Liquid Crystals for Luminescent Concentrators: A Review
Abstract
1. Introduction
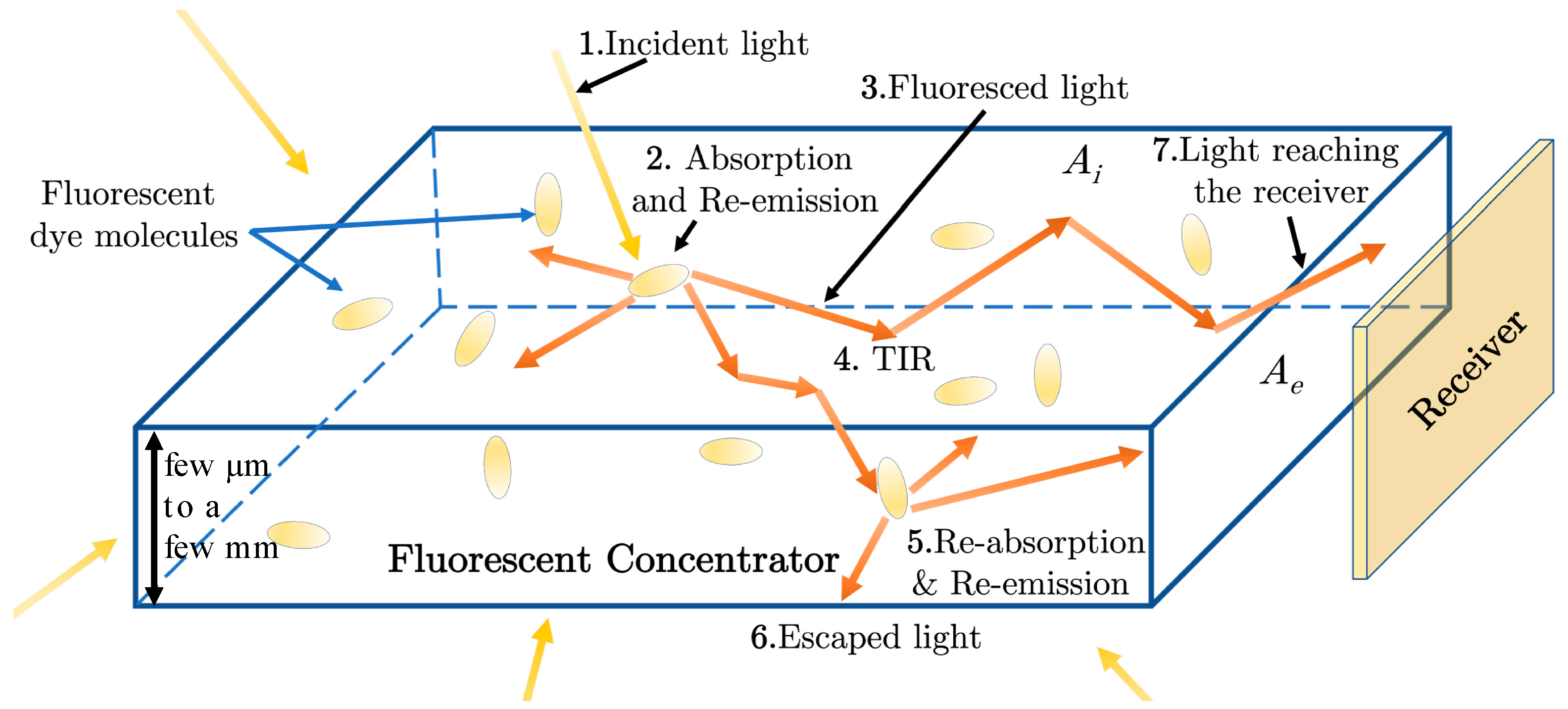
1.1. Luminescent Concentrators
1.2. Liquid Crystals as Host Materials
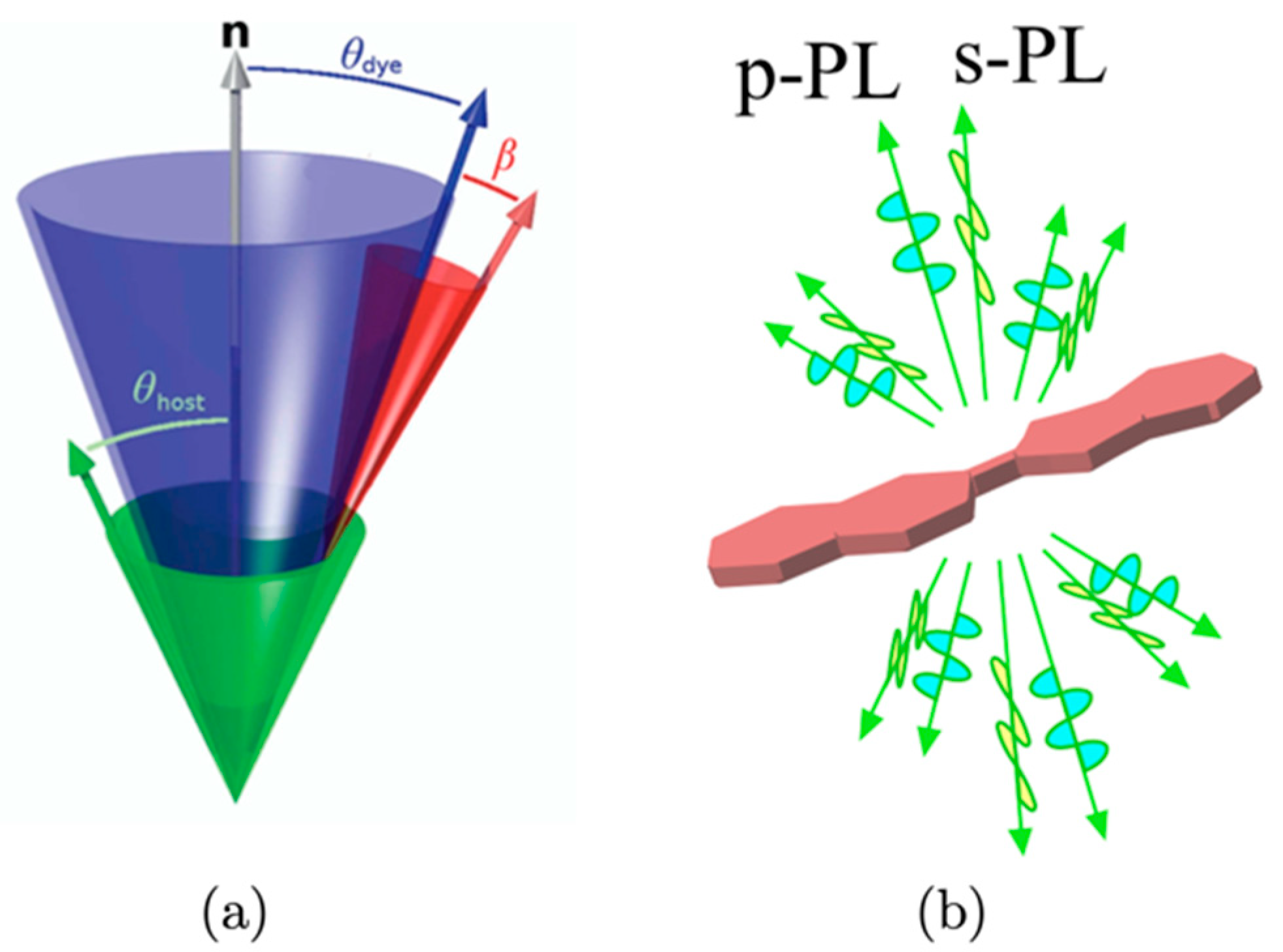
1.3. Organization of This Review Article
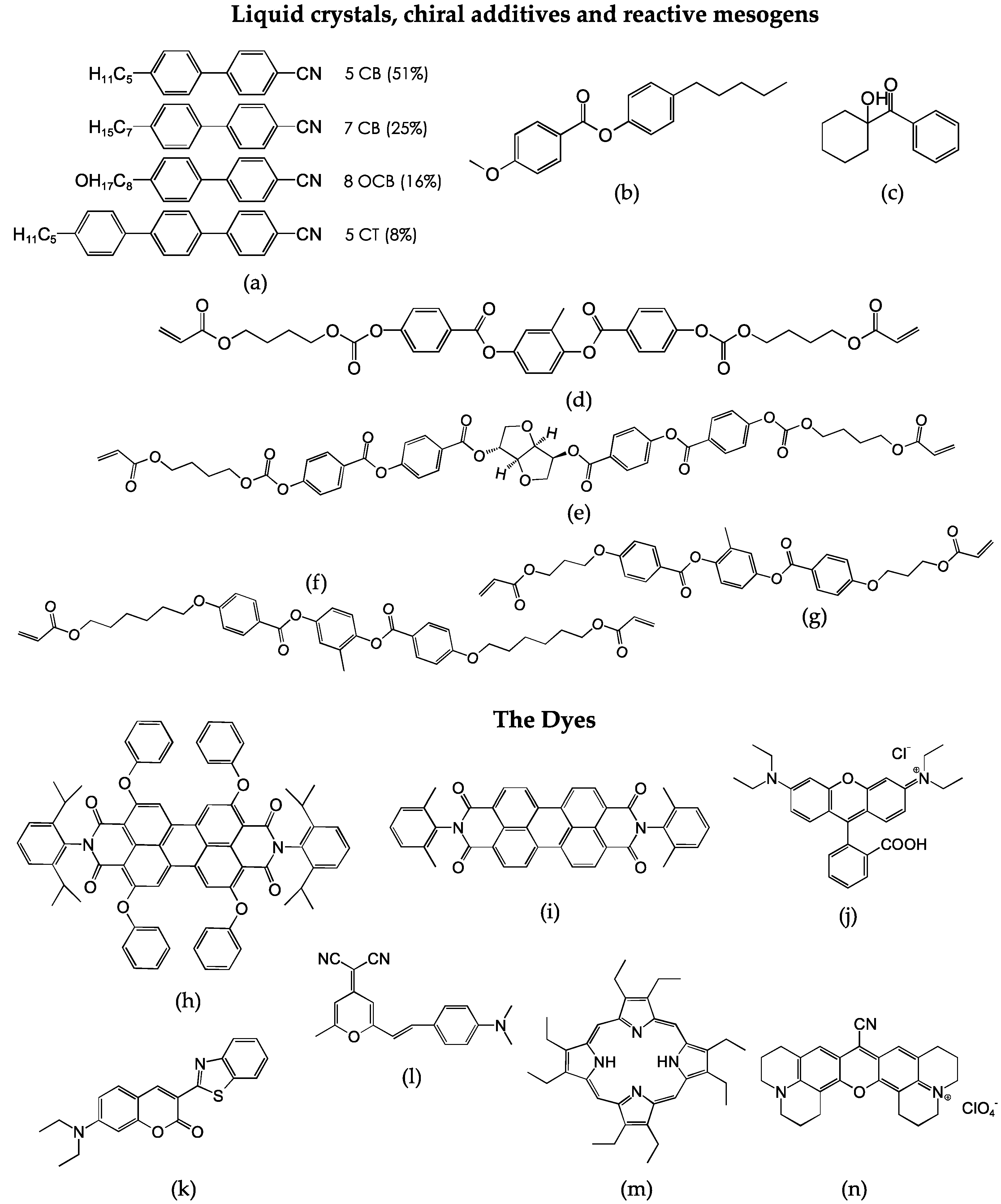
2. Material Combinations and Characterization Techniques
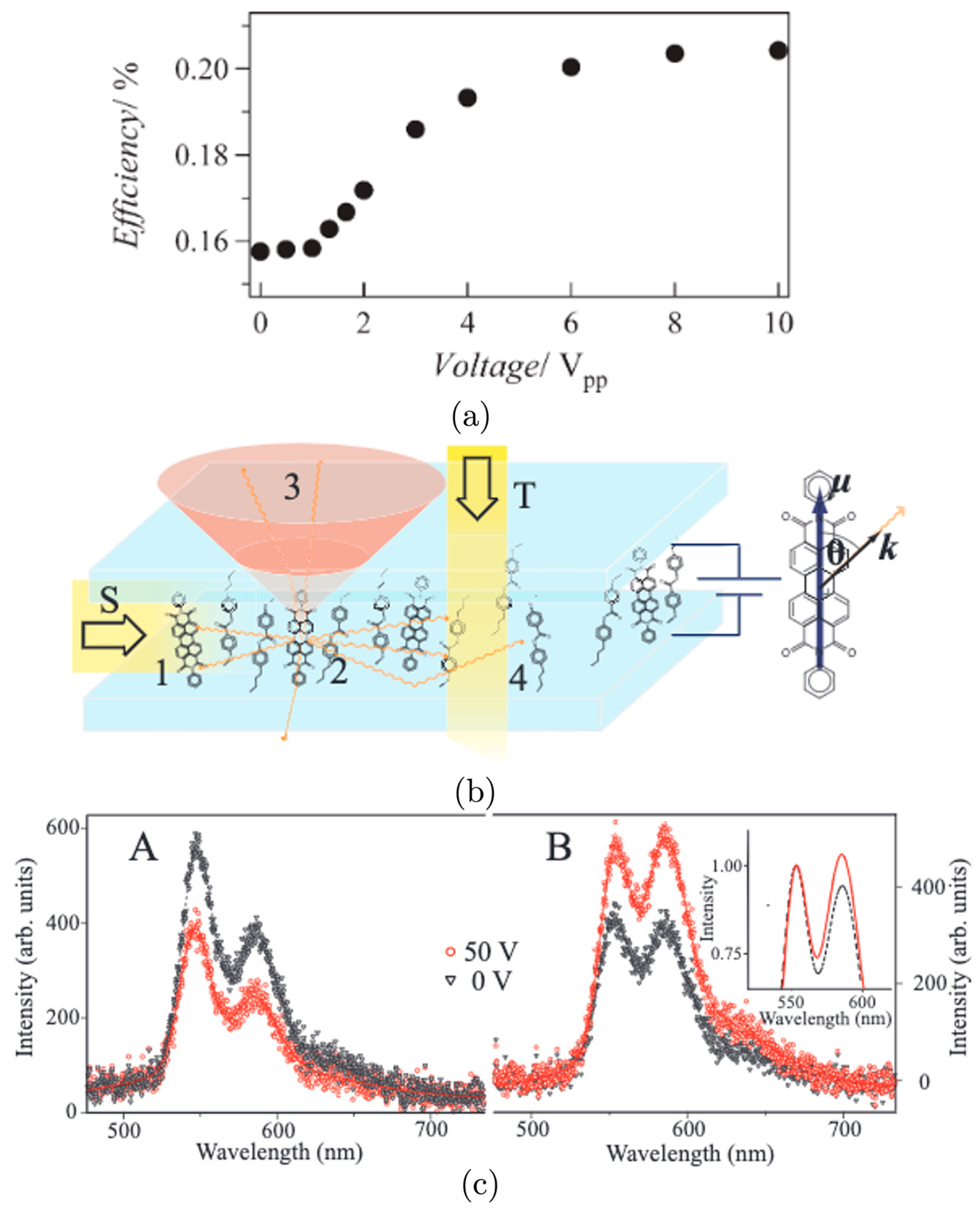
3. Liquid-Crystal Alignments
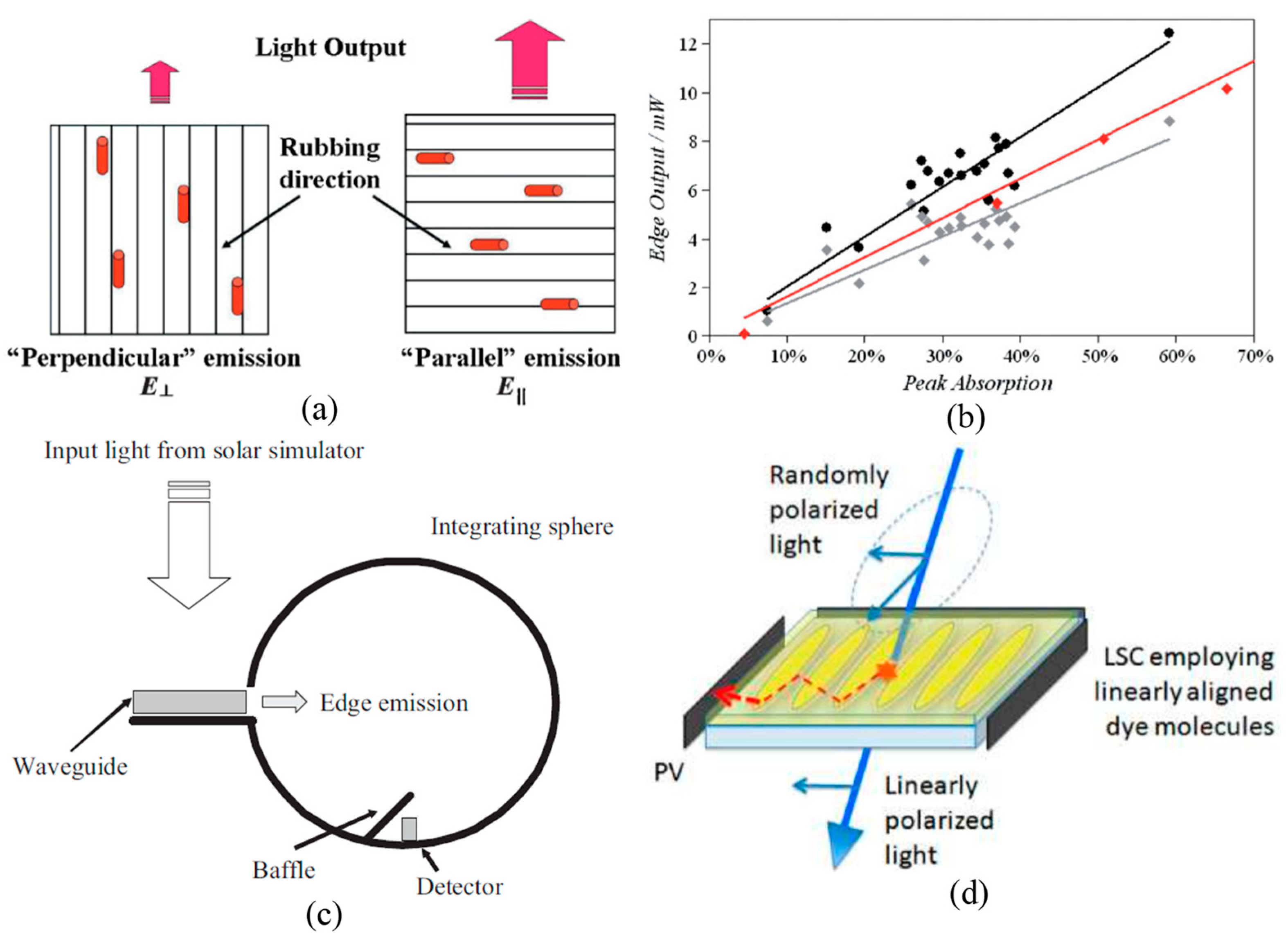
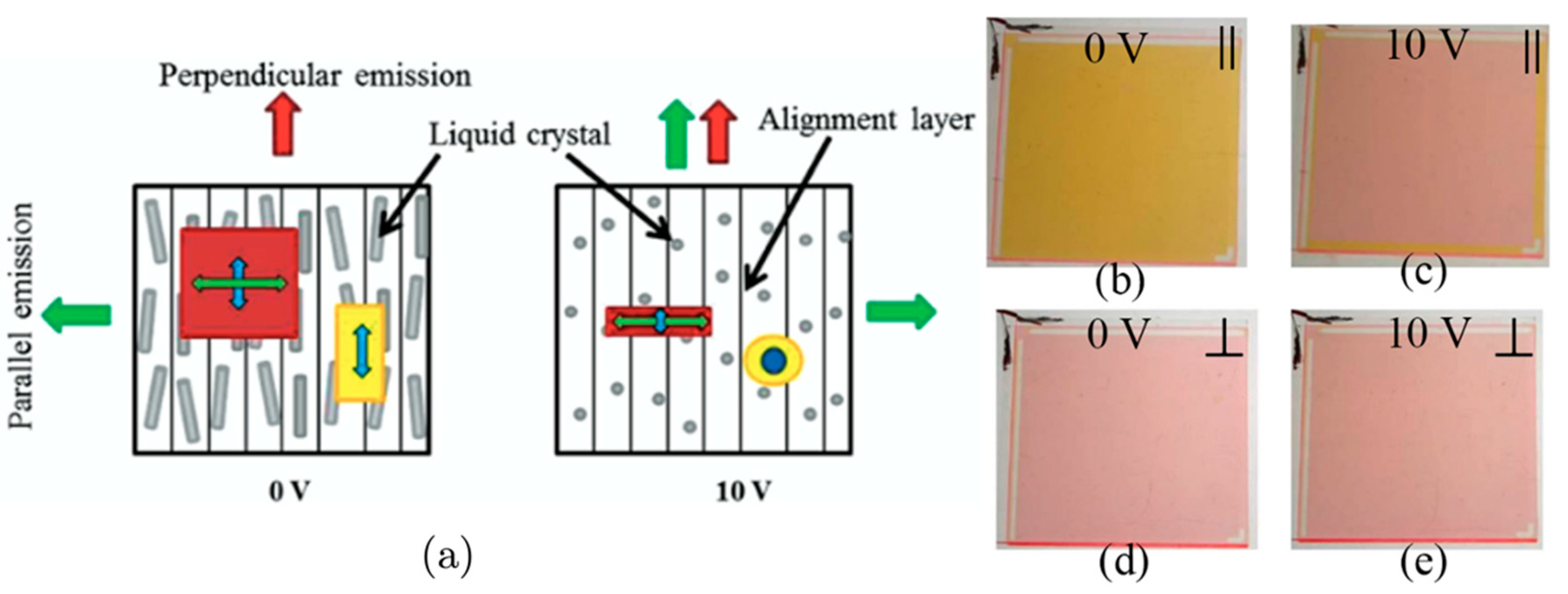
3.1. Planar Alignments
3.2. Homeotropic Alignments
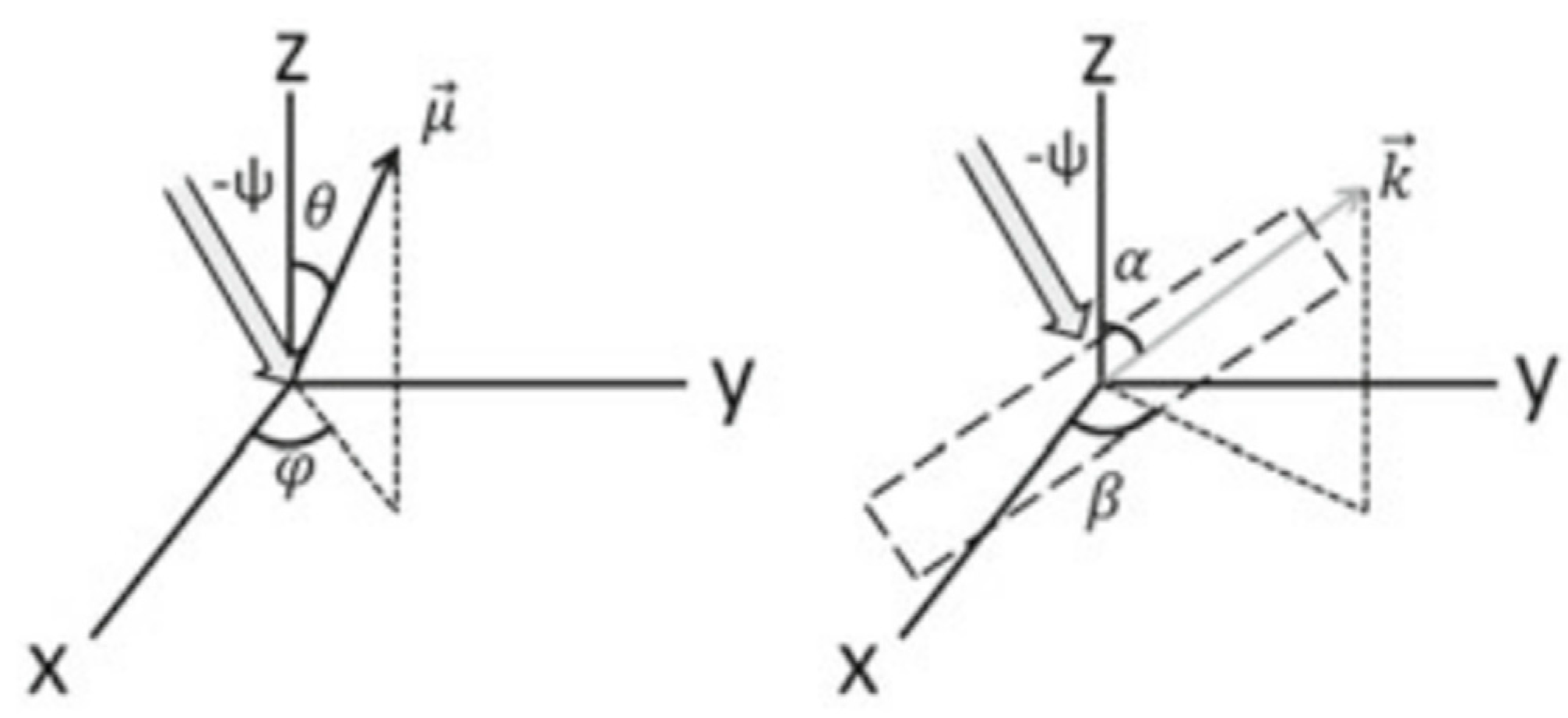
3.3. Theoretical Modelling
3.4. Dye-Doped Chiral Nematic and Twisted Nematic Phases
3.5. Combining Positive and Negative Dichroism
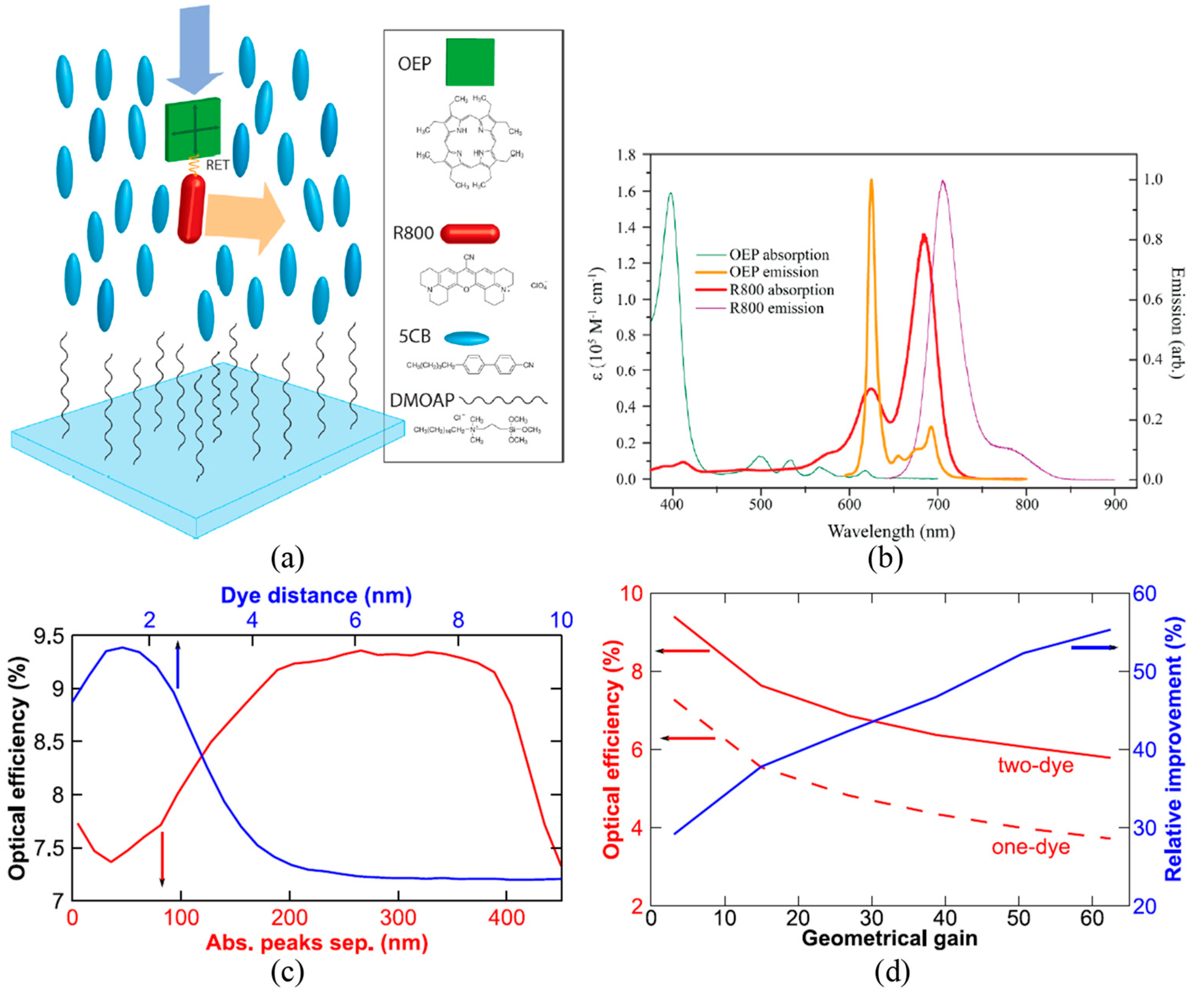
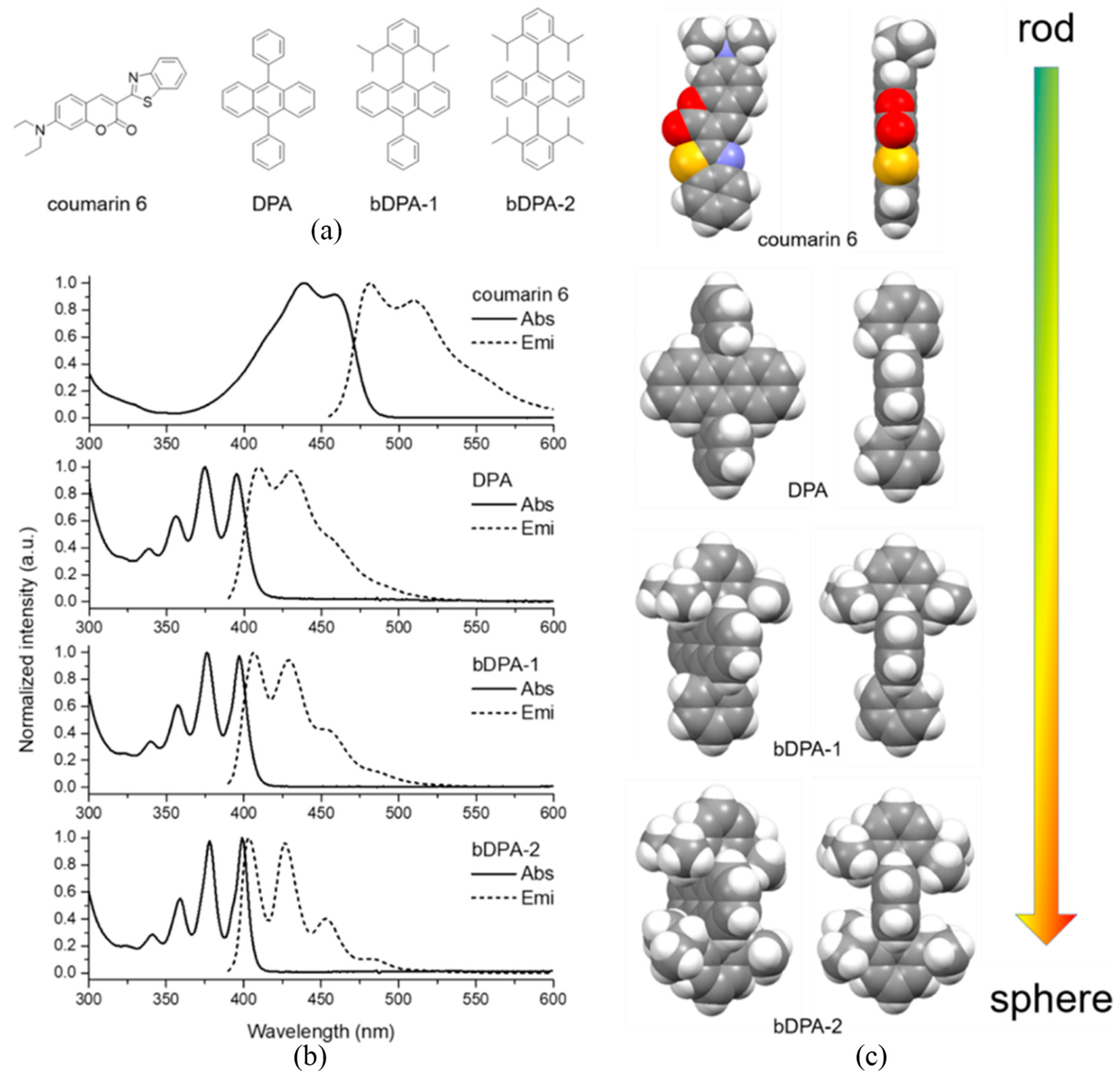
4. Optimizing the Dye Structures and Combinations
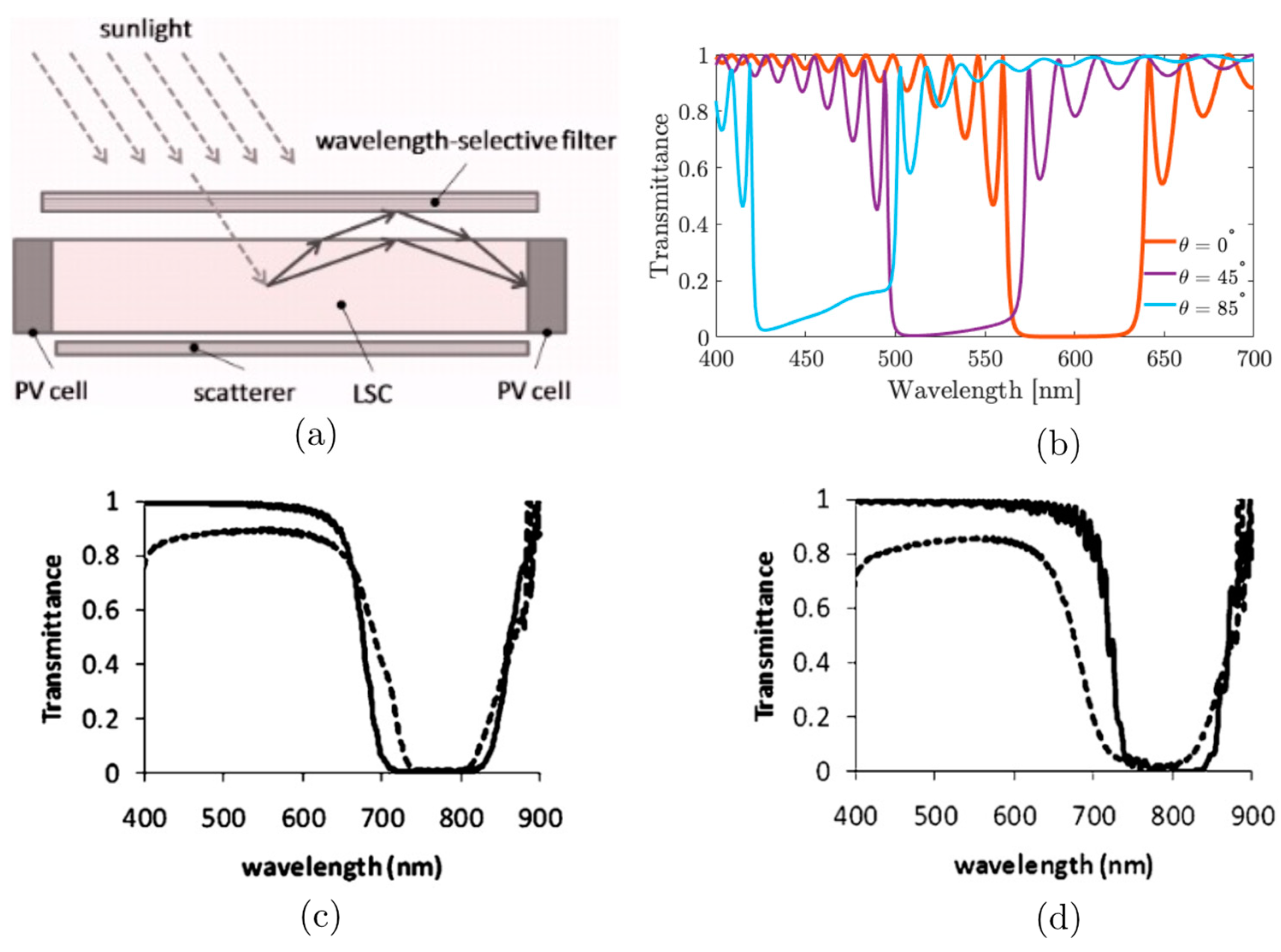
5. Chiral Nematic Reflectors
6. Conclusions and Further Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smets, A.H.M.; Jäger, K.; Isabella, O.; van Swaaij, R.A.; Zeman, M. Solar Energy: The Physics and Engineering of Photovoltaic Conversion, Technologies and Systems, 1st ed.; UIT Cambridge Ltd.: Cambridge, UK, 2016; Volume 1, ISBN 978 1 906860 32 5. [Google Scholar]
- Boxwell, M. Solar Electricity Handbook: 2017 Edition: A Simple, Practical Guide to Solar Energy—Designing and Installing Solar Photovoltaic Systems. (The Solar... and Installing Solar Photovoltaic Systems), 11th ed.; Greenstream Publishing Limited: Birmingham, UK, 2017; Volume 1. [Google Scholar]
- Winston, R. Dielectric Compound Parabolic Concentrators. Appl. Opt. 1976, 15, 291–292. [Google Scholar] [CrossRef]
- Winston, R.; Hinterberger, H. Principles of Cylindrical Concentrators for Solar Energy. Sol. Energy 1975, 17, 255–258. [Google Scholar] [CrossRef]
- Coffey, V.C. Solar Concentrators: Using Optics to Boost Photovoltaics. Opt. Photonics News 2011, 22, 22–27. [Google Scholar] [CrossRef]
- Zacharopoulos, A.; Eames, P.C.; McLarnon, D.; Norton, B. Linear Dielectric Non-Imaging Concentrating Covers for PV Integrated Building Facades. Sol. Energy 2000, 68, 439–452. [Google Scholar] [CrossRef]
- Apostoleris, H.; Stefancich, M.; Chiesa, M. Tracking-Integrated Systems for Concentrating Photovoltaics. Nat. Energy 2016, 1, 16018. [Google Scholar] [CrossRef]
- Madala, S.; Boehm, R.F. A Review of Nonimaging Solar Concentrators for Stationary and Passive Tracking Applications. Renew. Sustain. Energy Rev. 2017, 71, 309–322. [Google Scholar] [CrossRef]
- Winston, R. Principles of Solar Concentrators of a Novel Design. Sol. Energy 1974, 16, 89–95. [Google Scholar] [CrossRef]
- Lim, Y.S.; Lo, C.K.; Kee, S.Y.; Ewe, H.T.; Faidz, A.R. Design and Evaluation of Passive Concentrator and Reflector Systems for Bifacial Solar Panel on a Highly Cloudy Region—A Case Study in Malaysia. Renew. Energy 2014, 63, 415–425. [Google Scholar] [CrossRef]
- Batchelder, J.S.; Zewai, A.H.; Cole, T. Luminescent Solar Concentrators 1: Theory of Operation and Techniques for Performance Evaluation. Appl. Opt. 1979, 18, 3090–3110. [Google Scholar] [CrossRef]
- Weber, W.H.; Lambe, J. Luminescent Greenhouse Collector for Solar Radiation. Appl. Opt. 1976, 15, 2299–2300. [Google Scholar] [CrossRef]
- Levitt, J.A.; Weber, W.H. Materials for Luminescent Greenhouse Solar Collectors. Appl. Opt. 1977, 16, 2684–2689. [Google Scholar] [CrossRef]
- Goetzberger, A.; Greube, W. Solar Energy Conversion with Fluorescent Collectors. Appl. Phys. 1977, 14, 123–139. [Google Scholar] [CrossRef]
- Swartz, B.A.; Cole, T.; Zewail, A.H. Photon Trapping and Energy Transfer in Multiple-Dye Plastic Matrices: An Efficient Solar-Energy Concentrator. Opt. Lett. 1977, 1, 73–75. [Google Scholar] [CrossRef]
- Hermann, A.M. Luminescent Solar Concentrators—A Review. Sol. Energy 1982, 29, 323–329. [Google Scholar] [CrossRef]
- Barnham, K.; Marques, J.L.; Hassard, J.; O’Brien, P. Quantum-Dot Concentrator and Thermodynamic Model for the Global Redshift. Appl. Phys. Lett. 2000, 76, 1197–1199. [Google Scholar] [CrossRef]
- Baumberg, I.; Berezin, O.; Drabkin, A.; Gorelik, B.; Kogan, L.; Voskobojnik, M.; Zaidman, M. Effect of Polymer Matrix on Photo-Stability of Photo-Luminescent Dyes in Multi-Layer Polymeric Structures. Polym. Degrad. Stab. 2001, 73, 403–410. [Google Scholar] [CrossRef]
- Rau, U.; Einsele, F.; Glaeser, G.C. Efficiency Limits of Photovoltaic Fluorescent Collectors. Appl. Phys. Lett. 2005, 87, 171101. [Google Scholar] [CrossRef]
- Earp, A.A.; Smith, G.B.; Swift, P.D.; Franklin, J. Maximising the Light Output of a Luminescent Solar Concentrator. Sol. Energy 2004, 76, 655–667. [Google Scholar] [CrossRef]
- Chatten, A.J.; Barnham, K.W.J.; Buxton, B.F.; Ekins-Daukes, N.J.; Malik, M.A. Quantum Dot Solar Concentrators. Semiconductors 2004, 38, 909–917. [Google Scholar] [CrossRef]
- Rowan, B.C.; Wilson, L.R.; Richards, B.S. Advanced Material Concepts for Luminescent Solar Concentrators. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 1312–1322. [Google Scholar] [CrossRef]
- van Sark, W.G.J.H.M.; Barnham, K.W.J.; Slooff, L.H.; Chatten, A.J.; Büchtemann, A.; Meyer, A.; McCormack, S.J.; Koole, R.; Farrell, D.J.; Bose, R.; et al. Luminescent Solar Concentrators—A Review of Recent Results. Opt. Express 2008, 16, 21773–21792. [Google Scholar] [CrossRef]
- Debije, M.G.; Verbunt, P.P.C. Thirty Years of Luminescent Solar Concentrator Research: Solar Energy for the Built Environment. Adv. Energy Mater. 2012, 2, 12–35. [Google Scholar] [CrossRef]
- Corrado, C.; Leow, S.W.; Osborn, M.; Carbone, I.; Hellier, K.; Short, M.; Alers, G.; Carter, S.A. Power Generation Study of Luminescent Solar Concentrator Greenhouse. J. Renew. Sustain. Energy 2016, 8, 043502. [Google Scholar] [CrossRef]
- Griffini, G. Host Matrix Materials for Luminescent Solar Concentrators: Recent Achievements and Forthcoming Challenges. Front. Mater. 2019, 6, 1–8. [Google Scholar] [CrossRef]
- Frias, A.R.; Correia, S.F.H.; Martins, M.; Ventura, S.P.M.; Pecoraro, E.; Ribeiro, S.J.L.; André, P.S.; Ferreira, R.A.S.; Coutinho, J.A.P.; Carlos, L.D. Sustainable Liquid Luminescent Solar Concentrators. Adv. Sustain. Syst. 2019, 3, 1800134. [Google Scholar] [CrossRef]
- Papakonstantinou, I.; Portnoi, M.; Debije, M.G. The Hidden Potential of Luminescent Solar Concentrators. Adv. Energy Mater. 2021, 11, 2002883. [Google Scholar] [CrossRef]
- Castelletto, S.; Boretti, A. Luminescence Solar Concentrators: A Technology Update. Nano Energy 2023, 109, 108269. [Google Scholar] [CrossRef]
- Tummeltshammer, C.; Taylor, A.; Kenyon, A.J.; Papakonstantinou, I. Losses in Luminescent Solar Concentrators Unveiled. Sol. Energy Mater. Sol. Cells 2016, 144, 40–47. [Google Scholar] [CrossRef]
- Oliveto, V.J.; Boyd, C.; Smith, D.; Hughes, M.; Borca-Tasciuc, D.-A. Luminescent Solar Concentrators: A Review of Nanoengineering Opportunities for Reducing Surface Losses. IEEE Trans. Nanotechnol. 2022, 21, 360–366. [Google Scholar] [CrossRef]
- Pålsson, L.-O.; Vaughan, H.L.; Smith, A.; Szablewski, M.; Cross, G.H.; Roberts, T.; Masutani, A.; Yasuda, A.; Beeby, A.; Bloor, D. Guest–Host Interactions between Dichroic Dyes and Anisotropic Hosts. J. Lumin. 2006, 117, 113–122. [Google Scholar] [CrossRef]
- Stephen, M.J.; Straley, J.P. Physics of Liquid Crystals. Rev. Mod. Phys. 1974, 46, 617–704. [Google Scholar] [CrossRef]
- Andrienko, D. Introduction to Liquid Crystals. J. Mol. Liq. 2018, 267, 520–541. [Google Scholar] [CrossRef]
- Priestley, E.B. Liquid Crystal Mesophases. In Introduction to Liquid Crystals; Springer: Boston, MA, USA, 1975; pp. 1–13. [Google Scholar]
- Hahm, S.G.; Ko, Y.-G.; Rho, Y.; Ahn, B.; Ree, M. Liquid Crystal Alignment in Advanced Flat-Panel Liquid Crystal Displays. Curr. Opin. Chem. Eng. 2013, 2, 71–78. [Google Scholar] [CrossRef]
- Kowerdziej, R.; Parka, J.; Krupka, J.; Olifierczuk, M.; Nowinowski-Kruszelnicki, E.; Jaroszewicz, L.; Chojnowska, O. Dielectric Properties of Highly Anisotropic Nematic Liquid Crystals for Tunable Microwave Components. Appl. Phys. Lett. 2013, 103, 172902. [Google Scholar] [CrossRef]
- Zografopoulos, D.C.; Ferraro, A.; Beccherelli, R. Liquid-Crystal High-Frequency Microwave Technology: Materials and Characterization. Adv. Mater. Technol. 2019, 4, 1800447. [Google Scholar] [CrossRef]
- Lowe, A.M.; Abbott, N.L. Liquid Crystalline Materials for Biological Applications. Chem. Mater. 2012, 24, 746–758. [Google Scholar] [CrossRef]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Ma, J.; Choi, J.; Park, S.; Kong, I.; Kim, D.; Lee, C.; Youn, Y.; Hwang, M.; Oh, S.; Hong, W.; et al. Liquid Crystals for Advanced Smart Devices with Microwave and Millimeter-Wave Applications: Recent Progress for Next-Generation Communications. Adv. Mater. 2023, 35, 2302474. [Google Scholar] [CrossRef]
- Zou, J.; Yang, Z.; Mao, C.; Wu, S.-T. Fast-Response Liquid Crystals for 6G Optical Communications. Crystals 2021, 11, 797. [Google Scholar] [CrossRef]
- Heilmeier, G.H.; Zanoni, L.A. Guest-host interactions in nematic liquid crystals. A new electro-optic effect. Appl. Phys. Lett. 1968, 13, 91–92. [Google Scholar] [CrossRef]
- Goldschmidt, J.C.; Peters, M.; Bösch, A.; Helmers, H.; Dimroth, F.; Glunz, S.W.; Willeke, G. Increasing the Efficiency of Fluorescent Concentrator Systems. Sol. Energy Mater. Sol. Cells 2009, 93, 176–182. [Google Scholar] [CrossRef]
- Goldschmidt, J.C.; Peters, M.; Prönneke, L.; Steidl, L.; Zentel, R.; Bläsi, B.; Gombert, A.; Glunz, S.; Willeke, G.; Rau, U. Theoretical and Experimental Analysis of Photonic Structures for Fluorescent Concentrators with Increased Efficiencies. Phys. Status Solidi (a) 2008, 205, 2811–2821. [Google Scholar] [CrossRef]
- Portnoi, M.; Macdonald, T.J.; Sol, C.; Robbins, T.S.; Li, T.; Schläfer, J.; Guldin, S.; Parkin, I.P.; Papakonstantinou, I. All-Silicone-Based Distributed Bragg Reflectors for Efficient Flexible Luminescent Solar Concentrators. Nano Energy 2020, 70, 104507. [Google Scholar] [CrossRef]
- Coates, D. Development and Applications of Cholesteric Liquid Crystals. Liq. Cryst. 2015, 42, 653–665. [Google Scholar] [CrossRef]
- Taugerbeck, A.; Booth, C.J. Design and Synthesis of Chiral Nematic Liquid Crystals. In Handbook of Liquid Crystals; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–63. [Google Scholar]
- Sims, M.T. Dyes as Guests in Ordered Systems: Current Understanding and Future Directions. Liq. Cryst. 2016, 43, 2363–2374. [Google Scholar] [CrossRef]
- Fujieda, I.; Suzuki, D.; Masuda, T. Tilted Dipole Model for Bias-Dependent Photoluminescence Pattern. J. Appl. Phys. 2014, 116, 224507. [Google Scholar] [CrossRef]
- Verbunt, P.P.C.; Kaiser, A.; Hermans, K.; Bastiaansen, C.W.M.; Broer, D.J.; Debije, M.G. Controlling Light Emission in Luminescent Solar Concentrators Through Use of Dye Molecules Aligned in a Planar Manner by Liquid Crystals. Adv. Funct. Mater. 2009, 19, 2714–2719. [Google Scholar] [CrossRef]
- MacQueen, R.W.; Cheng, Y.Y.; Clady, R.G.C.R.; Schmidt, T.W. Towards an Aligned Luminophore Solar Concentrator. Opt. Express 2010, 18, A161. [Google Scholar] [CrossRef]
- Mulder, C.L.; Reusswig, P.D.; Beyler, A.P.; Kim, H.; Rotschild, C.; Baldo, M.A. Dye Alignment in Luminescent Solar Concentrators: II Horizontal Alignment for Energy Harvesting in Linear Polarizers. Opt. Express 2010, 18, A91. [Google Scholar] [CrossRef]
- Debije, M.G.; Van, M.-P.; Verbunt, P.P.C.; Kastelijn, M.J.; van der Blom, R.H.L.; Broer, D.J.; Bastiaansen, C.W.M. Effect on the Output of a Luminescent Solar Concentrator on Application of Organic Wavelength-Selective Mirrors. Appl. Opt. 2010, 49, 745–751. [Google Scholar] [CrossRef]
- Debije, M.G. Solar Energy Collectors with Tunable Transmission. Adv. Funct. Mater. 2010, 20, 1498–1502. [Google Scholar] [CrossRef]
- Mulder, C.L.; Reusswig, P.D.; Velázquez, A.M.; Kim, H.; Rotschild, C.; Baldo, M.A. Dye Alignment in Luminescent Solar Concentrators: I Vertical Alignment for Improved Waveguide Coupling. Opt. Express 2010, 18, A79. [Google Scholar] [CrossRef]
- MacQueen, R.W.; Schmidt, T.W. Molecular Polarization Switching for Improved Light Coupling in Luminescent Solar Concentrators. J. Phys. Chem. Lett. 2013, 4, 2874–2879. [Google Scholar] [CrossRef]
- Debije, M.G.; Verbunt, P.P.C.; Rowan, B.C.; Richards, B.S.; Hoeks, T.L. Measured Surface Loss from Luminescent Solar Concentrator Waveguides. Appl. Opt. 2008, 47, 6763–6768. [Google Scholar] [CrossRef]
- Verbunt, P.P.C.; Sánchez-Somolinos, C.; Broer, D.J.; Debije, M.G. Anisotropic Light Emissions in Luminescent Solar Concentrators–Isotropic Systems. Opt. Express 2013, 21, A485. [Google Scholar] [CrossRef]
- Verbunt, P.P.C.; de Jong, T.M.; de Boer, D.K.G.; Broer, D.J.; Debije, M.G. Anisotropic Light Emission from Aligned Luminophores. Eur. Phys. J. Appl. Phys. 2014, 67, 10201. [Google Scholar] [CrossRef]
- Bauman, D.; Moryson, H.; Wolarz, E. Orientational Behaviour of the Guest-Host Systems in the Smectic A and Nematic Phases. Acta Phys. Pol. A 1992, 81, 559–570. [Google Scholar] [CrossRef]
- Zannoni, C. Order Parameters and Orientational Distributions in Liquid Crystals. In Polarized Spectroscopy of Ordered Systems; Springer: Dordrecht, The Netherlands, 1988; pp. 57–83. [Google Scholar]
- van Ewyk, R.L.; O’Connor, I.; Mosley, A.; Cuddy, A.; Hilsum, C.; Blackburn, C.; Griffiths, J.; Jones, F. Anisotropic Fluorophors for Liquid Crystal Displays. Displays 1986, 7, 155–160. [Google Scholar] [CrossRef]
- Mulder, D.J.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. Chiral-Nematic Liquid Crystals as One Dimensional Photonic Materials in Optical Sensors. J. Mater. Chem. C 2014, 2, 6695–6705. [Google Scholar] [CrossRef]
- Rodarte, A.L.; Cisneros, F.; Hirst, L.S.; Ghosh, S. Dye-Integrated Cholesteric Photonic Luminescent Solar Concentrator. Liq. Cryst. 2014, 41, 1442–1447. [Google Scholar] [CrossRef][Green Version]
- Sol, J.A.H.P.; Timmermans, G.H.; Breugel, A.J.; Schenning, A.P.H.J.; Debije, M.G. Multistate Luminescent Solar Concentrator “Smart” Windows. Adv. Energy Mater. 2018, 8, 1702922. [Google Scholar] [CrossRef]
- Debije, M.G.; Menelaou, C.; Herz, L.M.; Schenning, A.P.H.J. Combining Positive and Negative Dichroic Fluorophores for Advanced Light Management in Luminescent Solar Concentrators. Adv. Opt. Mater. 2014, 2, 687–693. [Google Scholar] [CrossRef]
- Scheffer, T.J. Guest-Host Devices Using Anisotropic Dyes. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1983, 309, 189–201. [Google Scholar] [CrossRef]
- Wolarz, E.; Moryson, H.; Bauman, D. Dichroic Fluorescent Dyes for ‘Guest-Host’ Liquid Crystal Displays. Displays 1992, 13, 171–178. [Google Scholar] [CrossRef]
- Würthner, F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 35, 1564–1579. [Google Scholar] [CrossRef]
- Fritz, K.P.; Scholes, G.D. Alignment of Conjugated Polymers in a Nematic Liquid-Crystal Host. J. Phys. Chem. B 2003, 107, 10141–10147. [Google Scholar] [CrossRef]
- Kendhale, A.M.; Schenning, A.P.H.J.; Debije, M.G. Superior Alignment of Multi-Chromophoric Perylenebisimides in Nematic Liquid Crystals and Their Application in Switchable Optical Waveguides. J. Mater. Chem. A 2013, 1, 229–232. [Google Scholar] [CrossRef]
- Benjamin, W.E.; Veit, D.R.; Perkins, M.J.; Bain, E.; Scharnhorst, K.; McDowall, S.; Patrick, D.L.; Gilbertson, J.D. Sterically Engineered Perylene Dyes for High Efficiency Oriented Fluorophore Luminescent Solar Concentrators. Chem. Mater. 2014, 26, 1291–1293. [Google Scholar] [CrossRef]
- ter Schiphorst, J.; Kendhale, A.M.; Debije, M.G.; Menelaou, C.; Herz, L.M.; Schenning, A.P.H.J. Dichroic Perylene Bisimide Triad Displaying Energy Transfer in Switchable Luminescent Solar Concentrators. Chem. Mater. 2014, 26, 3876–3878. [Google Scholar] [CrossRef]
- Clegg, R.M. Chapter 1 Förster Resonance Energy Transfer—FRET What Is It, Why Do It, and How It’s Done. Lab. Tech. Biochem. Mol. Biol. 2009, 33, 1–57. [Google Scholar]
- Altan Bozdemir, O.; Erbas-Cakmak, S.; Ekiz, O.O.; Dana, A.; Akkaya, E.U. Towards Unimolecular Luminescent Solar Concentrators: Bodipy-Based Dendritic Energy-Transfer Cascade with Panchromatic Absorption and Monochromatized Emission. Angew. Chem. Int. Ed. 2011, 50, 10907–10912. [Google Scholar] [CrossRef]
- Jones, G.A.; Bradshaw, D.S. Resonance Energy Transfer: From Fundamental Theory to Recent Applications. Front. Phys. 2019, 7. [Google Scholar] [CrossRef]
- Zhang, B.; Lyu, G.; Kelly, E.A.; Evans, R.C. Förster Resonance Energy Transfer in Luminescent Solar Concentrators. Adv. Sci. 2022, 9, e2201160. [Google Scholar] [CrossRef]
- Tummeltshammer, C.; Taylor, A.; Kenyon, A.J.; Papakonstantinou, I. Homeotropic Alignment and Förster Resonance Energy Transfer: The Way to a Brighter Luminescent Solar Concentrator. J. Appl. Phys. 2014, 116, 173103. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, C.; Soleimaninejad, H.; White, J.M.; Smith, T.A.; Jones, D.J.; Ghiggino, K.P.; Wong, W.W.H. Highly Efficient Luminescent Solar Concentrators by Selective Alignment of Donor–Emitter Fluorophores. Chem. Mater. 2019, 31, 3001–3008. [Google Scholar] [CrossRef]
- de Boer, D.K.G.; Lin, C.-W.; Giesbers, M.P.; Cornelissen, H.J.; Debije, M.G.; Verbunt, P.P.C.; Broer, D.J. Polarization-Independent Filters for Luminescent Solar Concentrators. Appl. Phys. Lett. 2011, 98, 021111. [Google Scholar] [CrossRef]
- Slooff, L.H.; Burgers, A.R.; Debije, M.G. Reduction of Escape Cone Losses in Luminescent Solar Concentrators with Cholesteric Mirrors; Symko-Davies, M., Ed.; SPIE: San Diego, CA, USA, 2008; p. 704306. [Google Scholar]
- Debije, M.G.; Van der Blom, R.H.L.; Broer, D.J.; Bastiaansen, C.W.M. Using Selectively-Reflecting Organic Mirrors to Improve Light Output from a Luminescent Solar Concentrator. In Proceedings of the World Renewable Energy Congress IX, Florence, Italy, 19–25 August 2006. [Google Scholar]
- Debije, M.G.; Van, M.P.; Verbunt, P.P.C.; Broer, D.J.; Bastiaansen, C.W.M. The Effect of an Organic Selectively-Reflecting Mirror on the Performance of a Luminescent Solar Concentrator. In Proceedings of the 24th European Photovoltaic Solar Energy Conference, Hamburg, Germany, 21 September 2009; pp. 373–376. [Google Scholar]
- Debije, M.G.; Teunissen, J.-P.; Kastelijn, M.J.; Verbunt, P.P.C.; Bastiaansen, C.W.M. The Effect of a Scattering Layer on the Edge Output of a Luminescent Solar Concentrator. Sol. Energy Mater. Sol. Cells 2009, 93, 1345–1350. [Google Scholar] [CrossRef]
- Debije, M.G.; Verbunt, P.P.C. Using Liquid Crystals to Improve the Performance of Luminescent Solar Concentrators. In Nanotech 2011 Vol. 1: Advanced Materials, CNTs, Particles, Films and Composites; NSTI: Austin, TX, USA, 13 June 2011. [Google Scholar]
- Verbunt, P.P.C.; Debije, M.G.; Broer, D.J.; Bastiaansen, C.W.M.; de Boer, D.K.G. Organic Wavelength Selective Mirrors for Luminescent Solar Concentrators; Wehrspohn, R., Gombert, A., Eds.; SPIE: Brussels, Belgium, 2012; p. 843805. [Google Scholar]
- Verbunt, P.P.C.; Tsoi, S.; Debije, M.G.; Boer, D.J.; Bastiaansen, C.W.M.; Lin, C.-W.; de Boer, D.K.G. Increased Efficiency of Luminescent Solar Concentrators after Application of Organic Wavelength Selective Mirrors. Opt. Express 2012, 20, A655. [Google Scholar] [CrossRef]
- Verbunt, P.P.C.; de Boer, D.K.G.; Broer, D.J.; Debije, M.G. Special Dispersion Chiral Nematic Reflectors for Luminescent Solar Concentrators. In Proceedings of the 2015 IEEE 42nd Photovoltaic Specialist Conference (PVSC), New Orleans, LA, USA, 14–19 June 2015; pp. 1–6. [Google Scholar]
- Sol, J.A.H.P.; Dehm, V.; Hecht, R.; Würthner, F.; Schenning, A.P.H.J.; Debije, M.G. Temperature-Responsive Luminescent Solar Concentrators: Tuning Energy Transfer in a Liquid Crystalline Matrix. Angew. Chem. Int. Ed. 2018, 57, 1030–1033. [Google Scholar] [CrossRef]
- Mateen, F.; Ali, M.; Oh, H.; Hong, S.-K. Nitrogen-Doped Carbon Quantum Dot Based Luminescent Solar Concentrator Coupled with Polymer Dispersed Liquid Crystal Device for Smart Management of Solar Spectrum. Sol. Energy 2019, 178, 48–55. [Google Scholar] [CrossRef]
- Mateen, F.; Oh, H.; Jung, W.; Lee, S.Y.; Kikuchi, H.; Hong, S.-K. Polymer Dispersed Liquid Crystal Device with Integrated Luminescent Solar Concentrator. Liq. Cryst. 2018, 45, 498–506. [Google Scholar] [CrossRef]
- Gordon, C.K.; Browne, L.D.; Chan, S.; Brett, M.W.; Zemke-Smith, C.; Hardy, J.; Price, M.B.; Davis, N.J.L.K. Heterostructured Nanotetrapod Luminophores for Reabsorption Elimination within Luminescent Solar Concentrators. ACS Appl. Mater. Interfaces 2023, 15, 17914–17921. [Google Scholar] [CrossRef]
- de Clercq, D.M.; Chan, S.V.; Hardy, J.; Price, M.B.; Davis, N.J.L.K. Reducing Reabsorption in Luminescent Solar Concentrators with a Self-Assembling Polymer Matrix. J. Lumin. 2021, 236, 118095. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Giesselmann, F. Current Topics in Smectic Liquid Crystal Research. ChemPhysChem 2006, 7, 20–45. [Google Scholar] [CrossRef]
- Yoshizawa, A. Material Design for Blue Phase Liquid Crystals and Their Electro-Optical Effects. RSC Adv. 2013, 3, 25475–25497. [Google Scholar] [CrossRef]
- Beltran-Gracia, E.; Parri, O.L. A New Twist on Cholesteric Films by Using Reactive Mesogen Particles. J. Mater. Chem. C Mater. 2015, 3, 11335–11340. [Google Scholar] [CrossRef]
- Belmonte, A.; Bus, T.; Broer, D.J.; Schenning, A.P.H.J. Patterned Full-Color Reflective Coatings Based on Photonic Cholesteric Liquid-Crystalline Particles. ACS Appl. Mater. Interfaces 2019, 11, 14376–14382. [Google Scholar] [CrossRef]
- Surampudi, A. Coverage Analysis of a Thinned LiFi Optical Attocell Network. arXiv 2020, arXiv:2007.09724. [Google Scholar]
- Surampudi, A. Analyzing Optical TDMA to Mitigate Interference in Downlink LiFi Optical Attocell Networks. J. Opt. Commun. 2022, 1, 1–7. [Google Scholar] [CrossRef]
- Surampudi, A.; Ganti, R.K. Interference Characterization in Downlink Li-Fi Optical Attocell Networks. J. Light. Technol. 2018, 36, 3211–3228. [Google Scholar] [CrossRef]
- Collins, S.; O’Brien, D.C.; Watt, A. High Gain, Wide Field of View Concentrator for Optical Communications. Opt. Lett. 2014, 39, 1756–1759. [Google Scholar] [CrossRef]
- Riaz, A.; Faulkner, G.; O’Brien, D.; Collins, S. The Relationships between the Amplitude of Receiver Output Voltage and the Maximum Achievable OOK Data Rate. In Free-Space Laser Communications XXXII; Hemmati, H., Boroson, D.M., Eds.; SPIE: Bellingham, DC, USA, 2020; p. 43. [Google Scholar]
- Collins, S. A Receiver for Data Communications, a Receiver System, and a Data Communications System. US Patent Appl. 16/074,483, 7 February 2019. [Google Scholar]
- Peyronel, T.; Quirk, K.J.; Wang, S.C.; Tiecke, T.G. Luminescent Detector for Free-Space Optical Communication. Optica 2016, 3, 787. [Google Scholar] [CrossRef]
- Ali, W.; Manousiadis, P.P.; OaBrien, D.C.; Turnbull, G.A.; Samuel, I.D.W.; Collins, S. A Gigabit VLC Receiver That Incorporates a Fluorescent Antenna and a SiPM. J. Light. Technol. 2022, 40, 5369–5375. [Google Scholar] [CrossRef]
- Manousiadis, P.P.; Rajbhandari, S.; Mulyawan, R.; Vithanage, D.A.; Chun, H.; Faulkner, G.; O’Brien, D.C.; Turnbull, G.A.; Collins, S.; Samuel, I.D.W. Wide Field-of-View Fluorescent Antenna for Visible Light Communications beyond the Étendue Limit. Optica 2016, 3, 702. [Google Scholar] [CrossRef]
- Manousiadis, P.P.; Chun, H.; Rajbhandari, S.; Vithanage, D.A.; Mulyawan, R.; Faulkner, G.; Haas, H.; O’Brien, D.C.; Collins, S.; Turnbull, G.A.; et al. Optical Antennas for Wavelength Division Multiplexing in Visible Light Communications beyond the Étendue Limit. Adv. Opt. Mater. 2020, 8, 1901139. [Google Scholar] [CrossRef]
- Surampudi, A.; Singh, R.; Zhang, G.; Faulkner, G.; Booth, M.J.; Elston, S.J.; O’Brien, D.; Morris, S.M. A Polarization Sensitive Thin Film Optical Wireless Concentrator. In Proceedings of the 2022 IEEE Photonics Conference (IPC), Orlando, FL, USA, 12–16 November 2022; pp. 1–2. [Google Scholar]
- Portnoi, M.; Haigh, P.A.; Macdonald, T.J.; Ambroz, F.; Parkin, I.P.; Darwazeh, I.; Papakonstantinou, I. Bandwidth Limits of Luminescent Solar Concentrators as Detectors in Free-Space Optical Communication Systems. Light. Sci. Appl. 2021, 10, 3. [Google Scholar] [CrossRef]
- Surampudi, A.; Collins, S. Simple Digital Pre-Equalization of VLC Links. In Proceedings of the 2020 IEEE Photonics Conference (IPC), Vancouver, BC, Canada, 28 September–1 October 2020; pp. 1–2. [Google Scholar]
- Surampudi, A.; Singh, R.; Riaz, A.; Ali, W.; Faulkner, G.; OaBrien, D.; Collins, S. A Digital Pre-Equalizer for Optical Wireless Links. J. Light. Technol. 2022, 40, 961–967. [Google Scholar] [CrossRef]
- Surampudi, A.; Singh, R.; Faulkner, G.; O’Brien, D.; Collins, S. Raised Cosine Pulse Shaping for Pre-Equalized Optical Wireless Links. IEEE Photonics Technol. Lett. 2021, 33, 912–915. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Surampudi, A.; Zhang, G.; Singh, R.; Faulkner, G.; O’Brien, D.C.; Booth, M.J.; Morris, S.M. Liquid Crystals for Luminescent Concentrators: A Review. Crystals 2023, 13, 1615. https://doi.org/10.3390/cryst13121615
Surampudi A, Zhang G, Singh R, Faulkner G, O’Brien DC, Booth MJ, Morris SM. Liquid Crystals for Luminescent Concentrators: A Review. Crystals. 2023; 13(12):1615. https://doi.org/10.3390/cryst13121615
Chicago/Turabian StyleSurampudi, Atchutananda, Guanxiong Zhang, Ravinder Singh, Grahame Faulkner, Dominic C. O’Brien, Martin J. Booth, and Stephen M. Morris. 2023. "Liquid Crystals for Luminescent Concentrators: A Review" Crystals 13, no. 12: 1615. https://doi.org/10.3390/cryst13121615
APA StyleSurampudi, A., Zhang, G., Singh, R., Faulkner, G., O’Brien, D. C., Booth, M. J., & Morris, S. M. (2023). Liquid Crystals for Luminescent Concentrators: A Review. Crystals, 13(12), 1615. https://doi.org/10.3390/cryst13121615





