Abstract
A novel series of isatin-s-triazine hydrazone derivatives has been synthesized and reported herein. The synthetic methodology involved the reaction of s-triazine hydrazine precursors with isatin derivatives in the presence of CH3COOH as a catalyst and EtOH as solvent to afford the corresponding target products 6a-e in high yields and purities. The characterization data obtained from elemental analysis, FT-IR, NMR (1H- and 13C-) were in full agreement with the expected structures. Furthermore, an X-ray single crystal diffraction study of one of the target s-triazine hydrazone derivatives, 6c confirmed the structure of the desired compounds. It crystallized in the triclinic crystal system and P-1 space group with a = 10.3368(6) Å, b = 11.9804(8) Å, c = 12.7250(5) Å, α = 100.904(4)°, β = 107.959(4)° and γ = 109.638(6)°. The different non-covalent interactions which contributed in the molecular packing of 6c were analyzed using Hirshfeld analysis. The molecular packing of the organic part of the crystal structure showed important O…H (7.1%), C…H (16.4%), C…C (1.6%), H…H (34.8%), N…H (8.0%) and C…N (4.0%) interactions while for the crystal solvent, the O…H (21.3%), H…H (61.2%) and N…H (8.1%) contacts are the most significant. The studied compound 6c is polar and has a net dipole moment of 5.6072 Debye based on DFT study.
1. Introduction
s-Triazine derivatives have attracted the attention of many researchers due to their remarkable uses in a variety of sectors, including those in the pharmaceutical industry, polymers, and corrosion inhibitors [1,2,3]. The substantial biological activity of substituted s-triazine derivatives, such as their antibacterial, antifungal, antimalarial, anticancer, anti-inflammatory, and antileishmanial effects, have made them desirable candidates in medicinal chemistry [4,5,6,7,8,9,10].
On the other hand, isatin derivatives have diverse applications in biology and pharmaceutics [11,12]. Additionally, isatin derivatives are considered as starting materials for the synthesis of many heterocycles [13,14]. These compounds are also used as anticancer, antibiotic and antidepressant [15,16,17,18,19,20]. Shanmugakala et al. reported some metal complexes of indolin-3-one/1,3,5-triazine hybrid as organic ligand. The in vivo studies revealed a potential anticonvulsant as well as anti-inflammatory actions of these complexes [21].
In continuation to our interest in developing a new s-triazine hydrazone derivatives with isatin to explore new derivatives of compounds incorporated the two moieties, s-triazine and istain, with hydrazone linkage. The supramolecular structural properties of one of the synthesized s-triazine derivatives 6c were explored using single crystal X-ray diffraction analysis augmented with Hirshfeld calculations. Additionally, DFT calculations were performed to analyze the molecular and electronic aspects of this compound.
2. Experimental
2.1. Materials and Methods
All materials and methods details are given in Method S1 (Supplementary Data).
2.2. Synthetic Methodology
Synthesis of the compounds 2–4 was performed using the reported method [22] and their spectral characterizations are described in details in Method S2 (Supplementary Data).
2.2.1. General Method for the Synthesis of 1,3,5-Triazine-Hydrazone Derivatives, 6a–e
Substituted isatin 5a–e (2 mmoles) was dissolved in 30 mL ethanol and 2–3 drops of acetic acid (AcOH). Then s-triazine hydrazine 4 (2 mmoles) was added portion wise on the hot solution and stirred. After complete addition, the reaction mixture was refluxed for 3–4 h (the reaction followed by TLC using ethylacetate-hexane 2:1). At the end, the reaction was left to cool slowly to room temperature and then the product was collected by filtration, washed with cold ethanol, and dried to produce the pure products, as indicated from the spectral data.
Synthesis of 3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-one, 6a
The product was obtained as a white solid in yield 94%; mp 254–256 °C; IR (KBr, cm−1): 3281 (NH), 1581(C=N), 1488, 1412 (C=C); 1H NMR (DMSO-d6): δ = 1.49 (s, 4H, 2CH2), 1.59 (s, 2H, CH2), 3.72 (d, J = 62.1 Hz, 4H, 2CH2), 7.06–6.88 (m, 4H, 4CH), 7.47–7.46 (m, 3H, 3CH), 7.74 (s, 1H, CH), 9.75 (s, 1H, NH), 11.26 (s, 1H, NH-CO), 12.56 (s, 1H, NH-N=) ppm; 13C NMR (DMSO-d6): δ = 24.2, 25.4, 43.9, 64.3, 110.9, 119.9, 120.4, 121.2, 122.5, 125.6, 128.4, 130.4, 132.9, 138.9, 141.3, 163.1, 163.9 ppm. Anal. Calc. for C22H21ClN8O (448.91): C, 58.86; H, 4.72; N, 24.96. Found: C, 58.99; H, 4.65; N, 24.87.
5-Bromo-3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-one, 6b
The product was obtained as a yellow solid in yield 92%; mp 290–291 °C; IR (KBr, cm−1): 3151 (NH), 1581(C=N), 1486, 1412 (C=C); 1H NMR (DMSO-d6): δ = δ1.45 (d, J = 65.0 Hz, 4H, 2CH2), 1.66 (m, 2H, CH2),3.81–3.59 (m, 4H, 2CH2), 6.7 (m, 4H, 4CH), 7.38–7.15 (m, 2H, 2CH), 7.5 (s, 1H, CH), 9.5 (s, 1H, NH), 11.11 (s, 1H, NH-CO), 12.32 (s, 1H, NH-N=) ppm; 13C NMR (DMSO-d6): δ = 24.2, 25.4, 59.7, 112.9, 114.0, 121.1, 121.9, 122.6, 125.6, 128.3, 131.6, 132.4, 138.86, 140.3, 162.6, 163.7, 164.2, 170.3 ppm. Anal. Calc. for C22H20BrClN8O (527.80): C, 50.06; H, 3.82; N, 21.23. Found C, 50.23; H, 3.95; N, 21.51.
5-Chloro-3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)indolin-2-One, 6c
The product was obtained as a yellow solid in yield 99%; mp 291–293 °C; IR (KBr, cm−1): 3291 (NH), 1582(C=N), 1488, 1418 (C=C); 1H NMR (DMSO-d6): δ = 1.49 (s, 4H, 2CH2), 1.59 (s, 2H, CH2), 3.71 (m, 4H, 2CH2), 6.9–6.88 (m, 4H, 4CH), 7.38–7.2 (m, 2H, 2CH), 7.72 (s, 1H, CH), 9.73 (s, 1H, NH), 11.26 (s, 1H, NH-CO), 12.48 (s, 1H, NH-N=) ppm; 13C NMR (DMSO-d6): δ = 24.2, 25.5, 43.9, 112.44, 119.3, 121.1, 121.2, 122.2, 125.6, 126.5, 128.3, 129.7, 131.8, 138.9, 139.94, 162.9, 163.7, 164.2, 172.1 ppm. Anal. Calc. for C22H20Cl2N8O (483.35): C, 54.67; H, 4.17; N, 23.18. Found C, 54.89; H, 4.31; N, 23.36.
3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)-5-fluoroindolin-2-one, 6d
The product was obtained as a yellow solid in yield 93%; mp 276–278 °C; IR (KBr, cm−1): 3220 (NH), 1582(C=N), 1486, 1420 (C=C); 1H NMR (DMSO-d6): δ = 1.49 (s, 4H, 2CH2), 1.59 (s, 2H, CH2), 3.72 (m, 4H, 2CH2), 7.21–6.87 (m, 4H, 4CH), 7.31–7.29 (m, 2H, 2CH), 7.73 (s, 1H, CH), 9.73 (s, 1H, NH), 11.17 (s, 1H, NH-CO), 12.57 (s, 1H, NH-N=) ppm; 13C NMR (DMSO-d6): δ = 24.2, 25.4, 43.9, 106.8, 106.9, 111.9, 112.0, 116.6, 116.8, 121.2, 121.7, 125.6, 128.4, 132.4, 137.6, 138.9, 157.3, 159.2, 163.2, 163.8, 164.2,172.0 ppm. Anal. Calc. for C22H20ClFN8O (466.90): C, 56.59; H, 4.32; N, 24.00. Found C, 56.71; H, 4.48; N, 24.23.
3-(2-(4-((4-Chlorophenyl)amino)-6-(piperidin-1-yl)-1,3,5-triazin-2-yl)hydrazineylidene)-5-methylindolin-2-one, 6e
The product was obtained as a yellow solid in yield 77%; mp 296–297 °C; IR (KBr, cm−1): 3287 (NH), 1581 (C=N), 1494, 1422 (C=C); 1H NMR (DMSO-d6): δ = 1.49 (s, 4H, 2CH2), 1.59 (s, 2H, CH2), 2.26 (s, 3H, CH3), 3.72 (m, 4H, 2CH2), 7.26–6.76 (m, 4H, 4CH), 7.30–7.29 (m, 2H, 2CH), 7.72 (s, 1H, CH), 9.73 (s, 1H, NH), 11.05 (s, 1H, NH-CO), 12.55 (s, 1H, NH-N=) ppm; 13C NMR (DMSO-d6): δ = 24.2, 25.4, 43.9, 106.8, 107.0, 112.0, 112.0, 116.6, 116.8, 121.2, 121.8, 125.6, 128.4, 132.4, 137.6, 138.9, 157.4, 159.2, 163.2, 163.8, 164.2, 172.1 ppm. Anal. Calc. for C23H23FN8O (446.48): C, 61.87; H, 5.19; N, 25.10. Found C, 61.96; H, 5.32; N, 25.35.
2.3. X-ray Structure Determinations
The crystal of 6c was solved using the method described in Method S3 (Supplementary Data) [23,24,25,26]. The crystallographic details are summarized in Table 1.

Table 1.
Crystal data for 6c.
2.4. Hirshfeld and DFT Computations
The details of the topology [27] and DFT [28,29,30] calculations are summarized in Method S4 (Supplementary Data).
3. Results and Discussion
3.1. Synthesis and Characterizations
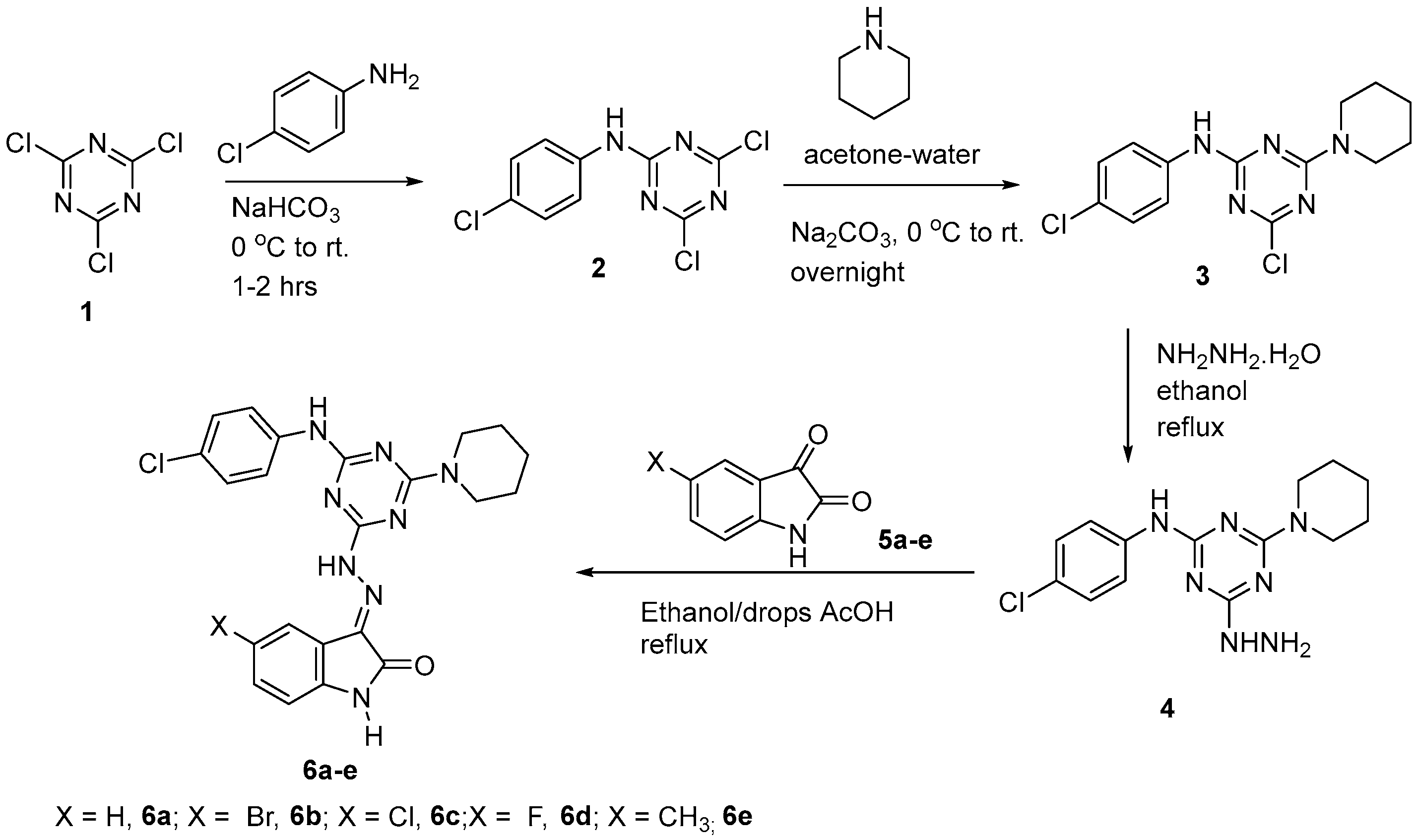
The initial step for the synthesis of the target products involved the reaction of cyanuric chloride 1 with p-chloroaninline via nucleophilic aromatic substitution of one of the chlorine atoms at 0 °C in the presence of the alkaline medium of NaHCO3 following the previously reported method [22] to afford the derivative 2. The second step involved a further displacement of the second chlorine atom by piperidine as a nucleophile at room temperature to give the derivative 3 [22] in a good yield and purity, as indicated from the spectral data (Supporting Information, Figures S1 and S2). Then, the derivative 3 was treated with hydrazine hydrate (80%) in ethanol overnight under reflux to afford the hydrazine derivative 4, which reacted directly without further purification with isatin derivatives 5a–e in ethanol as a solvent in the presence of catalytic amount of acetic acid to afford the products 6a–e in good yield and purities (Scheme 1).
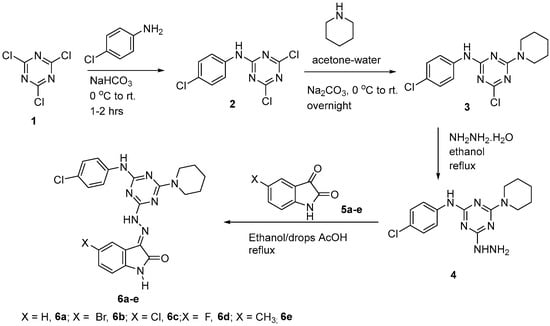
Scheme 1.
Synthetic route for preparation of s-triazine hydrazone derivatives.
3.2. X-ray Structure Description
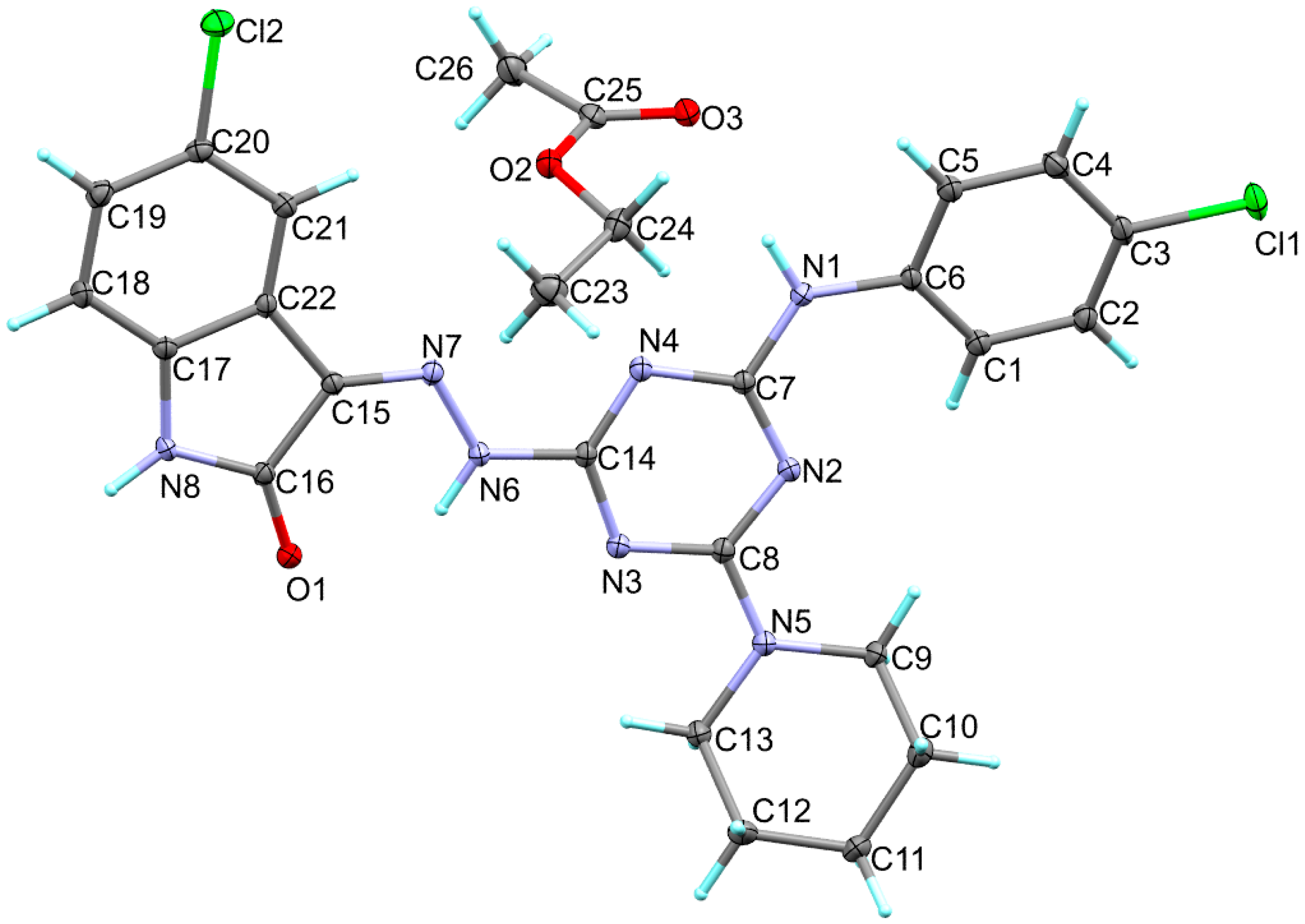
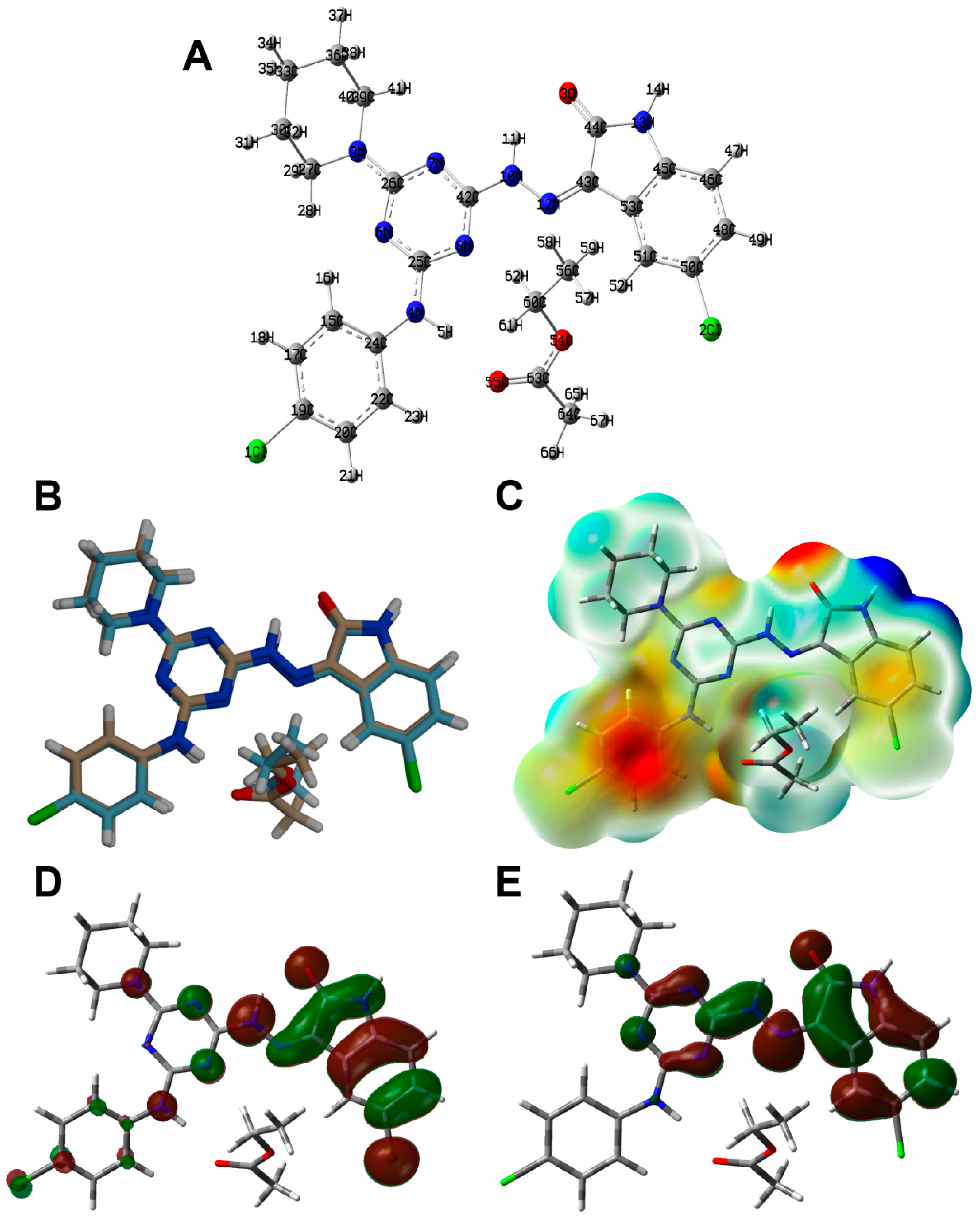
The structure of 6c is presented in Figure 1. Its crystal system is triclinic and the space group is P-1. The asymmetric unit comprised one molecule of the target compound and a co-crystallized ethyl acetate as a crystal solvent. The triclinic parameters are a = 10.3368(6) Å, b = 11.9804(8) Å, c = 12.7250(5) Å, α = 100.904(4)°, β =107.959(4)° and γ = 109.638(6)°. The unit cell volume is 1334.36 Å3 while the calculated density is 1.422 mg/m3 (Table 1). The target compound comprising one s-triazine core with three different substituents attached to the 2, 4 and 6 positions. The s-triazine core has almost planar structure where the CNCN dihedral angles do not exceed 5.1° (C14N3C8N2). The 1,3,5-triazine ring makes an angle of 14.78 and 5.99° with the aryl and indole moieties, respectively. The former showed a larger twist with respect to the triazine moiety compared to the latter. For the piperidine moiety, the ring showed a clear chair configuration. The most important geometrical parameters are listed in Table 2 and Table S1.
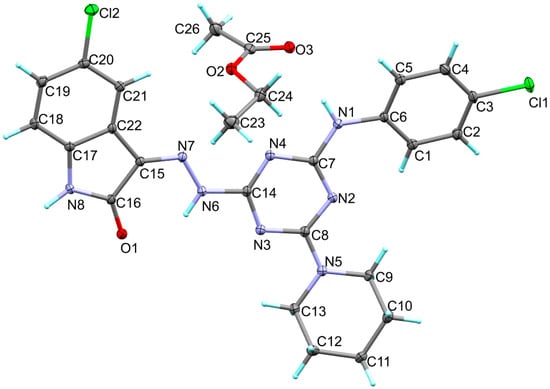
Figure 1.
X-ray structure of 6c.

Table 2.
Selected bond lengths [Å] for 6c a.
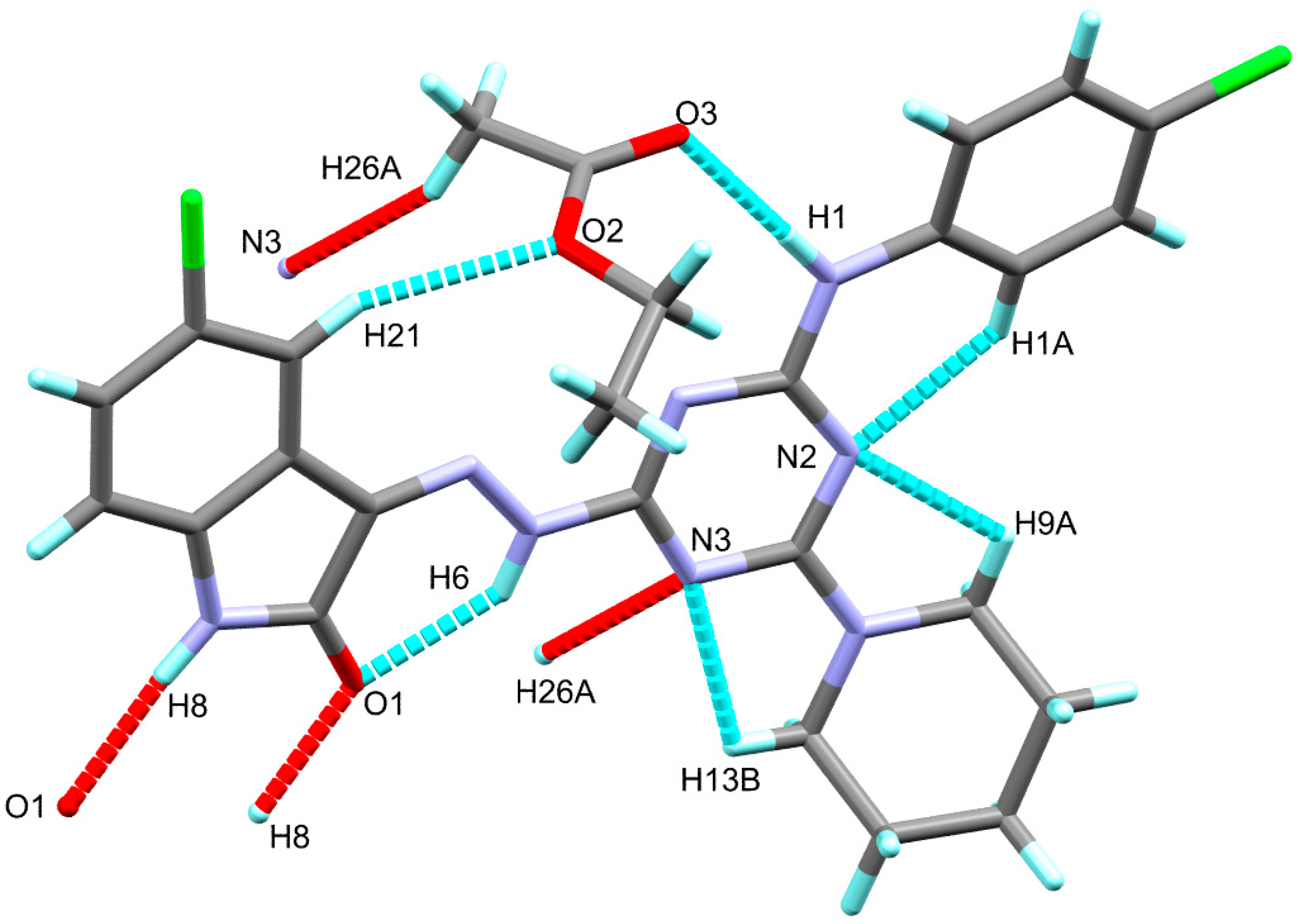
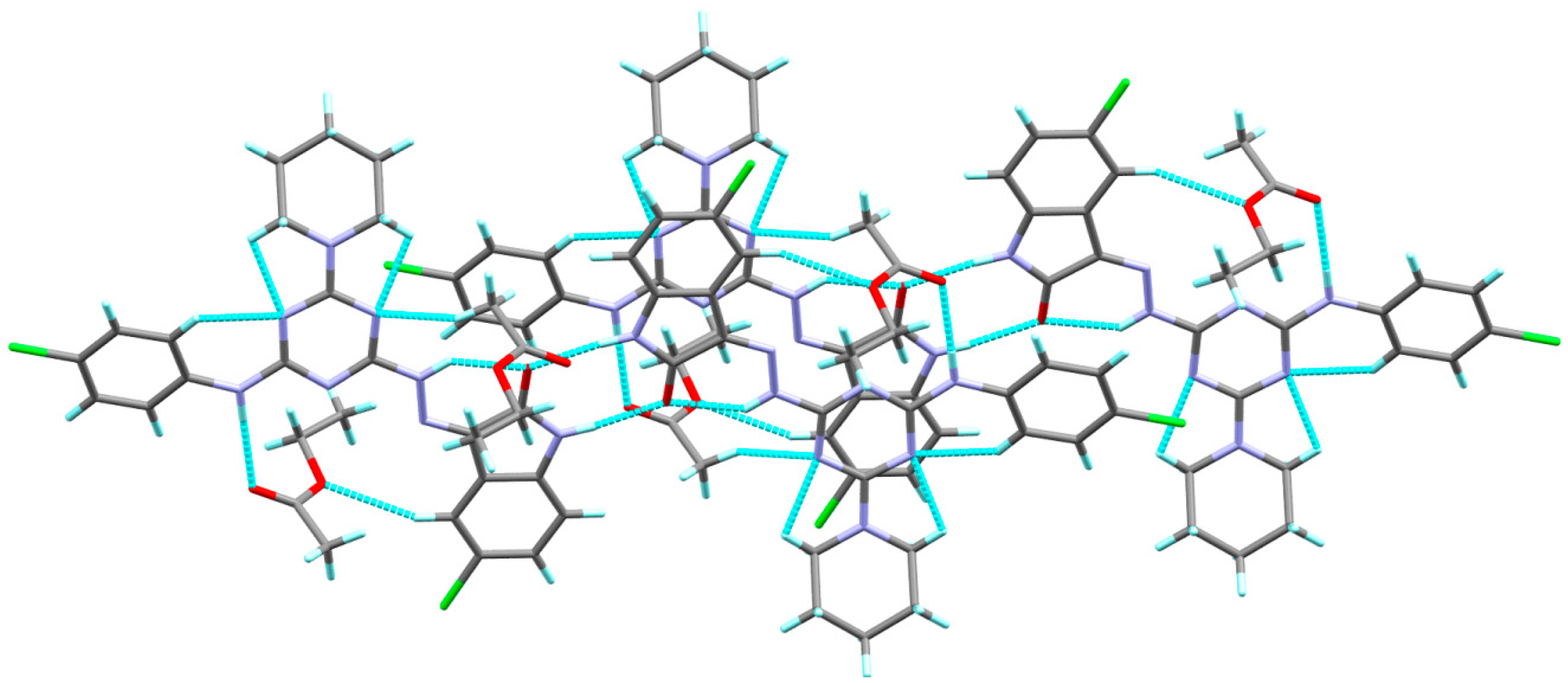
The X-ray structure of the titled compound showed a molecule of ethylacetate as a crystal solvent where both molecules are stabilized by some intra-molecular interactions including the classical N1-H1...O3 hydrogen bond and the non-classical C21-H21...O2 interactions, where the N1…O3 and C21...O2 distances are 2.931(2) and 3.433(3) Å, respectively. In addition, the crystal solvent shared in the packing scheme of 6c by the intermolecular C26-H26A...N3 interaction with C26...N3 distance of 3.461(3) Å. The donor(D)-hydrogen(H)…acceptor(A) angles are 149, 175(2) and 159°, respectively. Additionally, the molecules of the target compound are connected with each other via strong and classical N8-H8...O1 hydrogen bonds where the H…A and D…A distances are 1.98 and 2.854(2) Å, respectively, while the D-H…A angle is 170°. It is worth noting that the structure of the target organic molecule showed numerous intra-molecular interactions including the non-classical C-H…N interactions and the classical N6-H6…O1 hydrogen bond (Figure 2). List of these contacts are depicted in Table 3 while the packing scheme of this compound is shown in Figure 3.
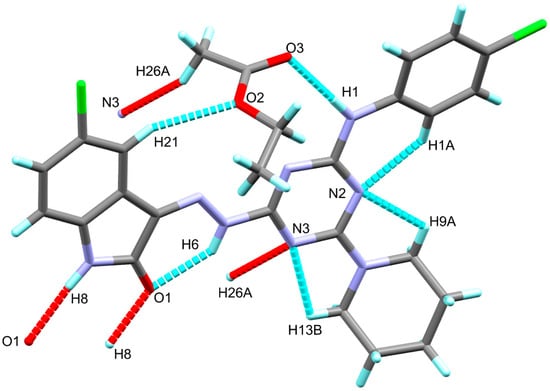
Figure 2.
Possible intra- and intermolecular contacts in 6c.

Table 3.
Hydrogen bonds for 6c [Å and °].
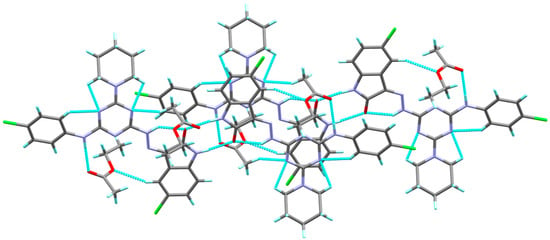
Figure 3.
Molecular packing showing the intra- and inter-molecular C-H…O and C-H…N interactions.
3.3. Hirshfeld Surface Analysis
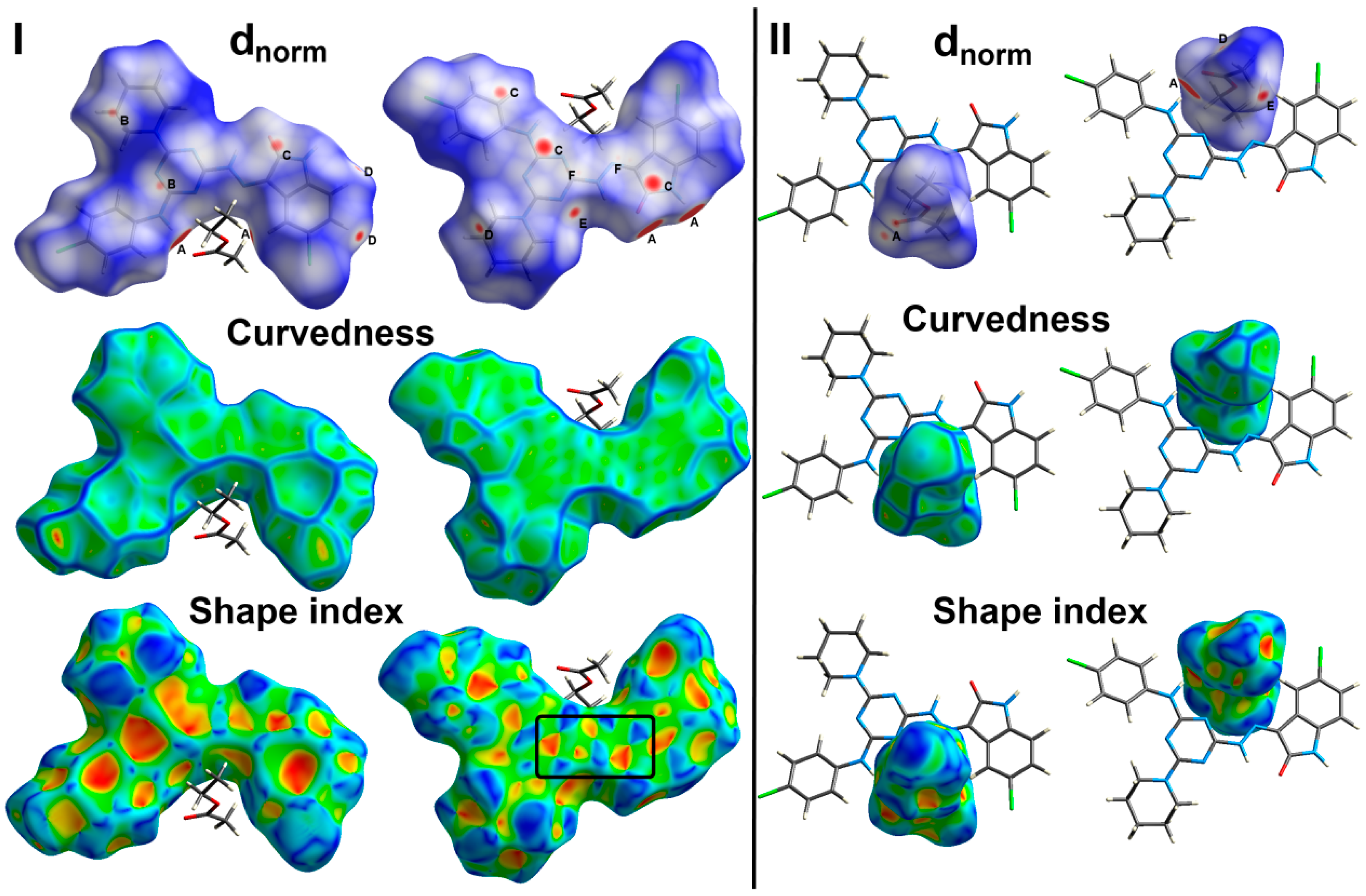
There are many weak and strong intra- and inter-molecular interactions in the crystal structure of 6c. These non-covalent interactions could be simply analyzed using Hirshfeld calculations. The resulting dnorm, shape index and curvedness maps as well as the fingerprint plot could give a full picture not only about all possible non-covalent interactions but also about the contributions of all these contacts. The studied crystal structure comprised one molecule of the target organic compound and one ethylacetate as a crystal solvent per asymmetric unit. Hence, the Hirshfeld surfaces were performed for each molecule and the resulting maps are collected in Figure 4.
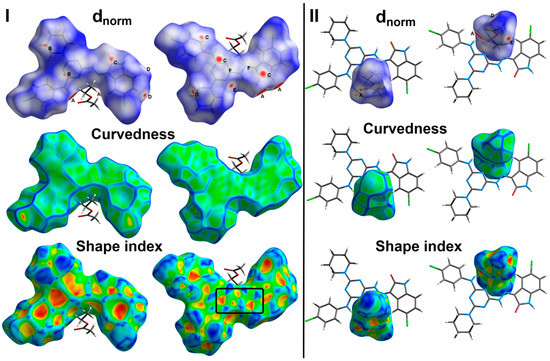
Figure 4.
Hirshfeld surfaces for the target molecule (I) and the crystal solvent (II) showing the most significant contacts; A, B, C, D, E and F refer to the short O…H, C…H, C…C, H…H, N…H and C…N interactions, respectively.
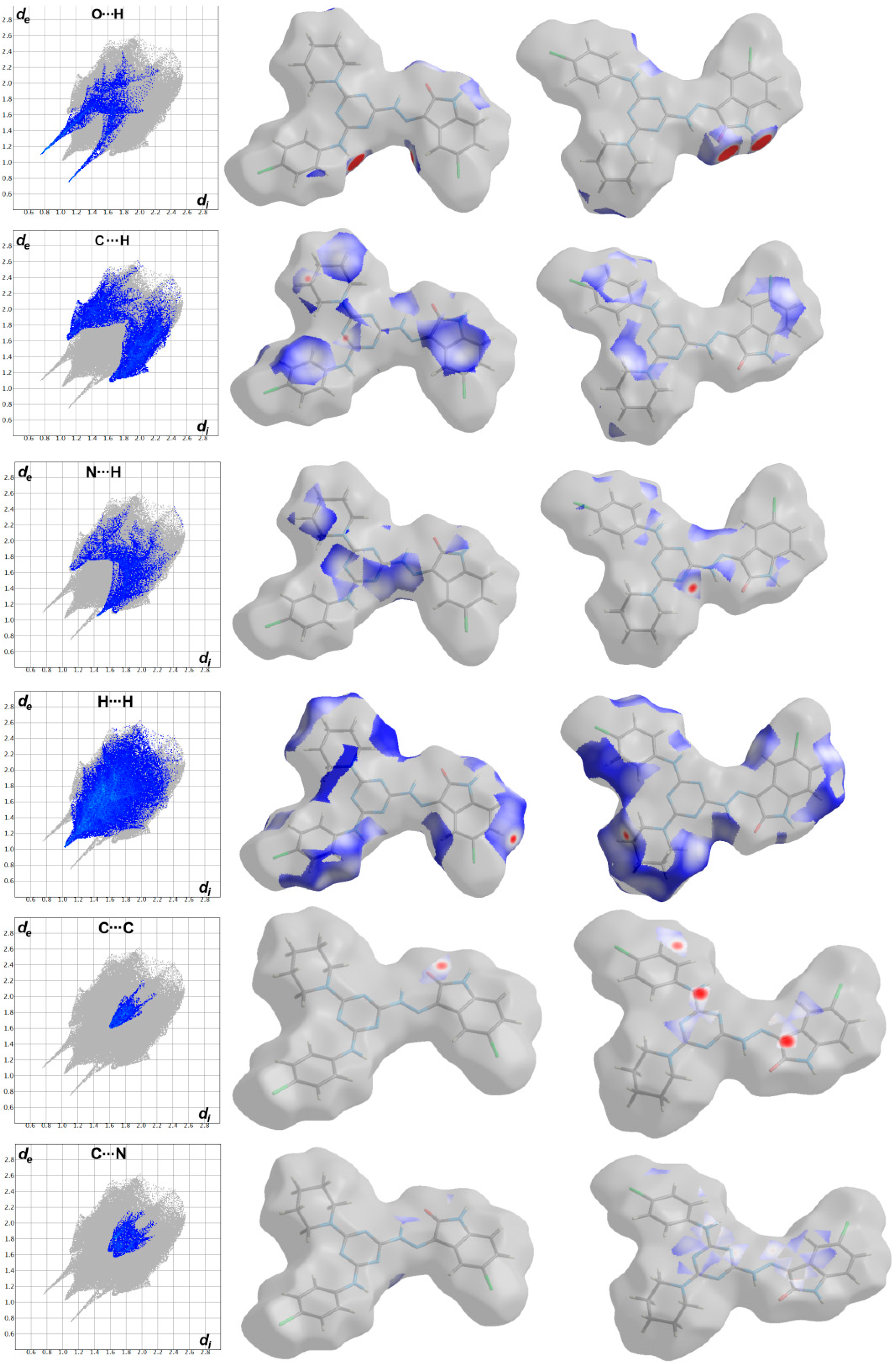
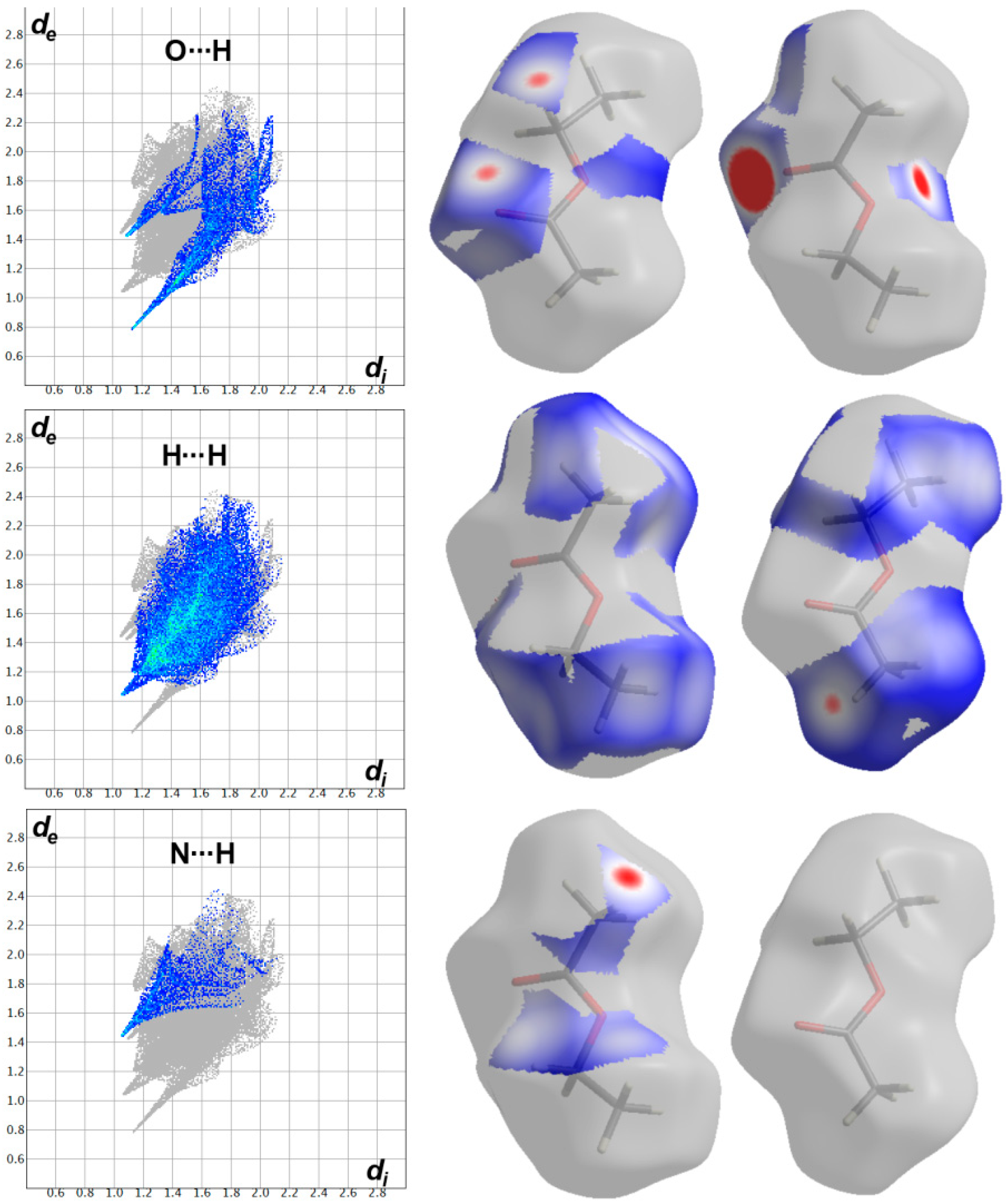
The most significant contacts appeared as red spots in the dnorm map. For simplicity, these contacts are labelled in Figure 4 by letters A to E which corresponds to the O…H, C…H, C…C, H…H, N…H and C…N contacts, respectively. The decomposed dnorm maps and fingerprint plots of these interactions are presented in Figure 5 and Figure 6 for the target organic molecule and the crystal solvent, respectively. The red regions could be detected close to the atoms which share significant interactions with other molecules outside the surface. For the target organic molecule, there are many O...H contacts occurred between the surface and the surrounding molecules. The O2…H21 (2.408 Å) and O3…H1 (1.925 Å) interactions occur between the organic target molecule and the crystal solvent. In addition, there are O3…H24A (2.513 Å) and O1…H8 (1.857 Å) short contacts. The former occurs between two molecules of the crystal solvent while the latter occurs between two of the target organic molecule. The N3…H26A contact (2.498 Å) is another polar interaction is found to occur between the N3 atom of the s-triazine core and H26A from the crystal solvent. In addition, other non-polar contacts such as C…H, H…H and C…C interactions were detected. The C…H contacts which belong to the C-H…π interaction occurred between C7 atom of the s-triazine core and H10B from the piperidine moiety where the corresponding C7…H10B interaction distance is 2.712 Å. On the other hand, the most important hydrogenic interactions are H18…H26B (2.103 Å) and H19…H9B (2.058 Å). The former occurs between H18 from the organic target molecule and H26B from the crystal solvent while the latter occurs between H19 and H9B from two neighboring units of the organic target molecule. Interestingly, the Hirshfeld analysis detected many C…C and C…N contacts which reveal the presence of some interactions between the π-system of the studied molecule. The C5…C16 (3.299 Å), C7…C16 (3.218 Å), C14…C15 (3.388 Å) and C14…N7 (3.230 Å) are the most important. All these π-π interactions occur between two of the organic target molecules. The π-π interactions appeared as red/blue triangles in the shape index map and green flat area in curvedness. For better clarity, the regions included in the π-π interactions are marked by black rectangle in Figure 4.
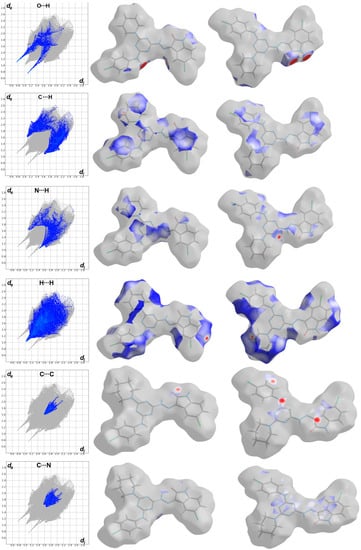
Figure 5.
The fingerprint plots and dnorm maps for the important contacts in the target molecule in the crystal structure of 6c.
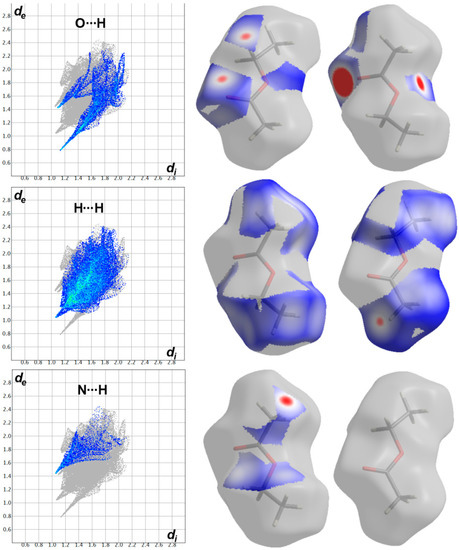
Figure 6.
The fingerprint plots and dnorm maps for the short contacts in the crystal solvent in the crystal structure of 6c.
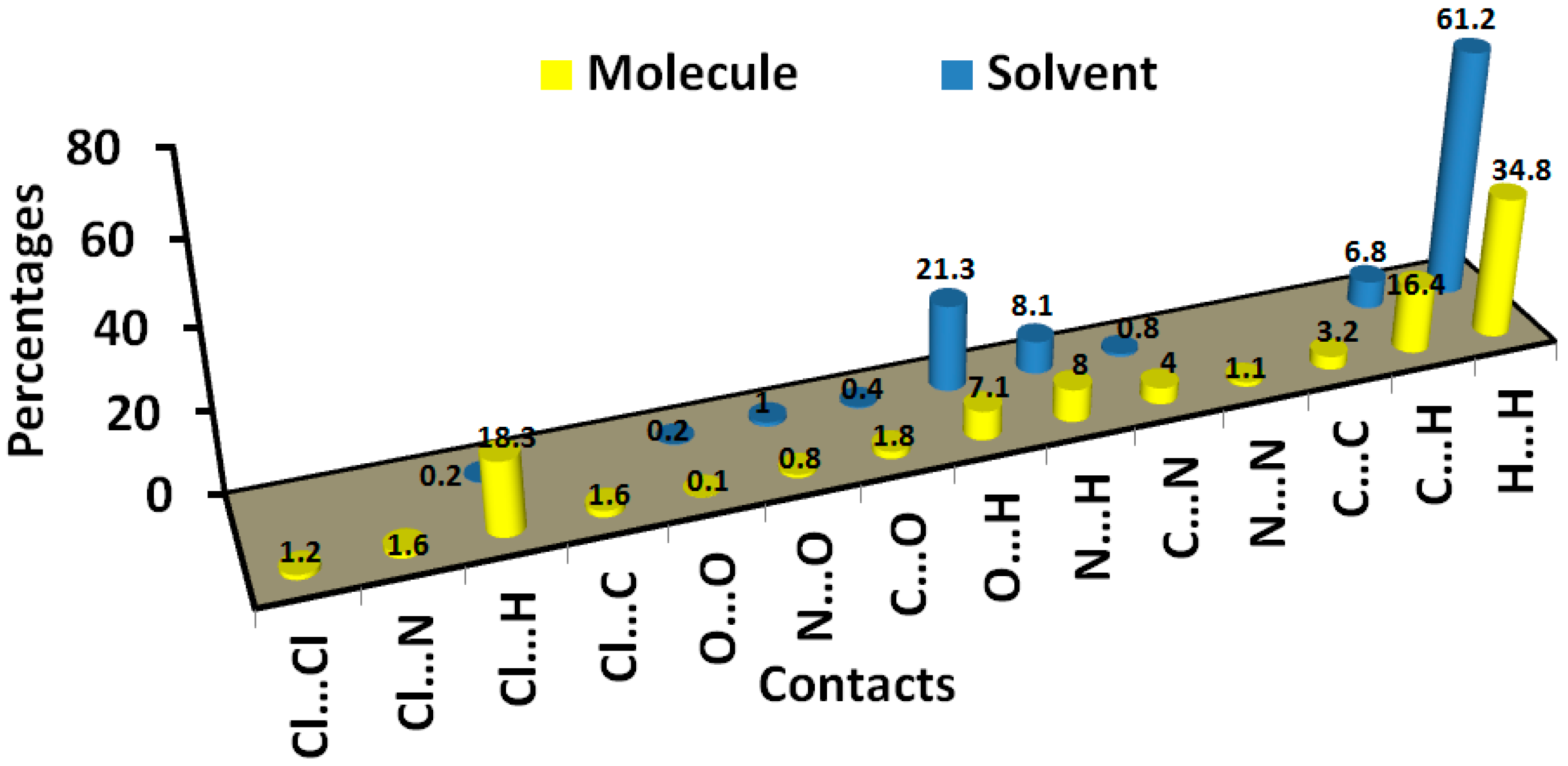
In addition, the area of the decomposed fingerprint plots of the O…H, C…H, C…C, H…H, N…H and C…N interactions represents the contribution of this contact in the molecular packing (Figure 5). For the organic target molecule, the percentages of these contacts are 7.1, 16.4, 1.6, 34.8, 8.0 and 4.0, respectively. In the case of the crystal solvent, the Hirshfeld analysis of this part of the crystal structure indicated the importance of the O…H, H…H and N…H contacts (Figure 6). Their percentages are 21.3, 61.2 and 8.1%, respectively. There are many other contracts presented along with their percentages are summarized in Figure 7. These interactions have low importance in the molecular packing compared to the above-mentioned contacts.
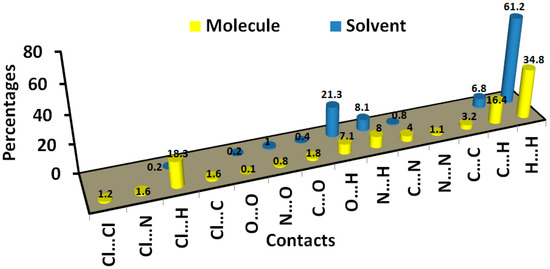
Figure 7.
Intermolecular contacts and their percentages in 6c.
3.4. DFT Analyses
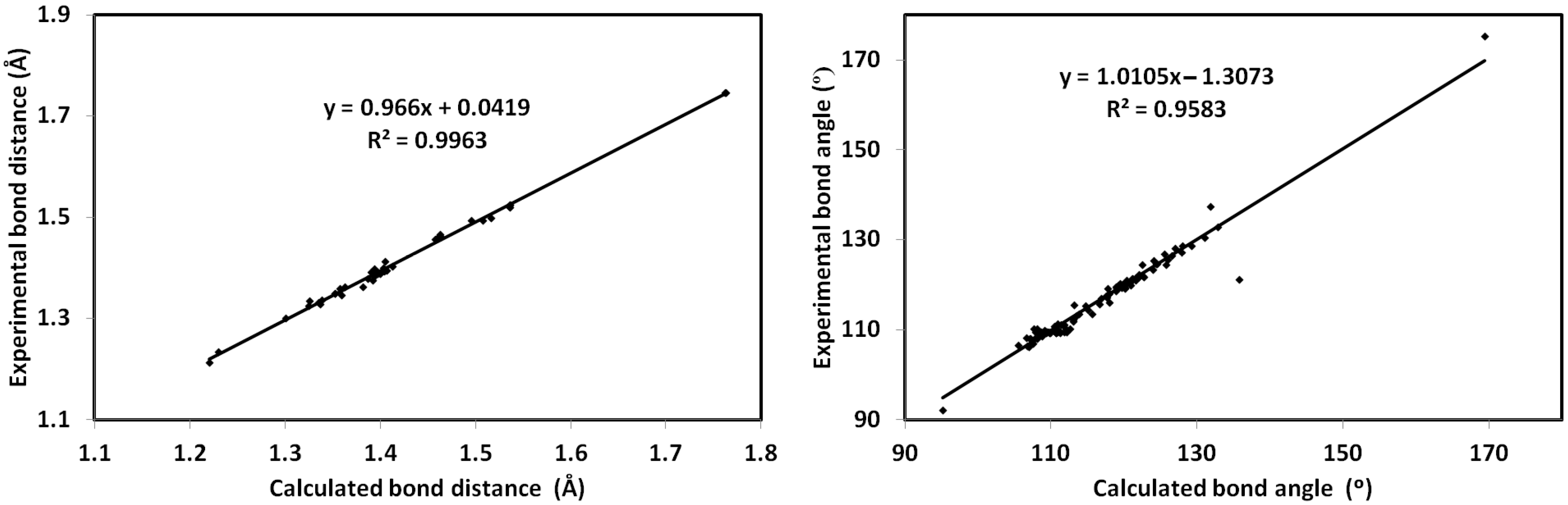
The calculated structure of 6c is shown in Figure 8A. In the same figure, the overlay of the X-ray structure over the calculated one is shown in Figure 8B. There is general great agreement between both structures. Additionally, there are good correlations between the calculated and optimized geometric parameters (Figure 9). List of the bond distances and angles are collected in Table S2 (Supplementary Data).
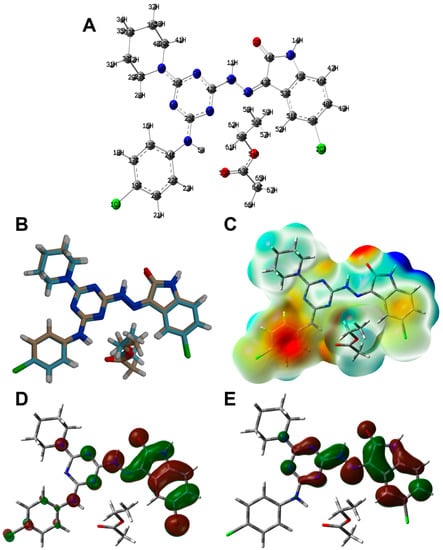
Figure 8.
Optimized geometry (A), overlay with the X-ray geometry (B), MEP (C), HOMO (D) and LUMO (E) of 6c.
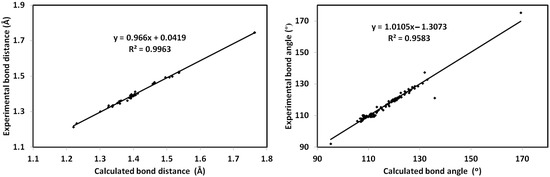
Figure 9.
Correlations of the calculated and experimental geometric parameters.
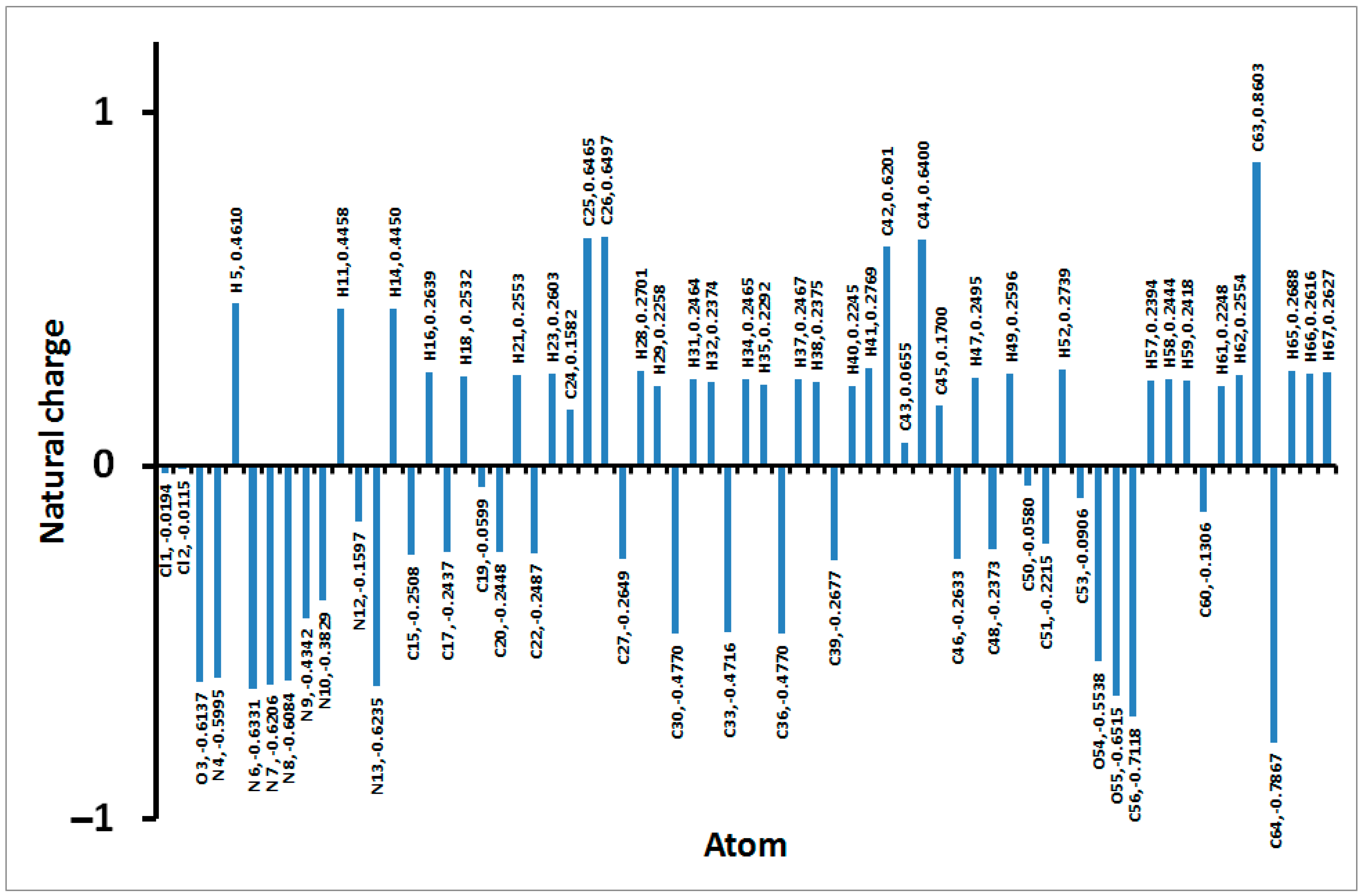
The natural atomic charges are presented in Figure 10. Detailed natural charges are shown in Table S3. In general, the O, N, Cl and most of C-atoms have negative charge while the opposite is true for the hydrogen atoms and other carbon atoms bonded to oxygen and nitrogen. The compound has a net dipole moment of 5.6072 Debye indicating a highly polar system. In addition, the electron density mapped over electrostatic potential showing the negatively charged regions colored by red and the positively charged regions colored by blue is shown in Figure 8C.
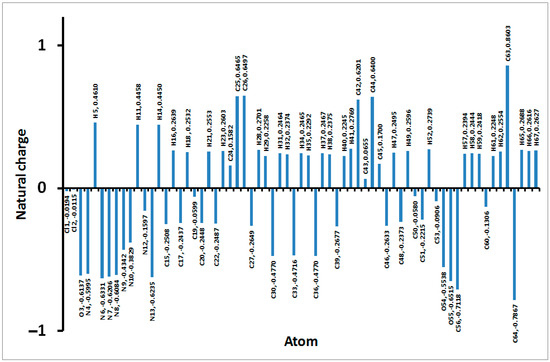
Figure 10.
Charges at the different atoms based on natural population analysis.
In the same figure, the HOMO and LUMO levels are presented (Figure 8D,E). These orbitals are mainly distributed over the π-systems of the indolone and s-triazine moieties. The energies of these molecular orbitals are calculated to be −5.5378 and −2.3097 eV, respectively. Hence, the HOMO-LUMO energy gap is 3.2281 eV. Based on the energies of the HOMO and LUMO levels, the ionization potential (I), electron affinity (A), hardness (η), electrophilicity index (ω) and chemical potential (μ) are calculated to be 5.5378, 2.3097, 3.2281, 2.3847 and −3.9238 eV, respectively [31,32,33,34,35,36,37].
4. Conclusions
The present work represents the synthesis of some s-triazine hydrazone derivatives incorporating isatin moiety. The structure of 6c was confirmed using X-ray diffraction of single crystal. The crystal comprised one of the organic target molecule in addition to a co-crystallized ethyl acetate as a crystal solvent. The supramolecular structure of the organic system and the crystal solvent is analyzed using Hirshfeld analysis. In the former, the molecular packing is controlled by the O…H (7.1%), C…H (16.4%), C…C (1.6%), H…H (34.8%), N…H (8.0%) and C…N (4.0%) interactions. On the other hand, the packing of the latter is dominated by O…H (21.3%), H…H (61.2%) and N…H (8.1%) contacts. DFT optimized geometry agreed very well with the reported X-ray structure. Different electronic and spectroscopic parameters for the studied system were predicted.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst13020305/s1, Figures S1–S7: 1H and 13C NMR spectral data for compounds 2,3 and 6a–e: title; Table S1: Selected bond angles [°] for 6c; Table S2: The calculated geometric parameters of 6c a and Table S3: The calculated natural charges of 6c a.
Author Contributions
Conceptualization, H.H.A.-R. and S.A.A.-k.; methodology, H.H.A.-R., S.M.S., M.H., A.E.-F. and A.B. software, S.M.S. and M.H.; formal analysis, H.H.A.-R., S.M.S. and M.H.; X-ray crystal structure: S.M.S. and M.H.; investigation, H.H.A.-R., S.M.S., M.H., A.E.-F. and A.B.; resources, H.H.A.-R.; writing—original draft preparation, H.H.A.-R., S.M.S., M.H., A.E.-F. and A.B.; writing—review and editing, A.E.-F., S.M.S. and A.B.; supervision, H.H.A.-R. and A.E.-F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
All thanks and respect to Research Center of the Female Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maliszewski, D.; Drozdowska, D. Recent Advances in the Biological Activity of s-Triazine Core Compounds. Pharmaceuticals 2022, 15, 221. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; AlOtaibi, B.S.; Thamer, B.M.; El-Faham, A. Synthesis of New S-Triazine Bishydrazino and Bishydrazido-Based Polymers and Their Application in Flame-Retardant Polypropylene Composites. Polymers 2022, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Ahmed, M.A.; Al-Lohedan, H.A.; El-Faham, A. Multi-Functional Cardanol Triazine Schiff Base Polyimine Additives for Self-Healing and Super-Hydrophobic Epoxy of Steel Coating. Coatings 2020, 10, 327. [Google Scholar] [CrossRef]
- Blotny, G. Recent Applications of 2,4,6-Trichloro-1,3,5-Triazine and Its Derivatives in Organic Synthesis. Tetrahedron 2006, 62, 9507–9522. [Google Scholar] [CrossRef]
- Gunasekaran, P.; Fan, M.; Kim, E.Y.; Shin, J.H.; Lee, J.E.; Son, E.J.; Kim, J.; Hwang, E.; Yim, M.S.; Kim, E.-H.; et al. Amphiphilic Triazine Polymer Derivatives as Antibacterial and Anti-atopic Agents in Mice Model. Sci. Rep. 2019, 9, 15161. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, S.; Utreja, D. Recent Advances in Synthesis and Antifungal Activity of 1,3,5-triazines. Curr. Org. Synth. 2016, 13, 484–503. [Google Scholar] [CrossRef]
- Adhikari, N.; Kashyap, A.; Shakya, A.; Ghosh, S.K.; Bhattacharyya, D.R.; Bhat, H.R.; Singh, U.P. Microwave Assisted Synthesis, Docking and Antimalarial Evaluation of Hybrid PABA-Substituted 1,3,5-Triazine Derivatives. J. Heterocyc. Chem. 2020, 57, 2389–2399. [Google Scholar] [CrossRef]
- Zacharie, B.; Abbott, S.D.; Duceppe, J.S.; Gagnon, L.; Grouix, B.; Geerts, L.; Laurin, P. Design and Synthesis of New 1,3,5- Trisubstituted Triazines for the Treatment of Cancer and Inflammation. ChemistryOpen 2018, 7, 737–749. [Google Scholar] [CrossRef]
- Baréa, P.; Barbosa, V.A.; Bidóia, D.L.; de Paula, J.C.; Stefanello, T.F.; da Costa, W.F.; Sarragiotto, M.H. Synthesis, Antileishmanial Activity and Mechanism of Action Studies of Novel Β-Carboline-1,3,5-Triazine Hybrids. Eur. J. Med. Chem. 2018, 150, 579–590. [Google Scholar] [CrossRef]
- Barakat, A.; El-Senduny, F.F.; Almarhoon, Z.; Al-Rasheed, H.H.; Badria, F.A.; Al-Majid, A.M.; El-Faham, A. Synthesis, X-ray Crystal Structures, and Preliminary Antiproliferative Activities of New s-Triazine-hydroxybenzylidene Hydrazone Derivatives. J. Chem. 2019, 2019, 9403908. [Google Scholar] [CrossRef]
- Shvekhgeimer, M.-G.A. Synthesis of Heterocyclic Compounds by the Cyclization of Isatin and Its Derivatives (Review). Chem. Heterocycl. Compd. 1996, 32, 249–276. [Google Scholar] [CrossRef]
- Da Silva, J.F.M.; Garden, S.J.; Pinto, A.C. The Chemistry of Isatins: A Review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Basedia, D.K.; Dubey, B.K.; Shrivastava, B. A Review on Synthesis and Biological Activity of Heterocyclic Compounds Bearing 1,3,5-Triazine Lead Moiety. Am. J. Pharm. Res. 2011, 1, 174–193. [Google Scholar]
- Shen, J.; Zhang, L.; Meng, X. Recent Advances in Cyclization Reactions of Isatins or Thioisatins via C–N Or C–S Bond Cleavage. Org. Chem. Front. 2021, 8, 6433. [Google Scholar] [CrossRef]
- Khan, F.A.; Maalik, A. Advances in Pharmacology of Isatin and its Derivatives: A Review. Trop. J. Pharm. Res. 2015, 14, 1937–1942. [Google Scholar] [CrossRef]
- Bhrigu, B.; Pathak, D.; Siddiqui, N.; Alam, M.S.; Ahsan, W. Search for Biological Active Isatins: A Short Review. Int. J. Pharm. Sci. Drug Res. 2010, 2, 229–235. [Google Scholar]
- Smitha, S.; Pandeya, S.N.; Stables, J.P.; Ganapathy, S. Anticonvulsant and Sedative-Hypnotic Activities of N-Acetyl/Methyl Isatin Derivatives. Sci. Pharm. 2008, 76, 621–636. [Google Scholar] [CrossRef]
- Srivastava, G.K.; Alonso-Alonso, M.L.; Fernandez-Bueno, I.; Garcia-Gutierrez, M.T.; Rull, F.; Medina, J.; Coco, R.M.; Pastor, J.C. Comparison between Direct Contact and Extract Exposure Methods for PFO Cytotoxicity Evaluation. Sci. Rep. 2018, 8, 1425. [Google Scholar] [CrossRef]
- Ghodsi, M.Z.; Zahra, P.; Fatemeh, M.; Mohammad, G.; Rajender, S.V. An Overview of Recent Advances in Isatin-Based Multicomponent Reactions. Curr. Org. Chem. 2022, 26, 1485–1502. [Google Scholar]
- Yan, L.J.; Wang, Y.C. Recent Advances in Green Synthesis of 3,3′-Spirooxindoles via Isatin–based One–pot Multicomponent Cascade Reactions in Aqueous Medium. ChemistrySelect 2016, 1, 6948–6960. [Google Scholar] [CrossRef]
- Shanmugakala, R.; Tharmaraj, P.; Sheela, C.D.; Chidambaranathan, N. Transition Metal Complexes of S-Triazine Derivative: New Class of Anticonvulsant, Anti-Inflammatory, and Neuroprotective Agents. Med. Chem. Res. 2014, 23, 329–342. [Google Scholar] [CrossRef]
- Shawish, I.; Barakat, A.; Aldalbahi, A.; Malebari, A.M.; Nafie, M.S.; Bekhit, A.A.; Albohy, A.; Khan, A.; Ul-Haq, Z.; Haukka, M.; et al. Synthesis and Antiproliferative Activity of a New Series of Monoand Bis(dimethylpyrazolyl)-s-triazine Derivatives Targeting EGFR/PI3K/AKT/mTOR Signaling Cascades. ACS Omega 2022, 7, 24858–24870. [Google Scholar] [CrossRef] [PubMed]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Oxfordshire, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17 (2017) University of Western Australia. Available online: https://crystalexplorer.net/download/ (accessed on 1 July 2017).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. GAUSSIAN 09, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.D., II; Keith, T.A.; Millam, J.M. GaussView, Version 4.1; Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from A Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Koopmans, T.A. Ordering of WaveFunctions and Eigenenergies to the Individual Electrons of an Atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpály, L.V.; Liu, S. Electrophilicity Index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A Combined Experimental and Quantum Chemical (DFT And AIM) Study on Molecular Structure, Spectroscopic Properties, NBO and Multiple Interaction Analysis in A Novel Ethyl 4-[2-(Carbamoyl) Hydrazinylidene]-3,5-Dimethyl-1H-Pyrrole-2-Carboxylate and Its Dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Hubert Joe, I.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Pinzaru, S.C. Theoretical and Vibrational Spectral Investigation of Sodium Salt of Acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar]
- Sebastian, S.; Sundaraganesan, N. The Spectroscopic (FT-IR, FT-IR Gas Phase, FT-Raman and UV) and NBO Analysis of 4-Hydroxypiperidine by Density Functional Method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).