Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
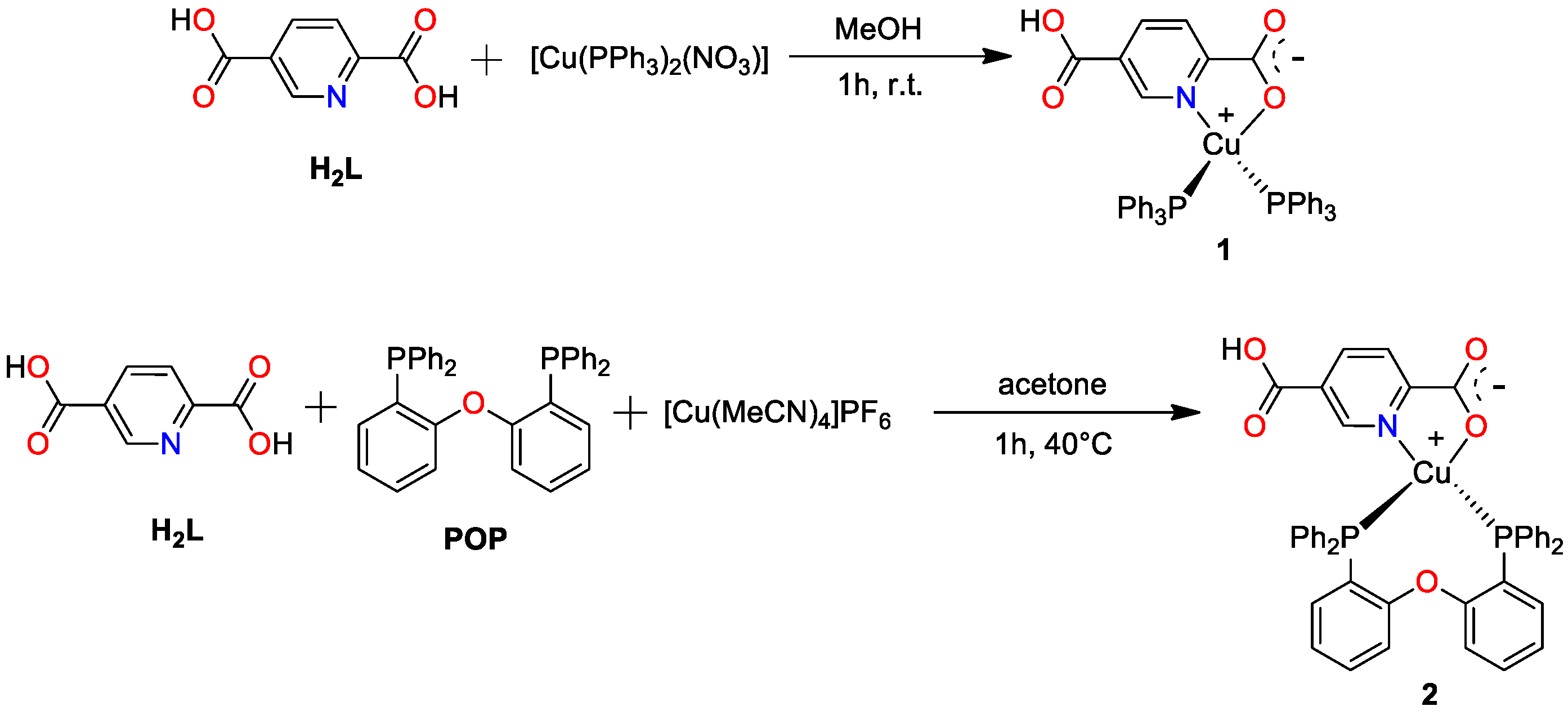
2.2. Preparation of [Cu(HL)(PPh3)2]·H2O (1)
2.3. Preparation of [Cu(HL)(POP)]·CH2Cl2 (2)
2.4. Crystallography
2.5. Solar Cell Construction
2.6. Computational Details
3. Results and Discussion
3.1. IR and NMR Spectroscopic Analysis
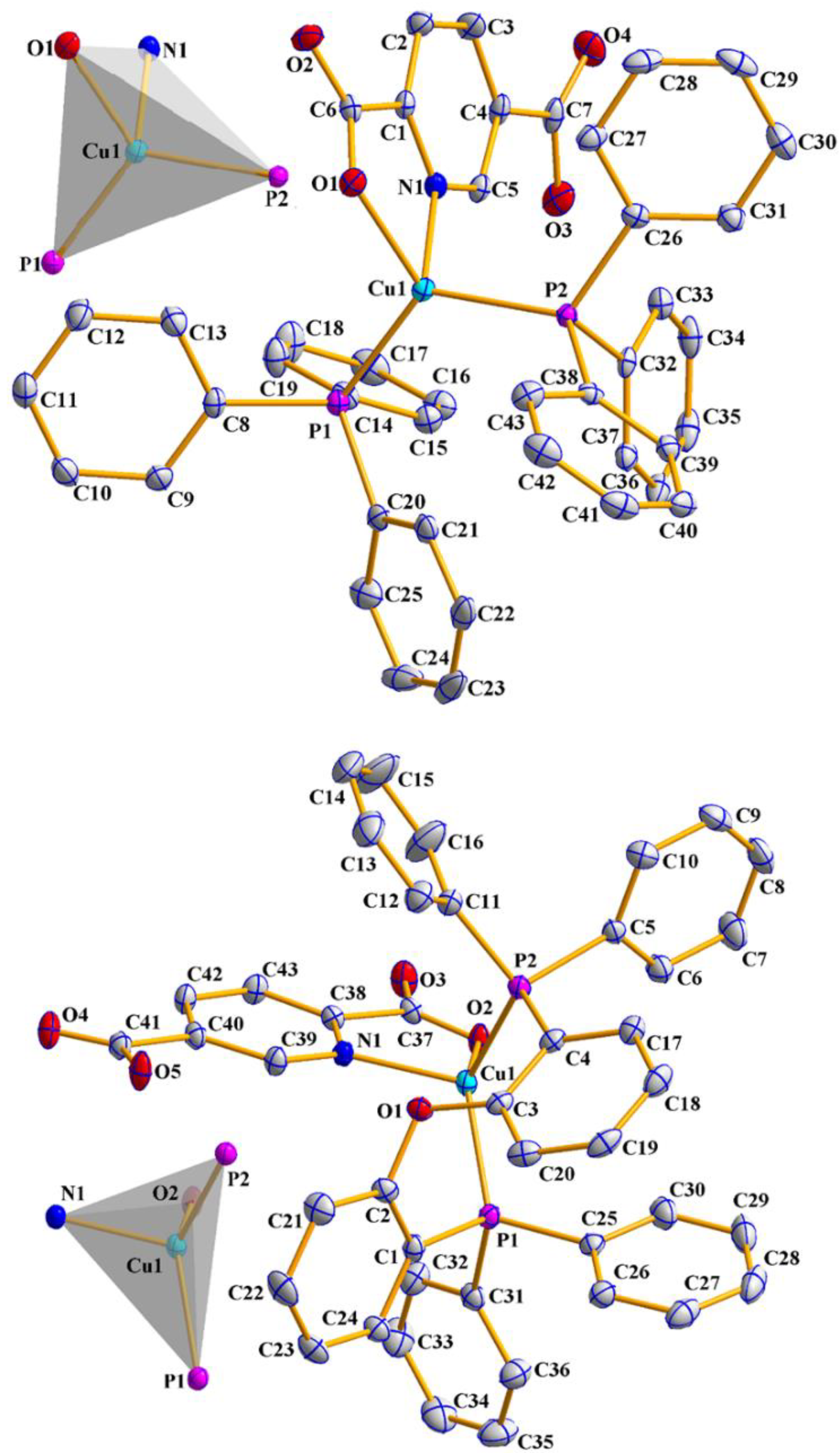
3.2. X-ray Crystallography
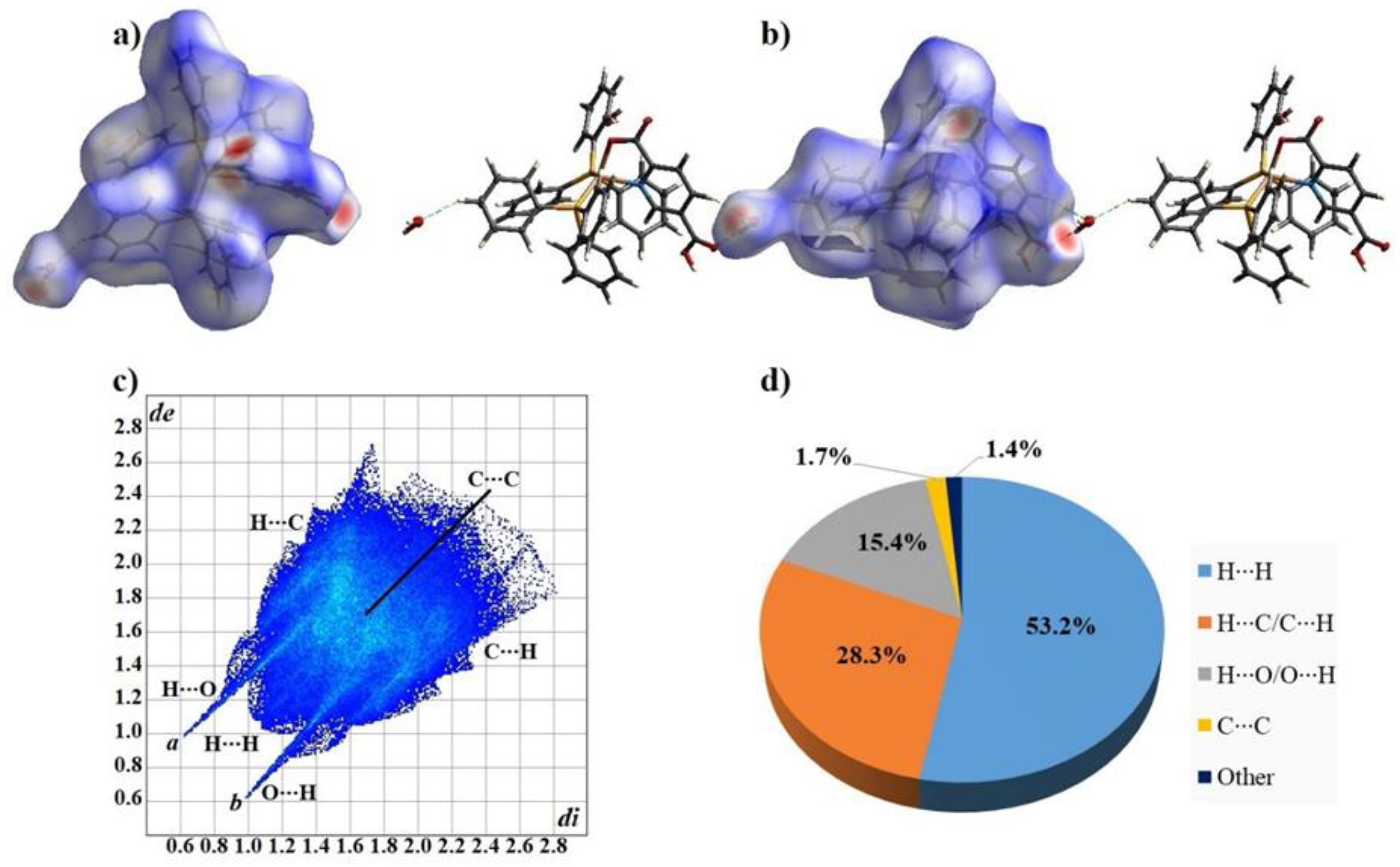
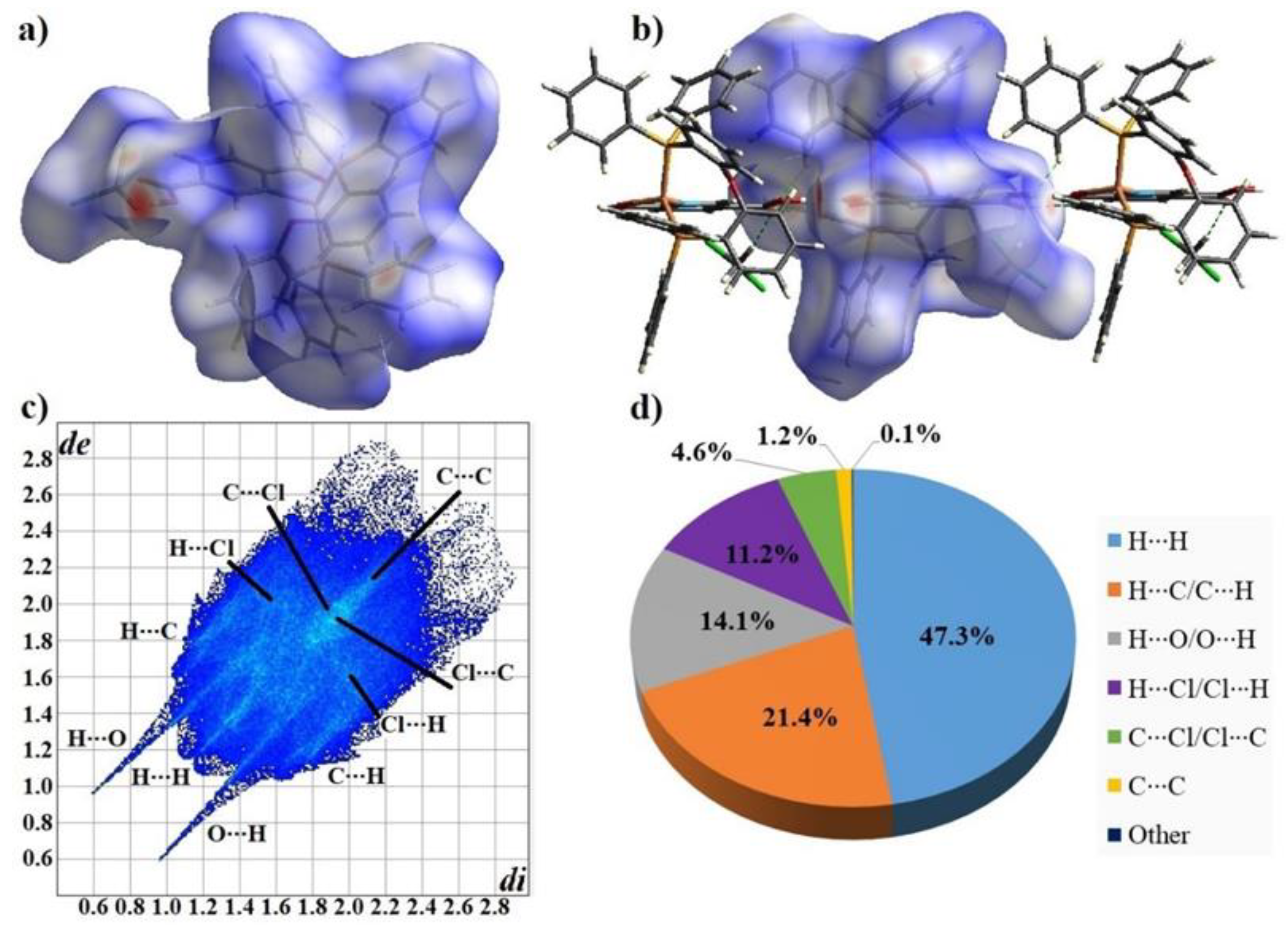
3.3. Analysis of the Hirshfeld Surface and Fingerprint Plots
3.4. DFT Calculations and UV−Vis Absorption
3.5. Electrochemistry
3.6. Application in DSSCs
3.7. Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffert, M.I.; Caldeira, K.; Jain, A.K.; Haites, E.F.; Harvey, L.D.D.; Potter, S.D.; Schlesinger, M.E.; Schneider, S.H.; Watts, R.G.; Wigley, T.M.L.; et al. Energy implications of future stabilization of atmospheric CO2 content. Nature 1998, 395, 881–884. [Google Scholar] [CrossRef]
- Caldeira, K.; Jain, A.K.; Hoffert, M.I. Climate Sensitivity Uncertainty and the Need for Energy Without CO2 Emission. Science 2003, 299, 2052–2054. [Google Scholar] [CrossRef]
- Eisenberg, R.; Nocera, D.G. Preface: Overview of the Forum on Solar and Renewable Energy. Inorg. Chem. 2005, 44, 6799–6801. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-García, A.B.; Benesperi, I.; Boschloo, G.; Concepcion, J.J.; Delcamp, J.H.; Gibson, E.A.; Meyer, G.J.; Pavone, M.; Pettersson, H.; Hagfeldt, A.; et al. Dye-sensitized solar cells strike back. Chem. Soc. Rev. 2021, 50, 12450–12550. [Google Scholar] [CrossRef]
- Polo, A.S.; Itokazu, M.K.; Iha, N.Y.M. Metal complex sensitizers in dye-sensitized solar cells. Chem. Rev. 2004, 248, 1343–1361. [Google Scholar] [CrossRef]
- Förster, C.; Heinze, K. Photophysics and photochemistry with Earth-abundant metals—Fundamentals and concepts. Chem. Soc. Rev. 2020, 49, 1057–1070. [Google Scholar] [CrossRef]
- Seo, J.; Noh, J.H.; Seok, S.I. Rational Strategies for Efficient Perovskite Solar Cells. Acc. Chem. Res. 2016, 49, 562–572. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 1–46. [Google Scholar] [CrossRef]
- Portillo-Cortez, K.; Martínez, A.; Dutt, A.; Santana, G. N719 Derivatives for Application in a Dye-Sensitized Solar Cell (DSSC): A Theoretical Study. J. Phys. Chem. A 2019, 123, 10930–10939. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.; Bouziani, A.; Ocak, C.; Seferoğlu, Z.; Sillanpää, M. Organic/metal-organic photosensitizers for dye-sensitized solar cells (DSSC): Recent developments, new trends, and future perceptions. Dye. Pigment. 2021, 192, 109227. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. Solar energy conversion using first row d-block metal coordination compound sensitizers and redox mediators. Chem. Sci. 2022, 13, 1225–1262. [Google Scholar] [CrossRef]
- Sandroni, M.; Pellegrin, Y.; Odobel, F. Heteroleptic bis-diimine copper(I) complexes for applications in solar energy conversion. Comptes Rendus Chim. 2016, 19, 79–93. [Google Scholar] [CrossRef]
- Lazorski, M.S.; Castellano, F.N. Advances in the light conversion properties of Cu(I)-based photosensitizers. Polyhedron 2014, 82, 57–70. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The emergence of copper(i)-based dye sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8386–8398. [Google Scholar] [CrossRef]
- Cao, Y.; Saygili, Y.; Ummadisingu, A.; Teuscher, J.; Luo, J.; Pellet, N.; Giordano, F.; Zakeeruddin, S.M.; Moser, J.-E.; Freitag, M.; et al. 11% efficiency solid-state dye-sensitized solar cells with copper(II/I) hole transport materials. Nat. Commun. 2017, 8, 15390. [Google Scholar] [CrossRef]
- Colombo, A.; Dragonetti, C.; Roberto, D.; Fagnani, F. Copper Complexes as Alternative Redox Mediators in Dye-Sensitized Solar Cells. Molecules 2021, 26, 194. [Google Scholar] [CrossRef]
- Franchi, D.; Leandri, V.; Pia Pizzichetti, A.R.; Xu, B.; Hao, Y.; Zhang, W.; Sloboda, T.; Svanström, S.; Cappel, U.B.; Kloo, L.; et al. Effect of the Ancillary Ligand on the Performance of Heteroleptic Cu(I) Diimine Complexes as Dyes in Dye-Sensitized Solar Cells. ACS Appl. Energy Mater. 2022, 5, 1460−1470. [Google Scholar] [CrossRef]
- Alonso-Vante, N.; Nierengarten, J.-F.; Sauvage, J.-P. Spectral sensitization of large-band-gap semiconductors (thin films and ceramics) by a carboxylated bis(1,10-phenanthroline)copper(I) complex. J. Chem. Soc. Dalton Trans. 1994, 1649–1654. [Google Scholar] [CrossRef]
- Fürer, S.O.; Luu, L.Y.; Bozic-Weber, B.; Constable, E.C.; Housecroft, C.E. Improving performance of copper(I)-based dye sensitized solar cells through I3−/I− electrolyte manipulation. Dye. Pigment. 2016, 132, 72–78. [Google Scholar] [CrossRef]
- Linfoot, C.L.; Richardson, P.; Hewat, T.E.; Moudam, O.; Forde, M.M.; Collins, A.; White, F.; Robertson, N. Substituted [Cu(i)(POP)(bipyridyl)] and related complexes: Synthesis, structure, properties and applications to dye-sensitised solar cells. Dalton Trans. 2010, 39, 8945–8956. [Google Scholar] [CrossRef]
- Brauchli, S.Y.; Malzner, F.J.; Constable, E.C.; Housecroft, C.E. Copper(i)-based dye-sensitized solar cells with sterically demanding anchoring ligands: Bigger is not always better. RSC Adv. 2015, 5, 48516–48525. [Google Scholar] [CrossRef]
- Fürer, S.O.; Bozic-Weber, B.; Neuburger, M.; Constable, E.C.; Housecroft, C.E. Heteroleptic copper(i) sensitizers with one versus two hole-transporting units in functionalized 2,9-dimethyl-1,10-phenanthroline ancillary ligands. RSC Adv. 2015, 5, 69430–69440. [Google Scholar] [CrossRef]
- Sandroni, M.; Kayanuma, M.; Planchat, A.; Szuwarski, N.; Blart, E.; Pellegrin, Y.; Daniel, C.; Boujtita, M.; Odobel, F. First application of the HETPHEN concept to new heteroleptic bis(diimine) copper(i) complexes as sensitizers in dye sensitized solar cells. Dalton Trans. 2013, 42, 10818–10827. [Google Scholar] [CrossRef]
- Sandroni, M.; Favereau, L.; Planchat, A.; Akdas-Kilig, H.; Szuwarski, N.; Pellegrin, Y.; Blart, E.; Le Bozec, H.; Boujtita, M.; Odobel, F. Heteroleptic copper(i)–polypyridine complexes as efficient sensitizers for dye sensitized solar cells. J. Mater. Chem. A 2014, 2, 9944–9947. [Google Scholar] [CrossRef]
- Selvaraj, B.; Shanmugam, G.; Kamaraj, S.; Gunasekeran, A.; Sambandam, A. Effect of 1-Substituted 2-(Pyridin-2-yl)-1H-Benzo[d]imidazole Ligand-Coordinated Copper and Cobalt Complex Redox Electrolytes on Performance of Ru(II) Dye-Based Dye-Sensitized Solar Cells. Inorg. Chem. 2021, 60, 1937–1947. [Google Scholar] [CrossRef]
- Magni, M.; Biagini, P.; Colombo, A.; Dragonetti, C.; Roberto, D.; Valore, A. Versatile copper complexes as a convenient springboard for both dyes and redox mediators in dyes sensiatized solar cells. Coord. Chem. Rev. 2016, 322, 69–93. [Google Scholar] [CrossRef]
- Báez-Castro, A.; Baldenebro-López, J.; Cruz-Enríquez, A.; Höpfl, H.; Glossman-Mitnik, D.; Miranda-Soto, V.; Parra-Hake, M.; Reynoso-Soto, E.; Campos-Gaxiola, J.J. Heteroleptic Cu(I) complexes containing polypyridyl ligands and triphenylphosphine: Synthesis, structure, photophysical properties, DFT studies and applications in co-sensitized solar cells. Inorg. Chim. Acta 2017, 466, 486–496. [Google Scholar] [CrossRef]
- Kubas, G.J.; Monzyk, B.; Crumbliss, A.L. Tetrakis(Acetonitrile)Copper(I) Hexafluorophosphate. Inorg. Synth. 1979, 19, 90–92. [Google Scholar] [CrossRef]
- CrysAlisPro; Version 1.171.37.35; Agilent Technologies: Yarnton, UK, 2014.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.C.; Reid, J.S. The analytical calculation of absorption in multifaceted crystals. Acta Crystallogr. Sect. A Found. Crystallogr. 1995, A51, 887–897. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, version 4.3.2; Crystal Impact GbR: Bonn, Germany, 2017. [Google Scholar]
- Malzner, F.J.; Prescimone, A.; Constable, E.C.; Housecroft, C.E.; Willgert, M. Exploring simple ancillary ligands in copper-based dye-sensitized solar cells: Effects of a heteroatom switch and of co-sensitization. J. Mater. Chem. A 2017, 5, 4671–4685. [Google Scholar] [CrossRef]
- Cruz-Gutiérrez, C.A.; Félix-Navarro, R.M.; Calva-Yañez, J.C.; Silva-Carrillo, C.; Lin-Ho, S.W.; Reynoso-Soto, E.A. Carbon nanotube-carbon black hybrid counter electrodes for dye-sensitized solar cells and the effect on charge transfer kinetics. J. Solid State Electrochem. 2021, 25, 1479–1489. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, WA, Australia, 2017. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Mennucci, V.B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; Revision A. 02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J.; Eppinnett, K.; Hovell, W.L.; Gilliland, R. GaussView, version 5.0.9; Semichem, Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Stratmann, R.E.; Scuseria, G.E.; Frisch, M.J. An efficient implementation of time-dependent density-functional theory for the calculation of excitation energies of large molecules. J. Chem. Phys. 1998, 109, 8218–8224. [Google Scholar] [CrossRef]
- Burke, K.; Werschnik, J.; Gross, E. Time-dependent density functional theory: Past, present, and future. J. Chem. Phys. 2005, 123, 062206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* basis set for third-row atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Sosa, C.; Andzelm, J.; Elkin, B.C.; Wimmer, E.; Dobbs, K.D.; Dixon, D.A. A local density functional study of the structure and vibrational frequencies of molecular transition-metal compounds. J. Phys. Chem. 1992, 96, 6630–6636. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. Electronic Molecular Structure, Reactivity and Intermolecular Forces: An Euristic Interpretation by Means of Electrostatic Molecular Potentials. Adv. Quantum Chem. 1978, 11, 115–193. [Google Scholar] [CrossRef]
- Andrés-Tomé, I.; Fyson, J.; Dias, F.B.; Monkman, A.P.; Iacobellis, G.; Coppo, P. Copper(i) complexes with bipyridyl and phosphine ligands: A systematic study. Dalton Trans. 2012, 41, 8669–8674. [Google Scholar] [CrossRef]
- Massoud, S.S.; Broussard, K.T.; Mautner, F.A.; Vicente, R.; Saha, M.K.; Bernal, I. Five-coordinate cobalt(II) complexes of tris(2-pyridylmethyl)amine (TPA): Synthesis, structural and magnetic characterization of a terephthalato-bridged dinuclear cobalt(II) complex. Inorg. Chim. Acta 2008, 361, 123–131. [Google Scholar] [CrossRef]
- Bora, J.S.; Chakrabortty, M.; Das, K.B. Ditopic carboxylate containing zigzag chain polymers with tetrahedral Co(II) and Zn(II) nodes. J. Mol. Struct. 2020, 1217, 128434. [Google Scholar] [CrossRef]
- Obaleye, J.A.; Lawal, M.; Jadeja, R.N.; Gupta, V.K.; Nnabuike, G.G.; Bamigboye, M.O.; Roy, H.; Yusuff, O.K.; Bhagariya, P. Crystal structure, spectroscopic, DFT calculations and antimicrobial study of the Cu(II) complex bearing second-generation quinolone ofloxacin and 2,2′-bipyridine. Inorg. Chim. Acta 2021, 519, 120264. [Google Scholar] [CrossRef]
- Báez-Castro, A.; Baldenebro-López, J.; Cruz-Enríquez, A.; Höpfl, H.; Glossman-Mitnik, D.; Valentín, M.-S.; Parra-Hake, M.; Campos-Gaxiola, J.J. Synthesis, structure, characterization and photophysical properties of copper(i) complexes containing polypyridyl ligands. RSC Adv. 2014, 4, 42624–42631. [Google Scholar] [CrossRef]
- Báez-Castro, A.; Baldenebro-López, J.; Ceballos-Mendivil, L.; Román-Bravo, P.P.; Höpfl, H.; Miranda-Soto, V.; Glossman-Mitnik, D.; Cruz-Enríquez, A.; Campos-Gaxiola, J.J. Synthesis, crystal structure, DFT studies and photophysical properties of a copper(I)–triphenylphosphane complex based on trans-(±)-2,4,5-tris(pyridin-2-yl)-2-imidazoline. Acta Crystallogr. C Struct. Chem. 2017, 73, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Peppas, A.; Papadaki, E.; Schnakenburg, G.; Magrioti, V.; Philippopoulos, A.I. Heteroleptic copper(I) complexes incorporating sterically demanding diazabutadiene ligands (DABs). Synthesis, spectroscopic characterization and solid state structural analysis. Polyhedron 2019, 171, 412–422. [Google Scholar] [CrossRef]
- Pavia, D.; Lampman, G.; Kriz, G.; Vyvyan, J. Introduction to Spectroscopy; Cengage Learning: Belmont, CA, USA, 2008. [Google Scholar]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Nohara, I.; Prescimone, A.; Häussinger, D.; Catherine, E.; Housecroft, C.E.; Constable, E.C. [Cu(POP)(N^S)][PF6] and [Cu(xantphos)(N^S)][PF6] compounds with 2-(thiophen-2-yl)pyridines. RSC Adv. 2019, 9, 13646–13657. [Google Scholar] [CrossRef]
- Rojas-León, I.; Guerrero-Alvarez, J.A.; Hernández-Paredes, J.; Höpfl, H. Solvent–solvent and solvent–solute interactions in a 3D chloroform clathrate with diorganotin macrocycles in the nano-sized pores. Chem. Commun. 2012, 48, 401–403. [Google Scholar] [CrossRef]
- Yong, G.-P.; Zhang, Y.-M.; She, W.-L. Anion–π interactions in new electron-deficient π systems: The relevance to solid phosphorescent colors. CrystEngComm 2012, 14, 3923–3929. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm. 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, B60, 627–668. [Google Scholar] [CrossRef]
- Woińska, M.; Jayatilaka, D.; Spackman, M.; Edwards, A.J.; Dominiak, P.; Woźniak, K.; Nishibori, E.; Sugimoto, K.; Grabowsky, S. Hirshfeld atom refinement for modelling strong hydrogen bonds. Acta Crystallogr. Sect. A Found. Adv. 2014, A70, 483–498. [Google Scholar] [CrossRef]
- Gao, S.; Fan, R.Q.; Wang, X.M.; Qiang, L.S.; Wei, L.G.; Wang, P.; Yang, Y.L.; Yu Lei Wang, Y.L.; Luan, T.Z. Multifunctional Zn(II)/Cd(II) metal complexes for tunable luminescence properties and highly efficient dye-sensitized solar cells. RSC Adv. 2015, 5, 43705–43716. [Google Scholar] [CrossRef]
- Lv, J.; Lu, Y.; Wang, J.; Zhao, F.; Wang, Y.; He, H.; Wu, Y. Schiff base-type copper(I) complexes exhibiting high molar extinction coefficients: Synthesis, characterization and DFT studies. J. Mol. Struct. 2022, 1249, 131638. [Google Scholar] [CrossRef]
- Li, C.; Mackenzie, C.F.R.; Said, S.A.; Pal, A.K.; Haghighatbin, M.A.; Babaei, A.; Sessolo, M.; Cordes, D.B.; Slawin, A.M.Z.; Kamer, P.C.J.; et al. Wide-Bite-Angle Diphosphine Ligands in Thermally Activated Delayed Fluorescent Copper(I) Complexes: Impact on the Performance of Electroluminescence Applications. Inorg. Chem. 2021, 60, 10323–10339. [Google Scholar] [CrossRef]
- Wang, J.; Chai, C.; Xu, S.; Zhao, F.; Xia, H.; Wang, Y. Modulation of photophysical properties of copper(I) complexes containing pyridyl-imidazole (PyIm) ligands functionalized by naphthyl, phenanthryl, and anthryl groups. Inorg. Chim. Acta 2019, 484, 237–244. [Google Scholar] [CrossRef]
- Brunner, F.; Babaei, A.; Pertegás, A.; Junquera-Hernández, J.M.; Prescimone, A.; Constable, E.C.; Bolink, H.J.; Sessolo, M.; Ortí, E.; Housecroft, C.E. Phosphane tuning in heteroleptic [Cu(N^N)(P^P)]+ complexes for light-emitting electrochemical cells. Dalton Trans. 2019, 48, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vashistha, V.K.; Tevatia, P.; Singh, R. Voltammetric Determination of Molecular Modeling Parameters for Pentaazamacrocyclic Complexes of Mn(II) and Co(II). Anal. Bioanal. Electrochem. 2016, 8, 848–861. [Google Scholar]
- Wei, L.; Yang, Y.; Fan, R.; Na, Y.; Wang, P.; Dong, Y.; Yang, B.; Cao, W. N, N′-Bis((6-methoxylpyridin-2-yl)methylene)-p-phenylenediimine based d10 transition metal complexes and their utilization in co-sensitized solar cells. Dalton Trans. 2014, 43, 11361–11370. [Google Scholar] [CrossRef]
- Wang, D.-D.; Song, L.; Wang, Y.Y.; Guo, J.Y.; Shen, H.Y.; Wang, X.R.; Chai, W.X. Heteroleptic [Cu(NN)P2]+-type cuprous complexes and their structural modulation on phosphorescent color: Synthesis, structural characterization, properties, and theoretical calculations. Appl. Organomet. Chem. 2020, 34, e5561. [Google Scholar] [CrossRef]
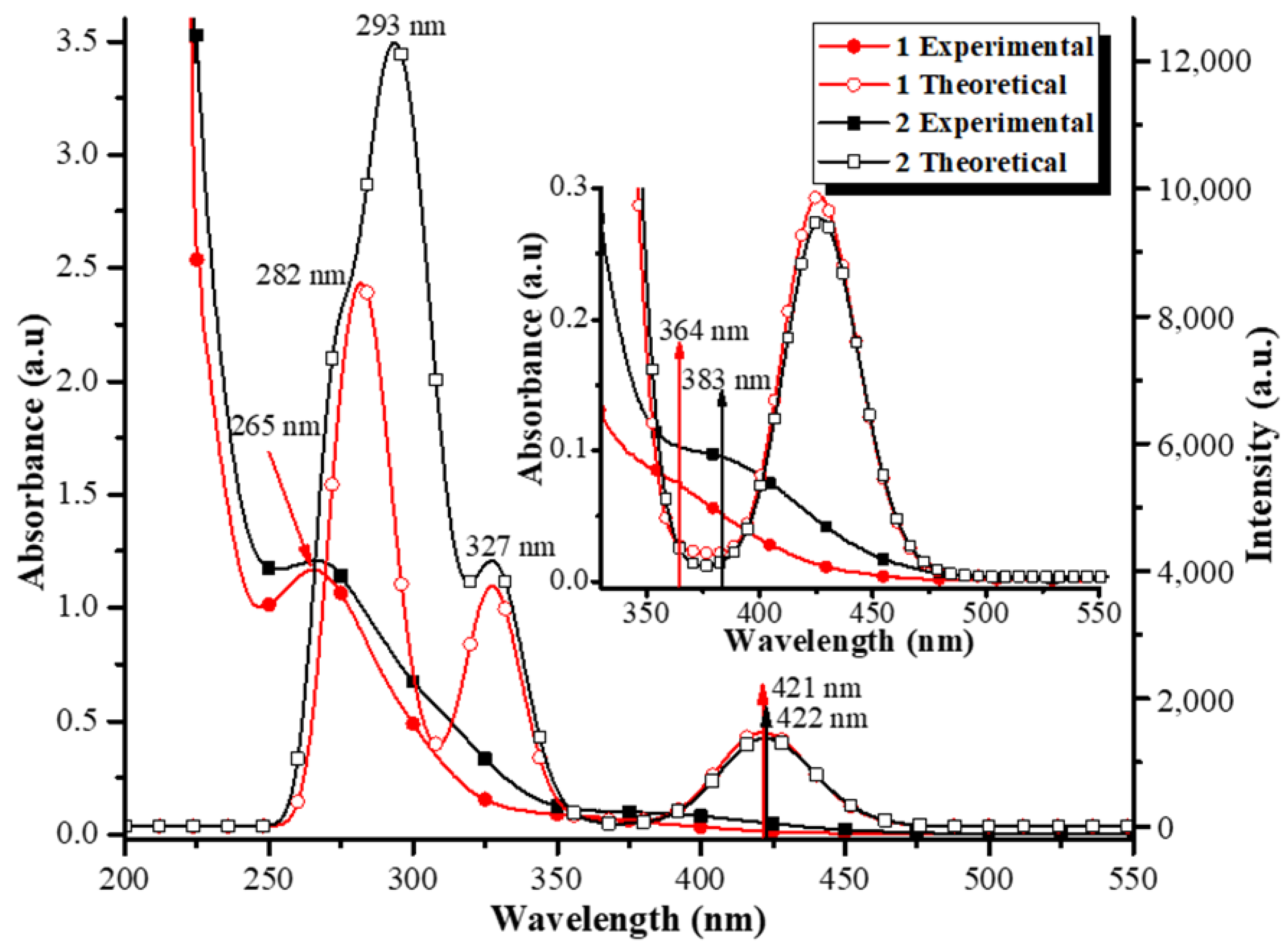

| Compound | ν(C=O) | ν(COO−)asy | ν(COO−)sym | ν(C=N) | ν(P−C) |
|---|---|---|---|---|---|
| H2L | 1733 (s) | - | - | 1595 (m) | - |
| PPh3 | - | - | - | - | 1095 (s) |
| 1 | 1715 (m) | 1597 (m) | 1265 (s) | 1554 (m) | 1094 (m) |
| POP | - | - | - | - | 1092 (w) |
| 2 | 1722 (w) | 1589 (w) | 1261 (m) | 1566 (w) | 1093 (m) |
| Complex | Bond (Ǻ) DFT Data | Angle (°) DFT Data | ||||
|---|---|---|---|---|---|---|
| 1 | Cu1−N1 | 2.0974(15) | 2.130 | N1−Cu1−O1 | 79.03(5) | 79.60 |
| Cu1−O1 | 2.0991(13) | 2.080 | N1−Cu1−P1 | 108.50(5) | 106.06 | |
| Cu1−P1 | 2.2420(6) | 2.264 | N1−Cu1−P2 | 105.35(4) | 108.40 | |
| Cu1−P2 | 2.2574(5) | 2.258 | O1−Cu1−P1 | 116.69(4) | 117.35 | |
| O1−Cu1−P2 | 115.71(4) | 119.57 | ||||
| P1−Cu1−P2 | 121.40(2) | 117.02 | ||||
| 2 | Cu1−N1 | 2.0483(15) | 2.130 | N1−Cu1−O2 | 79.41(5) | 80.07 |
| Cu1−O2 | 2.1305(13) | 2.073 | N1−Cu1−P1 | 115.16(4) | 113.08 | |
| Cu1−P1 | 2.2261(5) | 2.253 | N1−Cu1−P2 | 109.92(4) | 102.65 | |
| Cu1−P2 | 2.2457(5) | 2.268 | O2−Cu1−P1 | 122.07(4) | 119.33 | |
| O2−Cu1−P2 | 110.98(4) | 119.27 | ||||
| P1−Cu1−P2 | 114.25(2) | 114.78 | ||||
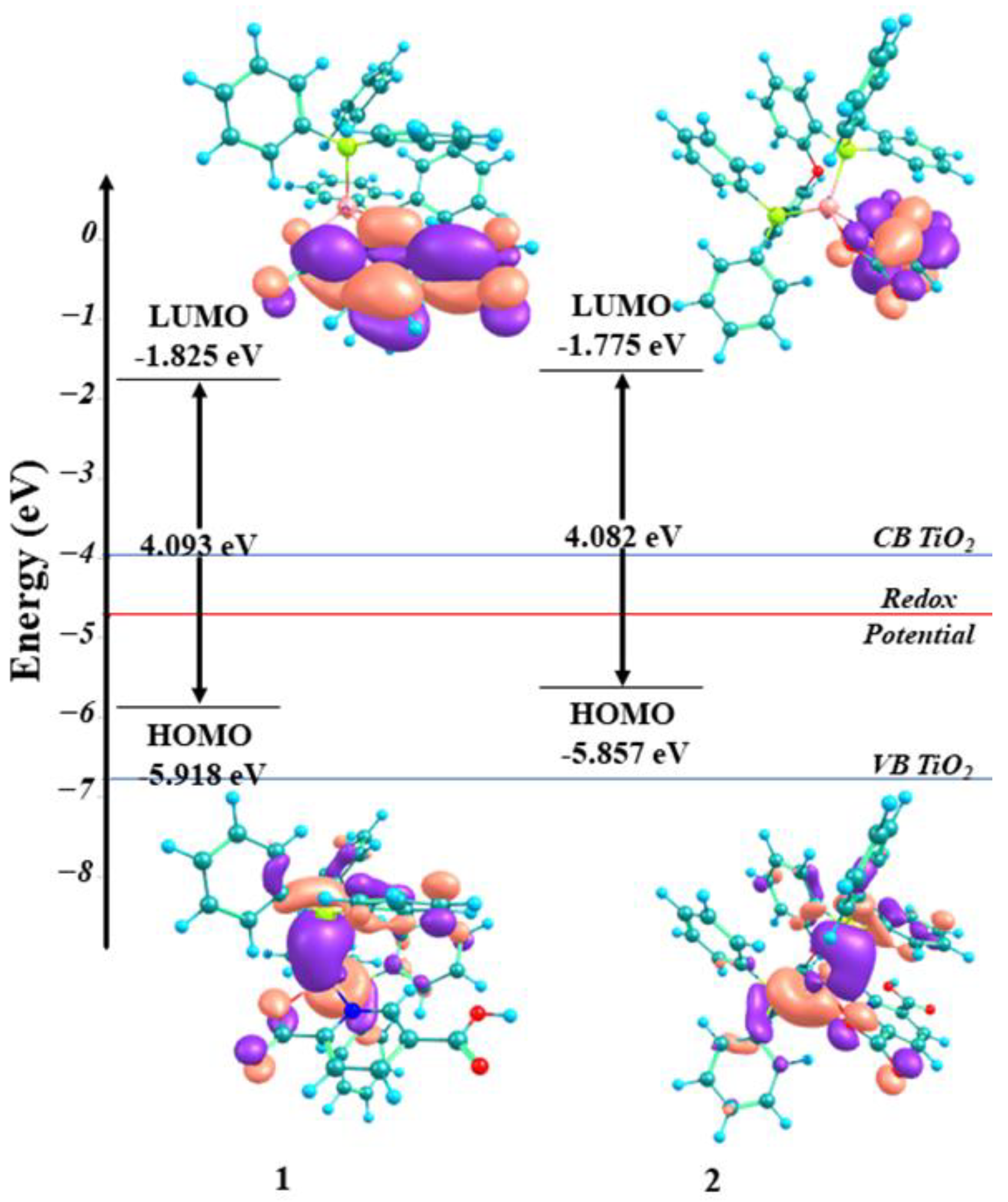
| Complex | (V) | (V) | (V) | HOMO (eV) | LUMO (eV) | a ΔE (eV) |
|---|---|---|---|---|---|---|
| 1 | −1.89 | 0.70 | 1.47 | −5.85 | −2.49 | 3.36 |
| 2 | −1.94 | 0.51 | 1.56 | −5.94 | −2.44 | 3.50 |
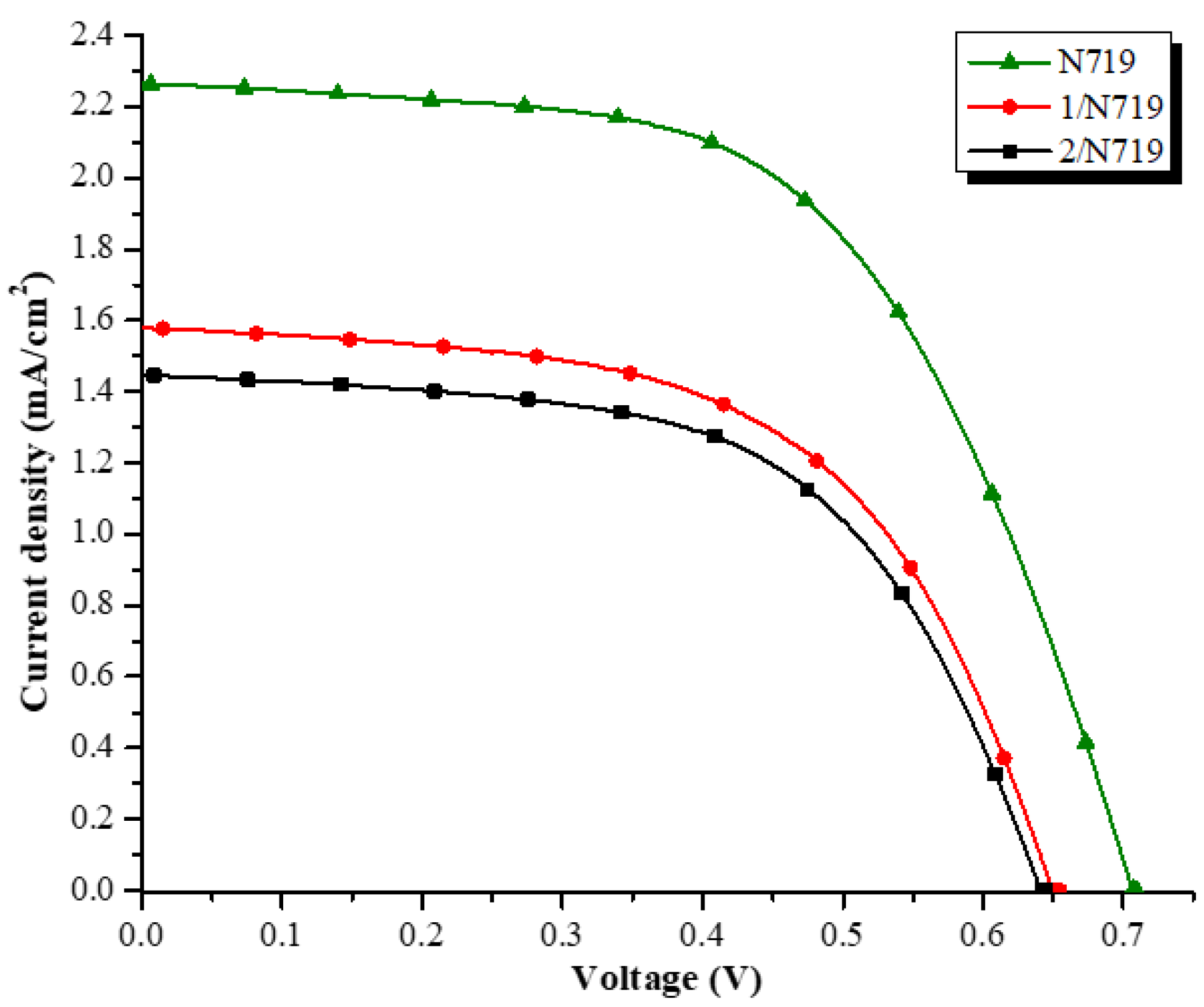
| Dyes | jsc (mA/cm2) | Voc (V) | ff (%) | ƞ (%) | ƞRelative (%) |
|---|---|---|---|---|---|
| [a]1/N719 (1:1) | 1.580 | 0.652 | 56.6 | 2.92 | 63.62 |
| [a]2/N719 (1:1) | 1.446 | 0.643 | 57.9 | 2.69 | 58.61 |
| N719 | 2.265 | 0.708 | 57.2 | 4.59 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto-Acosta, S.; Campos-Gaxiola, J.J.; Reynoso-Soto, E.A.; Cruz-Enríquez, A.; Baldenebro-López, J.; Höpfl, H.; García, J.J.; Flores-Álamo, M.; Miranda-Soto, V.; Glossman-Mitnik, D. Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC. Crystals 2022, 12, 1240. https://doi.org/10.3390/cryst12091240
Soto-Acosta S, Campos-Gaxiola JJ, Reynoso-Soto EA, Cruz-Enríquez A, Baldenebro-López J, Höpfl H, García JJ, Flores-Álamo M, Miranda-Soto V, Glossman-Mitnik D. Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC. Crystals. 2022; 12(9):1240. https://doi.org/10.3390/cryst12091240
Chicago/Turabian StyleSoto-Acosta, Samuel, José J. Campos-Gaxiola, Edgar A. Reynoso-Soto, Adriana Cruz-Enríquez, Jesús Baldenebro-López, Herbert Höpfl, Juventino J. García, Marcos Flores-Álamo, Valentín Miranda-Soto, and Daniel Glossman-Mitnik. 2022. "Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC" Crystals 12, no. 9: 1240. https://doi.org/10.3390/cryst12091240
APA StyleSoto-Acosta, S., Campos-Gaxiola, J. J., Reynoso-Soto, E. A., Cruz-Enríquez, A., Baldenebro-López, J., Höpfl, H., García, J. J., Flores-Álamo, M., Miranda-Soto, V., & Glossman-Mitnik, D. (2022). Synthesis, Crystal Structure, DFT Studies and Optical/Electrochemical Properties of Two Novel Heteroleptic Copper(I) Complexes and Application in DSSC. Crystals, 12(9), 1240. https://doi.org/10.3390/cryst12091240









