Monoammonium Trimetaphosphimate (NH4)H2(PO2NH)3
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal Structure Analysis
2.3. Powder Diffraction
3. Results and Discussion
3.1. Synthesis and Characterization
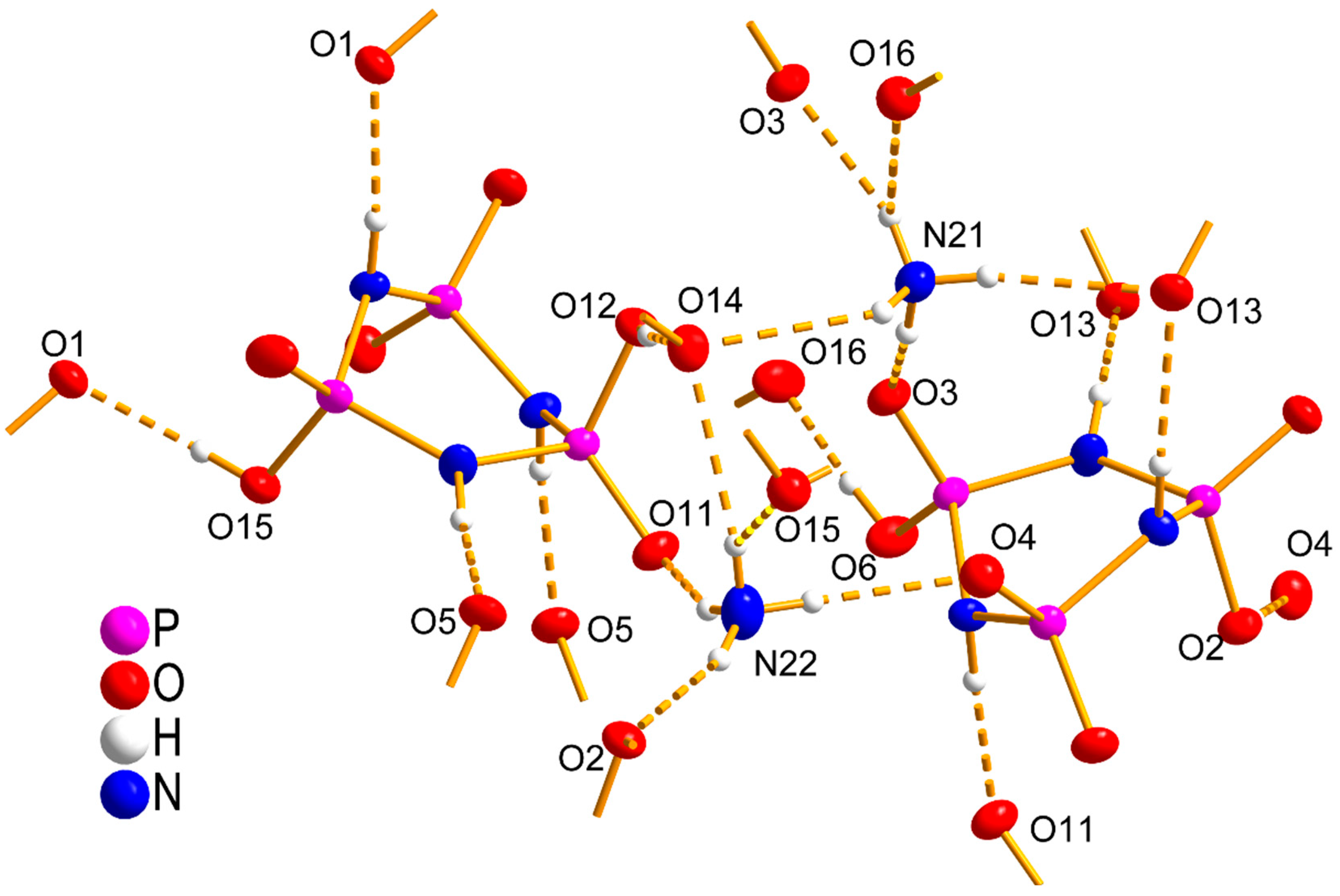
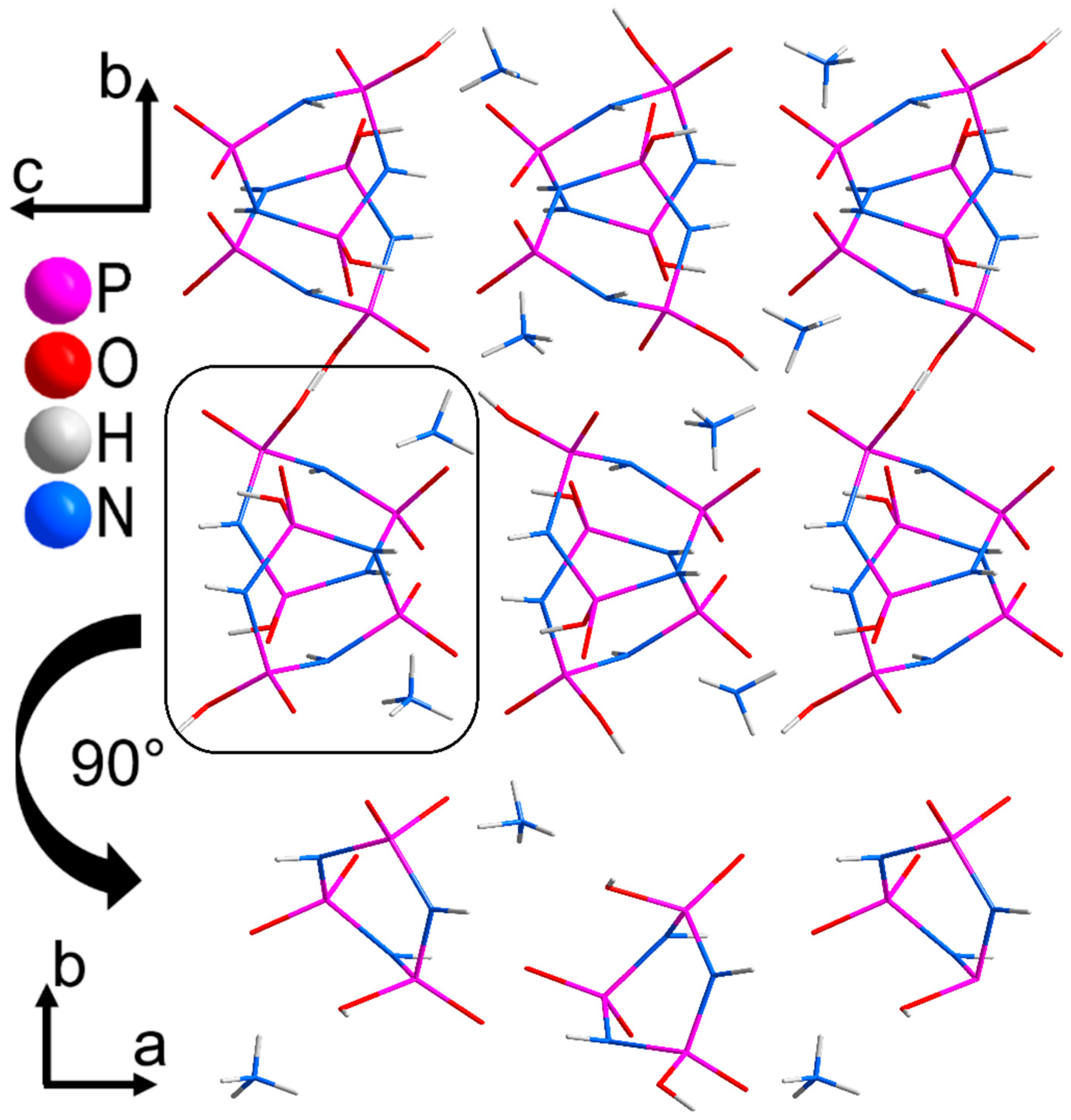
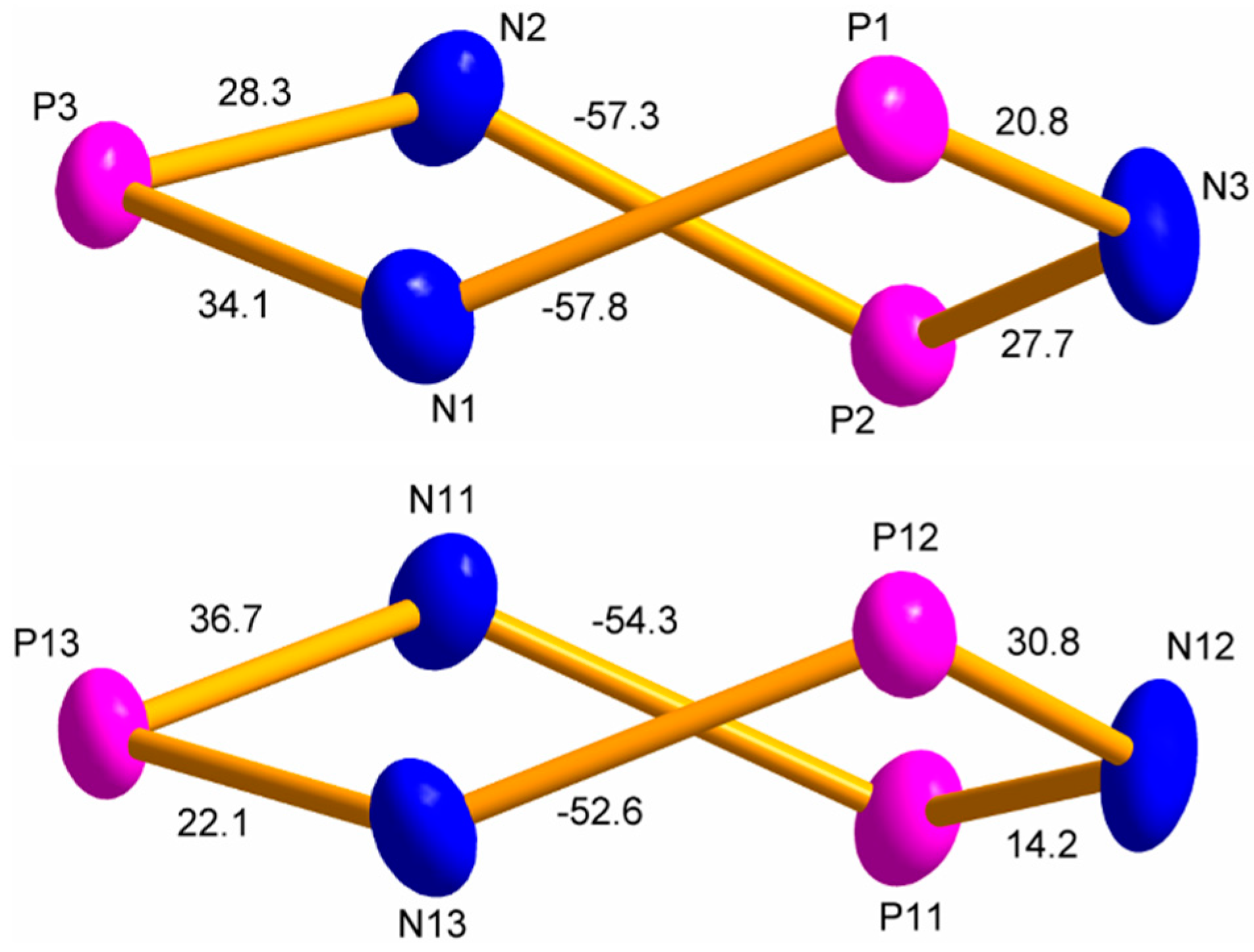
3.2. Crystal Structure
3.3. Thermal Behavior
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stokes, H.N. On the Chloronitrides of Phosphorus. Am. Chem. J. 1895, 17, 275–290. [Google Scholar]
- Olthof, R.; Migchelsen, T.; Vos, A. The Crystal Structure of Compounds with (N-P)n Rings. II. Sodium Trimetaphosphimate Terahydrate Na3(NHPO2)3·4H2O. Acta Crystallogr. 1965, 19, 596–603. [Google Scholar] [CrossRef]
- Stock, N.; Irran, E.; Schnick, W. Synthese und Kristallstruktur der Übergangsmetalltrimetaphosphimate Zn3[(PO2NH)3]·14H2O und Co3[(PO2NH)3]·14H2O. Z. Anorg. Allg. Chem. 1999, 625, 555–561. [Google Scholar] [CrossRef]
- Stock, N.; Schnick, W. Synthesen, Kristallstrukturen und Eigenschaften von Trisilber und Trikalium-tri-μ-imido- cyclotriphosphat, Ag3(PO2NH)3 und K3(PO2NH)3. Z. Naturforsch. B 1997, 52, 251–255. [Google Scholar] [CrossRef]
- Jacobs, H.; Nymwegen, R. Darstellung und Strukturbestimmung zweier Salze der Trimetaphosphimsäure, K3(PO2NH)3 und Rb3(PO2NH)3. Z. Anorg. Allg. Chem. 1998, 624, 199–204. [Google Scholar] [CrossRef]
- Correll, S. Dreierringe in molekulerionischen Imidophosphaten und kondensierten Oxonitridophosphaten mit NPO Zeolithstruktur. Ph.D. Thesis, Ludwig-Maximilians-Universität München, Munich, Germany, 2006. [Google Scholar]
- Corell, S.; Sedlmaier, S.J.; Schnick, W. Synthesis, crystal structures and properties of the trimetaphosphimates NaBa(PO2NH)3, KSr(PO2NH)3·4H2O and NH4Sr(PO2NH)3·4H2O. Solid State Sci. 2005, 7, 1261–1271. [Google Scholar] [CrossRef]
- Stock, N.; Herrendorf, W.; Beck, J.; Schnick, W. The Synthesis and Structure of Trimetaphophimato Complexes of Hafnium and Zirconium. Eur. J. Inorg. Chem. 1998, 4, 469–476. [Google Scholar] [CrossRef]
- Stock, N.; Schnick, W. Trisodium Trimetaphosphimate Monohydrate. Acta Crystallogr. Sect. C 1997, 53, 532–534. [Google Scholar] [CrossRef]
- Stock, N.; Jürgens, B.; Schnick, W. Synthese, Kristallstruktur und Eigenschaften von Triguanidinium-tri-μ-imido- cyclotriphosphat-Monohydrat und Tetraguanidinium-tri-μ-imidocyclotriphosphat-Tetrahydrat, [C(NH2)3]3(PO2NH)3·H2O und [C(NH2)3]4(PO2NH)4·4H2O. Z. Naturforsch. B 1998, 53, 1115–1126. [Google Scholar] [CrossRef]
- Sedlmaier, S.J.; Johrendt, D.; Oeckler, O.; Schnick, W. Synthesis, Crystal Structures and Properties of the Trimetaphosphimates Na2M(PO2NH)3·2H2O with M = K, Tl. Z. Anorg. Allg. Chem. 2007, 633, 2217–2222. [Google Scholar] [CrossRef]
- Correll, S.; Stock, N.; Schnick, W. Synthesis, crystal structures and properties of the bis-(trimetaphosphimato)-metallatesNa4{Co[(PO2NH)3]2}·12H2O and Na4{Ni[(PO2NH)3]2}·12H2O. Solid State Sci. 2004, 6, 953–965. [Google Scholar] [CrossRef]
- Rozanov, I.A.; Medvedeva, L.Y.; Beresnev, E.N.; Sokolov, Y.A.; Sokol, V.I. Some bivalent d-element trimetaphosphimates. Zh. Neorg. Khimii 1981, 3, 668–676. [Google Scholar]
- Stock, N. Phosphor(V)-oxidenitride: Von molekularen und molekularionischen Vorstufen zu kondensierten Festkörpern. Ph.D. Thesis, Universität Bayreuth, Bayreuth, Germany, 1998. [Google Scholar]
- Correll, S.; Schnick, W. Synthese, Kristallstruktur und Eigenschaften von Tetranatrium-bis(trimetaphosphimato) cuprat(II)-Decahydrat Na4{Cu[(PO2NH)3]2}·10H2O. Z. Anorg. Allg. Chem. 2000, 626, 2347–2352. [Google Scholar] [CrossRef]
- Attig, R.; Mootz, D. Dihydrat und primäres Ammoniumsalz der Trimetaphosphimsäure. Z. Anorg. Allg. Chem. 1976, 419, 139–156. [Google Scholar] [CrossRef]
- Günther, D.; Kalischer, C.; Oeckler, O. A new modification of oxonium trimetaphosphimate monohydrate. Z. Anorg. Allg. Chem. 2022, 648, e202200259. [Google Scholar] [CrossRef]
- Stock, N.; Schnick, W. Triammonium Trimetaphosphimate Monohydrate. Acta Crystallogr. Sect. C 1996, 52, 2645–2647. [Google Scholar] [CrossRef]
- Correll, S.; Sedlmaier, S.J.; Schnick, W. Synthese, Kristallstruktur und Eigenschaften von Chrom(III)-trimephosphimat-Heptahydrat Cr(PO2NH)3·7H2O. Z. Anorg. Allg. Chem. 2005, 631, 1359–1364. [Google Scholar] [CrossRef]
- Yi, J.; Fu, Z.; Liao, S.; Song, D.; Dai, J. Nitrogen-containing porous cerium trimetaphosphimate as a new efficient base catalyst. J. Mater. Chem. 2011, 21, 6144–6147. [Google Scholar] [CrossRef]
- Sokol, V.I.; Porai-Koshits, M.A.; Berdnikov, V.R.; Rozanov, I.A.; Butman, L.A. Crystal structure refinement of potassium ammonium bis(trimetaphosphinato)prasedymate octahydrate of the composition K1.3(NH4)1.7{Pr[PO2NH)3]2}·8H2O. Koord. Khimiya 1979, 5, 1093–1102. [Google Scholar]
- Sokol, V.I.; Porai-Koshits, M.A.; Berdnikov, V.R.; Rozanov, I.A. Butman, L.A. Crystal structure of gallium sodium trimetaphosphimate and structure of its complex anion. Koord. Khimiya 1975, 1, 429–434. [Google Scholar]
- Schmidt, G.; Fenesan, M. Über komplexe Kupfer(II)-Salze der Trimetaphosphimsäure. Stud. Univ. Babes-Bolyai Chem. 1972, 1, 29–36. [Google Scholar]
- Rozanov, N.A.; Medvedeva, L.Y.; Beresnev, E.N. Synthesis of iron(II) and iron(III) trimetaphosphimates. Zh. Neorg. Khimii 1978, 8, 2259–2260. [Google Scholar]
- Agilent. CrysAlis PRO, Version 171.38.41; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2015.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver an molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. DIAMOND 4.6.4; Crystal Impact GbR: Bonn, Germany, 2020. [Google Scholar]
- Steiner, T. The hydrogen bond in the solid state. Angew. Chem. Int. Ed. 2002, 41, 48–76. [Google Scholar] [CrossRef]
- Cremer, D.; Pople, J.A. Genral Definition of Ring Puckering Coordinates. J. Am. Chem. Soc. 1975, 97, 1354–1358. [Google Scholar] [CrossRef]
- Zschunke, A. Molekülstruktur; Spektrum Akademischer Verlag: Heidelberg, Australia, 1993. [Google Scholar]
- Bucourt, R. The Torsion Angle Concept in Conformational Analysis. Top. Stereochem. 1975, 8, 159–224. [Google Scholar] [CrossRef]
| Sum Formula | Space Group | Lattice Parameters | Cation Coordination | Anion Arrangement |
|---|---|---|---|---|
| (H3O)H2(PO2NH)3·H2O [16] | P21/c | a = 7.022(1) Å b = 14.008(1) Å c = 9.353(2) Å β = 93.34(2)° | irregular coordination of H3O+/H5O2+ connected via hydrogen bonds | hexagonal pattern of anion stacks along [001] |
| (H3O)H2(PO2NH)3·H2O [17] | P21/c | a = 7.0289(3) Å b = 14.1700(7) Å c = 11.3378(6) Å β = 124.225° | irregular coordination of H3O+/H5O2+ connected via hydrogen bonds | rectangular pattern of anion stacks along [001] |
| (NH4)H2(PO2NH)3·CH3OH [18] | Pbca | a = 15.025(3) Å b = 7.264(1) Å c = 19.252(1) Å | distorted tetrahedral coordination of NH4+ ions by O atoms from anions via hydrogen bonds | distorted hexagonal pattern of double chains along [010], solvent between the chains |
| (NH4)3(PO2NH)3·H2O [18] | P21 | a = 6.5712(6) Å b = 12.9917(6) Å c = 6.9237(3) Å β = 102.133(6)° | irregular coordination of NH4+ ion (H atom positions not determined) | crankshaft-like anion stacks along [010] |
| Na3(PO2NH)3·H2O [9] | C2 | a = 9.8797(7) Å b = 12.2119(8) Å c = 7.6464(6) Å β = 104.394(6)° | distorted NaO6 octahedra with all O atoms from anions or O atoms of 4 anions and 2 water molecules | hexagonal pattern of anion pairs stacked along [001] |
| Na3(PO2NH)3⋅H2O:Rh [6] | C2/m | a = 9.976(2) Å b = 12.105(3) Å c = 7.641(2) Å β = 104.95(3)° | disordered distorted NaO6 octahedra with O atoms only from 4 anions and 2 water molecules | hexagonal pattern of anion pairs stacked along [001] |
| Na3(PO2NH)3·4H2O [2] | P21/n | a = 16.976(9) Å b = 7.834(3) Å c = 8.918(6) Å β = 97.08(7)° | distorted NaO6 octahedra with O atoms from 5 anions and 1 water or from 3 anions and 3 water molecules | rectangular pattern of anion stacks along [010] |
| Ag3(PO2NH)3 [4] | P21/c | a = 11.666(1) Å b = 7.864(1) Å c = 9.978(1) Å β = 106.91(1)° | distorted AgO4 or AgO3N tetrahedra | crankshaft-like anion stacks along [001] |
| K3(PO2NH)3 [4,5] | R | a = 12.714(2) Å c = 10.179(2) Å | ninefold K coordination | hexagonal pattern of anion stacks along [001] |
| Rb3(PO2NH)3 [5] | R | a = 12.9971(5) Å c = 10.5485(5) Å | ninefold Rb coordination | hexagonal pattern of anion stacks along [001] |
| [C(NH2)3]3(PO2NH)3·H2O [10] | Pbca | a = 15.656(2) Å b = 10.6831(6) Å c = 20.918(2) Å | irregular coordination of C(NH2)3+ involving 6 hydrogen bonds | layers of anion pairs in (001) plane |
| Zn3[(PO2NH)3]·14H2O [3] | P | a = 7.443(5) Å b = 9.613(6) Å c = 9.834(5) Å α = 102.60(4)° β = 90.31(4)° γ = 99.92(4)° | distorted ZnO6 octahedra, O atoms from 3 anions and 3 water or from 2 anions and 4 water molecules | distorted hexagonal pattern of anion stacks along [100], solvent between the chains |
| Co3[(PO2NH)3]·14H2O [3] | p | a = 7.4605(1) Å b = 9.5706(2) Å c = 9.8851(2) Å α = 102.162(1)° β = 90.044(1)° γ = 99.258(1)° | distorted CoO6 octahedra, O atoms from 3 anions and 3 water or from 2 anions and 4 water molecules | distorted hexagonal pattern of anion stacks along [100], solvent between the chains |
| Cr(PO2NH)3·7H2O [19] | P21/n | a = 9.461(2) Å b = 10.760(2) Å c = 12.954(3) Å β = 92.68(3)° | distorted Cr(H2O)6 octahedra | anion stacks along [100] separated by solvent water |
| Ce(PO2NH)3·5H2O [6,20] | P21212 | a = 7.3040(15) Å b = 9.1675(18) Å c = 9.5663(19) Å | CeO8 quadratic antiprism, O atoms from 6 anions and 2 water molecules | layers of anions in (001) plane |
| Pr(PO2NH)3·5H2O [6] | P21212 | a = 7.307(1) Å b = 9.159(1) Å c = 9.585(2) Å | PrO8 quadratic antiprism, O atoms from 6 anions and 2 water molecules | layers of anions in (001) plane |
| NaBa(PO2NH)3 [7] | C2/m | a = 10.845(2) Å b = 10.250(2) Å c = 7.962(2) Å β = 115.18(3)° | distorted NaO6 octahedra, Ba: 8 + 2 coordination | staggered arrangement of chains along [010] |
| KSr(PO2NH)3·4H2O [7] | P21/n | a = 10.872(2) Å b = 10.496(2) Å c = 11.912(2) Å β = 111.98(3)° | Sr: sevenfold coordination by O atoms from 5 anions and 2 water molecules, K: nine-fold coordination by 4 O and 2 N anion atoms and 3 water molecules | layers parallel (100) of anion pairs stacked along [010] |
| NH4Sr(PO2NH)3·4H2O [7] | P21/n | a = 10.884(2) Å b = 10.485(2) Å c = 11.969(2) Å β = 111.43(3)° | Sr: sevenfold coordination by O atoms from 5 anions and 2 water molecules, NH4+: nine-fold coordination by O/N (H atom positions not determined) | layers parallel (100) of anion pairs stacked along [010] |
| K1.3(NH4)1.7Pr [(PO2NH)3]2·8H2O [21] | Pna21 | a = 25.623(8) Å b = 10.577(3) Å c = 8.632(2) Å | distorted PrO8 quadratic antiprism, all O atoms from anions; K coordinated by 4 O atoms of anions and 3 water molecules | staggered stacks along [010] forming layer parallel (100) and additional anions in between |
| Na2K(PO2NH)3·2H2O [11] | P | a = 7.948(2) Å b = 8.034(2) Å c = 8.515(2) Å α = 75.84(3)° β = 83.54(3)° γ = 77.37(3)° | NaO6 octahedra with O atoms from 3 anions and 3 water molecules, K: tenfold coordination by 5 O and 2 N atoms from anions and 3 H2O | anion pairs stacked along [10] |
| Na2Tl(PO2NH)3·2H2O [11] | P | a = 7.991(2) Å b = 7.993(2) Å c = 8.646(2) Å α = 76.83(3)° β = 83.81(3)° γ = 77.57(3)° | NaO6 octahedra with O atoms from 3 anions and 3 water molecules, Tl: tenfold coordination by 5 O and 2 N atoms from anions and 3 H2O | anion pairs stacked along [10] |
| Na4{Co[(PO2NH)3]2}·12H2O high temperature-phase [12,13] | C2/c | a = 8.889(1) Å b = 19.018(2) Å c = 17.112(2) Å β = 104.59(1)° | disordered NaO6 octahedra with O atoms from 2 anions and 4 water molecules, CoO6 tetrahedra with O atoms of anions | layers of complex {Co[(PO2NH)3]2}4−·ions in (010) plane |
| Na4{Co[(PO2NH)3]2}·12H2O low temperature-phase [12,13] | P21/n | a = 8.887(2) Å b = 19.001(4) Å c = 17.072(3) Å β = 104.59(3)° | ordered NaO6 octahedra, O atoms: 2 anions + 4 crystal water; CoO6 tetrahedra with O atoms of anions | layers of complex {Co[(PO2NH)3]2}4−·ions in (010) plane |
| Na4{Ni[(PO2NH)3]2}·12H2O high temperature-phase [12,13] | C2/c | a = 8.944(1) Å b = 18.906(1) Å c = 17.236(1) Å β = 104.49(1)° | disordered NaO6 octahedra with O atoms from 2 anions and 4 water molecules, NiO6 tetrahedra with O atoms of anions | layers of complex {Ni[(PO2NH)3]2}4−·ions in (010) plane |
| Na4{Ni[(PO2NH)3]2}·12H2O low temperature-phase [12,13] | P21/n | a = 8.903(1) Å b = 18.902(2) Å c = 17.133(2) Å β = 104.62(1)° | ordered NaO6 octahedra, O atoms: 2 anions + 4 crystal water; NiO6 tetrahedra with O atoms of anions | layers of complex {Ni[(PO2NH)3]2}4−·ions in (010) plane |
| Na4{Zn[(PO2NH)3]2} ·12H2O [13,14] | C2/c | a = 8.883(1) Å b = 18.994(2) Å c = 17.126(2) Å β = 104.54(1)° | disordered NaO6 octahedra with O atoms from 2 anions and 4 water molecules, ZnO6 tetrahedra with O atoms of anions | layers of complex {Zn[(PO2NH)3]2}4−· ions in (010) plane |
| NaZn[(PO2NH)3]2}·7H2O [13] | P | a = 10.209(3) Å b = 9.256(2) Å c = 9.539(2) Å α = 116.02(4)° β = 97.7(3)° γ = 103.16(3)° | ZnO6 octahedra with O atoms from 4 anions and 2 water or from 2 anions and 4 water molecules; (Na coordination not given in literature) | chains of comple X[Zn(PO2NH)3(H2O)3]− ions along [010] |
| NaCo[(PO2NH)3]2}·7H2O [13] | P | a = 10.207(3) Å b = 9.253(2) Å c = 9.530(2) Å α = 116.00(5)° β = 97.61(3)° γ = 103.17(5)° | CoO6 octahedra with O atoms from 4 anions and 2 water or from 2 anions and 4 water molecules; (Na coordination not given in literature) | chains of comple X[Co(PO2NH)3(H2O)3]− ions along [010] |
| Na4{Cu[(PO2NH)3]2}·10H2O [13] | P | a = 9.1251(6) Å b = 9.3214(6) Å c = 9.6610(6) Å α = 94.840(5)° β = 108.652(6)° γ = 118.588(6)° | distorted CuO6 bipyramids with O atoms from 4 anions and 2 water molecules; Na: distorted octahedra with O atoms from 2 anions and 4 water or trigonal bipyramidal coordination with O atoms from 2 anions and 3 water molecules | chains of complex {Cu[(PO2NH)3]2}4− ions along [010] |
| Na4{Hf(μ-O)(μ4-OH)6 [(PO2NH)3]4}·18H2O [15] | Pa | a = 22.678(3) Å | HfO4 tetrahedra with O atoms from anions; NaO6 octahedra with O atoms from 3 anions and 3 water or sevenfold coordination with O atoms from 2 anions and 5 water molecules | complex {Hf(μ-O)(μ4-OH)6 [(PO2NH)3]4}4− ions forming a 3D network |
| Na4{Hf(μ-O)(μ4-OH)6 [(PO2NH)3]4}·21H2O [8] | R | a = 14.350(2) Å c = 50.348(10) Å | HfO4 tetrahedra with O atoms from anions; NaO6 octahedra with O atoms from 3 anions and 3 water or from 2 anions and 4 water molecules | bilayers of complex {Hf(μ-O)(μ4-OH)6 [(PO2NH)3]4}4− ions in (001) plane |
| Na4{Zr(μ-O)(μ4-OH)6 [(PO2NH)3]4}·18H2O [8] | Pa | a = 22.693(3) Å | ZrO4 tetrahedra with O atoms from anions; NaO6 octahedra with O atoms from 3 anions and 3 water or sevenfold coordination with O atoms from 2 anions and 5 water molecules | complex {Zr(μ-O)(μ4-OH)6 [(PO2NH)3]4}4− ions forming a 3D network |
| Na4{Zr(μ-O)(μ4-OH)6 [(PO2NH)3]4}·21H2O [8] | R | a = 14.303(2) Å c = 50.284(10) Å | ZrO4 tetrahedra with O atoms from anions; NaO6 octahedra with O atoms from 3 anions and 3 water or from 2 anions and 4 water molecules | bilayers of complex {Zr(μ-O)(μ4-OH)6 [(PO2NH)3]4}4− ions in (001) plane |
| NaCa(PO2NH)3⋅8H2O [8] | P21/c | a = 7.8138(16) Å b = 6.8026(14) Å c = 29.693(6) Å β = 92.18(3)° | Ca: seven-fold coordination to 5 anions and 2 water molecules; Na(H2O)6 octahedra | bilayers parallel (001) of anion stacks along [010] |
| NaCa(PO2NH)3⋅4H2O [6] | R | a = 17.863(3) Å c = 20.548(4) Å | Ca: seven-fold coordination to 6 anions and 1 water molecule; NaO6 octahedra with all O atoms from anions | anions interconnected by Ca2+ and H bonds form zeolite-like framework |
| NaCa(PO2NH)3 [6] | P213 | a = 9.34023(10) Å | distorted CaO6 and NaO6 octahedra | cubic arrangement of anions that form no stacks |
| K6{Cu3[(PO2NH)3]4}·6H2O [6] | P | a = 8.498(2) Å b = 9.552(2) Å c = 13.222(3) Å α = 86.72(3)° β = 75.09(3)° γ = 70.95(3)° | Cu2+: square-pyramidal and octahedral coordination with all O atoms from anions; K: seven- or tenfold coordination by O/N from anions and water molecules | alternating stacks of {Cu[(PO2NH)3]2}4− and {Cu2[(PO2NH)3]2·2H2O}4− complex ions along [10] |
| Na3{Al[(PO2NH)3]2}⋅12H2O [6] | P | a = 8.6824(15) Å b = 8.7399(16) Å c = 9.1973(14) Å α = 82.131(12)° β = 86.692(9)° γ = 87.505(12)° | AlO6 octahedra with all O atoms from anions; NaO6 octahedra with O atoms from 1 anion and 5 water molecules | honeycomb-like arrangement of chains of {Al[(PO2NH)3]2}3− ions along [111] |
| Na3{Fe[(PO2NH)3]2}⋅12H2O [6] | P | a = 8.7584(4) Å b = 8.7283(3) Å c = 9.2182(4) Å α = 82.286(3)° β = 86.240(4)° γ = 88.365(3)° | FeO6 octahedra with all O atoms from anions; NaO6 octahedra with O atoms from 1 anion and 5 water molecules | honeycomb-like arrangement of chains of {Al[(PO2NH)3]2}3− ions along [111] |
| Na3{Ga[(PO2NH)3]2}⋅12H2O [22] | P | a = 8.729(5) Å b = 9.902(5) Å c = 8.716(5) Å α = 97.84(2)° β = 87.88(2)° γ = 93.45(2)° | GaO6 octahedra with all O atoms from anions; NaO6 octahedra with O atoms from 1 anion and 5 water or from 6 water molecules | hexagonal pattern of chains of complex {Ga[(PO2NH)3]2}3− ions along [111] |
| Sum Formula | (NH4)H2(PO2NH)3 |
|---|---|
| Formula weight | 254.02 |
| Temperature (°C) | 25 |
| Crystal system | monoclinic |
| Space group | P21/c (no. 14) |
| Lattice parameters (Å, °) | a = 10.2511(5) b = 14.0170(9) c = 11.9423(7) β = 101.633(3) |
| V (Å3) | 1680.74(17) |
| 2.008 | |
| Z | 8 |
| F(000) | 1040 |
| Wave length (Å) | 0.500 |
| No. of reflections (unique) | 31,031 (4152) |
| Rint | 0.0758 |
| Rσ | 0.0388 |
| μ (mm−1) | 0.271 |
| Absorption correction | semiempirical [26] |
| No. of parameters | 290 |
| Weighting scheme | |
| R1/wR2 [I > 2σ (I)] | 0.0291/0.0831 |
| R1/wR2 (all reflections) | 0.0302/0.0844 |
| GooF | 1.073 |
| −0.53/0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Günther, D.; Paulmann, C.; Oeckler, O. Monoammonium Trimetaphosphimate (NH4)H2(PO2NH)3. Crystals 2023, 13, 111. https://doi.org/10.3390/cryst13010111
Günther D, Paulmann C, Oeckler O. Monoammonium Trimetaphosphimate (NH4)H2(PO2NH)3. Crystals. 2023; 13(1):111. https://doi.org/10.3390/cryst13010111
Chicago/Turabian StyleGünther, Daniel, Carsten Paulmann, and Oliver Oeckler. 2023. "Monoammonium Trimetaphosphimate (NH4)H2(PO2NH)3" Crystals 13, no. 1: 111. https://doi.org/10.3390/cryst13010111
APA StyleGünther, D., Paulmann, C., & Oeckler, O. (2023). Monoammonium Trimetaphosphimate (NH4)H2(PO2NH)3. Crystals, 13(1), 111. https://doi.org/10.3390/cryst13010111






