashikaa, P.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2020, 264, 128580. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Saied, E.; Hasanin, M.S. Green and ecofriendly bio-removal of methylene blue dye from aqueous solution using biologically activated banana peel waste. Sustain. Chem. Pharm. 2020, 18, 100333. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Hemavathy, R.; Jeevanantham, S.; Jawahar, M.J.; Neshaanthini, J. A review on synthesis methods and recent applications of nanomaterial in wastewater treatment: Challenges and future perspectives. Chemosphere 2022, 307, 135713. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J.-Q. A new 3D 8-connected Cd(ii) MOF as a potent photocatalyst for oxytetracycline antibiotic degradation. Crystengcomm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, J.; Zhang, L.; Cheng, Y.; Lu, C.; Liu, Y.; Singh, A.; Trivedi, M.; Kumar, A.; Liu, J. Metal organic frameworks as efficient adsorbents for drugs from wastewater. Mater. Today Commun. 2022, 31, 103514. [Google Scholar] [CrossRef]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, V.P.; Meera, S.; Maria, C.G.A.; Nidhin, M. Green synthesis of nanoparticles from biodegradable waste extracts and their applications: A critical review. Nanotechnol. Environ. Eng. 2022, 1–21. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Anegbe, B. Synthesis, photocatalytic degradation and antibacterial properties of selenium or silver doped zinc oxide nanoparticles: A detailed review. Opennano 2022, 8, 100082. [Google Scholar] [CrossRef]
- Somu, P.; Paul, S. Protein assisted one pot controlled synthesis of monodispersed and multifunctional colloidal silver-gold alloy nanoparticles. J. Mol. Liq. 2019, 291, 111303. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; Lashin, I.; Hassan, S.A.M. In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Convers. Biorefinery 2021, 1–11. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Hashem, A.H.; Wassel, A.R.; Hasanin, M. Antimicrobial and Antiviral Activities of Durable Cotton Fabrics Treated with Nanocomposite Based on Zinc Oxide Nanoparticles, Acyclovir, Nanochitosan, and Clove Oil. Appl. Biochem. Biotechnol. 2021, 194, 783–800. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.S.; Mohamed, R.M.S.R.; Noman, E.A.; Kassim, A.H.M. Prospects of Fresh Market Wastes Management in Developing Countries; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lashin, I.; Hasanin, M.; Hassan, S.A.M.; Hashem, A.H. Green biosynthesis of zinc and selenium oxide nanoparticles using callus extract of Ziziphus spina-christi: Characterization, antimicrobial, and antioxidant activity. Biomass Convers. Biorefinery 2021, 1–14. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.A.; El-Wakil, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. Biometals 2022, 35, 601–616. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Hashem, A.H. Eco-Friendly Synthesis of Superhydrophobic Antimicrobial Film Based on Cellulose Acetate/Polycaprolactone Loaded with the Green Biosynthesized Copper Nanoparticles for Food Packaging Application. J. Polym. Environ. 2021, 30, 1820–1832. [Google Scholar] [CrossRef]
- Hashem, A.H.; Al Abboud, M.A.; Alawlaqi, M.M.; Abdelghany, T.M.; Hasanin, M. Synthesis of Nanocapsules Based on Biosynthesized Nickel Nanoparticles and Potato Starch: Antimicrobial, Antioxidant, and Anticancer Activity. Starch-Stärke 2021, 74, 2100165. [Google Scholar] [CrossRef]
- Hashem, A.H.; Selim, T.A.; Alruhaili, M.H.; Selim, S.; Alkhalifah, D.H.M.; Al Jaouni, S.K.; Salem, S.S. Unveiling Antimicrobial and Insecticidal Activities of Biosynthesized Selenium Nanoparticles Using Prickly Pear Peel Waste. J. Funct. Biomater. 2022, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.M.; Hasanin, M.S.; Suleiman, W.B.; Helal, E.E.-H.; Hashem, A.H. Green biosynthesis of titanium dioxide quantum dots using watermelon peel waste: Antimicrobial, antioxidant, and anticancer activities. Biomass Convers. Biorefinery 2022, 1–12. [Google Scholar] [CrossRef]
- Rahuman, H.B.H.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal plants mediated the green synthesis of silver nanoparticles and their biomedical applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef]
- Jayarambabu, N.; Rao, T.V.; Kumar, R.R.; Akshaykranth, A.; Shanker, K.; Suresh, V. Anti-hyperglycemic, pathogenic and anticancer activities of Bambusa arundinacea mediated Zinc Oxide nanoparticles. Mater. Today Commun. 2020, 26, 101688. [Google Scholar] [CrossRef]
- Simon, S.; Sibuyi, N.R.S.; Fadaka, A.O.; Meyer, S.; Josephs, J.; Onani, M.O.; Meyer, M.; Madiehe, A.M. Biomedical Applications of Plant Extract-Synthesized Silver Nanoparticles. Biomedicines 2022, 10, 2792. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Saied, E.; Amin, B.H.; Alotibi, F.O.; Al-Askar, A.A.; Arishi, A.A.; Elkady, F.M.; Elbahnasawy, M.A. Antifungal Activity of Biosynthesized Silver Nanoparticles (AgNPs) against Aspergilli Causing Aspergillosis: Ultrastructure Study. J. Funct. Biomater. 2022, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Saied, E.; Ali, O.M.; Selim, S.; Al Jaouni, S.K.; Elkady, F.M.; El-Sayyad, G.S. Pomegranate Peel Extract Stabilized Selenium Nanoparticles Synthesis: Promising Antimicrobial Potential, Antioxidant Activity, Biocompatibility, and Hemocompatibility. Appl. Biochem. Biotechnol. 2023, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elghait, M.; Hasanin, M.; Hashem, A.H.; Salem, S.S. Ecofriendly novel synthesis of tertiary composite based on cellulose and myco-synthesized selenium nanoparticles: Characterization, antibiofilm and biocompatibility. Int. J. Biol. Macromol. 2021, 175, 294–303. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Hashem, A.H.; El-Sayed, E.S.A.; El-Saied, H. Green ecofriendly bio-deinking of mixed office waste paper using various enzymes from Rhizopus microsporus AH3: Efficiency and characteristics. Cellulose 2020, 27, 4443–4453. [Google Scholar] [CrossRef]
- Hashem, A.H.; Suleiman, W.B.; Abu-Elreesh, G.; Shehabeldine, A.M.; Khalil, A.M.A. Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresour. Technol. Rep. 2020, 12, 100569. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abu-Elreesh, G.; El-Sheikh, H.H.; Suleiman, W.B. Isolation, identification, and statistical optimization of a psychrotolerant Mucor racemosus for sustainable lipid production. Biomass Convers. Biorefinery 2022, 13, 3415–3426. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khattab, A.M.; Abdelraof, M. A facile one-pot bioconversion of frying oil waste to single cell oils and related products using fungi via response surface methodology. Biomass Convers. Biorefinery 2022, 1–11. [Google Scholar] [CrossRef]
- Peramune, D.; Manatunga, D.C.; Dassanayake, R.S.; Premalal, V.; Liyanage, R.N.; Gunathilake, C.; Abidi, N. Recent advances in biopolymer-based advanced oxidation processes for dye removal applications: A review. Environ. Res. 2022, 215, 114242. [Google Scholar] [CrossRef]
- Jeon, J.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J. Enhancing the Photocatalytic Activity of TiO 2 Catalysts. Adv. Sustain. Syst. 2020, 4, 202000197. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Transition metal selenides and diselenides: Hydrothermal fabrication, investigation of morphology, particle size and and their applications in photocatalyst. Adv. Colloid Interface Sci. 2021, 287, 102321. [Google Scholar] [CrossRef] [PubMed]
- Morán-Serradilla, C.; Angulo-Elizari, E.; Henriquez-Figuereo, A.; Sanmartín, C.; Sharma, A.K.; Plano, D.J.M. Seleno-metabolites and their precursors: A new dawn for several illnesses? Metabolites 2022, 12, 874. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Bhattacharya, S. Protective Role of the Essential Trace Elements in the Obviation of Cadmium Toxicity: Glimpses of Mechanisms. Biol. Trace Element Res. 2021, 200, 2239–2246. [Google Scholar] [CrossRef]
- Bisht, N.; Phalswal, P.; Khanna, P.K. Selenium nanoparticles: A review on synthesis and biomedical applications. Mater. Adv. 2021, 3, 1415–1431. [Google Scholar] [CrossRef]
- Sarkar, J.; Mridha, D.; Davoodbasha, M.A.; Banerjee, J.; Chanda, S.; Ray, K.; Roychowdhury, T.; Acharya, K.; Sarkar, J. A State-of-the-Art Systemic Review on Selenium Nanoparticles: Mechanisms and Factors Influencing Biogenesis and Its Potential Applications. Biol. Trace Element Res. 2023, 1–37. [Google Scholar] [CrossRef]
- Stater, E.P.; Sonay, A.Y.; Hart, C.; Grimm, J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat. Nanotechnol. 2021, 16, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Truong, L.; Medina-Cruz, D.; Mostafavi, E.; Rabiee, N. Selenium Nanomaterials to Combat Antimicrobial Resistance. Molecules 2021, 26, 3611. [Google Scholar] [CrossRef] [PubMed]
- Makhlof, M.E.M.; Albalwe, F.M.; Al-Shaikh, T.M.; El-Sheekh, M.M. Suppression Effect of Ulva lactuca Selenium Nanoparticles (USeNPs) on HepG2 Carcinoma Cells Resulting from Degradation of Epidermal Growth Factor Receptor (EGFR) with an Evaluation of Its Antiviral and Antioxidant Activities. Appl. Sci. 2022, 12, 11546. [Google Scholar] [CrossRef]
- Kondaparthi, P.; Flora, S.; Naqvi, S. Selenium nanoparticles: An insight on its Pro-oxidant and antioxidant properties. Front. Nanosci. Nanotechnol. 2019, 6, 1000189. [Google Scholar] [CrossRef]
- Khurana, A.; Tekula, S.; Saifi, M.A.; Venkatesh, P.; Godugu, C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019, 111, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Wang, C.Y.; Zare, E.N.; Borzacchiello, A.; Niu, L.N.; Tay, F.R. Metal-Based Nanomaterials in Biomedical Applications: Antimicrobial Activity and Cytotoxicity Aspects. Adv. Funct. Mater. 2020, 30, 1910021. [Google Scholar] [CrossRef]
- Hashem, A.H.; Khalil, A.M.A.; Reyad, A.M.; Salem, S.S. Biomedical Applications of Mycosynthesized Selenium Nanoparticles Using Penicillium expansum ATTC 36200. Biol. Trace Element Res. 2021, 199, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Saied, E.; Salem, S.S.; Al-Askar, A.A.; Elkady, F.M.; Arishi, A.A.; Hashem, A.H.J.B. Mycosynthesis of hematite (α-Fe2O3) nanoparticles using Aspergillus niger and their antimicrobial and photocatalytic activities. Bioengineering 2022, 9, 397. [Google Scholar] [CrossRef]
- Mohmed, A.A.; Hassan, S.E.-D.; Fouda, A.; Elgamal, M.S.; Salem, S.S. Extracellular Biosynthesis of Silver Nanoparticles Using Aspergillus sp. and Evaluation of their Antibacterial and Cytotoxicity. J. Appl. Life Sci. Int. 2017, 11, 1–12. [Google Scholar] [CrossRef]
- Albalawi, M.A.; Abdelaziz, A.M.; Attia, M.S.; Saied, E.; Elganzory, H.H.; Hashem, A.H.J.A. Mycosynthesis of Silica Nanoparticles Using Aspergillus niger: Control of Alternaria solani Causing Early Blight Disease, Induction of Innate Immunity and Reducing of Oxidative Stress in Eggplant. Antioxidants 2022, 11, 2323. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, S.E.-D.; Saied, E.; Azab, M.S. An eco-friendly approach to textile and tannery wastewater treatment using maghemite nanoparticles (γ-Fe2O3-NPs) fabricated by Penicillium expansum strain (K-w). J. Environ. Chem. Eng. 2020, 9, 104693. [Google Scholar] [CrossRef]
- Perez, C. Antibiotic assay by agar-well diffusion method. Acta. Biol. Med. Exp. 1990, 15, 113–115. [Google Scholar]
- Bataghva, F.; Sajjadi, S.M.; Daraei, B. Simultaneous Spectrophotometric Quantification of Crystal Violet and Malachite Green in Aqueous Samples: Combination of Multivariate Calibration Method and Solid Phase Extraction Based on Sodium Dodecyl Sulfate (SDS) Grafted Chitosan Nano-composite. Anal. Bioanal. Chem. Res. 2020, 7, 525–539. [Google Scholar] [CrossRef]
- Saied, E.; Hashem, A.H.; Ali, O.M.; Selim, S.; Almuhayawi, M.S.; Elbahnasawy, M.A. Photocatalytic and Antimicrobial Activities of Biosynthesized Silver Nanoparticles Using Cytobacillus firmus. Life 2022, 12, 1331. [Google Scholar] [CrossRef]
- Bafghi, M.H.; Nazari, R.; Darroudi, M.; Zargar, M.; Zarrinfar, H.J.B.P. The effect of biosynthesized selenium nanoparticles on the expression of CYP51A and HSP90 antifungal resistance genes in Aspergillus fumigatus and Aspergillus flavus. Biotechnol. Prog. 2022, 38, e3206. [Google Scholar] [CrossRef]
- Zikalala, N.; Matshetshe, K.; Parani, S.; Oluwafemi, O.S. Biosynthesis protocols for colloidal metal oxide nanoparticles. Nano-Struct. Nano-Objects 2018, 16, 288–299. [Google Scholar] [CrossRef]
- Khandel, P.; Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: Current status and future challenges. J. Nanostruct. Chem. 2018, 8, 369–391. [Google Scholar] [CrossRef]
- Islam, S.N.; Naqvi, S.M.A.; Raza, A.; Jaiswal, A.; Singh, A.K.; Dixit, M.; Barnwal, A.; Gambhir, S.; Ahmad, A. Mycosynthesis of highly fluorescent selenium nanoparticles from Fusarium oxysporum, their antifungal activity against black fungus Aspergillus niger, and in-vivo biodistribution studies. 3 Biotech 2022, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.-S.R.; Abdelhakim, H.K.; Ahmed, A.S.J.B. Solid-state fermentation for enhanced production of selenium nanoparticles by gamma-irradiated Monascus purpureus and their biological evaluation and photocatalytic activities. Bioprocess Biosyst. Eng. 2020, 43, 797–809. [Google Scholar] [CrossRef]
- Teeling, E.C.; Springer, M.S.; Madsen, O.; Bates, P.; O’Brien, S.J.; Murphy, W.J. A Molecular Phylogeny for Bats Illuminates Biogeography and the Fossil Record. Science 2005, 307, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Saroha, J.; Lalla, N.; Kumar, M.; Sharma, S.N. Ultrafast transient absorption spectroscopic studies on the Impact of growth time on size, stability, and optical characteristics of colloidal gold nanoparticles. Optik 2022, 268, 169759. [Google Scholar] [CrossRef]
- Hassan, H.U.; Raja, N.I.; Abasi, F.; Mehmood, A.; Qureshi, R.; Manzoor, Z.; Shahbaz, M.; Proćków, J. Comparative Study of Antimicrobial and Antioxidant Potential of Olea ferruginea Fruit Extract and Its Mediated Selenium Nanoparticles. Molecules 2022, 27, 5194. [Google Scholar] [CrossRef]
- Zahra, S.E.; Iqbal, M.S.; Abbas, K.; Qadir, M.I. Synthesis, characterization and evaluation of biological properties of selenium nanoparticles from Solanum lycopersicum. Arab. J. Chem. 2022, 15, 103901. [Google Scholar] [CrossRef]
- Ranjitha, V.; Ravishankar, V.R. Extracellular synthesis of selenium nanoparticles from an actinomycetes streptomyces griseoruber and evaluation of its cytotoxicity on HT-29 cell line. Pharm. Nanotechnol. 2018, 6, 61–68. [Google Scholar] [CrossRef]
- A Wadhwani, S.; Gorain, M.; Banerjee, P.; Shedbalkar, U.U.; Singh, R.; Kundu, G.C.; A Chopade, B. Green synthesis of selenium nanoparticles using Acinetobacter sp. SW30: Optimization, characterization and its anticancer activity in breast cancer cells. Int. J. Nanomed. 2017, 12, 6841–6855. [Google Scholar] [CrossRef]
- Fouda, A.; Al-Otaibi, W.A.; Saber, T.; AlMotwaa, S.M.; Alshallash, K.S.; Elhady, M.; Badr, N.F.; Abdel-Rahman, M.A. Antimicrobial, antiviral, and in-vitro cytotoxicity and mosquitocidal activities of Portulaca oleracea-based green synthesis of selenium nanoparticles. J. Funct. Biomater. 2022, 13, 157. [Google Scholar] [CrossRef]
- Anmol, A.; Jaiswal, S.K.; Prakash, R.; Mihara, H.; Prakash, N.T. Concomitant synthesis and stabilization of selenium nanoparticles using extract of endophytic fungus, Nigrospora gullinensis. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Arumugham, T.; Alagumuthu, M.; Amimodu, R.G.; Munusamy, S.; Iyer, S.K. A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain. Mater. Technol. 2020, 23, e00138. [Google Scholar] [CrossRef]
- A Hernández-Díaz, J.; Garza-García, J.J.; León-Morales, J.M.; Zamudio-Ojeda, A.; Arratia-Quijada, J.; Velázquez-Juárez, G.; López-Velázquez, J.C.; García-Morales, S. Antibacterial Activity of Biosynthesized Selenium Nanoparticles Using Extracts of Calendula officinalis against Potentially Clinical Bacterial Strains. Molecules 2021, 26, 5929. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Qin, L.; Li, Y.; Liang, F.; Li, L.; Lan, Y.; Li, Z.; Lu, X.; Yang, M.; Ma, D. A microporous 2D cobalt-based MOF with pyridyl sites and open metal sites for selective adsorption of CO2. Microporous Mesoporous Mater. 2022, 341, 112098. [Google Scholar] [CrossRef]
- Alam, H.; Khatoon, N.; Khan, M.A.; Husain, S.A.; Saravanan, M.; Sardar, M. Synthesis of Selenium Nanoparticles Using Probiotic Bacteria Lactobacillus acidophilus and Their Enhanced Antimicrobial Activity Against Resistant Bacteria. J. Clust. Sci. 2019, 31, 1003–1011. [Google Scholar] [CrossRef]
- Li, S.; Ren, X.; Mezari, B.; Liu, Y.; Pornsetmetakul, P.; Liutkova, A.; Kosinov, N.; Hensen, E.J. Direct synthesis of Al-rich ZSM-5 nanocrystals with improved catalytic performance in aromatics formation from methane and methanol. Microporous Mesoporous Mater. 2023, 351, 112485. [Google Scholar] [CrossRef]
- Hassanien, R.; Abed-Elmageed, A.A.I.; Husein, D.Z. Eco-Friendly Approach to Synthesize Selenium Nanoparticles: Photocatalytic Degradation of Sunset Yellow Azo Dye and Anticancer Activity. Chemistryselect 2019, 4, 9018–9026. [Google Scholar] [CrossRef]
- Al Jahdaly, B.A.; Al-Radadi, N.S.; Eldin, G.M.; Almahri, A.; Ahmed, M.; Shoueir, K.; Janowska, I. Selenium nanoparticles synthesized using an eco-friendly method: Dye decolorization from aqueous solutions, cell viability, antioxidant, and antibacterial effectiveness. J. Mater. Res. Technol. 2021, 11, 85–97. [Google Scholar] [CrossRef]
- Qian, F.; Li, X.; Tang, L.; Lai, S.K.; Lu, C.; Lau, S.P. Selenium quantum dots: Preparation, structure, and properties. Appl. Phys. Lett. 2017, 110, 053104. [Google Scholar] [CrossRef]
- Jena, J.; Pradhan, N.; Dash, B.P.; Sukla, L.B.; Panda, P.K. Biosynthesis and characterization of silver nanoparticles using microalga Chlorococcum humicola and its antibacterial activity. Int. J. Nanomater. Biostruct 2013, 3, 1–8. [Google Scholar]
- Mekky, A.E.; Farrag, A.A.; Hmed, A.A.; Sofy, A.R. Preparation of Zinc Oxide Nanoparticles using Aspergillus niger as Antimicrobial and Anticancer Agents. J. Pure Appl. Microbiol. 2021, 15, 1547–1566. [Google Scholar] [CrossRef]
- Garza-García, J.J.O.; Hernández-Díaz, J.A.; Zamudio-Ojeda, A.; León-Morales, J.M.; Guerrero-Guzmán, A.; Sánchez-Chiprés, D.R.; López-Velázquez, J.C.; García-Morales, S. The Role of Selenium Nanoparticles in Agriculture and Food Technology. Biol. Trace Element Res. 2021, 200, 2528–2548. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Kizek, R. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef]
- Ikram, M.; Javed, B.; Raja, N.I.; Mashwani, Z.-U. Biomedical Potential of Plant-Based Selenium Nanoparticles: A Comprehensive Review on Therapeutic and Mechanistic Aspects. Int. J. Nanomed. 2021, 16, 249–268. [Google Scholar] [CrossRef]
- Muthu, S.; Raju, V.; Gopal, V.B.; Gunasekaran, A.; Narayan, K.S.; Malairaj, S.; Lakshmikanthan, M.; Duraisamy, N.; Krishnan, K.; Perumal, P. A rapid synthesis and antibacterial property of selenium nanoparticles using egg white lysozyme as a stabilizing agent. SN Appl. Sci. 2019, 1, 1543. [Google Scholar] [CrossRef]
- Shubharani, R.; Mahesh, M.; Yogananda Murthy, V. Biosynthesis and characterization, antioxidant and antimicrobial activities of selenium nanoparticles from ethanol extract of Bee Propolis. J. Nanomed. Nanotechnol. 2019, 10, 1000522. [Google Scholar]
- Hernández-Díaz, J.A.; Garza-García, J.J.; Zamudio-Ojeda, A.; León-Morales, J.M.; López-Velázquez, J.C.; García-Morales, S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric. 2021, 101, 1270–1287. [Google Scholar] [CrossRef]
- Zafar, M.N.; Dar, Q.; Nawaz, F.; Zafar, M.N.; Iqbal, M.; Nazar, M.F. Effective adsorptive removal of azo dyes over spherical ZnO nanoparticles. J. Mater. Res. Technol. 2019, 8, 713–725. [Google Scholar] [CrossRef]
- Bayram, O.; Köksal, E.; Moral, E.; Göde, F.; Pehlivan, E. Efficient decolorization of cationic dye (malachite green) by natural-based biosorbent (nano-magnetic Sophora Japonica fruit seed biochar). J. Dispers. Sci. Technol. 2022, 1–12. [Google Scholar] [CrossRef]
- Cittrarasu, V.; Kaliannan, D.; Dharman, K.; Maluventhen, V.; Easwaran, M.; Liu, W.C.; Balasubramanian, B.; Arumugam, M. Green synthesis of selenium nanoparticles mediated from Ceropegia bulbosa Roxb extract and its cytotoxicity, antimicrobial, mosquitocidal and photocatalytic activities. Sci. Rep. 2021, 11, 1032. [Google Scholar] [CrossRef]
- Akinola, P.; Lateef, A.; Asafa, T.; Beukes, L.; Hakeem, A.; Irshad, H. Multifunctional titanium dioxide nanoparticles biofabricated via phytosynthetic route using extracts of Cola nitida: Antimicrobial, dye degradation, antioxidant and anticoagulant activities. Heliyon 2020, 6, e04610. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Shoueir, K. Recent advances in polymer/metal/metal oxide hybrid nanostructures for catalytic applications: A review. J. Environ. Chem. Eng. 2020, 8, 104175. [Google Scholar]
- Sackey, J.; Bashir, A.; Ameh, A.; Nkosi, M.; Kaonga, C.; Maaza, M. Date pits extracts assisted synthesis of magnesium oxides nanoparticles and its application towards the photocatalytic degradation of methylene blue. J. King Saud Univ. Sci. 2020, 32, 2767–2776. [Google Scholar] [CrossRef]
- Bhadra, J.; Parangusan, H.; Popelka, A.; Lehocky, M.; Humpolicek, P.; Al-Thani, N. Electrospun Polystyrene/PANI-Ag fibers for organic dye removal and antibacterial application. J. Environ. Chem. Eng. 2020, 8, 103746. [Google Scholar] [CrossRef]
- Neves, T.D.F.; Dalarme, N.B.; da Silva, P.M.M.; Landers, R.; Picone, C.S.F.; Prediger, P. Novel magnetic chitosan/quaternary ammonium salt graphene oxide composite applied to dye removal. J. Environ. Chem. Eng. 2020, 8, 103820. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Du, Z.; Li, Z. Recent advances in metal halide perovskite photocatalysts: Properties, synthesis and applications. J. Energy Chem. 2020, 54, 770–785. [Google Scholar] [CrossRef]



| Microbial Strain | Inhibition Zone/mm | ||
|---|---|---|---|
| Se (1000 µg/mL) | SeNPs (1000 µg/mL) | CAZ/FLU | |
| E. coli | ND | 16 | ND |
| K. pneumonia | ND | 13 | ND |
| S. haemolyticus | ND | 14 | ND |
| S. aureus | ND | 20 | 8 |
| C. albicans | ND | 12 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saied, E.; Mekky, A.E.; Al-Askar, A.A.; Hagag, A.F.; El-bana, A.A.; Ashraf, M.; Walid, A.; Nour, T.; Fawzi, M.M.; Arishi, A.A.; et al. Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals 2023, 13, 450. https://doi.org/10.3390/cryst13030450
Saied E, Mekky AE, Al-Askar AA, Hagag AF, El-bana AA, Ashraf M, Walid A, Nour T, Fawzi MM, Arishi AA, et al. Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals. 2023; 13(3):450. https://doi.org/10.3390/cryst13030450
Chicago/Turabian StyleSaied, Ebrahim, Alsayed E. Mekky, Abdulaziz A. Al-Askar, Abdelrahman F. Hagag, Abdullah A. El-bana, Mohamed Ashraf, Abdelrahman Walid, Taha Nour, Mahmoud M. Fawzi, Amr A. Arishi, and et al. 2023. "Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities" Crystals 13, no. 3: 450. https://doi.org/10.3390/cryst13030450
APA StyleSaied, E., Mekky, A. E., Al-Askar, A. A., Hagag, A. F., El-bana, A. A., Ashraf, M., Walid, A., Nour, T., Fawzi, M. M., Arishi, A. A., & Hashem, A. H. (2023). Aspergillus terreus-Mediated Selenium Nanoparticles and Their Antimicrobial and Photocatalytic Activities. Crystals, 13(3), 450. https://doi.org/10.3390/cryst13030450








