High pO2 Flux Growth and Characterization of NdNiO3 Crystals
Abstract
1. Introduction
2. Materials and Synthesis
2.1. High-Pressure Flux Growth of NdNiO3 Crystals
2.2. In-House X-ray Powder Diffraction (PXRD)
2.3. Synchrotron X-ray Single-Crystal Diffraction (SXRD)
2.4. High-Resolution Synchrotron X-ray Powder Diffraction (HRPXRD)
2.5. Scanning Electron Microscopy (SEM)
2.6. Energy Dispersive Spectrometer (EDS)
2.7. Electrical Transport
2.8. Magnetic Susceptibility
3. Results and Discussion
3.1. High pO2 Single Crystal Growth
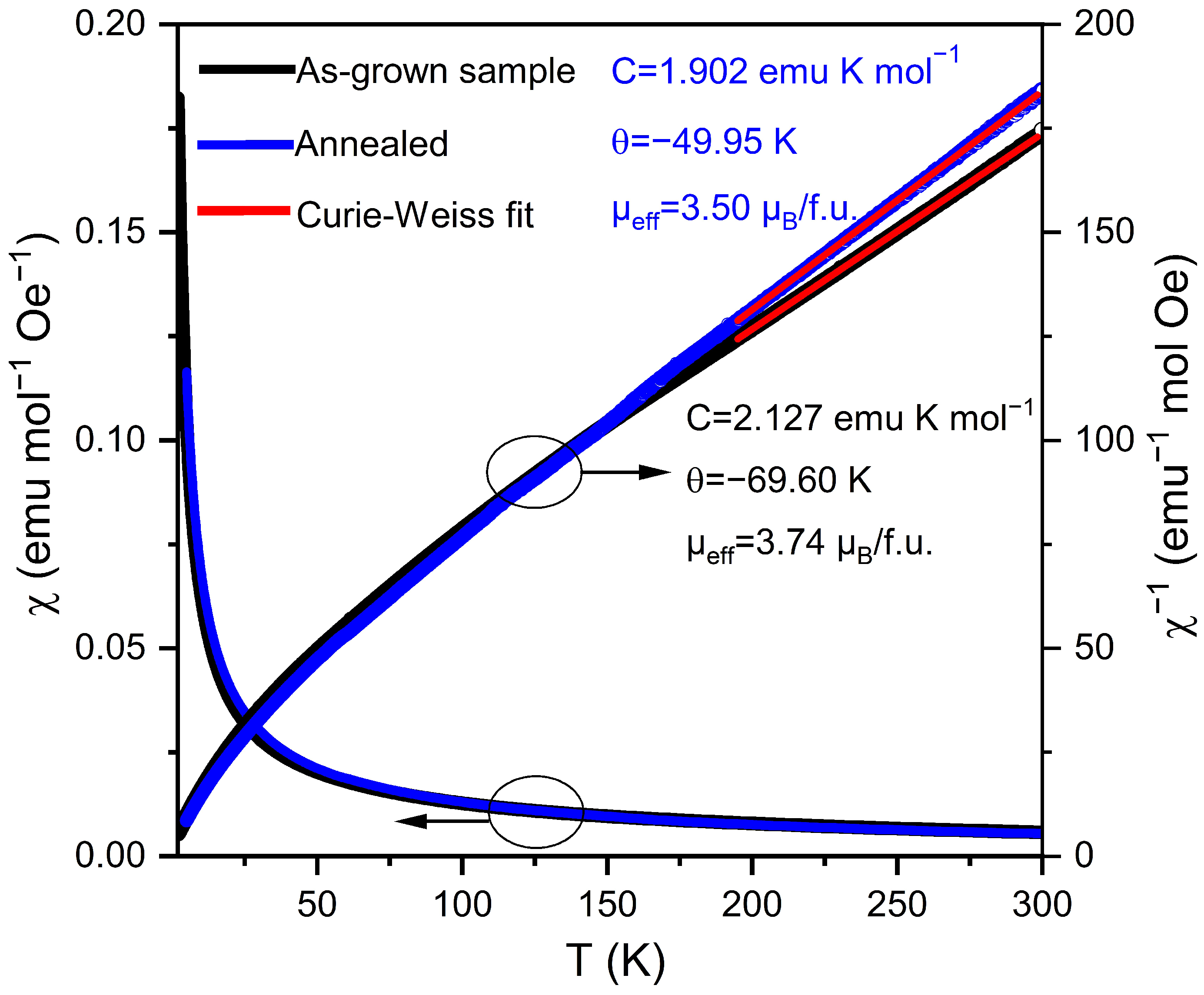
3.2. Physical Properties
3.3. Structural Study at Various Temperatures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, D.; Lee, K.; Wang, B.Y.; Osada, M.; Crossley, S.; Lee, H.R.; Cui, Y.; Hikita, Y.; Hwang, H.Y. Superconductivity in an infinite-layer nickelate. Nature 2019, 572, 624–627. [Google Scholar] [CrossRef]
- Chow, L.E.; Ariando, A. Infinite-Layer Nickelate Superconductors: A Current Experimental Perspective of the Crystal and Electronic Structures. Front. Phys. 2022, 10, 834658. [Google Scholar] [CrossRef]
- Mitchell, J.F. A Nickelate Renaissance. Front. Phys. 2021, 9, 813483. [Google Scholar] [CrossRef]
- Bernardini, F.; Iglesias, L.; Bibes, M.; Cano, A. Thin-Film Aspects of Superconducting Nickelates. Front. Phys. 2022, 10, 828007. [Google Scholar] [CrossRef]
- Botana, A.S.; Bernardini, F.; Cano, A. Nickelate superconductors: An ongoing dialog between theory and experiments. J. Exp. Theor. Phys 2021, 132, 618–627. [Google Scholar] [CrossRef]
- Botana, A.S.; Lee, K.-W.; Norman, M.R.; Pardo, V.; Pickett, W.E. Low Valence Nickelates: Launching the Nickel Age of Superconductivity. Front. Phys. 2022, 9, 813532. [Google Scholar] [CrossRef]
- Gu, Q.; Wen, H.-H. Superconductivity in nickel-based 112 systems. Innovation 2022, 3, 100202. [Google Scholar] [CrossRef] [PubMed]
- Hepting, M.; Dean, M.P.M.; Lee, W.-S. Soft X-ray Spectroscopy of Low-Valence Nickelates. Front. Phys. 2021, 9, 808683. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, J.; Li, L.; Liao, Z. Superconductivity in infinite layer nickelates. J. Appl. Phys. 2021, 130, 060901. [Google Scholar] [CrossRef]
- Jiang, M. Relevance of 3D multiplet structure in nickelate and cuprate superconductors. Chin. Phys. B 2021, 30, 107103. [Google Scholar] [CrossRef]
- Li, D. The discovery and research progress of the nickelate superconductors. Sci. Sin. Phys. Mech. Astron. 2021, 51, 047405. [Google Scholar] [CrossRef]
- Nomura, Y.; Arita, R. Superconductivity in infinite-layer nickelates. Rep. Prog. Phys. 2022, 85, 052501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tao, X. Review on quasi-2D square planar nickelates. CrystEngComm 2021, 23, 3249–3264. [Google Scholar] [CrossRef]
- Zhou, X.; Qin, P.; Feng, Z.; Yan, H.; Wang, X.; Chen, H.; Meng, Z.; Liu, Z. Experimental progress on the emergent infinite-layer Ni-based superconductors. Mater. Today 2022, 55, 170–185. [Google Scholar] [CrossRef]
- Li, D.; Wang, B.Y.; Lee, K.; Harvey, S.P.; Osada, M.; Goodge, B.H.; Kourkoutis, L.F.; Hwang, H.Y. Superconducting Dome in Nd1−xSrxNiO2 Infinite Layer Films. Phys. Rev. Lett. 2020, 125, 027001. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, C.; Chow, L.E.; Cao, Y.; Zhang, Z.; Tang, C.S.; Yin, X.; Lim, Z.S.; Hu, J.; Yang, P.; et al. Superconductivity in infinite-layer nickelate La1−xCaxNiO2 thin films. Sci. Adv. 2022, 8, eabl9927. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Tang, C.S.; Yin, X.; Li, C.; Li, M.; Huang, Z.; Hu, J.; Liu, W.; Omar, G.J.; Jani, H.; et al. Phase Diagram and Superconducting Dome of Infinite-Layer Nd1-xSrxNiO2 Thin Films. Phys. Rev. Lett. 2020, 125, 147003. [Google Scholar] [CrossRef] [PubMed]
- Osada, M.; Wang, B.Y.; Goodge, B.H.; Lee, K.; Yoon, H.; Sakuma, K.; Li, D.; Miura, M.; Kourkoutis, L.F.; Hwang, H.Y. A superconducting praseodymium nickelate with infinite layer structure. Nano Lett. 2020, 20, 5735–5740. [Google Scholar] [CrossRef]
- Osada, M.; Wang, B.Y.; Goodge, B.H.; Harvey, S.P.; Lee, K.; Li, D.; Kourkoutis, L.F.; Hwang, H.Y. Nickelate Superconductivity without Rare-Earth Magnetism: (La,Sr)NiO2. Adv. Mater. 2021, 33, e2104083. [Google Scholar] [CrossRef]
- Pan, G.A.; Ferenc Segedin, D.; LaBollita, H.; Song, Q.; Nica, E.M.; Goodge, B.H.; Pierce, A.T.; Doyle, S.; Novakov, S.; Cordova Carrizales, D.; et al. Superconductivity in a quintuple-layer square-planar nickelate. Nat. Mater. 2022, 21, 160–164. [Google Scholar] [CrossRef]
- Wang, N.N.; Yang, M.W.; Yang, Z.; Chen, K.Y.; Zhang, H.; Zhang, Q.H.; Zhu, Z.H.; Uwatoko, Y.; Gu, L.; Dong, X.L.; et al. Pressure-induced monotonic enhancement of Tc to over 30 K in superconducting Pr0.82Sr0.18NiO2 thin films. Nat. Commun. 2022, 13, 4367. [Google Scholar] [CrossRef]
- Catalano, S.; Gibert, M.; Fowlie, J.; Íñiguez, J.; Triscone, J.M.; Kreisel, J. Rare-earth nickelates RNiO3: Thin films and heterostructures. Rep. Prog. Phys. 2018, 81, 046501. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Zhang, H.-T. Quantum nickelate platform for future multidisciplinary research. J. Appl. Phys. 2022, 131, 120901. [Google Scholar] [CrossRef]
- Ardizzone, I.; Teyssier, J.; Crassee, I.; Kuzmenko, A.B.; Mazzone, D.G.; Gawryluk, D.J.; Medarde, M.; van der Marel, D. Raman spectroscopic evidence for multiferroicity in rare earth nickelate single crystals. Phys. Rev. Res. 2021, 3, 033007. [Google Scholar] [CrossRef]
- Giovannetti, G.; Kumar, S.; Khomskii, D.; Picozzi, S.; van den Brink, J. Multiferroicity in rare-earth nickelates RNiO3. Phys. Rev. Lett. 2009, 103, 156401. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, H.; Ren, Y.; Mitchell, J.F. High-pressure floating-zone growth of perovskite nickelate LaNiO3 single crystals. Cryst. Growth Des. 2017, 17, 2730–2735. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Wang, B.; Phelan, D.; Krogstad, M.J.; Ren, Y.; Phelan, W.A.; Chmaissem, O.; Poudel, B.; Mitchell, J.F. High pO2 Floating Zone Crystal Growth of the Perovskite Nickelate PrNiO3. Crystals 2019, 9, 324. [Google Scholar] [CrossRef]
- Phelan, W.A.; Zahn, J.; Kennedy, Z.; McQueen, T.M. Pushing boundaries: High pressure, supercritical optical floating zone materials discovery. J. Solid State Chem. 2018, 270, 705–709. [Google Scholar] [CrossRef]
- Azuma, M.; Saito, T.; Ishiwata, S.; Yamada, I.; Kohsaka, Y.; Takagi, H.; Takano, M. Single crystal growth of transition metal oxides at high pressures of several GPa. Physica C 2003, 392–396 Pt 1, 22–28. [Google Scholar] [CrossRef]
- Masaki, A.; Takashi, S.; Shintaro, I.; Hirofumi, Y.; Mikio, T.; Yuhki, K.; Hidenori, T.; Wataru, U. Single-crystal growth of transition metal oxides at high pressures of several GPa. J. Phys. Condens. Matter 2002, 14, 11321. [Google Scholar]
- Alonso, J.A.; Muñoz, A.; Largeteau, A.; Demazeau, G. Crystal growth of NdNiO3 perovskite under high oxygen pressure. J. Phys. Condens. Matter 2004, 16, S1277. [Google Scholar] [CrossRef]
- Puphal, P.; Wu, Y.-M.; Fürsich, K.; Lee, H.; Pakdaman, M.; Bruin, J.A.N.; Nuss, J.; Suyolcu, Y.E.; Aken, P.A.v.; Keimer, B.; et al. Topotactic transformation of single crystals: From perovskite to infinite-layer nickelates. Sci. Adv. 2021, 7, eabl8091. [Google Scholar] [CrossRef]
- Klein, Y.M.; Kozłowski, M.; Linden, A.; Lacorre, P.; Medarde, M.; Gawryluk, D.J. RENiO3 Single Crystals (RE = Nd, Sm, Gd, Dy, Y, Ho, Er, Lu) Grown from Molten Salts under 2000 bar of Oxygen Gas Pressure. Cryst. Growth Des. 2021, 21, 4230–4241. [Google Scholar] [CrossRef]
- Bruker APEX2; Bruker Analytical X-ray Instruments Inc: Madison, WI, USA, 2014.
- Prakash, J.; Blakely, C.K.; Poltavets, V.V. Low temperature high-pressure synthesis of LnNiO3 (Ln = Eu, Gd) in molten salts. Solid State Sci. 2013, 17, 72–75. [Google Scholar] [CrossRef]
- Song, Y.T.; Peng, J.B.; Wang, X.; Sun, G.L.; Lin, C.T. Ambient-condition growth of superconducting YBa2Cu4O8 single crystals using KOH flux. J. Cryst. Growth 2007, 300, 263–266. [Google Scholar] [CrossRef]
- de Brion, S.; Bonda, M.; Darie, C.; Bordet, P.; Sheikin, I. Magnetic phase diagram of the S = 1/2 triangular layered compound NaNiO2: A single crystal study. J. Phys. Condens. Matter 2010, 22, 126001. [Google Scholar] [CrossRef]
- Wang, B.-X.; Zheng, H.; Krivyakina, E.; Chmaissem, O.; Lopes, P.P.; Lynn, J.W.; Gallington, L.C.; Ren, Y.; Rosenkranz, S.; Mitchell, J.F.; et al. Synthesis and characterization of bulk Nd1−xSrxNiO2 and Nd1−xSrxNiO3. Phys. Rev. Mater. 2020, 4, 084409. [Google Scholar] [CrossRef]
- Fujihara, S.; Murakami, G.; Kimura, T. Oxygen deficiency and electrical conductivity of Nd1−xAxNiO3−y (A = alkaline earth) prepared by the low-temperature process. J. Alloys Compd. 1996, 243, 70–76. [Google Scholar] [CrossRef]
- Catalan, G. Progress in perovskite nickelate research. Phase Transit. 2008, 81, 729–749. [Google Scholar] [CrossRef]
- Lorenzo, J.E.; Hodeau, J.L.; Paolasini, L.; Lefloch, S.; Alonso, J.A.; Demazeau, G. Resonant X-ray scattering experiments on electronic orderings in NdNiO3 single crystals. Phys. Rev. B 2005, 71, 045128. [Google Scholar] [CrossRef]
- García-Muñoz, J.L.; Aranda, M.A.G.; Alonso, J.A.; Martínez-Lope, M.J. Structure and charge order in the antiferromagnetic band-insulating phase of NdNiO3. Phys. Rev. B 2009, 79, 134432. [Google Scholar] [CrossRef]






| T (°C) | pO2 (Bar) | Dwelling Time (h) | Cooling Rate (°C/h) | Phase (s) | Crystal Size (μm) |
|---|---|---|---|---|---|
| 400 | 5 | 24 | 10 | NdNiO3 + NiO (trace) | <5 |
| 400 | 20 | 24 | 10 | NdNiO3 + NiO (trace) | <5 |
| 400 | 20 | 48 | 10 | NdNiO3 | <5 |
| 400 | 100 | 48 | 10 | NdNiO3 | 5–15 |
| 400 | 200 | 48 | 10 | NdNiO3 | 15–25 |
| 400 | 300 | 48 | 10 | NdNiO3 | 15–28 |
| 500 | 300 | 48 | 10 | NdNiO3 + Na0.6Ni3O5.4 | 15–28 |
| 400 | 200 | 48 | 1 | NdNiO3 | 20–50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, S.; Liu, C.; Fan, C.; Han, L.; Li, F.; Chang, T.; Chen, Y.-S.; Wang, S.; Tao, X.; et al. High pO2 Flux Growth and Characterization of NdNiO3 Crystals. Crystals 2023, 13, 180. https://doi.org/10.3390/cryst13020180
Wang X, Wang S, Liu C, Fan C, Han L, Li F, Chang T, Chen Y-S, Wang S, Tao X, et al. High pO2 Flux Growth and Characterization of NdNiO3 Crystals. Crystals. 2023; 13(2):180. https://doi.org/10.3390/cryst13020180
Chicago/Turabian StyleWang, Xiaoli, Shilei Wang, Chao Liu, Chuanyan Fan, Lu Han, Feiyu Li, Tieyan Chang, Yu-Sheng Chen, Shanpeng Wang, Xutang Tao, and et al. 2023. "High pO2 Flux Growth and Characterization of NdNiO3 Crystals" Crystals 13, no. 2: 180. https://doi.org/10.3390/cryst13020180
APA StyleWang, X., Wang, S., Liu, C., Fan, C., Han, L., Li, F., Chang, T., Chen, Y.-S., Wang, S., Tao, X., & Zhang, J. (2023). High pO2 Flux Growth and Characterization of NdNiO3 Crystals. Crystals, 13(2), 180. https://doi.org/10.3390/cryst13020180








