Abstract
To promote the use of γ-TiAl alloys in various domains, such as the aerospace industry, it is pivotal to investigate the unusual phase transformation from rapidly solidified and metastable γ-TiAl toward the equilibrium state. In this study, the microstructure characteristics of gas-atomized β-solidifying Ti-44Al-6Nb-1.2Cr alloy powder, in terms of the effect of rapid solidification on microstructure evolution, were explored in comparison with cast materials. The phase constitution, morphology, and crystallographic orientation between phases were noted to be distinct. Furthermore, subsequent heat treatment was conducted at different temperatures using gas-atomized powder. The transition from the metastable to equilibrium state was observed, wherein firstly, the γ phase precipitated from the retained α2 phase, forming an α2/γ lamellar microstructure. In intensified heat-treatment conditions adequate for cellular reaction, β/γ cells were formed at the grain boundaries of α2/γ lamellar colonies. The findings highlight the overall phase transformation during rapid solidification and continuous microstructural evolution from the nonequilibrium to the equilibrium state. This research can bridge the gap in understanding the effect of the solidification rate on microstructural evolution and contribute to enhanced comprehension of the microstructure in other domains involving rapid solidification, such as the additive manufacturing of γ-TiAl alloys.
1. Introduction
Gamma (γ)-titanium aluminide alloys (γ-TiAl) are promising alternative structural materials to the superalloys currently used in the aerospace industry, owing to their low density, high creep resistance, and excellent oxidation resistance [1,2,3]. Although conventional Ti-48Al-2Nb-2Cr (at.%) alloy has been successfully used in aero-engine low-pressure turbine blades [1,4], it has become necessary to attain superior temperature capabilities and expand the application scope of γ-TiAl alloys. Therefore, considerable research has been focused on exploring different compositions, and various pathways for microstructure optimization and manufacturing have been proposed [4,5]. Notably, additive manufacturing (AM) has emerged as a promising manufacturing strategy owing to the unique microstructure characteristics involved, and its competitiveness relative to traditional manufacturing processes has been demonstrated [6,7,8].
The microstructure characteristics in AM depend on the composition of TiAl alloys and the cooling rates which can be controlled with AM process parameters [6,7,9]. In addition, the cooling rates during the AM process have been estimated to be 105–107 K/s [10,11,12]. Moreover, this process involves multiple reheating and cooling cycles, resulting in rapid solidification and complex thermal variations, leading to the unique microstructures observed during AM. However, most experimental data have been derived under low cooling rates, such as those typically used during casting and subsequent heat treatment to control the microstructure [13]. Although certain studies have furnished data pertaining to the quenching [14,15,16], the effect of rapid solidification on the microstructure remains to be clarified.
In the domain of powder metallurgy, gas atomization is a powder manufacturing method that yields spherical particles [17]. The cooling rate during gas atomization is similar to that in the AM process [18]. Thus, equilibrium phase transformation is likely suppressed during solidification, resulting in the dominance of the α/α2 phase in the final microstructure [19,20,21,22]. Despite controversial results concerning the ordering in the α/α2 phase, it is clear that the microstructure of γ-TiAl alloys depends on the cooling rate. Several researchers have attempted to illustrate the evolution from the metastable α/α2 phase toward equilibrium [21,23]. These studies highlighted that the γ phase precipitated in a nano α2/γ lamellar structure during subsequent heat treatment, then the α2/γ disappeared and fully recrystallized. There was no plastic deformation during the subsequent heat treatment that could have triggered the recrystallization. Thus, it is necessary to investigate the unusual phase transformation of the rapidly solidified, metastable γ-TiAl toward the equilibrium state.
Therefore, this study was aimed at exploring the microstructure development of metastable alloys, especially during the transition from the metastable to equilibrium state. We observed the microstructure characteristics of gas-atomized Ti-44Al-6Nb-1.2Cr (at.%) powder subjected to heat treatment, in comparison with those of cast materials. The findings are expected to fill the gap between the different microstructural developments depending on the cooling rate. In addition, the direct observation after rapid solidification with subsequent heat treatment provides further understanding of the enhancement in the microstructure after rapid solidification and complex thermal cycling, such as those typically involved in the AM of γ-TiAl alloys, and useful guidance for microstructure optimization.
2. Materials and Methods
Ti-44Al-6Nb-1.2Cr (at.%) alloy powder was fabricated using the gas-atomization process (Osaka Titanium Technologies, Amagasaki, Hyogo, Japan). The mean particle diameter was measured to be 36.0 µm using a laser-diffraction-type particle size distribution measuring device (Mastersize 3000E, Malvern Panalytical, Malvern, UK). To investigate the evolution of the microstructure during the subsequent heat treatment, two temperatures were selected: The raw powders were isothermally exposed at 800 °C and 900 °C for 3 h followed by quenching in water to room temperature. All samples were sealed in an Ar atmosphere to prevent oxidation. For comparison, the same alloy composition of the ingot (as-cast) was attained using arc melting, and identical heat treatment procedures were conducted.
The phase constitution of the powder and as-cast samples was identified through X-ray diffractometry (XRD; X’pert PRO, Philips, Amsterdam, The Netherlands) using Cu K-alpha radiation. Other microstructural characteristics, such as the phase morphology, composition distributions, and crystallographic orientations, were investigated from backscattered electron (BSE) images, energy dispersive X-ray spectroscopy (EDS; Aztec 4.4, X-MaxN, Oxford Instruments, Oxfordshire, UK), and electron backscatter diffraction (EBSD; Aztec 4.4, NordlysMax3, Oxford Instruments, Oxfordshire, UK) images obtained with through field-emission scanning electron microscopy (FE-SEM; JEM-6500F, Tokyo, JEOL, Japan). The powder samples for BSE, EDS, and EBSD characterization were embedded in acrylic resin (KM-CO, PRESI, Eybens, France) and polished to a mirror finish.
3. Results and Discussion
3.1. Differences in Microstructures of As-Cast Alloys and Gas-Atomized Powder
Figure 1 shows the XRD profiles and SEM-BSE images of the as-cast alloy and gas-atomized powder, respectively. The XRD analysis (Figure 1a) revealed three different phases, β, α2, and γ, in the as-cast sample. In contrast, the powder consisted of only the β and α2 phases, and no γ phase was detected. Furthermore, the SEM-BSE image of the as-cast sample showed an intermediate contrast of coarse α2/γ colonies surrounded by the brightest β phase (clumps indicated by white ridges) and small amounts of γ phase precipitated with dark contrast inside the β phase (Figure 1b). From the EDS results listed in Table 1, the β phase indicated a lower Al concentration and much higher Nb and Cr concentration compared to the α2/γ colonies and γ phase. Therefore, the different contrasts are determined from their compositional differences, which are commonly observed microstructural characteristics in β-solidifying γ-TiAl alloys manufactured via casting [13,24]. In contrast, the cross-section of the powder revealed a dendritic and interdendritic morphology with gray and dark contrasts, respectively (Figure 1c). The chemical distribution represented that the dendrite region is rich in Ti and Nb, while the interdendrite is rich in Al and Cr (Table 2). This segregation phenomenon occurs during the solidification because the Al and Cr repelled to the interdendritic regions when the dendrite solidified from the liquid [22]. Furthermore, irregular morphology of grain boundaries across the dendritic and interdendritic regions can be observed (red arrows).
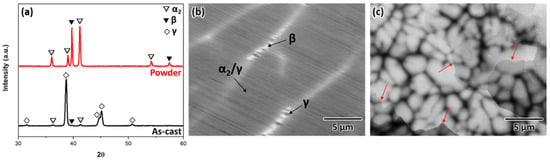
Figure 1.
(a) Typical XRD patterns of as-cast samples and gas-atomized powder, (b) SEM-BSE microstructure images of the as-cast sample, and (c) powder in the cross-sections. The red arrows indicate the grain boundaries across the dendritic and interdendritic regions.

Table 1.
The EDS results of different phases in cast materials.

Table 2.
The EDS results of different regions in the cross-section of gas-atomized powder.
To better understand the microstructural characteristics, additional observations were performed using SEM-EBSD. Consistent with the results of the SEM-BSE analysis, coarse α2/γ colonies and an elongated β phase were observed in the phase map (Figure 2a), with each α2/γ colony exhibiting a different crystallographic orientation in the inverse pole figure (IPF) map (Figure 2b). These different orientations resulted due to the solid phase transformation from the β to α phase during solidification, a key concept in the design of β-solidifying γ-TiAl alloys. In general, solid phase transformation through the complete β phase region without peritectic reactions can suppress chemical inhomogeneities and grain growth in the casting process for different γ-TiAl alloys [25]. Pole figures (PFs) for the β, α2, and γ phases obtained from within the black rectangular box in Figure 2a,b show the orientation relationship between each phase (Figure 2c). The PFs demonstrate that the (β and α2) and (α2 and γ) phases are crystallographically aligned according to the Burgers orientation relationship (Burgers OR) and Blackburn orientation relationship (Blackburn OR), respectively, as previously reported [16,26].
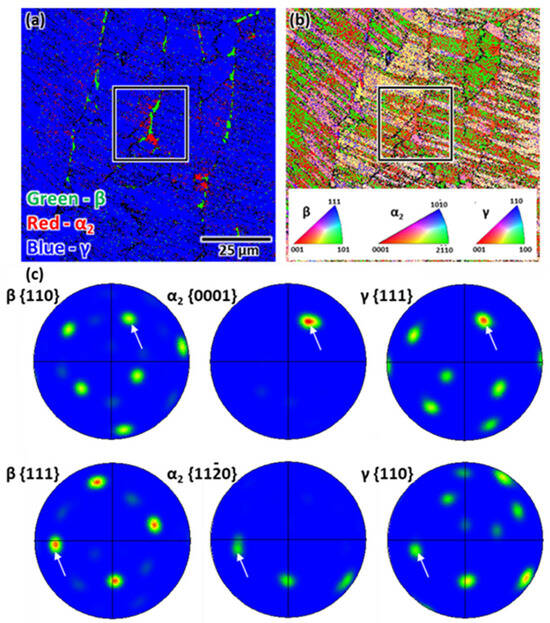
Figure 2.
SEM-EBSD analysis of the as-cast sample: (a) phase map, (b) IPF map, and (c) PFs. The PFs pertaining to the phases corresponding to the black rectangular area and white arrows indicate that the (β and α2) and (α2 and γ) phases are aligned according to the Burgers OR and Blackburn OR, respectively.
In contrast, the phase map showed a small amount of the β phase and a predominant α2 phase in the powder cross-sections (Figure 3a). The volume fraction of the β phase increased as the powder size decreased, owing to the higher cooling rate of smaller particles, consistent with observations pertaining to powders of different sizes [27]. In addition, the irregular morphology of the α2 phases was randomly distributed (Figure 3b). Detailed observation in a specific region (black rectangular box) including the β and α2 phases revealed no specific orientation relationship between the two phases (Figure 3c), conflicting with the result of the as-cast sample. This difference was attributable to the massive α grain formation owing to rapid solidification [28].
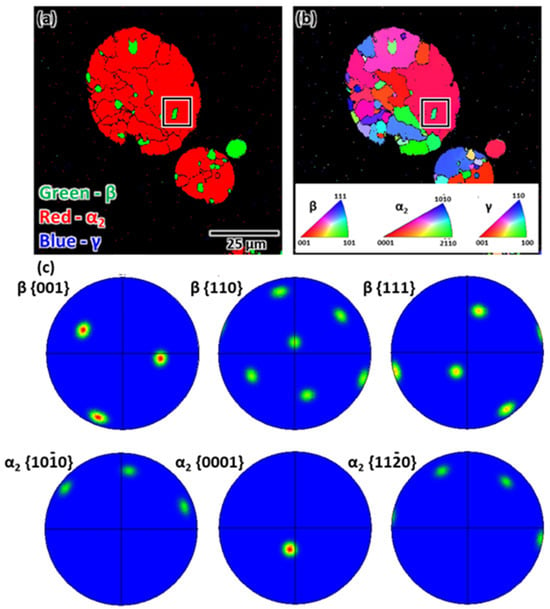
Figure 3.
SEM-EBSD analysis of the gas-atomized powder: (a) phase map, (b) IPF map, and (c) PFs. The PFs correspond to the black rectangular area. The alignment of the β and α2 phases does not comply with the Burgers OR.
As widely recognized, the phase transformation sequence of β-solidifying γ-TiAl alloys can be expressed as L → L + β → β → α + β → α, where L represents the liquid state [24]. During solidification, the primary β phase is completely solidified from the liquid, followed by the separation of the α phase in different orientations within a single β grain. Subsequent cooling induces the precipitation of the γ phase in the α phase, resulting in the formation of α2/γ colonies. However, in the case of rapidly solidified gas-atomized powder, the cooling rate is considerably higher than the reaction rate, which impedes phase separation in the α + β phase region. In other words, the high cooling rate results in the massive transformation of the α2 phase, suppressing the formation of the α2/γ microstructure [20]. These discrepancies in microstructural characteristics imply that the solidification rate associated with β-solidifying γ-TiAl alloys considerably affects the microstructure development.
3.2. Microstructural Evolution after Subsequent Heat Treatment
The microstructure of the as-cast sample consisted of coarse α2/γ colonies and an elongated β phase, consistent with the near equilibrium microstructure of β-solidifying γ-TiAl alloys, given the low cooling rate typically experienced during casting. Thus, minimal transitions were observed in the microstructure of the as-cast samples after subsequent heat treatments at 800 °C and 900 °C (Figure 4). Only the volume fraction of the γ phase and α2/γ lamellar spacing exhibited variations. Notably, the selected temperatures were similar to those used to control the α2/γ lamellar spacing during the subsequent heat treatment [29].
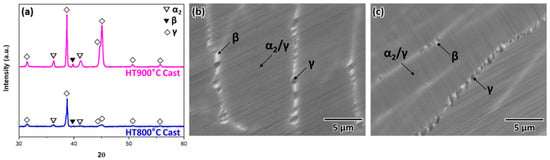
Figure 4.
(a) Typical XRD patterns of the as-cast samples subjected to heat treatment and (b,c) SEM-BSE images showing the microstructure after heat treatment at 800 °C and 900 °C, respectively.
Conversely, the powders contained only β and α2 phases because the high cooling rate suppressed the precipitation of the γ phase. Thus, heat treatment at the same temperatures was conducted to induce α2 → γ phase transformation, aiming to return to the equilibrium microstructure. As shown in Figure 5a, the XRD peaks of the γ phase noticeably intensified, and the fraction of the γ phase sequentially increased with rising heat-treatment temperature. Figure 5b,c show highly magnified SEM-BSE images of the powders subjected to heat treatment at 800 °C and 900 °C, respectively. The α2 phase grains were observed in extremely thin lamellae, with varying orientations. Moreover, small β/γ cells started precipitating at the grain boundaries of the α2/γ colonies (Figure 5b), which is due to a cellular reaction. In addition, coarse β/γ cells were observed when the temperature increased to 900 °C, and the β/γ cells tended to grow away from the grain boundaries and toward the α2/γ lamellar colonies (Figure 5c). Unlike the cast samples, the microstructure of the powder clearly transitioned during the subsequent heat treatment, owing to rapid solidification.
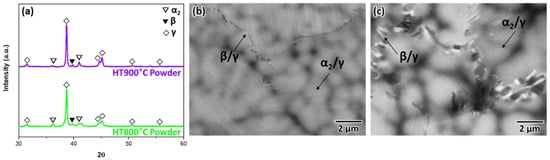
Figure 5.
(a) Typical XRD patterns of the gas-atomized powder subjected to heat treatment, and (b,c) SEM-BSE images showing the microstructure post heat treatment at 800 °C and 900 °C, respectively.
TiAl alloys typically exhibit the α2/γ lamellar microstructure. According to the mechanisms of γ phase transformation reported in a prior study [30], the development of stacking faults in the disordered α or ordered α2 matrix induces the metastable face-centered cubic phase. Through a change in the chemical composition via atom transfer, the ordering reaction forms the final γ phase. However, the rapidly solidifying gas-atomization process causes solute supersaturation due to the high cooling rate. As shown in Table 1 and Table 2, the β phase is a commonly observed phase in the casting and powder materials, but the EDS results showed a significant difference. Although the EDS analysis was carried out in the dendrite and interdendritic regions in this study, the β phase is located in the dendrite regions which is deduced from a previous report [20]. In addition, after rapid solidification or quenching and subsequent aging treatment, the induced α2/γ lamellar microstructure transforms into an ultrafine nano-lamellar structure [22,31]. The chemical disequilibrium in the α2 phase and high interfacial energy in the α2/γ lamellar are the driving forces of the cellular reaction [32]. In fact, Table 3 shows the EDS results of phases in the powder after heat treatment at 900 °C. It can be found that the chemical distribution is changed toward equilibrium, which is a similar tendency compared to casting (Table 1). Meanwhile, higher temperature promotes atomic diffusion during the subsequent heat treatment, which results in a higher volume fraction of the β/γ cells in the 900 °C than 800 °C heat treatment condition.

Table 3.
The EDS results of different phases in gas-atomized powder after the subsequent heat treatment at 900 °C.
Additionally, SEM-EBSD observations were conducted to address the crystallographic orientation of the powder after heat treatment at 900 °C. The phase map indicated a dominant γ phase (Figure 6a), and the IPF map suggested different crystallographic orientation distributions of the grains (Figure 6b). However, the α2/γ lamellar colony followed the Blackburn OR when considering each grain (Figure 6c). While different types of crystallographic orientation between β/γ cells and α2/γ lamellar colonies have been reported [32,33], the first type possesses the same crystallographic orientation relationship between the β/γ cells and α2/γ lamellar, and the lamellar interfaces are the low energy habit plane. Second, the β/γ cells are perpendicular to α2/γ lamellar, with a high energy faceted lamellar interface. Third, the β/γ cells and α2/γ lamellar have different crystallographic orientations, but they form a low-energy habit plane as their interface. Thus, the β/γ cells can represent the same crystallographic orientation with the α2/γ lamellar interfaces or not. The mechanical properties of γ-TiAl alloys are sensitive to changes in phase constitution and/or α2/γ lamellar microstructure. The appropriate volume fraction of the β/γ cells can enhance the mechanical properties due to the twinning-induced plasticity effect [34], and ultrafine α2/γ lamellar also contribute to increased hardness [35]. Therefore, the observed β/γ cells and ultrafine α2/γ lamellar microstructure after rapid solidification in this study may have the potential to improve the mechanical properties of γ-TiAl alloys.
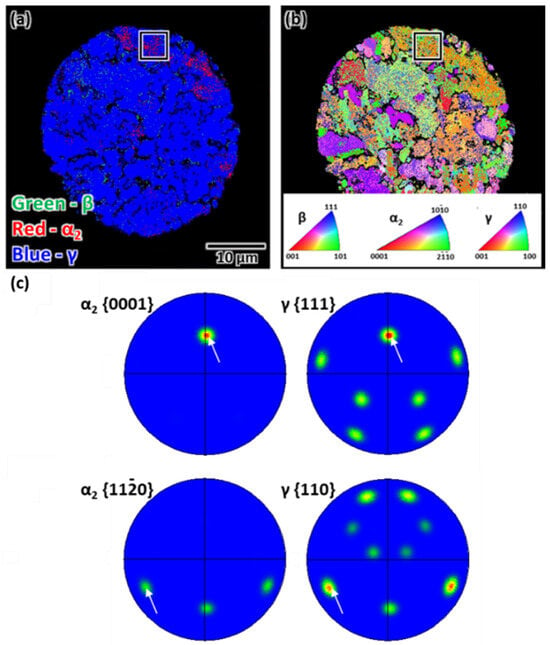
Figure 6.
SEM-EBSD analysis of the powder subjected to heat treatment at 900 °C: (a) phase map, (b) IPF map, and (c) PFs. The PFs obtained from the black rectangular area indicate compliance of the Blackburn OR between the α2 and γ phases as shown with white arrows indicating the (α2 and γ) phases are aligned according to the Blackburn OR.
Lastly, understanding how this microstructure can be achieved on a bulk scale is crucial. The AM process, characterized by rapid solidification and repeated thermal cycling, can enable unique microstructure control. Additionally, this process affords control over the crystallographic orientation [36,37] and can potentially be used to regulate the α2/γ lamellar orientation [38], which can significantly improve the mechanical properties. Overall, this study suggests that preheating and/or subsequent heat treatment at high temperatures during AM can yield precipitation of fine β/γ cell microstructures. However, appropriate temperature control is crucial to avoid cellular reaction and control the orientation of the α2/γ lamellar structure through crystallographic texture formation.
4. Conclusions
This study was aimed at providing a comprehensive understanding of the microstructure evolution of metastable gas-atomized Ti-44Al-6Nb-1.2Cr (at.%), especially during the transition from the metastable to equilibrium state, in comparison with cast materials. The following conclusions can be drawn:
- The cast material with a relatively low solidification rate demonstrated α2/γ colonies surrounded by the β phase. In contrast, the powder consisted of a small amount of the β phase and predominant massive α2 phases with high chemical disequilibrium owing to the high cooling rate.
- The β phase and massive α2 phases have no specific crystallographic orientation relationship with each other.
- After subsequent heat treatment, there were no distinguishing differences in the cast materials; only the volume fraction of the γ phase was changed. However, ultrafine α2/γ lamellar microstructure emerged from the α2 phase, and β/γ cell microstructures were formed at α2/γ lamellar grain boundaries via cellular reaction.
Author Contributions
Conceptualization, S.-H.P. and T.N.; methodology, S.-H.P., O.G. and R.O.; validation, O.G., R.O., K.C., H.Y.Y. and T.N.; formal analysis, S.-H.P. and O.G.; data curation, O.G. and R.O.; writing—original draft preparation, S.-H.P.; writing—review and editing, M.-H.O. and T.N.; visualization, S.-H.P. and R.O.; supervision, T.N.; project administration, R.O. and T.N.; funding acquisition, R.O. and T.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Grant-in-Aid for Transformative Research Area A (21H05198, 22H05288) and Scientific Research (22H01812 and 23H00235) from the Japan Society for the Promotion of Science (JSPS), and CREST-Nanomechanics: Elucidation of macroscale mechanical properties based on understanding nanoscale dynamics of innovative mechanical materials (Grant Number: JPMJCR2194) from the Japan Science and Technology Agency (JST).
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Clemens, H.; Wallgram, W.; Kremmer, S.; Güther, V.; Otto, A.; Bartels, A. Design of novel β-solidifying TiAl alloys with adjustable β/B2-phase fraction and excellent hot-workability. Adv. Eng. Mater. 2008, 10, 707–713. [Google Scholar] [CrossRef]
- Kim, S.W.; Hong, J.K.; Na, Y.S.; Yeom, J.T.; Kim, S.E. Development of TiAl alloys with excellent mechanical properties and oxidation resistance. Mater. Des. 2014, 54, 814–819. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Kim, S.-L. Advances in gammalloy materials–processes–application technology: Successes, dilemmas, and future. JOM 2018, 70, 553–560. [Google Scholar] [CrossRef]
- Duan, B.; Yang, Y.; He, S.; Feng, Q.; Mao, L.; Zhang, X.; Jiao, L.; Lu, X.; Chen, G.; Li, C. History and development of γ-TiAl alloys and the effect of alloying elements on their phase transformations. J. Alloys Compd. 2022, 909, 164811. [Google Scholar] [CrossRef]
- Todai, M.; Nakano, T.; Liu, T.; Yasuda, H.Y.; Hagihara, K.; Cho, K.; Ueda, M.; Takeyama, M. Effect of building direction on the microstructure and tensile properties of Ti-48Al-2Cr-2Nb alloy additively manufactured by electron beam melting. Addit. Manuf. 2017, 13, 61–70. [Google Scholar] [CrossRef]
- Cho, K.; Kawabata, H.; Hayashi, T.; Yasuda, H.Y.; Nakashima, H.; Takeyama, M.; Nakano, T. Peculiar microstructural evolution and tensile properties of β-containing γ-TiAl alloys fabricated by electron beam melting. Addit. Manuf. 2021, 46, 102091. [Google Scholar] [CrossRef]
- Gao, P.; Huang, W.; Yang, H.; Jing, G.; Liu, Q.; Wang, G.; Wang, Z.; Zeng, X. Cracking behavior and control of β-solidifying Ti-40Al-9V-0.5Y alloy produced by selective laser melting. J. Mater. Sci. Technol. 2020, 39, 144–154. [Google Scholar] [CrossRef]
- Kan, W.; Chen, B.; Jin, C.; Peng, H.; Lin, J. Microstructure and mechanical properties of a high Nb-TiAl alloy fabricated by electron beam melting. Mater. Des. 2018, 160, 611–623. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, B.; Mushongera, L.; Kundin, J.; Liao, Y. Laser Powder bed fusion of titanium aluminides: An investigation on site-specific microstructure evolution mechanism. Mater. Des. 2021, 201, 109501. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, H.; Peng, G.; Yin, J.; Zeng, X. Microstructure prediction of selective laser melting AlSi10Mg using finite element analysis. Mater. Des. 2018, 142, 319–328. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Cui, R.; Wang, B.; Luo, L.; Su, Y. Differences in microstructure and nano-hardness of selective laser melted Inconel 718 single tracks under various melting modes of molten pool. J. Mater. Res. Technol. 2020, 9, 10401–10410. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Clemens, H.; Mayer, S.; Lindemann, J.; Klose, J.; Smarsly, W.; Güther, V. Microstructural design and mechanical properties of a cast and heat-treated Intermetallic multi-phase γ-TiAl based alloy. Intermetallics 2014, 44, 128–140. [Google Scholar] [CrossRef]
- Takeyama, M.; Kobayashi, S. Physical metallurgy for wrought gamma titanium aluminides: Microstructure control through phase transformations. Intermetallics 2005, 13, 993–999. [Google Scholar] [CrossRef]
- Hu, D.; Jiang, H. Martensite in a TiAl alloy quenched from beta phase field. Intermetallics 2015, 56, 87–95. [Google Scholar] [CrossRef]
- Mayer, S.; Petersmann, M.; Fischer, F.D.; Clemens, H.; Waitz, T.; Antretter, T. Experimental and theoretical evidence of displacive martensite in an intermetallic Mo-containing γ-TiAl based alloy. Acta. Mater. 2016, 115, 242–249. [Google Scholar] [CrossRef]
- Martín, A.; Cepeda-Jiménez, C.M.; Pérez-Prado, M.T. Gas atomization of γ-TiAl alloy powder for additive manufacturing. Adv. Eng. Mater. 2020, 22, 1900594. [Google Scholar] [CrossRef]
- Gerling, R.; Clemens, H.; Schimansky, F.P. Powder metallurgical processing of intermetallic gamma titanium aluminides. Adv. Eng. Mater. 2004, 6, 23–38. [Google Scholar] [CrossRef]
- Yang, D.Y.; Guo, S.; Peng, H.X.; Cao, F.Y.; Liu, N.; Sun, J.F. Size dependent phase transformation in atomized TiAl powders. Intermetallics 2015, 61, 72–79. [Google Scholar] [CrossRef]
- Kastenhuber, M.; Klein, T.; Rashkova, B.; Weißensteiner, I.; Clemens, H.; Mayer, S. Phase transformations in a β-solidifying γ-TiAl based alloy during rapid solidification. Intermetallics 2017, 91, 100–109. [Google Scholar] [CrossRef]
- Guyon, J.; Hazotte, A.; Bouzy, E. Evolution of metastable α phase during heating of Ti48Al2Cr2Nb intermetallic alloy. J. Alloys Compd. 2015, 656, 667–675. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Wu, M.; Ye, Z.; Wang, Q.; Gu, J. Atypical pathways for lamellar and twinning transformations in rapidly solidified TiAl alloy. Acta Mater. 2022, 227, 117718. [Google Scholar] [CrossRef]
- Guyon, J.; Hazotte, A.; Wagner, F.; Bouzy, E. Recrystallization of coherent nanolamellar structures in Ti48Al2Cr2Nb intermetallic alloy. Acta Mater. 2016, 103, 672–680. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, L.; He, J.; Zhang, F.; Feng, X.; Nan, H.; Lin, J.; Kim, Y.W. As-cast microstructure characteristics dependent on solidification mode in TiAl-Nb alloys. J. Alloys Compd. 2019, 809, 151862. [Google Scholar] [CrossRef]
- Imayev, R.M.; Imayev, V.M.; Oehring, M.; Appel, F. Alloy design concepts for refined gamma titanium aluminide based alloys. Intermetallics 2007, 15, 451–460. [Google Scholar] [CrossRef]
- Yang, J.; Cao, B.; Wu, Y.; Gao, Z.; Hu, R. Continuous cooling transformation (CCT) behavior of a high Nb-containing TiAl alloy. Materialia 2019, 5, 100169. [Google Scholar] [CrossRef]
- Laipple, D.; Stark, A.; Schimansky, F.P.; Schwebke, B.; Pyczak, F.; Schreyer, A. Microstructure of Ti-45Al-5Nb and Ti-45Al-10Nb powders. Key Eng. Mater. 2016, 704, 214–222. [Google Scholar] [CrossRef]
- Massalski, T.B. Massive transformations revisited. Metall. Mater. Trans. A 2002, 33, 2277–2283. [Google Scholar] [CrossRef]
- Panov, D.O.; Sokolovsky, V.S.; Stepanov, N.D.; Zherebtsov, S.V.; Panin, P.V.; Volokitina, E.I.; Nochovnaya, N.A.; Salishchev, G.A. Effect of interlamellar spacing on strength-ductility combination of β-solidified γ-TiAl based alloy with fully lamellar structure. Mater. Sci. Eng. A 2023, 862, 144458. [Google Scholar] [CrossRef]
- Denquint, A.; Naka, S. Phase transformation mechanisms involved in two-phase TiAl-based alloys-I. lambellar structure formation. Acta Mater. 1996, 44, 343–352. [Google Scholar] [CrossRef]
- Sun, Y.Q. Nanometer-scale, fully lamellar microstructure in anaged TiAl-based alloy. Metall. Mater. Trans. A 1998, 29, 2679–2685. [Google Scholar] [CrossRef]
- Kastenhuber, M.; Rashkova, B.; Clemens, H.; Mayer, S. Effect of microstructural instability on the creep resistance of an advanced intermetallic γ-TiAl based alloy. Intermetallics 2017, 80, 1–9. [Google Scholar] [CrossRef]
- Mitaof, S.; Bendersky, L.A. Morphology and growth kinetics of discontinuous coarsening in fully lamellar Ti-44 Al (at.%) alloy. Acta Mater. 1997, 45, 4475–4489. [Google Scholar] [CrossRef]
- Zheng, G.; Tang, B.; Zhao, S.; Wang, W.Y.; Chen, X.; Zhu, L.; Li, J. Evading the strength-ductility trade-off at room temperature and achieving ultrahigh plasticity at 800 °C in a TiAl alloy. Acta Mater. 2022, 225, 117585. [Google Scholar] [CrossRef]
- Cha, L.; Scheu, C.; Clemens, H.; Chladil, H.F.; Dehm, G.; Gerling, R.; Bartels, A. Nanometer-scaled lamellar microstructures in Ti-45Al-7.5Nb-(0; 0.5)C alloys and their influence on hardness. Intermetallics 2008, 16, 868–875. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ishimoto, T.; Hibino, S.; Yasutomi, J.; Narushima, T.; Nakano, T. Unique crystallographic texture formation in Inconel 718 by laser powder bed fusion and its effect on mechanical anisotropy. Acta Mater. 2021, 212, 116876. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ishimoto, T.; Nishikawa, Y.; Kim, Y.S.; Matsugaki, A.; Ozasa, R.; Weinmann, M.; Schnitter, C.; Stenzel, M.; Kim, H.S.; et al. Novel single crystalline-like non-equiatomic TiZrHfNbTaMo Bio-high entropy alloy (BioHEA) developed by laser powder bed fusion. Mater. Res. Lett. 2023, 11, 274–280. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Zheng, M.; Ye, Z.; Yang, X.; Gu, J. Anisotropic tensile behavior of Ti-47Al-2Cr-2Nb alloy fabricated by direct laser deposition. Addit. Manuf. 2020, 32, 101087. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).