Synthesis and Characterizations of Novel Isatin-s-Triazine Hydrazone Derivatives; X-ray Structure, Hirshfeld Analysis and DFT Calculations
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthetic Methodology
2.2.1. General Method for the Synthesis of 1,3,5-Triazine-Hydrazone Derivatives, 6a–e
Synthesis of 3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-one, 6a
5-Bromo-3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-one, 6b
5-Chloro-3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-One, 6c
3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)-5-fluoroindolin-2-one, 6d
3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)-5-methylindolin-2-one, 6e
2.3. X-ray Structure Determinations
2.4. Hirshfeld and DFT Computations
3. Results and Discussion
3.1. Synthesis and Characterizations
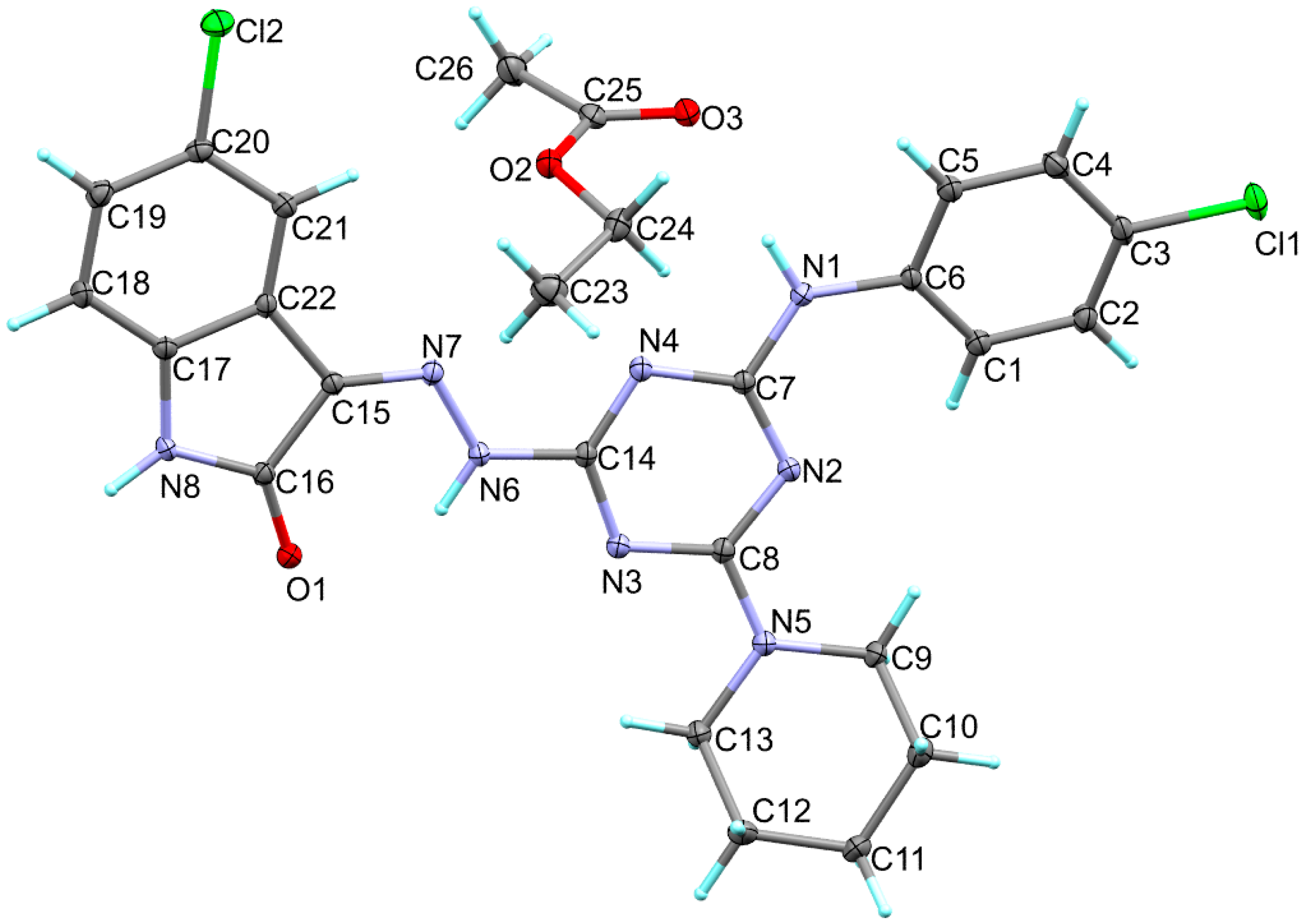
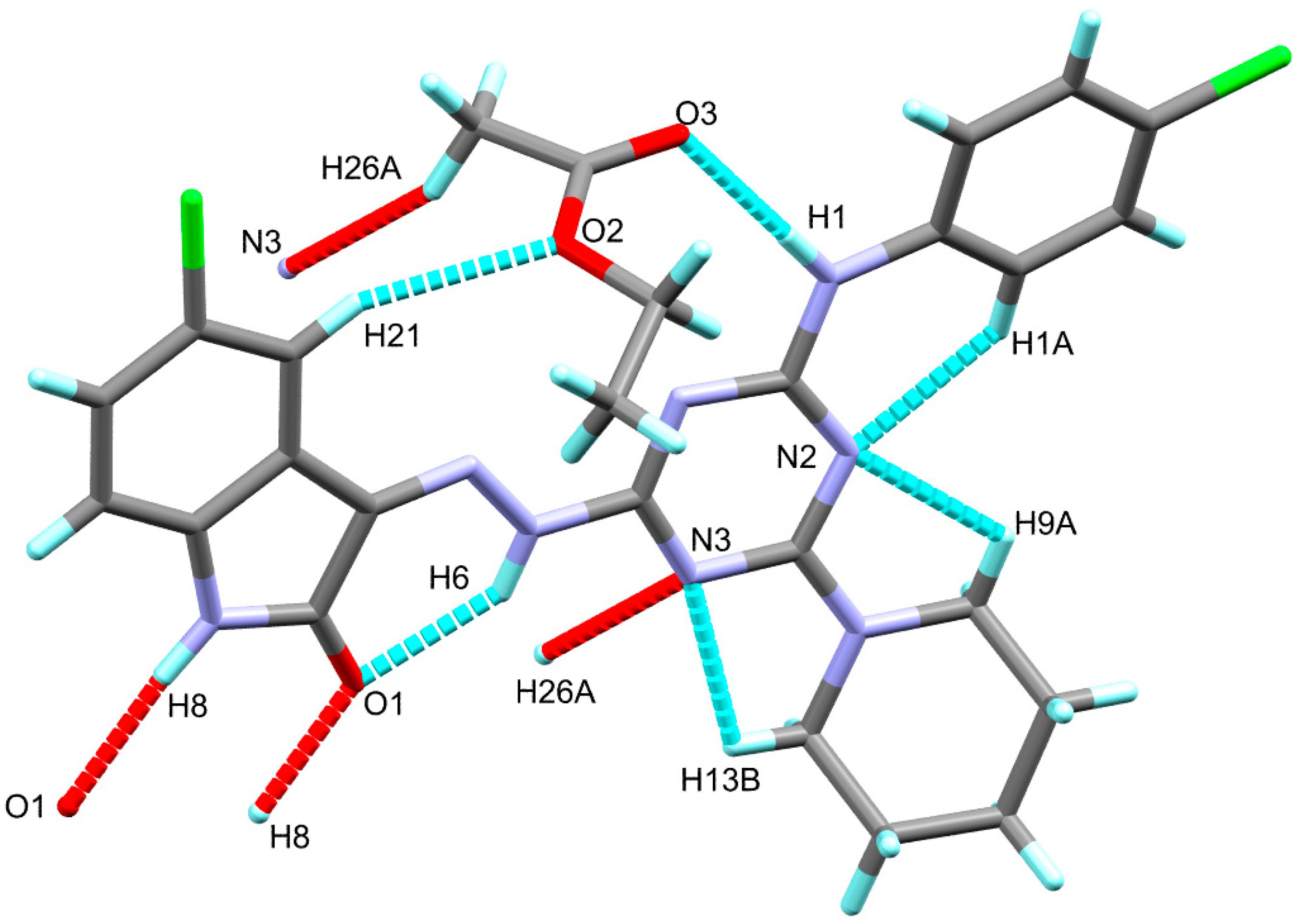
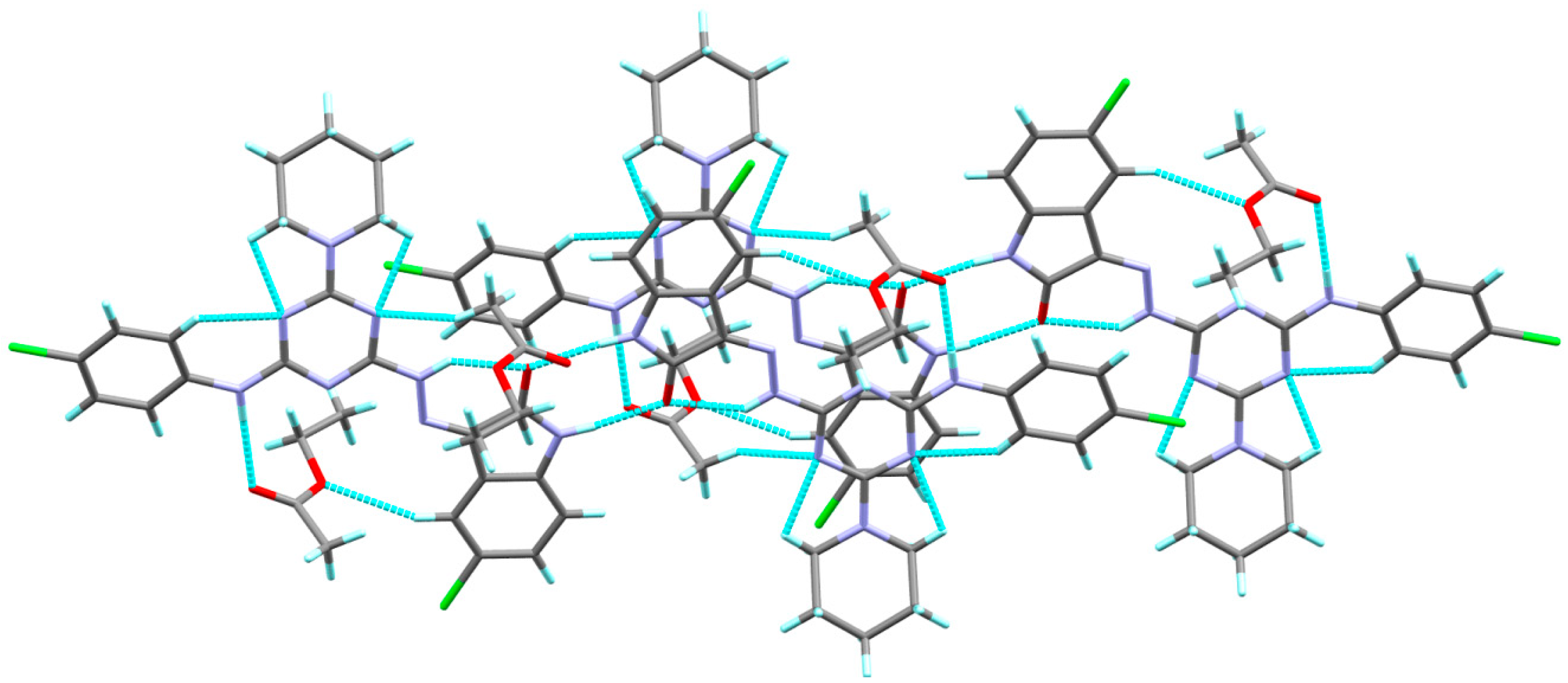
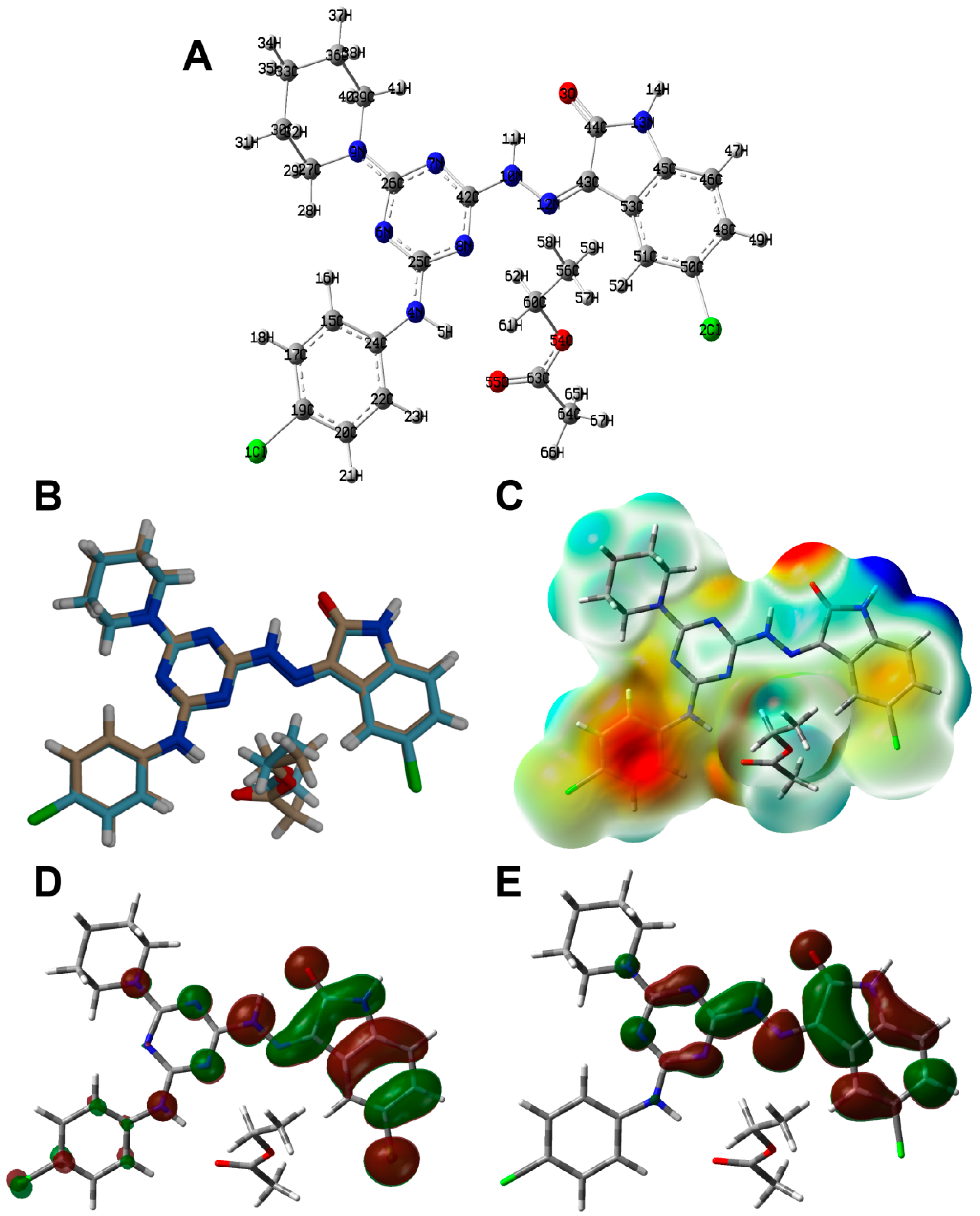
3.2. X-ray Structure Description
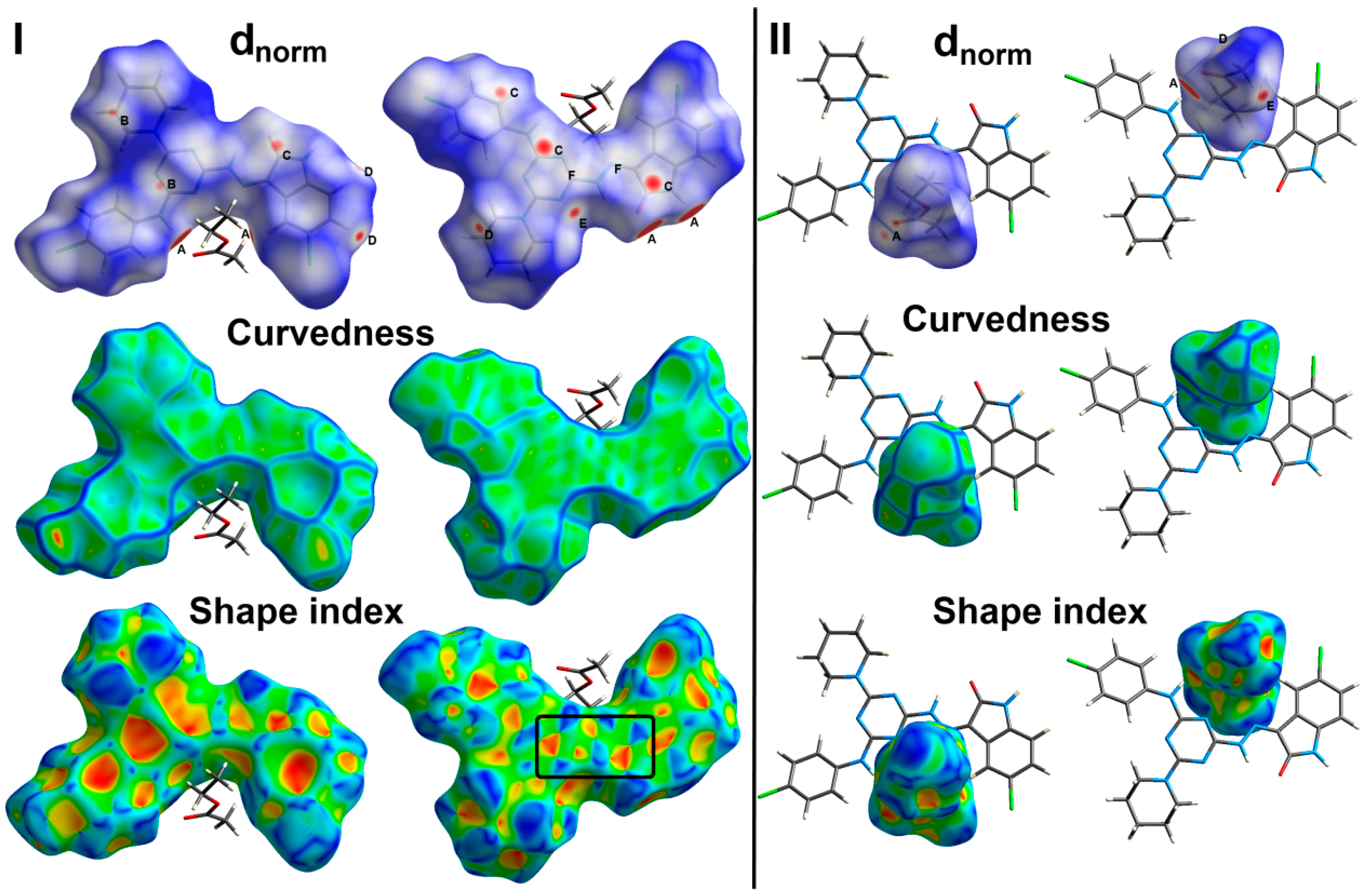
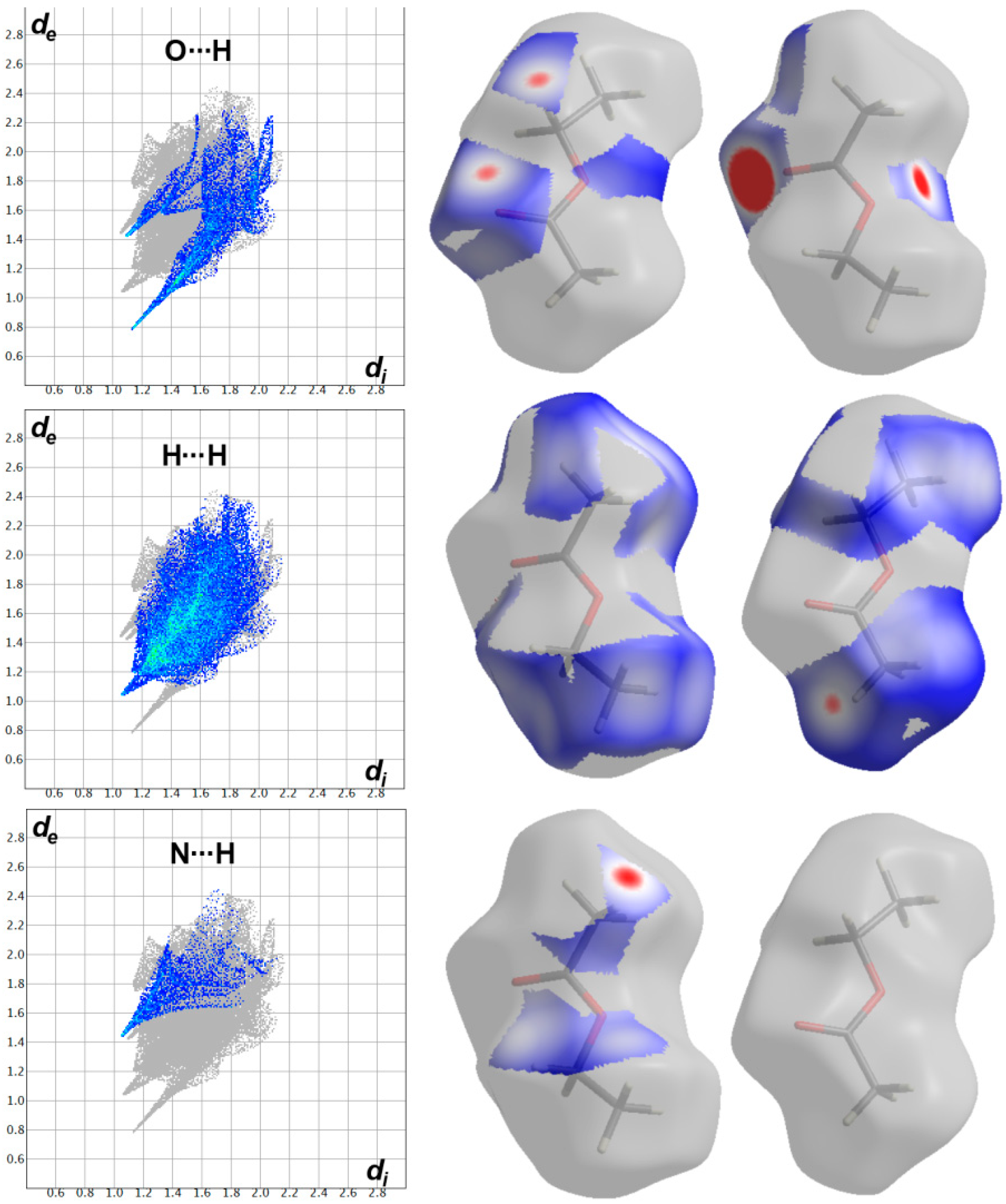
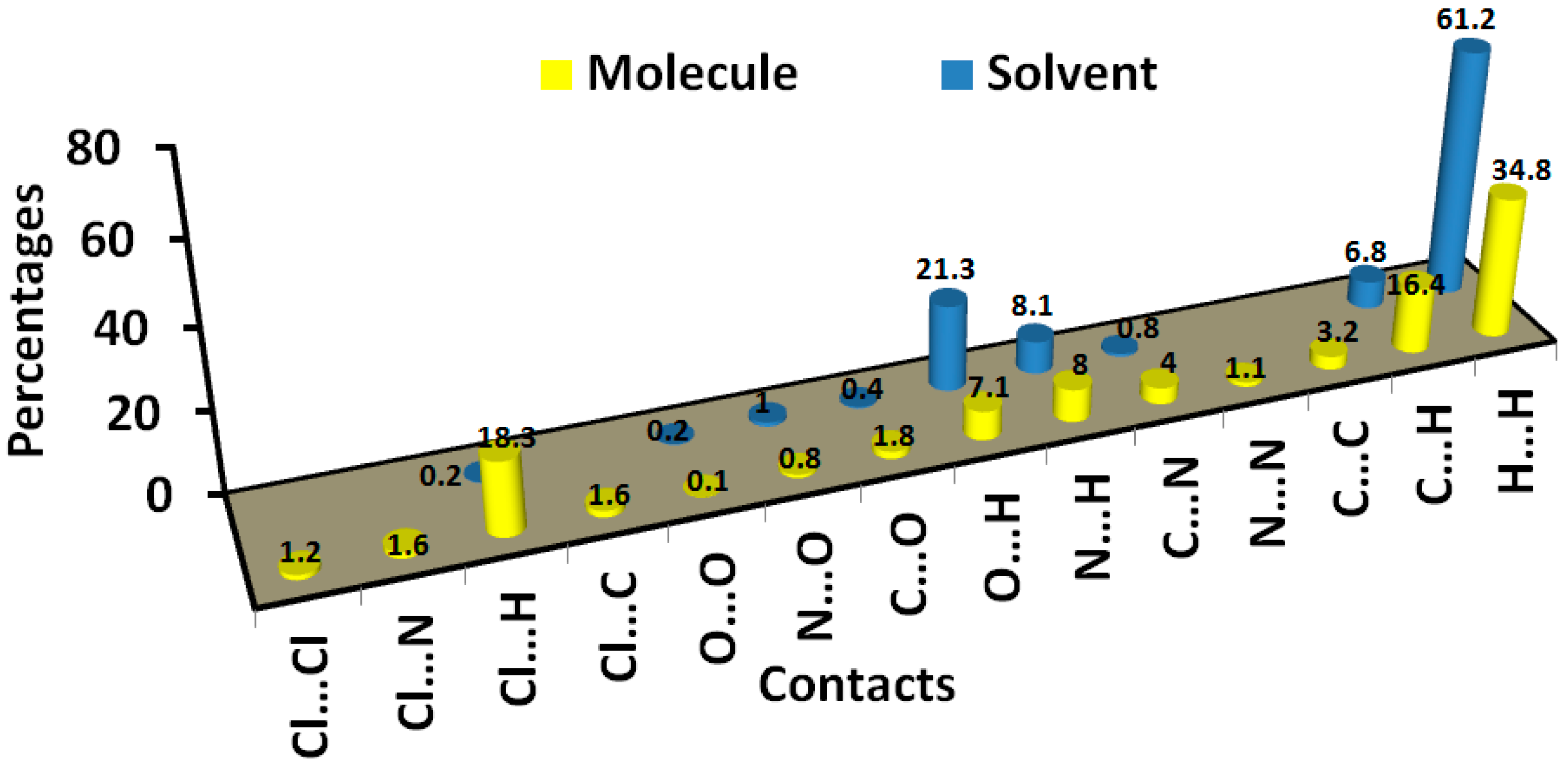
3.3. Hirshfeld Surface Analysis
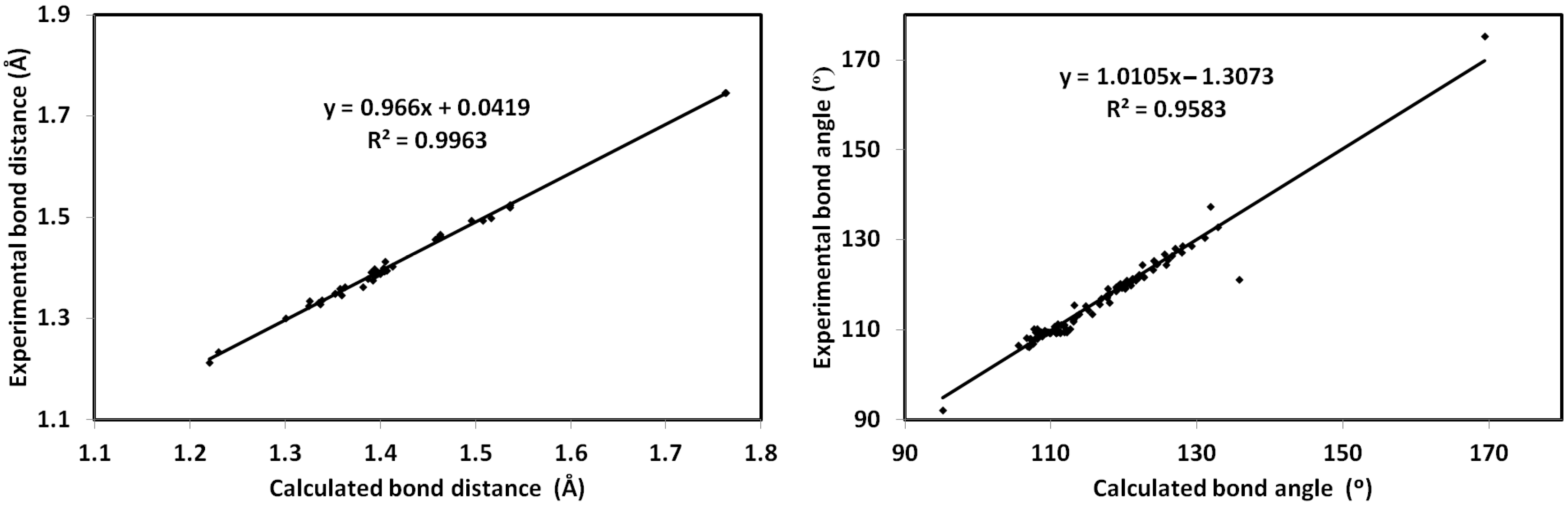
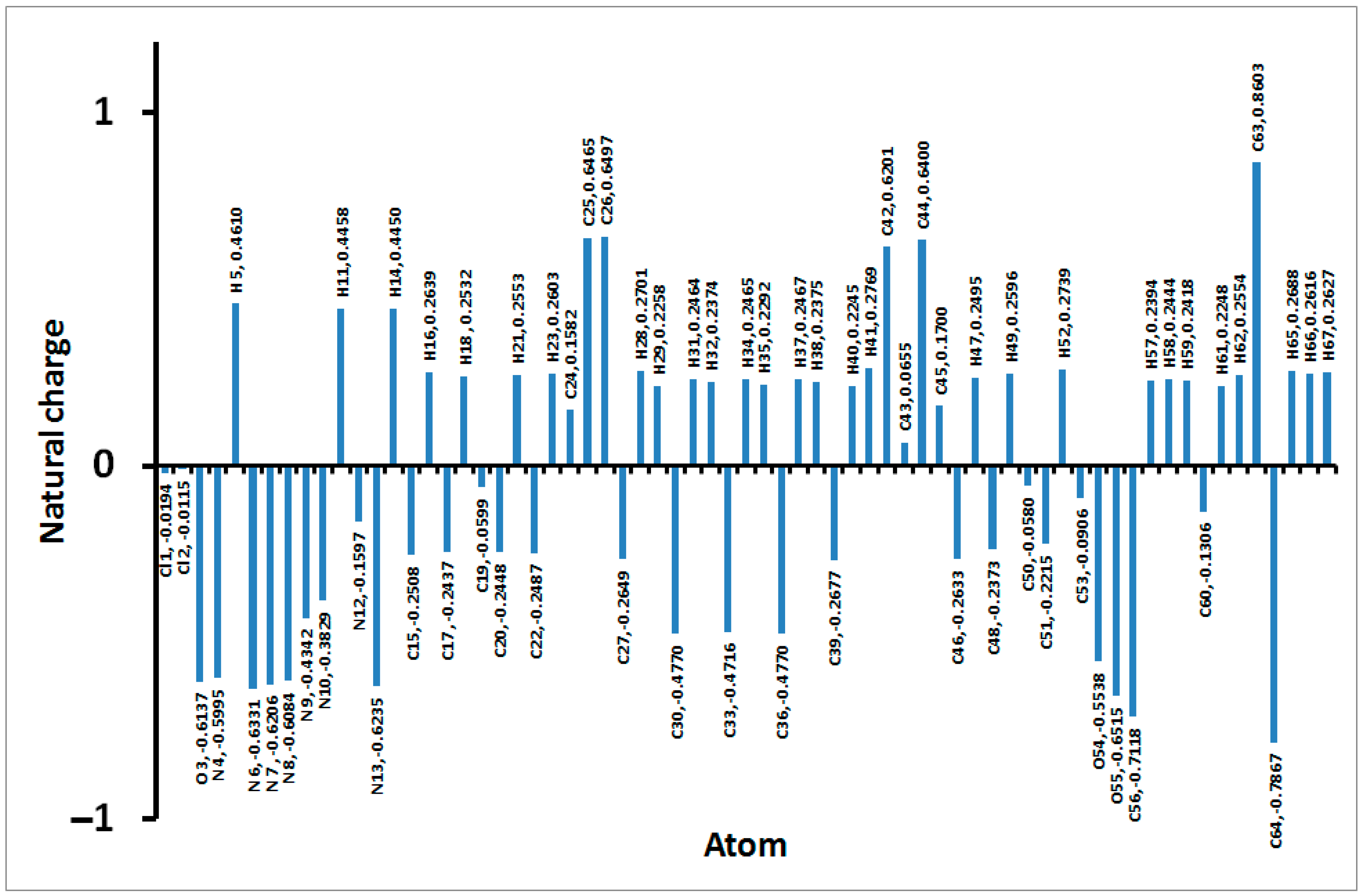
3.4. DFT Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maliszewski, D.; Drozdowska, D. Recent Advances in the Biological Activity of s-Triazine Core Compounds. Pharmaceuticals 2022, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; AlOtaibi, B.S.; Thamer, B.M.; El-Faham, A. Synthesis of New S-Triazine Bishydrazino and Bishydrazido-Based Polymers and Their Application in Flame-Retardant Polypropylene Composites. Polymers 2022, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Ahmed, M.A.; Al-Lohedan, H.A.; El-Faham, A. Multi-Functional Cardanol Triazine Schiff Base Polyimine Additives for Self-Healing and Super-Hydrophobic Epoxy of Steel Coating. Coatings 2020, 10, 327. [Google Scholar] [CrossRef]
- Blotny, G. Recent Applications of 2,4,6-Trichloro-1,3,5-Triazine and Its Derivatives in Organic Synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Fan, M.; Kim, E.Y.; Shin, J.H.; Lee, J.E.; Son, E.J.; Kim, J.; Hwang, E.; Yim, M.S.; Kim, E.-H.; et al. Amphiphilic Triazine Polymer Derivatives as Antibacterial and Anti-atopic Agents in Mice Model. Sci. Rep. 2019, 9, 15161. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Utreja, D. Recent Advances in Synthesis and Antifungal Activity of 1,3,5-triazines. Curr. Org. Synth. 2016, 13, 484–503. [Google Scholar] [CrossRef]
- Adhikari, N.; Kashyap, A.; Shakya, A.; Ghosh, S.K.; Bhattacharyya, D.R.; Bhat, H.R.; Singh, U.P. Microwave Assisted Synthesis, Docking and Antimalarial Evaluation of Hybrid PABA-Substituted 1,3,5-Triazine Derivatives. J. Heterocyc. Chem. 2020, 57, 2389–2399. [Google Scholar] [CrossRef]
- Zacharie, B.; Abbott, S.D.; Duceppe, J.S.; Gagnon, L.; Grouix, B.; Geerts, L.; Laurin, P. Design and Synthesis of New 1,3,5- Trisubstituted Triazines for the Treatment of Cancer and Inflammation. ChemistryOpen 2018, 7, 737–749. [Google Scholar] [CrossRef]
- Baréa, P.; Barbosa, V.A.; Bidóia, D.L.; de Paula, J.C.; Stefanello, T.F.; da Costa, W.F.; Sarragiotto, M.H. Synthesis, Antileishmanial Activity and Mechanism of Action Studies of Novel Β-Carboline-1,3,5-Triazine Hybrids. Eur. J. Med. Chem. 2018, 150, 579–590. [Google Scholar] [CrossRef]
- Barakat, A.; El-Senduny, F.F.; Almarhoon, Z.; Al-Rasheed, H.H.; Badria, F.A.; Al-Majid, A.M.; El-Faham, A. Synthesis, X-ray Crystal Structures, and Preliminary Antiproliferative Activities of New s-Triazine-hydroxybenzylidene Hydrazone Derivatives. J. Chem. 2019, 2019, 9403908. [Google Scholar] [CrossRef]
- Shvekhgeimer, M.-G.A. Synthesis of Heterocyclic Compounds by the Cyclization of Isatin and Its Derivatives (Review). Chem. Heterocycl. Compd. 1996, 32, 249–276. [Google Scholar] [CrossRef]
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The Chemistry of Isatins: A Review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Basedia, D.K.; Dubey, B.K.; Shrivastava, B. A Review on Synthesis and Biological Activity of Heterocyclic Compounds Bearing 1,3,5-Triazine Lead Moiety. Am. J. Pharm. Res. 2011, 1, 174–193. [Google Scholar]
- Shen, J.; Zhang, L.; Meng, X. Recent Advances in Cyclization Reactions of Isatins or Thioisatins via C–N Or C–S Bond Cleavage. Org. Chem. Front. 2021, 8, 6433. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A. Advances in Pharmacology of Isatin and its Derivatives: A Review. Trop. J. Pharm. Res. 2015, 14, 1937–1942. [Google Scholar] [CrossRef]
- Bhrigu, B.; Pathak, D.; Siddiqui, N.; Alam, M.S.; Ahsan, W. Search for Biological Active Isatins: A Short Review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 229–235. [Google Scholar]
- Smitha, S.; Pandeya, S.N.; Stables, J.P.; Ganapathy, S. Anticonvulsant and Sedative-Hypnotic Activities of N-Acetyl/Methyl Isatin Derivatives. Sci. Pharm. 2008, 76, 621–636. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between Direct Contact and Extract Exposure Methods for PFO Cytotoxicity Evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Ghodsi, M.Z.; Zahra, P.; Fatemeh, M.; Mohammad, G.; Rajender, S.V. An Overview of Recent Advances in Isatin-Based Multicomponent Reactions. Curr. Org. Chem. 2022, 26, 1485–1502. [Google Scholar]
- Yan, L.J.; Wang, Y.C. Recent Advances in Green Synthesis of 3,3′-Spirooxindoles via Isatin–based One–pot Multicomponent Cascade Reactions in Aqueous Medium. ChemistrySelect 2016, 1, 6948–6960. [Google Scholar] [CrossRef]
- Shanmugakala, R.; Tharmaraj, P.; Sheela, C.D.; Chidambaranathan, N. Transition Metal Complexes of S-Triazine Derivative: New Class of Anticonvulsant, Anti-Inflammatory, and Neuroprotective Agents. Med. Chem. Res. 2014, 23, 329–342. [Google Scholar] [CrossRef]
- Shawish, I.; Barakat, A.; Aldalbahi, A.; Malebari, A.M.; Nafie, M.S.; Bekhit, A.A.; Albohy, A.; Khan, A.; Ul-Haq, Z.; Haukka, M.; et al. Synthesis and Antiproliferative Activity of a New Series of Monoand Bis(dimethylpyrazolyl)-s-triazine Derivatives Targeting EGFR/PI3K/AKT/mTOR Signaling Cascades. ACS Omega 2022, 7, 24858–24870. [Google Scholar] [CrossRef] [PubMed]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Oxfordshire, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 (2017) University of Western Australia. Available online: https://crystalexplorer.net/download/ (accessed on 1 July 2017).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. GAUSSIAN 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.D., II; Keith, T.A.; Millam, J.M. GaussView, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from A Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Koopmans, T.A. Ordering of WaveFunctions and Eigenenergies to the Individual Electrons of an Atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A Combined Experimental and Quantum Chemical (DFT And AIM) Study on Molecular Structure, Spectroscopic Properties, NBO and Multiple Interaction Analysis in A Novel Ethyl 4-[2-(Carbamoyl) Hydrazinylidene]-3,5-Dimethyl-1H-Pyrrole-2-Carboxylate and Its Dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Hubert Joe, I.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoretical and Vibrational Spectral Investigation of Sodium Salt of Acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar]
- Sebastian, S.; Sundaraganesan, N. The Spectroscopic (FT-IR, FT-IR Gas Phase, FT-Raman and UV) and NBO Analysis of 4-Hydroxypiperidine by Density Functional Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef]

| 6c | |
|---|---|
| CCDC | 2214508 |
| empirical formula | C26H28Cl2N8O3 |
| Fw | 571.46 |
| temp (K) | 120(2) |
| λ (Å) | 0.71073 |
| cryst syst | Triclinic |
| space group | P |
| a (Å) | 10.3368(6) |
| b (Å) | 11.9804(8) |
| c (Å) | 12.7250(5) |
| α (deg) | 100.904(4) |
| β (deg) | 107.959(4) |
| γ(deg) | 109.638(6) |
| V (Å3) | 1334.36(14) |
| Z | 2 |
| ρcalc (Mg/m3) | 1.422 |
| μ (Mo Kα) (mm−1) | 0.289 |
| No. reflns. | 12754 |
| Unique reflns. | 7212 |
| Completeness to θ = 67.684° | 100% |
| GOOF (F2) | 1.031 |
| Rint | 0.0286 |
| R1 a (I ≥ 2σ) | 0.0482 |
| wR2 b (I ≥ 2σ) | 0.0953 |
| Atoms | Distance | Atoms | Distance |
|---|---|---|---|
| Cl1-C3 | 1.746 | N4-C7 | 1.3592 |
| Cl2-C20 | 1.7472 | N5-C8 | 1.3462 |
| O1-C16 | 1.2352 | N5-C13 | 1.4632 |
| N1-C7 | 1.3632 | N5-C9 | 1.4652 |
| N1-C6 | 1.4132 | N6-N7 | 1.3352 |
| N2-C7 | 1.3292 | N6-C14 | 1.3862 |
| N2-C8 | 1.3502 | N7-C15 | 1.3012 |
| N3-C14 | 1.3322 | N8-C16 | 1.3622 |
| N3-C8 | 1.3502 | N8-C17 | 1.3982 |
| N4-C14 | 1.3252 |
| D-H...A | d(D-H) | d(H…A) | d(D…A) | <(DHA) | Symm. Codes |
|---|---|---|---|---|---|
| N1-H1…O3 | 0.88(2) | 2.05(2) | 2.931(2) | 175(2) | |
| C21-H21…O2 | 0.95 | 2.53 | 3.433(3) | 159 | |
| N6-H6…O1 | 0.88 | 2.04 | 2.755(2) | 137 | |
| N8-H8…O1 | 0.88 | 1.98 | 2.854(2) | 170 | 2-x, 1-y, 1-z |
| C1-H1A…N2 | 0.95 | 2.35 | 2.922(3) | 118 | |
| C9-H9A…N2 | 0.99 | 2.31 | 2.750(3) | 106 | |
| C13-H13B…N3 | 0.99 | 2.31 | 2.747(3) | 106 | |
| C26-H26A…N3 | 0.98 | 2.59 | 3.461(3) | 149 | 1-x, 1-y, 1-z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rasheed, H.H.; AL-khamis, S.A.; Barakat, A.; El-Faham, A.; Haukka, M.; Soliman, S.M. Synthesis and Characterizations of Novel Isatin-s-Triazine Hydrazone Derivatives; X-ray Structure, Hirshfeld Analysis and DFT Calculations. Crystals 2023, 13, 305. https://doi.org/10.3390/cryst13020305
Al-Rasheed HH, AL-khamis SA, Barakat A, El-Faham A, Haukka M, Soliman SM. Synthesis and Characterizations of Novel Isatin-s-Triazine Hydrazone Derivatives; X-ray Structure, Hirshfeld Analysis and DFT Calculations. Crystals. 2023; 13(2):305. https://doi.org/10.3390/cryst13020305
Chicago/Turabian StyleAl-Rasheed, Hessa H., Sarah A. AL-khamis, Assem Barakat, Ayman El-Faham, Matti Haukka, and Saied M. Soliman. 2023. "Synthesis and Characterizations of Novel Isatin-s-Triazine Hydrazone Derivatives; X-ray Structure, Hirshfeld Analysis and DFT Calculations" Crystals 13, no. 2: 305. https://doi.org/10.3390/cryst13020305
APA StyleAl-Rasheed, H. H., AL-khamis, S. A., Barakat, A., El-Faham, A., Haukka, M., & Soliman, S. M. (2023). Synthesis and Characterizations of Novel Isatin-s-Triazine Hydrazone Derivatives; X-ray Structure, Hirshfeld Analysis and DFT Calculations. Crystals, 13(2), 305. https://doi.org/10.3390/cryst13020305










