Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries
Abstract
:1. Introduction
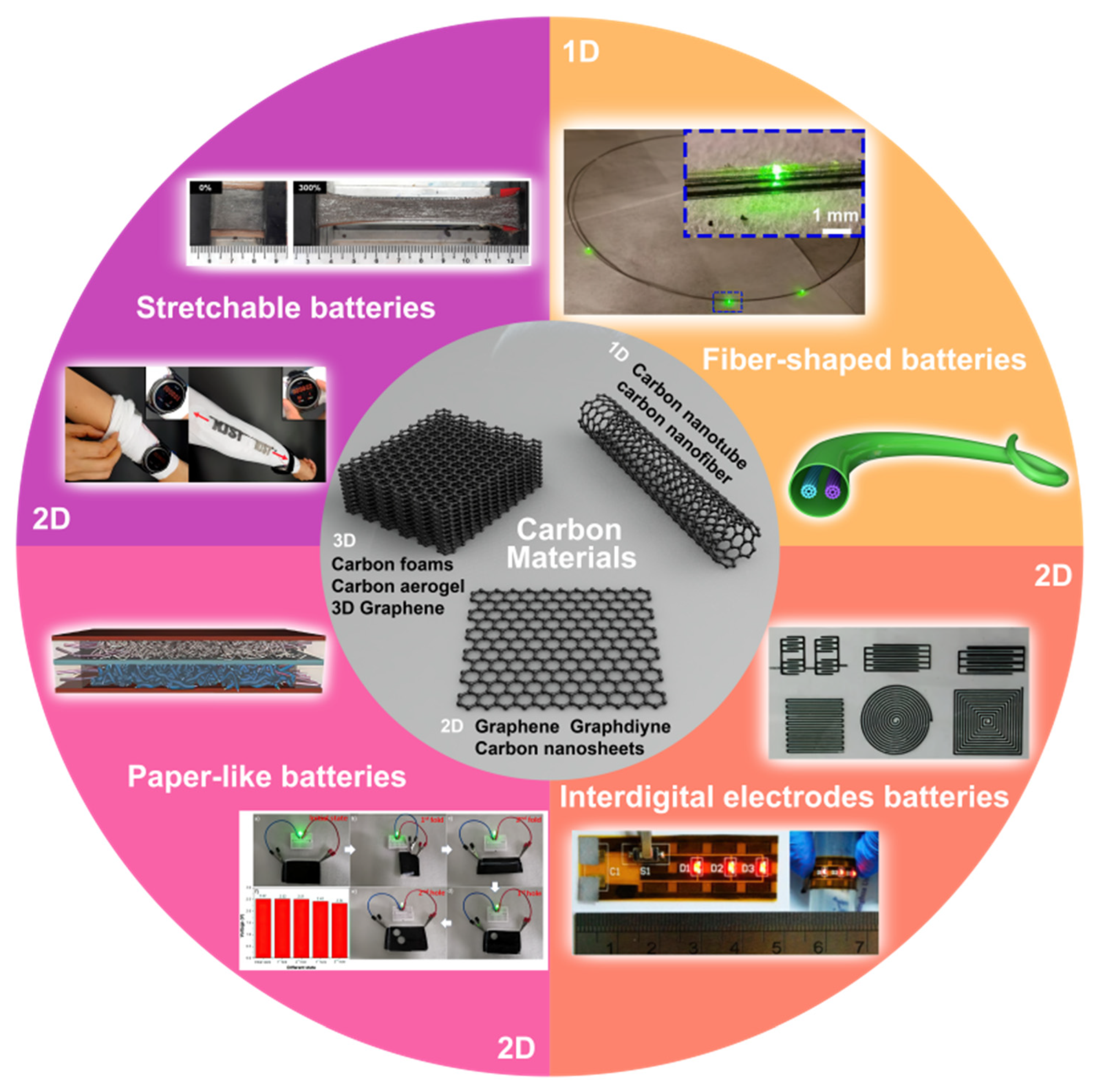
2. Carbon Materials
2.1. 1D Carbon Materials
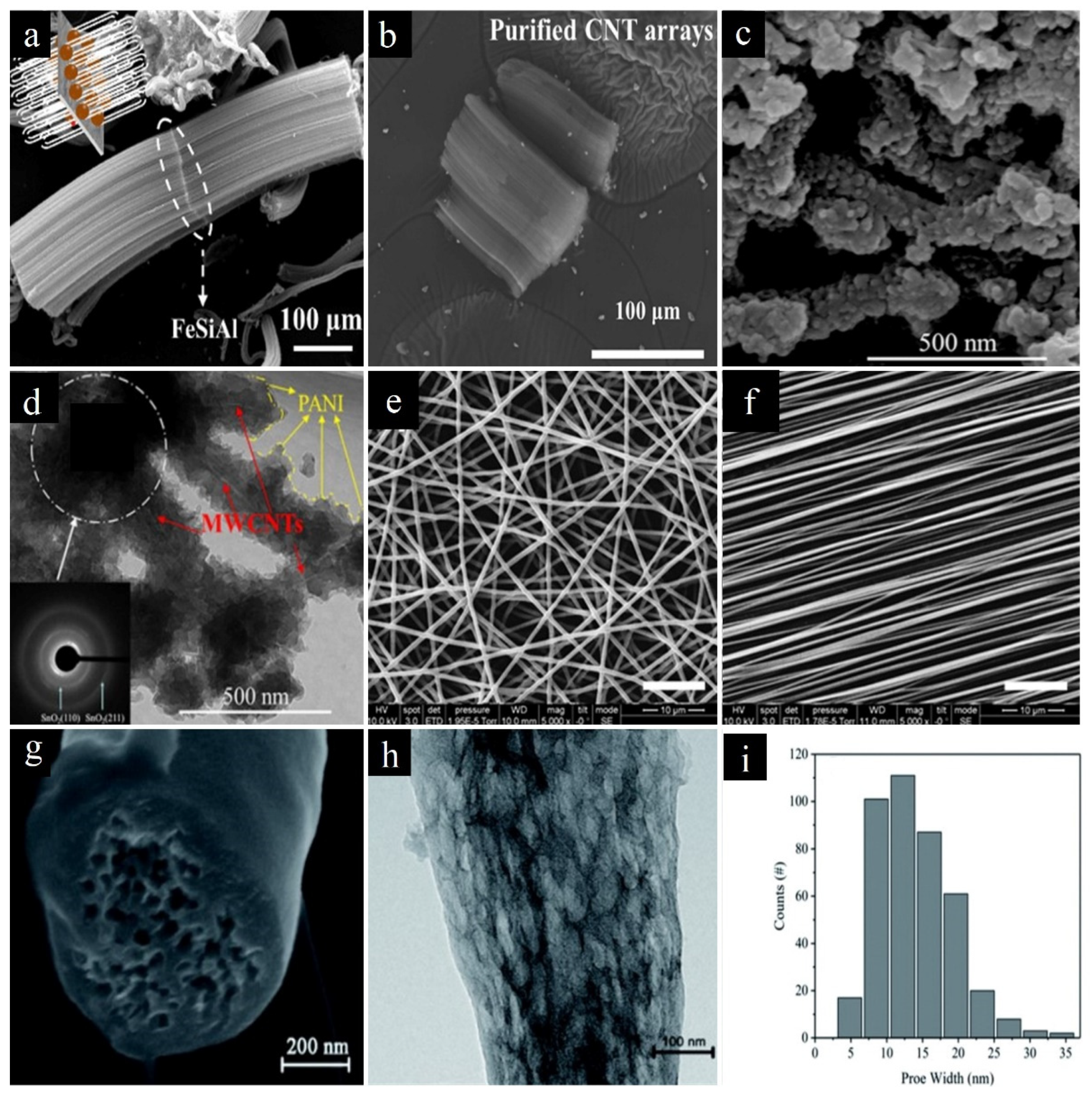
2.2. 2D Carbon Materials
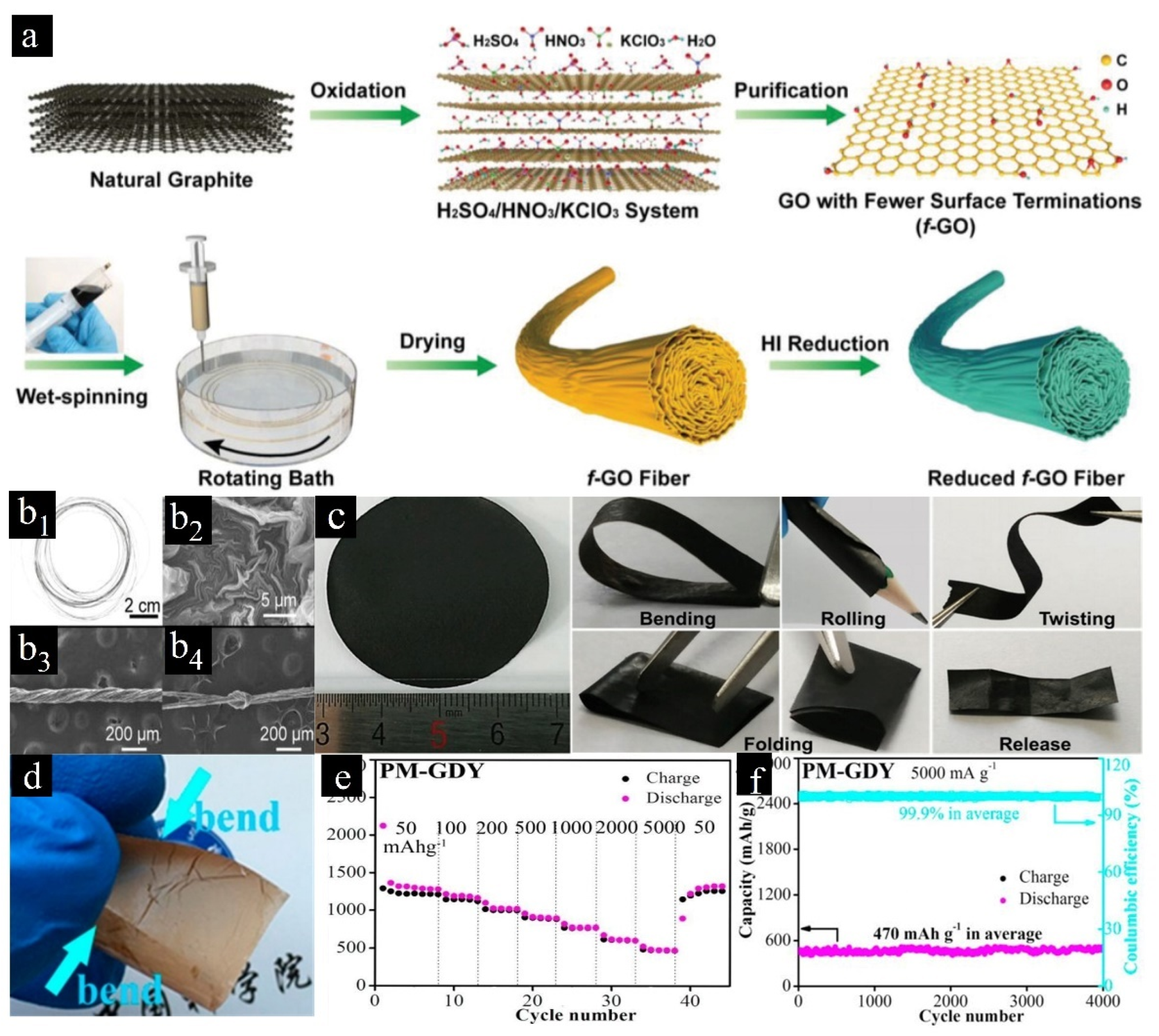
2.3. 3D Carbon Materials

3. Application in Flexible Lithium-Ion Batteries
3.1. 1D Fiber-Shaped Batteries
3.2. 2D Paper-like Batteries

3.3. Interdigital Batteries
3.4. Stretchable Batteries
4. Conclusions and Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wu, Z.; Wang, Y.; Liu, X.; Lv, C.; Li, Y.; Wei, D.; Liu, Z. Carbon-Nanomaterial-Based Flexible Batteries for Wearable Electronics. Adv. Mater. 2019, 31, 1800716. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Ul-Islam, M.; Ahmad, M.W.; Khan, M.S.; Imran, M.; Siyal, S.H.; Javed, M.S. Synthetic Methodologies and Energy Storage/Conversion Applications of Porous Carbon Nanosheets: A Systematic Review. Energy Fuels 2022, 36, 3420–3442. [Google Scholar] [CrossRef]
- Zheng, Z.; Xue, Y.; Li, Y. A new carbon allotrope: Graphdiyne. Trends Chem. 2022, 4, 754–768. [Google Scholar] [CrossRef]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Shi, S.; Wang, G.; Wan, G.; Tang, Y.; Zhao, G.; Deng, Z.; Chai, J.; Wei, C.; Wang, G. Titanium niobate (Ti2Nb10O29) anchored on nitrogen-doped carbon foams as flexible and self-supported anode for high-performance lithium ion batteries. J. Colloid Interface Sci. 2021, 587, 622–632. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, Y.; Mei, L. Diffusion and condensation of lithium atoms in single-walled carbon nanotubes. Phys. Rev. B 2005, 71, 165413. [Google Scholar] [CrossRef]
- Huang, L.; Guan, Q.; Cheng, J.; Li, C.; Ni, W.; Wang, Z.; Zhang, Y.; Wang, B. Free-standing N-doped carbon nanofibers/carbon nanotubes hybrid film for flexible, robust half and full lithium-ion batteries. Chem. Eng. J. 2018, 334, 682–690. [Google Scholar] [CrossRef]
- Sun, N.; Wang, X.; Dong, X.; Huang, H.; Qi, M. PVP-grafted synthesis for uniform electrospinning silica@carbon nanofibers as flexible free-standing anode for Li-ion batteries. Solid State Ion. 2021, 374, 115817. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Jian, X.; Zhang, S.; Shang, Y.; Xu, T.; Dai, S.; Xu, J.; Kong, D.; Wang, Y.; et al. Free-standing and flexible CNT/(Fe@Si@SiO2) composite anodes with kernel-pulp-skin nanostructure for high-performance lithium-ion batteries. J. Alloys Compd. 2021, 878, 160396. [Google Scholar] [CrossRef]
- Yu, H.; Chen, L.; Li, W.; Dirican, M.; Liu, Y.; Zhang, X. Root-whisker structured 3D CNTs-CNFs network based on coaxial electrospinning: A free-standing anode in lithium-ion batteries. J. Alloys Compd. 2021, 863, 158481. [Google Scholar] [CrossRef]
- Wang, B.; Xia, Y.; Deng, Z.; Zhang, Y.; Wu, H. Three-dimensional cross-linked MnO/Sb hybrid nanowires co-embedded nitrogen-doped carbon tubes as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2020, 835, 155239. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.; Hou, J.; Meng, B.; Ali, R.; Liu, Y.; Jian, X. Symmetrical growth of carbon nanotube arrays on FeSiAl micro-flake for enhancement of lithium-ion battery capacity. Carbon 2022, 189, 93–103. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, Z.-Q.; Chen, Z.; Yang, Z.-L.; Tang, Z.-L.; Luo, H.-Y.; Huang, Y.; Li, X.; Xu, W. One dimensional and coaxial polyaniline@tin dioxide@multi-wall carbon nanotube as advanced conductive additive free anode for lithium ion battery. Chem. Eng. J. 2018, 334, 162–171. [Google Scholar] [CrossRef]
- Guo, W.; Si, W.; Zhang, T.; Han, Y.; Wang, L.; Zhou, Z.; Lu, P.; Hou, F.; Liang, J. Ultrathin NixCoy-silicate nanosheets natively anchored on CNTs films for flexible lithium ion batteries. J. Energy Chem. 2021, 54, 746–753. [Google Scholar] [CrossRef]
- Liang, P.; Zhu, K.; Rao, Y.; Zheng, H.; Yao, Z.; Wu, M.; Zhang, J.; Liu, J.; Yan, K.; Wang, J.; et al. Flexible and Self-Standing Urchinlike V2O3@Carbon Nanofibers toward Ultralong Cycle Lifespan Lithium-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 3242–3251. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, M.; Du, D.; Tang, X.; Wang, H.; Zhang, M.; Zhao, T.; Liu, F.; Wang, Z.; Ma, Y. Affinity-Engineered Flexible Scaffold toward Energy-Dense, Highly Reversible Na Metal Batteries. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Joshi, B.; Samuel, E.; Kim, Y.-I.; Yarin, A.L.; Swihart, M.T.; Yoon, S.S. Progress and potential of electrospinning-derived substrate-free and binder-free lithium-ion battery electrodes. Chem. Eng. J. 2022, 430, 132876. [Google Scholar] [CrossRef]
- Gao, M.; Liu, B.; Zhang, X.; Zhang, Y.; Li, X.; Han, G. Ultrathin MoS2 nanosheets anchored on carbon nanofibers as free-standing flexible anode with stable lithium storage performance. J. Alloys Compd. 2021, 894, 162550. [Google Scholar] [CrossRef]
- Zhou, X.-H.; Gao, Y.; Lyu, L.-H.; Liang, Y.-Y.; Li, Z.-H. General construction of molybdenum-based compounds embedded in flexible 3D interconnected porous carbon nanofibers with protective porous shell for high-performance lithium-ion battery. Carbon 2021, 179, 142–150. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, Y.; Huang, X.; Huang, L.; Cao, M.; Song, G.; Guo, X.; Sui, X.; Ren, R.; Chen, J. Self-healing liquid metal nanoparticles encapsulated in hollow carbon fibers as a free-standing anode for lithium-ion batteries. Nano Energy. 2019, 62, 883–889. [Google Scholar] [CrossRef]
- Yadav, A.; De, B.; Singh, S.K.; Sinha, P.; Kar, K.K. Facile Development Strategy of a Single Carbon-Fiber-Based All-Solid-State Flexible Lithium-Ion Battery for Wearable Electronics. ACS Appl. Mater. Interfaces 2019, 11, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-G.; Lin, J.; Fan, C.-Y.; Li, Y.-F.; Zhang, J.-P.; Wu, X.-L.; Sun, H.-Z.; Deng, M.-X.; Su, Z.-M. Target encapsulating NiMoO4 nanocrystals into 1D carbon nanofibers as free-standing anode material for lithium-ion batteries with enhanced cycle performance. J. Alloys Compd. 2020, 830, 154648. [Google Scholar] [CrossRef]
- Yu, C.C.; Chen, Y.W.; Yeh, P.Y.; Hsiao, Y.S.; Lin, W.T.; Kuo, C.W.; Chueh, D.Y.; You, Y.W.; Shyue, J.J.; Chang, Y.C.; et al. Random and aligned electrospun PLGA nanofibers embedded in microfluidic chips for cancer cell isolation and integration with air foam technology for cell release. J. Nanobiotechnol. 2019, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, W.; Zhang, X.; Huo, L.; Liang, J.; Wu, L.; Liu, Y.; Gao, J.; Pang, H.; Xue, H. Copolymer derived micro/meso-porous carbon nanofibers with vacancy-type defects for high-performance supercapacitors. J. Mater. Chem. A 2020, 8, 2463–2471. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Li, X.; Jin, H.; Ali, W.; Song, Z.; Ding, S. Metal–organic-framework derived Co@CN modified horizontally aligned graphene oxide array as free-standing anode for lithium-ion batteries. J. Mater. Chem. A 2022, 10, 699–706. [Google Scholar] [CrossRef]
- He, D.; Sun, M.; Cao, D.; Ding, Y.; Chen, H.; He, G. Flexible Free-Standing Fe2O3 Nanoparticle/Carbon Shells/Graphene Films for Advanced Lithium-Ion Batteries. ACS Appl. Nano Mater. 2022, 5, 5017–5024. [Google Scholar] [CrossRef]
- Dai, C.; Sun, G.; Hu, L.; Xiao, Y.; Zhang, Z.; Qu, L. Recent progress in graphene-based electrodes for flexible batteries. InfoMat 2019, 2, 509–526. [Google Scholar] [CrossRef]
- Zhai, S.; Wei, L.; Karahan, H.E.; Chen, X.; Wang, C.; Zhang, X.; Chen, J.; Wang, X.; Chen, Y. 2D materials for 1D electrochemical energy storage devices. Energy Storage Mater. 2019, 19, 102–123. [Google Scholar] [CrossRef]
- Tang, P.; Deng, Z.; Zhang, Y.; Liu, L.X.; Wang, Z.; Yu, Z.Z.; Zhang, H.-B. Tough, Strong, and Conductive Graphene Fibers by Optimizing Surface Chemistry of Graphene Oxide Precursor. Adv. Funct. Mater. 2022, 32, 2112156. [Google Scholar] [CrossRef]
- Mahmood, A.; Yuan, Z.; Sui, X.; Riaz, M.A.; Yu, Z.; Liu, C.; Chen, J.; Wang, C.; Zhao, S.; Mahmood, N.; et al. Foldable and scrollable graphene paper with tuned interlayer spacing as high areal capacity anodes for sodium-ion batteries. Energy Storage Mater. 2021, 41, 395–403. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, Y.; Zhao, Z.; Yuan, C.; Lou, X.W.D. A Ternary Fe1−xS@Porous Carbon Nanowires/Reduced Graphene Oxide Hybrid Film Electrode with Superior Volumetric and Gravimetric Capacities for Flexible Sodium Ion Batteries. Adv. Energy Mater. 2019, 9, 1803052. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, X.; Yang, N. Graphdiyne Electrochemistry: Progress and Perspectives. Small 2022, 18, e2201135. [Google Scholar] [CrossRef] [PubMed]
- Febrian, R.; Septiani, N.L.W.; Iqbal, M.; Yuliarto, B. Review—Recent Advances of Carbon-Based Nanocomposites as the Anode Materials for Lithium-Ion Batteries: Synthesis and Performance. J. Electrochem. Soc. 2021, 168, 110520. [Google Scholar] [CrossRef]
- Kong, F.; Yue, Y.; Li, Q.; Ren, S. Sulfur-Doped Graphdiyne as a High-Capacity Anode Material for Lithium-Ion Batteries. Nanomaterials 2021, 11, 1161. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shen, X.; Wang, N.; He, J.; Li, X.; Wang, X.; Hou, Z.; Wang, K.; Gao, J.; Jiu, T.; et al. Graphdiyne Containing Atomically Precise N Atoms for Efficient Anchoring of Lithium Ion. ACS Appl. Mater. Interfaces 2019, 11, 2608–2617. [Google Scholar] [CrossRef]
- Yang, H.-R.; Yang, Y.; Seo, H.; Kim, K.; Lee, H.S.; Lee, J.; Lee, J.; Kim, J.-H. SnS nanosheets on carbon foam as a flexible anode platform for rechargeable Li- and Na-ion batteries. Appl. Surf. Sci. 2021, 544, 148837. [Google Scholar] [CrossRef]
- Wang, C.; Xiong, Y.; Wang, H.; Jin, C.; Sun, Q. Naturally three-dimensional laminated porous carbon network structured short nano-chains bridging nanospheres for energy storage. J. Mater. Chem. A 2017, 5, 15759–15770. [Google Scholar] [CrossRef]
- Peydayesh, M.; Vogt, J.; Chen, X.; Zhou, J.; Donat, F.; Bagnani, M.; R. Müller, C.; Mezzenga, R. Amyloid-based carbon aerogels for water purification. Chem. Eng. J. 2022, 449, 137703. [Google Scholar] [CrossRef]
- Inagaki, M.; Qiu, J.; Guo, Q. Carbon foam: Preparation and application. Carbon 2015, 87, 128–152. [Google Scholar] [CrossRef]
- Huang, R.; Li, Y.; Song, Y.; Wang, L. Facial preparation of N-doped carbon foam supporting Co3O4 nanorod arrays as free-standing lithium-ion batteries’ anode. J. Alloys Compd. 2019, 818, 152839. [Google Scholar] [CrossRef]
- Wang, X.; Fei, S.; Huang, S.; Wu, C.; Zhao, J.; Chen, Z.; Uvdal, K.; Hu, Z. MoS2 nanosheets inlaid in 3D fibrous N-doped carbon spheres for lithium-ion batteries and electrocatalytic hydrogen evolution reaction. Carbon 2019, 150, 363–370. [Google Scholar] [CrossRef]
- Liu, W.; Fu, Y.; Li, Y.; Chen, S.; Song, Y.; Wang, L. Three-dimensional carbon foam surrounded by carbon nanotubes and Co-Co3O4 nanoparticles for stable lithium-ion batteries. Compos. Part B Eng. 2019, 163, 464–470. [Google Scholar] [CrossRef]
- Yuan, W.; Wang, B.; Wu, H.; Xiang, M.; Wang, Q.; Liu, H.; Zhang, Y.; Liu, H.; Dou, S. A flexible 3D nitrogen-doped carbon foam@CNTs hybrid hosting TiO2 nanoparticles as free-standing electrode for ultra-long cycling lithium-ion batteries. J. Power Source 2018, 379, 10–19. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, S.; Liu, B.; Guo, P.; Lv, M.; Liu, D.; He, D. Interfacial modification of a lightweight carbon foam current collector for high-energy density Si/LCO lithium-ion batteries. J. Mater. Chem. A 2017, 5, 13168–13175. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Tan, Q.; Chen, Y. Designed synthesis of ultrafine NiO nanocrystals bonded on a three dimensional graphene framework for high-capacity lithium-ion batteries. New J. Chem. 2018, 42, 9901–9910. [Google Scholar] [CrossRef]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, J.; Feng, J.; Jiang, Y.; Ding, F. Carbon aerogels by pyrolysis of TEMPO-oxidized cellulose. Appl. Surf. Sci. 2018, 440, 873–879. [Google Scholar] [CrossRef]
- Guo, F.; Jiang, Y.; Xu, Z.; Xiao, Y.; Fang, B.; Liu, Y.; Gao, W.; Zhao, P.; Wang, H.; Gao, C. Highly stretchable carbon aerogels. Nat. Commun. 2018, 9, 881. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Xie, H.; Gao, T.; Yao, Y.; Pastel, G.; Han, X.; Li, Y.; Zhao, J.; Fu, K.; et al. 3D-Printed All-Fiber Li-Ion Battery toward Wearable Energy Storage. Adv. Funct. Mater. 2017, 27, 1703140. [Google Scholar] [CrossRef]
- Khudiyev, T.; Grena, B.; Loke, G.; Hou, C.; Jang, H.; Lee, J.; Noel, G.; Alain, J.; Joannopoulos, J.; Li, K.; et al. Thermally drawn rechargeable battery fiber enables pervasive power. Mater. Today 2022, 52, 80–89. [Google Scholar] [CrossRef]
- Praveen, S.; Sim, G.S.; Ho, C.W.; Lee, C.W. 3D-printed twisted yarn-type Li-ion battery towards smart fabrics. Energy Storage Mater. 2021, 41, 748–757. [Google Scholar] [CrossRef]
- Ji, D.; Zheng, H.; Zhang, H.; Liu, W.; Ding, J. Coaxial 3D-printing constructing all-in-one fibrous lithium-, sodium-, and zinc-ion batteries. Chem. Eng. J. 2022, 433, 133815. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yang, X.Y.; Liu, T.; Zhang, X.B. Flexible 1D Batteries: Recent Progress and Prospects. Adv. Mater. 2020, 32, e1901961. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, C.H.; Lu, W.; Dai, L. Recent Advances in Fiber-Shaped Supercapacitors and Lithium-Ion Batteries. Adv. Mater. 2020, 32, e1902779. [Google Scholar] [CrossRef]
- Qu, H.; Lu, X.; Skorobogatiy, M. All-Solid Flexible Fiber-Shaped Lithium Ion Batteries. J. Electrochem. Soc. 2018, 165, A688–A695. [Google Scholar] [CrossRef]
- Man, P.; He, B.; Zhang, Q.; Li, C.; Zhou, Z.; Li, Q.; Xu, W.; Hong, G.; Yao, Y. High-Performance and Ultraflexible Aqueous Rechargeable Lithium-Ion Batteries Developed by Constructing All Binder-free Electrode Materials. ACS Appl. Mater. Interfaces 2020, 12, 25700–25708. [Google Scholar] [CrossRef]
- Song, H.; Jeon, S.-Y.; Jeong, Y. Fabrication of a coaxial high performance fiber lithium-ion battery supported by a cotton yarn electrolyte reservoir. Carbon 2019, 147, 441–450. [Google Scholar] [CrossRef]
- Rao, J.; Liu, N.; Zhang, Z.; Su, J.; Li, L.; Xiong, L.; Gao, Y. All-fiber-based quasi-solid-state lithium-ion battery towards wearable electronic devices with outstanding flexibility and self-healing ability. Nano Energy 2018, 51, 425–433. [Google Scholar] [CrossRef]
- Zhang, T.; Han, S.; Guo, W.; Hou, F.; Liu, J.; Yan, X.; Chen, S.; Liang, J. Continuous carbon nanotube composite fibers for flexible aqueous lithium-ion batteries. Sustain. Mater. Technol. 2019, 20, e00096. [Google Scholar] [CrossRef]
- He, J.; Lu, C.; Jiang, H.; Han, F.; Shi, X.; Wu, J.; Wang, L.; Chen, T.; Wang, J.; Zhang, Y.; et al. Scalable production of high-performing woven lithium-ion fiber batteries. Nature 2021, 597, 57–63. [Google Scholar] [CrossRef]
- Hoshide, T.; Zheng, Y.; Hou, J.; Wang, Z.; Li, Q.; Zhao, Z.; Ma, R.; Sasaki, T.; Geng, F. Flexible Lithium-Ion Fiber Battery by the Regular Stacking of Two-Dimensional Titanium Oxide Nanosheets Hybridized with Reduced Graphene Oxide. Nano Lett. 2017, 17, 3543–3549. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, K.; Lv, C.; Zhong, S.; Wang, Q.; Liu, T.; Liu, X.; Yin, Y.; Hu, Y.; Wei, D.; et al. Ultrahigh-Energy Density Lithium-Ion Cable Battery Based on the Carbon-Nanotube Woven Macrofilms. Small 2018, 14, e1800414. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.T.; Jang, J.; Lee, Y.; Choi, S.T.; In, J.B. Laser-assisted fabrication of flexible monofilament fiber supercapacitors. J. Mater. Chem. A 2021, 9, 4841–4850. [Google Scholar] [CrossRef]
- Yuan, H.; Wang, G.; Zhao, Y.; Liu, Y.; Wu, Y.; Zhang, Y. A stretchable, asymmetric, coaxial fiber-shaped supercapacitor for wearable electronics. Nano Res. 2020, 13, 1686–1692. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Lin, H.; Chen, Y. Flexible fiber-shaped supercapacitors with high energy density based on self-twisted graphene fibers. J. Power Source 2019, 433, 226711. [Google Scholar] [CrossRef]
- Gong, W.; Fugetsu, B.; Wang, Z.; Ueki, T.; Sakata, I.; Ogata, H.; Han, F.; Li, M.; Su, L.; Zhang, X.; et al. Thicker carbon-nanotube/manganese-oxide hybridized nanostructures as electrodes for the creation of fiber-shaped high-energy-density supercapacitors. Carbon 2019, 154, 169–177. [Google Scholar] [CrossRef]
- Xie, C.; Xu, N.; Shi, P.; Lv, Y.; Maleki Kheimeh Sari, H.; Shi, J.W.; Xiao, W.; Qin, J.; Yang, H.; Li, W.; et al. Flexible and robust silicon/carbon nanotube anodes exhibiting high areal capacities. J. Colloid Interface Sci. 2022, 625, 871–878. [Google Scholar] [CrossRef]
- Kang, Y.; Deng, C.; Chen, Y.; Liu, X.; Liang, Z.; Li, T.; Hu, Q.; Zhao, Y. Binder-Free Electrodes and Their Application for Li-Ion Batteries. Nanoscale Res. Lett. 2020, 15, 112. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Qi, S.; Ge, W.; Ma, W.; Zhao, Y.; Huang, R.; Xu, L.; Qian, Y. Ultrahigh-Areal-Capacity Battery Anodes Enabled by Free-Standing Vanadium Nitride@N-Doped Carbon/Graphene Architecture. ACS Appl. Mater. Interfaces 2020, 12, 49607–49616. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, F.; Yang, S.; Wang, G.; Yu, M.; Feng, X. Flexible in-plane micro-supercapacitors: Progresses and challenges in fabrication and applications. Energy Storage Mater. 2020, 28, 160–187. [Google Scholar] [CrossRef]
- Zeng, Y.; Huang, Y.; Liu, N.; Wang, X.; Zhang, Y.; Guo, Y.; Wu, H.-H.; Chen, H.; Tang, X.; Zhang, Q. N-doped porous carbon nanofibers sheathed pumpkin-like Si/C composites as free-standing anodes for lithium-ion batteries. J. Energy Chem. 2021, 54, 727–735. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, X.; Yang, Y.; Wang, X.; Yao, J. Electrospinning fabrication of flexible, foldable, and twistable Sb2S3/TiO2/C nanofiber anode for lithium ion batteries. Chem. Eng. J. 2021, 413, 127400. [Google Scholar] [CrossRef]
- Peng, J.; Tao, J.; Liu, Z.; Yang, Y.; Yu, L.; Zhang, M.; Wang, F.; Ding, Y. Ultra-stable and high capacity flexible lithium-ion batteries based on bimetallic MOFs derivatives aiming for wearable electronic devices. Chem. Eng. J. 2021, 417, 129200. [Google Scholar] [CrossRef]
- Lian, X.; Xu, N.; Ma, Y.; Hu, F.; Wei, H.; Chen, H.-Y.; Wu, Y.; Li, L.; Peng, S. In-situ formation of Co1−xS hollow polyhedrons anchored on multichannel carbon nanofibers as self-supporting anode for lithium/sodium-ion batteries. Chem. Eng. J. 2020, 421, 127755. [Google Scholar] [CrossRef]
- Li, X.; Deng, C.; Wang, H.; Si, J.; Zhang, S.; Huang, B. Iron Nitride@C Nanocubes Inside Core-Shell Fibers to Realize High Air-Stability, Ultralong Life, and Superior Lithium/Sodium Storages. ACS Appl. Mater. Interfaces 2021, 13, 7297–7307. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Shen, W.; Xu, T.; Li, K.; Yang, L.; Zhou, Y.; Zhong, M.; Yang, F.; Xu, X.; Wang, Y.; et al. Ultra-flexible and foldable gel polymer lithium–ion batteries enabling scalable production. Mater. Today Energy 2021, 23, 100889. [Google Scholar] [CrossRef]
- Yang, Y.; Fu, W.; Bell, C.; Lee, D.C.; Drexler, M.; Nuli, Y.; Ma, Z.-F.; Magasinski, A.; Yushin, G.; Alamgir, F.S. Iron Phosphide Confined in Carbon Nanofibers as a Free-Standing Flexible Anode for High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34074–34083. [Google Scholar] [CrossRef]
- Liu, J.; Liang, J.; Wang, C.; Ma, J. Electrospun CoSe@N-doped carbon nanofibers with highly capacitive Li storage. J. Energy Chem. 2019, 33, 160–166. [Google Scholar] [CrossRef]
- Jiang, H.; Gan, Y.; Liu, J.; Wang, X.; Ma, R.; Liu, J.; Wang, J. Boosting the transport kinetics of free-standing SnS2@Carbon nanofibers by electronic structure modulation for advanced lithium storage. J. Mater. Chem. A 2022, 10, 9468–9681. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, Z.; Hu, X.; Liu, X.; Wang, H. Silicon in Hollow Carbon Nanospheres Assembled Microspheres Cross-linked with N-doped Carbon Fibers toward a Binder Free, High Performance, and Flexible Anode for Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101487. [Google Scholar] [CrossRef]
- Luiso, S.; Petrecca, M.J.; Williams, A.H.; Christopher, J.; Velev, O.D.; Pourdeyhimi, B.; Fedkiw, P.S. Structure-Performance Relationships of Li-Ion Battery Fiber-Based Separators. ACS Appl. Polym. Mater. 2022, 4, 3676–3686. [Google Scholar] [CrossRef]
- Zhang, T.; Qiu, D.; Hou, Y. Free-standing and consecutive ZnSe@carbon nanofibers architectures as ultra-long lifespan anode for flexible lithium-ion batteries. Nano Energy 2022, 94, 106909. [Google Scholar] [CrossRef]
- Li, B.; Yu, M.; Li, Z.; Yu, C.; Wang, H.; Li, Q. Constructing Flexible All-Solid-State Supercapacitors from 3D Nanosheets Active Bricks via 3D Manufacturing Technology: A Perspective Review. Adv. Funct. Mater. 2022, 32, 2201166. [Google Scholar] [CrossRef]
- Jiang, X.; Gao, R.; Liu, G.; Luo, H.; Zhao, X.; Jiang, L. Construction of Graphene-Based “In-Paper” 3D Interdigital Microelectrodes for High Performance Metal-Free Flexible Supercapacitors. Small Methods 2022, 6, e2101454. [Google Scholar] [CrossRef]
- Zhang, M.; Bai, R.; King, S.; Yin, F.; Peng, H.; Wang, G. Dual Active and Kinetically Inter-Promoting Li3VO4/Graphene Anode Enabling Printable High Energy Density Lithium Ion Micro Capacitors. Energy Storage Mater. 2021, 43, 482–491. [Google Scholar] [CrossRef]
- Shi, X.; Tian, L.; Wang, S.; Wen, P.; Su, M.; Xiao, H.; Das, P.; Zhou, F.; Liu, Z.; Sun, C.; et al. Scalable and fast fabrication of graphene integrated micro-supercapacitors with remarkable volumetric capacitance and flexibility through continuous centrifugal coating. J. Energy Chem. 2021, 52, 284–290. [Google Scholar] [CrossRef]
- Shi, X.; Pei, S.; Zhou, F.; Ren, W.; Cheng, H.-M.; Wu, Z.-S.; Bao, X. Ultrahigh-voltage integrated micro-supercapacitors with designable shapes and superior flexibility. Energy Environ. Sci. 2019, 12, 1534–1541. [Google Scholar] [CrossRef]
- Zheng, S.; Wu, Z.-S.; Zhou, F.; Wang, X.; Ma, J.; Liu, C.; He, Y.-B.; Bao, X. All-solid-state planar integrated lithium ion micro-batteries with extraordinary flexibility and high-temperature performance. Nano Energy 2018, 51, 613–620. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, B.; Xu, Q.; Hou, Y.; Seyedin, S.; Qin, S.; Wallace, G.; Beirne, B.; Razal, J.; Chen, J. Development of Graphene Oxide/Polyaniline Inks for High Performance Flexible Microsupercapacitors via Extrusion Printing. Adv. Funct. Mater. 2018, 28, 1706592. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Jiang, Y.; Tang, X.; Pan, Y.; Hu, S. Fully Packaged Carbon Nanotube Supercapacitors by Direct Ink Writing on Flexible Substrates. ACS Appl. Mater. Interfaces 2017, 9, 28433–28440. [Google Scholar] [CrossRef]
- Zheng, S.; Ma, J.; Wu, Z.-S.; Zhou, F.; He, Y.-B.; Kang, F.; Cheng, H.-M.; Bao, X. All-solid-state flexible planar lithium ion micro-capacitors. Energy Environ. Sci. 2018, 11, 2001–2009. [Google Scholar] [CrossRef]
- Ye, J.; Tan, H.; Wu, S.; Ni, K.; Pan, F.; Liu, J.; Tao, Z.; Qu, Y.; Ji, H.; Simon, P.; et al. Direct Laser Writing of Graphene Made from Chemical Vapor Deposition for Flexible, Integratable Micro-Supercapacitors with Ultrahigh Power Output. Adv. Mater. 2018, 30, e1801384. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Chen, H.; Wang, W.; Lu, L. Laser-Induced Graphene/MoO2 Core-Shell Electrodes on Carbon Cloth for Integrated, High-Voltage, and In-Planar Microsupercapacitors. Adv. Mater. Technol. 2021, 6, 2000991. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Meng, Y.; Plamthottam, R.; Tjiu, W.W.; Zhang, C.; Liu, T. Ultrathin Polypyrrole Layers Boosting MoO3 as Both Cathode and Anode Materials for a 2.0 V High-Volt. Aqueous Supercapacitor. ACS Appl. Mater. Interfaces 2022, 14, 4490–4499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, R.; Zheng, H.; Bao, J.; Tang, Y.; Zhou, K. Laser-Assisted Printing of Electrodes Using Metal–Organic Frameworks for Micro-Supercapacitors. Adv. Funct. Mater. 2021, 31, 2009057. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Q.; Li, L.; Yang, L.; Wang, Y.; Wang, X.; Fang, H.-T. Femtosecond Laser-Etched MXene Microsupercapacitors with Double-Side Configuration via Arbitrary On- and Through-Substrate Connections. Adv. Energy Mater. 2020, 10, 2000470. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, F.; Peng, J.; Wu, R.; Wu, Z.S.; Bao, X. One-Step Scalable Fabrication of Graphene-Integrated Micro-Supercapacitors with Remarkable Flexibility and Exceptional Performance Uniformity. Adv. Funct. Mater. 2019, 29, 1902860. [Google Scholar] [CrossRef]
- Hong, S.Y.; Jee, S.M.; Ko, Y.; Cho, J.; Lee, K.H.; Yeom, B.; Kim, H.; Son, J.G. Intrinsically Stretchable and Printable Lithium-Ion Battery for Free-Form Configuration. ACS Nano 2022, 16, 2271–2281. [Google Scholar] [CrossRef]
- Chen, D.; Lou, Z.; Jiang, K.; Shen, G. Device Configurations and Future Prospects of Flexible/Stretchable Lithium-Ion Batteries. Adv. Funct. Mater. 2018, 28, 1805596. [Google Scholar] [CrossRef]
- Song, W.-J.; Lee, S.; Song, G.; Park, S. Stretchable Aqueous Batteries: Progress and Prospects. ACS Energy Lett. 2018, 4, 177–186. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Q.; Hong, M.; Xie, W.; Jing, S.; Bao, Y.; Chen, G.; Pang, Z.; Hu, L.; Li, T. Toward stretchable batteries: 3D-printed deformable electrodes and separator enabled by nanocellulose. Mater. Today 2022, 54, 18–26. [Google Scholar] [CrossRef]
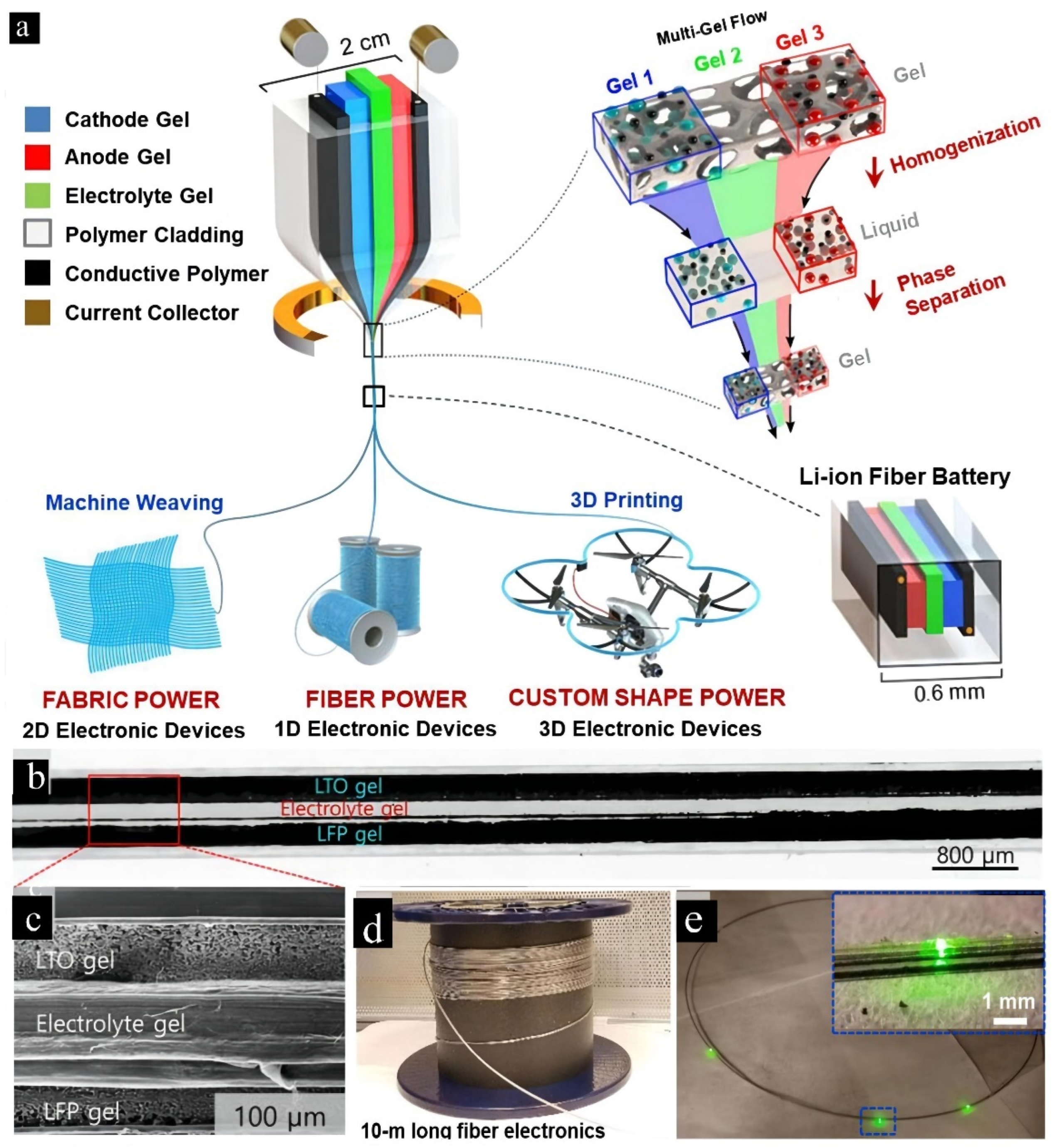
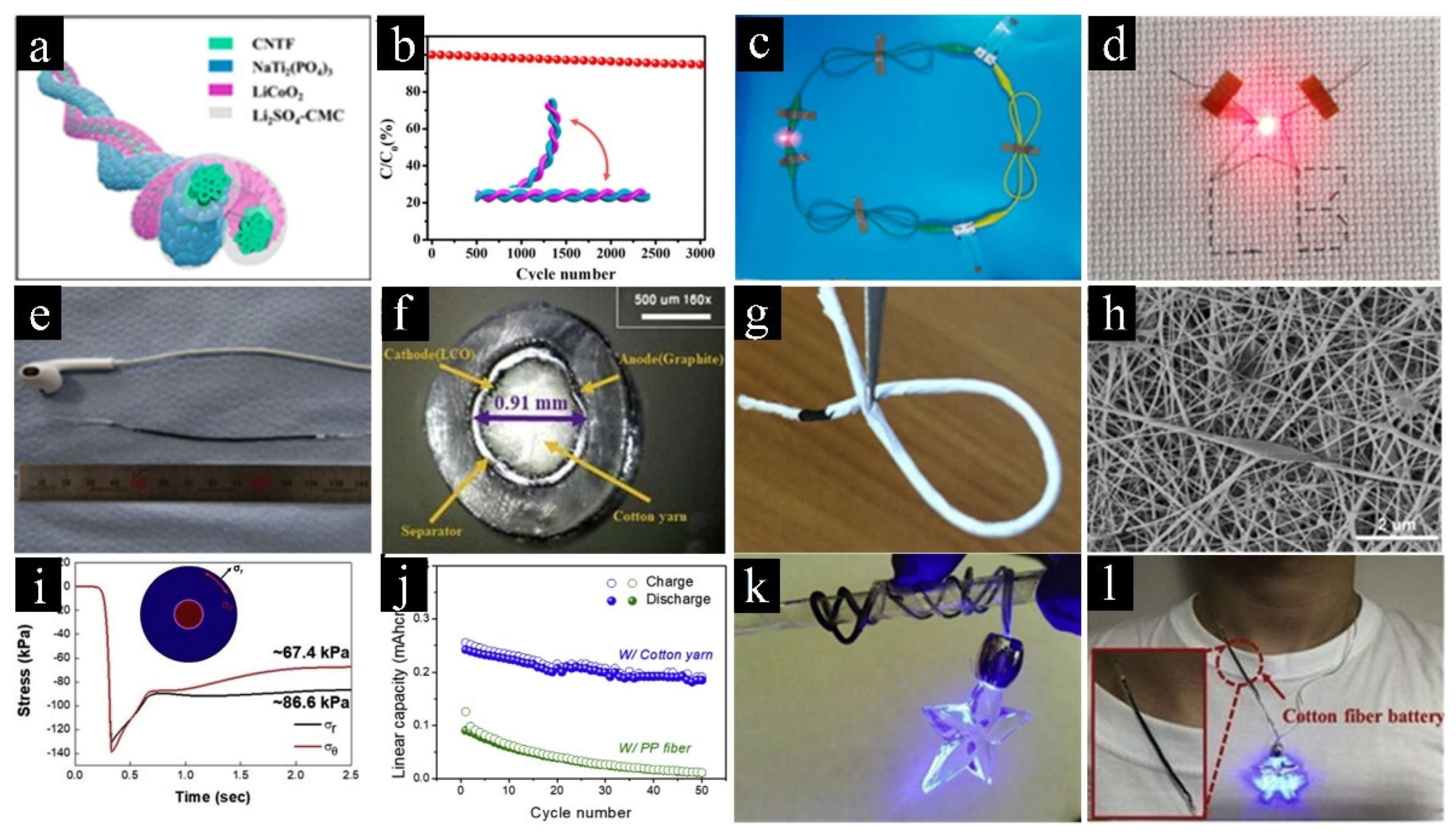
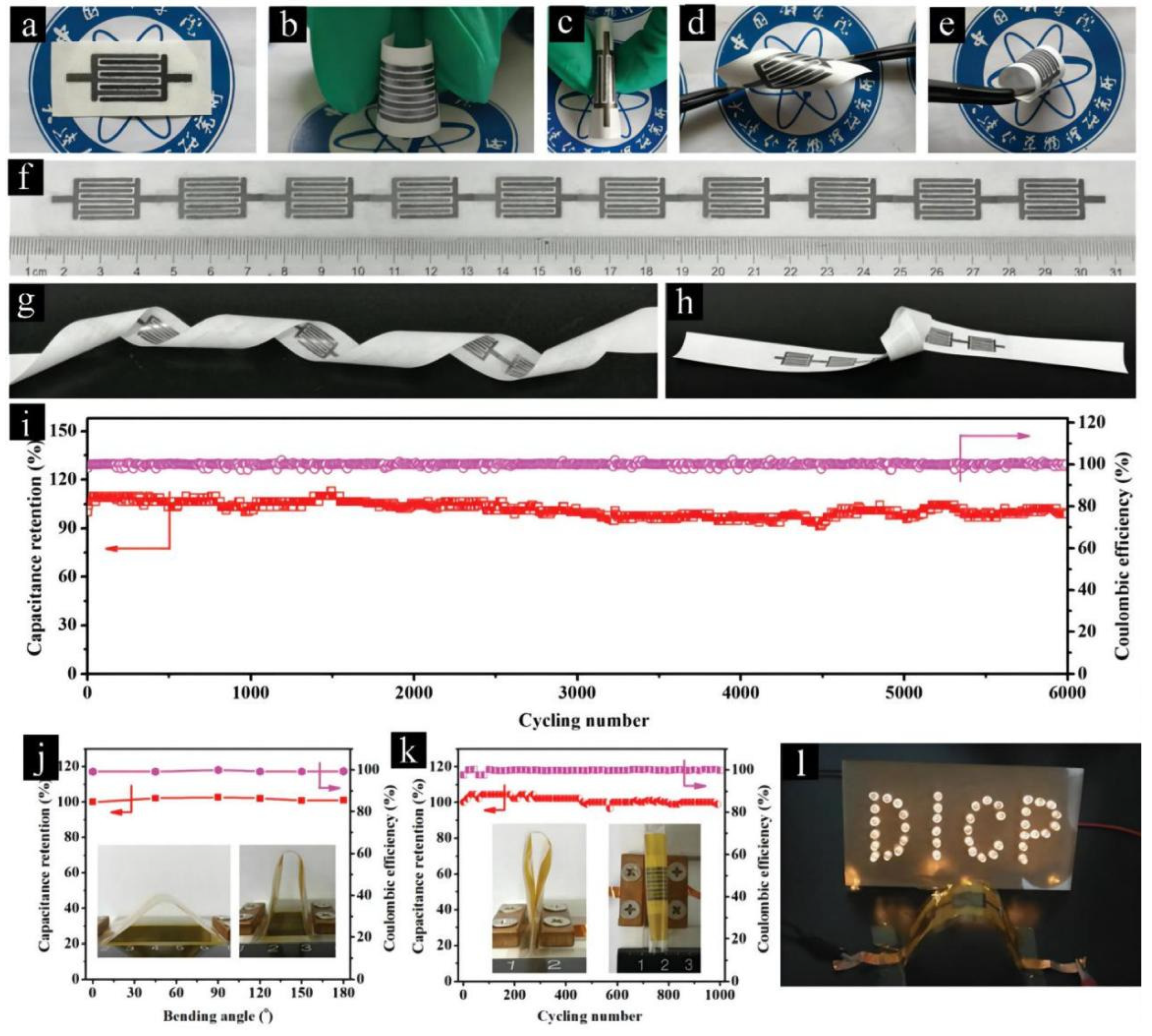

| Carbon-Based Electrodes | Specific Capacity | Current Density | Cycling Life | Capacity Retention | Reference |
|---|---|---|---|---|---|
| SnO2@rGO/LiCoO2@rGO | 82.6 mAh g−1 | 0.1 A g−1 | [59] | ||
| LTP@CNT/LFP@CNT | 29.1 mAh g−1 | 0.25 A g−1 | [60] | ||
| graphite/LCO | 170 mAh g−1 | 0.017 A g−1 | 500 | 93% | [61] |
| graphite@CNT/LiNi0.6 Co0.2Mn0.2O2@CNT | 166 mAh g−1 | 0.1C | 50 | 99% | [53] |
| NTP@CNT/LCO@CNT | 45.2 mAh cm−3 | 0.2 A cm−3 | 3000 | 94.7% | [57] |
| graphite@CNT/LCO@CNT | 141.8 mAh g−1 | 0.145 A g−1 | 50 | 84% | [58] |
| TiO2@rGO/LiMn2O4 | 126 mAh g−1 | 0.017 mA g−1 | 100 | 80% | [62] |
| LTO@CNT/LCO@CNT | 135 mAh g−1 | 0.15 A g−1 | 100 | 86.2% | [63] |
| Ag@rGo/MnO2@rGO | 24.5 mF cm−2 | 0.1 mA cm−2 | 3000 | 94.3% | [64] |
| CNT@Fe2O3/CNT@NiO @MnOx | 10.4 F·cm−3 | 30 mA cm−3 | 2000 | 95% | [65] |
| Graphene fibers | 973.1 mF cm−3 | 161.6 mA cm−3 | 10,000 | 91% | [66] |
| CNT/MnO2@CF | 91.6 F cm −3 | 104.7 mA cm−3 | 7000 | 95.3% | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Wei, T.; Liu, J.; Zhan, L.; Chen, W.; Cao, J. Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries. Crystals 2022, 12, 1279. https://doi.org/10.3390/cryst12091279
Deng L, Wei T, Liu J, Zhan L, Chen W, Cao J. Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries. Crystals. 2022; 12(9):1279. https://doi.org/10.3390/cryst12091279
Chicago/Turabian StyleDeng, Ling, Tongye Wei, Jie Liu, Li Zhan, Wei Chen, and Juexian Cao. 2022. "Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries" Crystals 12, no. 9: 1279. https://doi.org/10.3390/cryst12091279
APA StyleDeng, L., Wei, T., Liu, J., Zhan, L., Chen, W., & Cao, J. (2022). Recent Developments of Carbon-Based Anode Materials for Flexible Lithium-Ion Batteries. Crystals, 12(9), 1279. https://doi.org/10.3390/cryst12091279






