Impact of Mo-Doping on the Structural, Optical, and Electrocatalytic Degradation of ZnO Nanoparticles: Novel Approach
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of Mo-Doped ZnO Nanostructures
2.2. Devices and Measurements
3. Results and Discussion
3.1. X-ray Diffraction Studies
3.2. SEM Studies
3.3. Diffuse Reflectance Analysis of Mo-Doped ZnO Nanostructures
3.4. Raman Spectroscopy of Mo-Doped ZnO Nanostructures
3.5. FT-IR Spectroscopy of Mo-Doped ZnO Nanostructures
3.6. Dielectric Properties of Mo-Doped ZnO Nanostructures
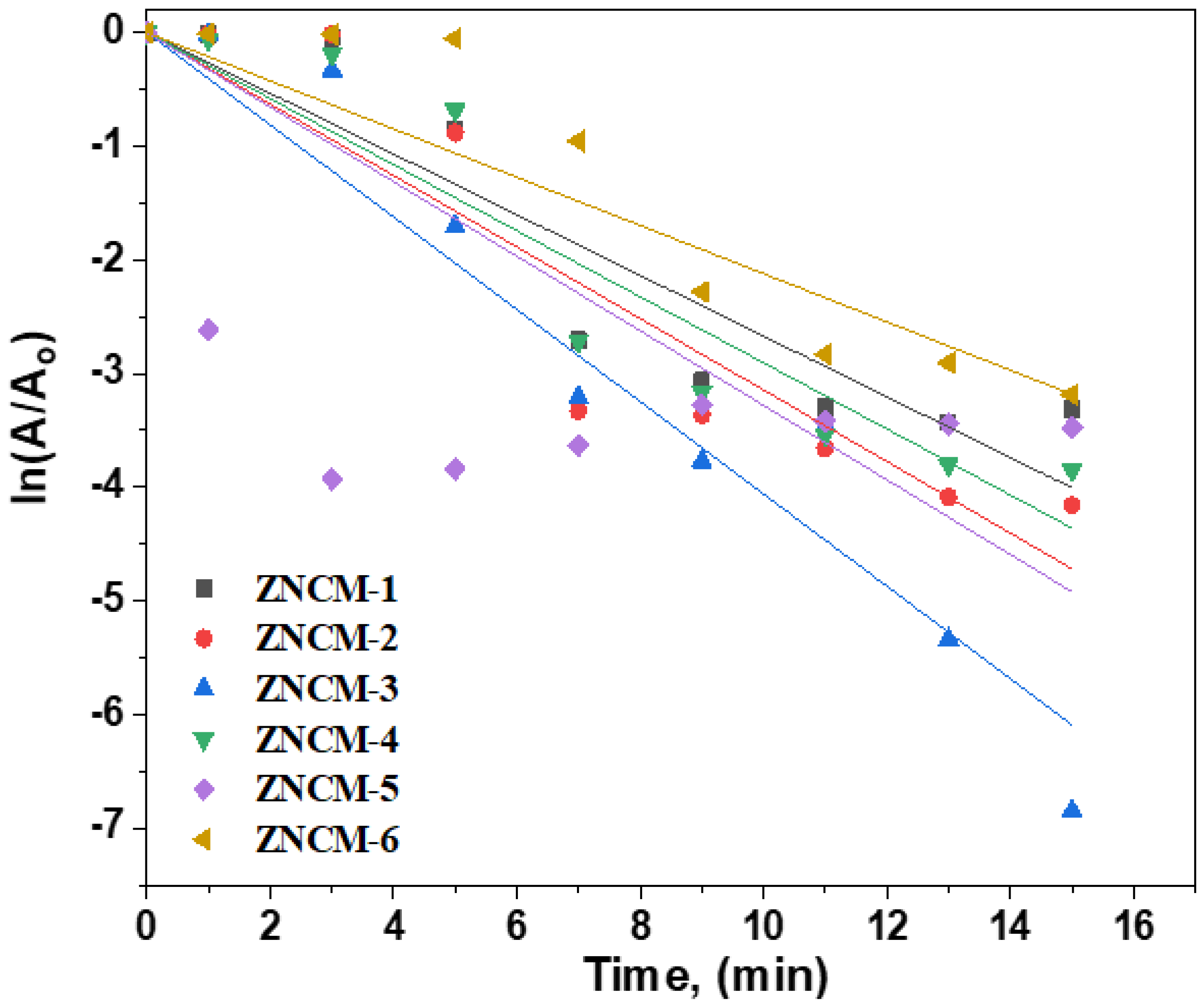
3.7. Kinetic Study of Electrocatalysis of RhB of Mo-Doped ZnO Nanostructures
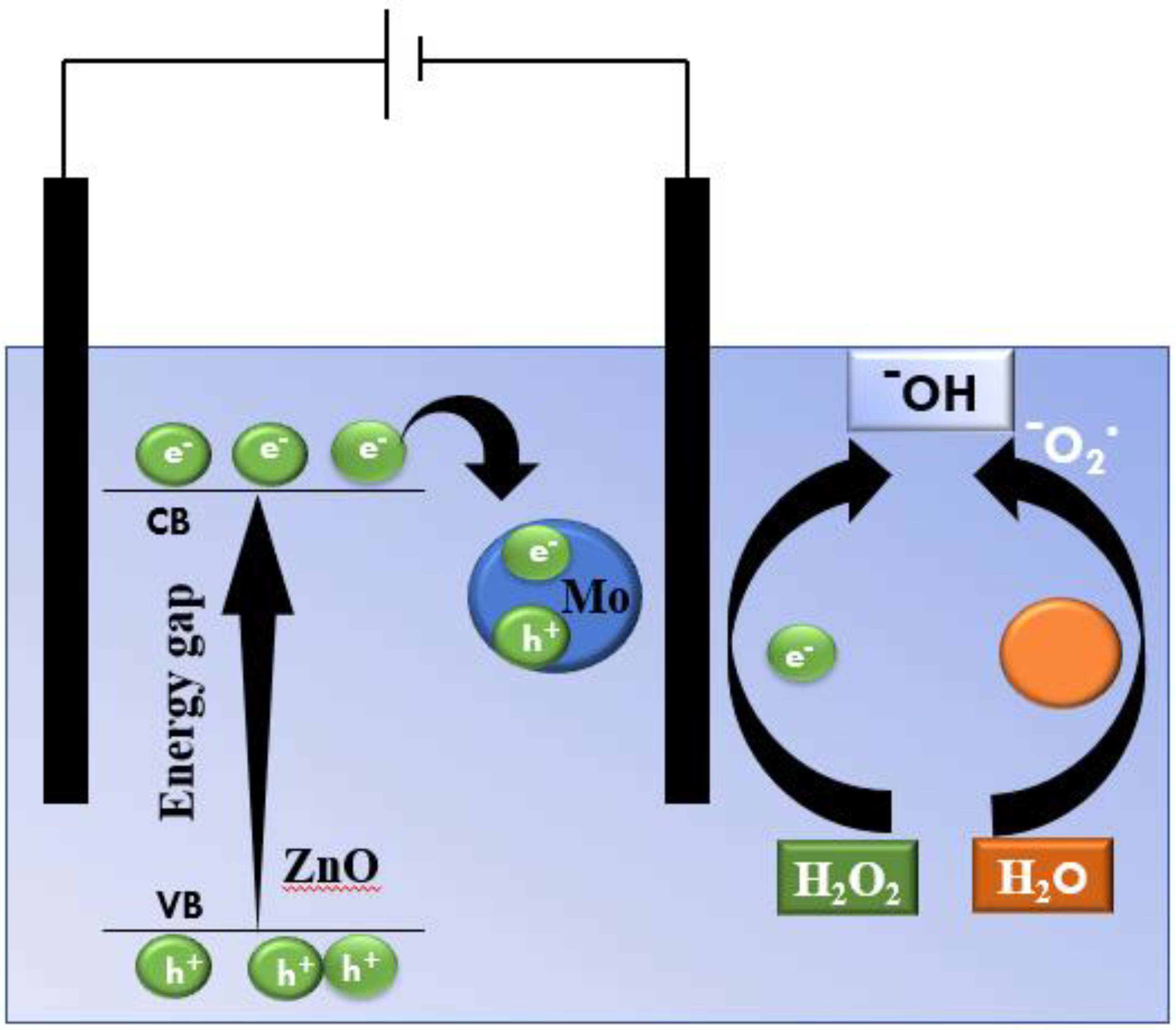
3.7.1. Mechanism of EC of RhB in the Presence of ZNCM
3.7.2. Comparison of PEC of RhB in the Presence of ZNCM with Previous Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravichandran, K.; Dhanraj, C.; Ibrahim, M.M.; Kavitha, P. Enhancing the electrocatalytic efficiency of ZnO thin films by adding Mo and rGO. Mater. Today Proc. 2022, 48, 234–244. [Google Scholar] [CrossRef]
- Saravanan, R.; Mansoob Khan, M.; Gupta, V.K.; Mosquera, E.; Gracia, F.; Narayanan, V.; Stephen, A. ZnO/Ag/CdO nanocomposite for visible light-induced electrocatalytic degradation of industrial textile effluents. J. Colloid Interface Sci. 2015, 452, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Thejaswini, T.V.L.; Prabhakaran, D.; Maheswari, M.A. Soft synthesis of potassium co-doped Al–ZnO nanocomposites: A comprehensive study on their visible light driven electrocatalytic activity on dye degradation. J. Mater. Sci. 2016, 51, 8187–8208. [Google Scholar] [CrossRef]
- Bizarro, M. High electrocatalytic activity of ZnO and ZnO: Al nanostructured films deposited by spray pyrolysis. Appl. Catal. B Environ. 2010, 97, 198–203. [Google Scholar] [CrossRef]
- Bizarro, M.; Martínez-Padilla, E. Visible light responsive electrocatalytic ZnO: Al films decorated with Ag nanoparticles. Thin Solid Films. 2014, 553, 179–183. [Google Scholar] [CrossRef]
- Luo, Y.; Wu, Y.; Huang, C.; Menon, C.; Feng, S.-P.; Chu1, P.K. Plasma modified and tailored defective electrocatalysts for water electrolysis and hydrogen fuel cells. EcoMat 2022, 4, e12197. [Google Scholar] [CrossRef]
- Shaik, U.P.; Kumar, P.A.; Krishna, M.G.; Rao, S.V. Morphological manipulation of the nonlinear optical response of ZnO thin films grown by thermal evaporation. Mater. Res. Express 2014, 1, 046201. [Google Scholar] [CrossRef]
- Yan, R.; Gargas, D.; Yang, P. Nanowire photonics. Nat. Photon. 2009, 3, 569. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.J.; Morkoç, H.J. A comprehensive review of ZnO materials and devices. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Panda, D.; Tseng, T.-Y. One-dimensional ZnO nanostructures: Fabrication, optoelectronic properties, and device applications. J. Mater. Sci. 2013, 48, 6849. [Google Scholar] [CrossRef]
- Dhara, S.; Giri, P.K. ZnO nanowire heterostructures: Intriguing photophysics and emerging applications. Rev. Nanosci. Nanotech. 2013, 2, 1. [Google Scholar] [CrossRef]
- Shaik, U.P.; Krishna, G.M. Single-step formation of indium and tin-doped ZnO nanowires by thermal oxidation of indium–zinc and tin–zinc metal films: Growth and optical properties. Ceram. Int. 2014, 40, 13611–13620. [Google Scholar] [CrossRef]
- Jose, A.; Devi, K.R.S.; Pinheiro, S.; Narayana, S.L. Electrochemical synthesis, photodegradation and antibacterial properties of PEG capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 187, 25–34. [Google Scholar] [CrossRef]
- Fan, L.; Song, H.; Li, T.; Yu, L.; Liu, Z.; Pan, G.; Lei, Y.; Bai, X.; Wang, T.; Zheng, Z.; et al. Hydrothermal synthesis and photoluminescent properties of ZnO nanorods. J. Lumin. 2007, 122, 819–821. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Boonpavanitchakul, K.; Kangwansupamonkon, W. Preparation of ZnO Nanostructures by Solvothermal Method. J. Microsc. Soc. 2009, 23, 75–78. [Google Scholar]
- Singhal, M.; Cbbabra, V.; Kang, P.; Shah, D.O. Pergamon PI1 SOO2s-5408(96)00175-4 synthesis of ZnO nanoparticles for varistor application using zn-substituted aerosol ot microemulsion. Mater. Res. Bull. 1997, 32, 239–247. [Google Scholar] [CrossRef]
- Rataboul, F.; Nayral, C.; Casanove, M.-J.; Maisonnat, A.; Chaudret, B. Synthesis and characterization of monodisperse zinc and zinc oxide nanoparticles from the organometallic. J. Organomet. Chem. 2002, 643, 307–312. [Google Scholar] [CrossRef]
- Moghaddam, A.B.; Nazari, T.; Badraghi, J.; Kazemzad, M. Synthesis of ZnO nanoparticles and electrodeposition of polypyrrole/ZnO nanocomposite film. Int. J. Electrochem. Sci. 2009, 4, 247–257. [Google Scholar]
- Hu, X.L.; Zhu, Y.J.; Wang, S.W. Sonochemical and microwave-assisted synthesis of linked single-crystalline ZnO rods. Mater. Chem. Phys. 2004, 88, 421–426. [Google Scholar] [CrossRef]
- Wu, J.J.; Wu, J.J.; Liu, S.C.; Liu, S.C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Synth. Met. 1999, 102, 1091. [Google Scholar] [CrossRef]
- Okuyama, K.; Lenggoro, W.W. Preparation of nanoparticles via spray route. Chem. Eng. Sci. 2003, 58, 537–547. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. Int. Sch. Res. Not. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Ananda, S.S. Synthesis and Characterization of Mo-Doped ZnO Nanoparticles by Electrochemical Method: Photodegradation Kinetics of Methyl Violet Dye and Study of Antibacterial Activities of Mo-Doped ZnO Nanoparticles. Int. J. Nanomater. Biostruct. 2015, 5, 7–14. [Google Scholar]
- Tachikawa, S.; Noguchi, A.; Tsuge, T.; Hara, M.; Odawara, O.; Wada, H. Optical properties of ZnO nanoparticles capped with polymers. Materials 2011, 4, 1132–1143. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rivera-Muñoz, E.M.; Diosa, J.E.; Mosquera, E.E.; Rodríguez-Páez, J.E. Nanoparticles of ZnO and Mg-doped ZnO: Synthesis, characterization and efficient removal of methyl orange (MO) from aqueous solution. Ceram. Int. 2021, 47, 15668–15681. [Google Scholar] [CrossRef]
- Ren, K.; Shu, H.; Huo, W.; Cui, Z.; Xu, Y. Tuning electronic, magnetic and catalytic behaviors of biphenylene network by atomic doping. Nanotechnology 2022, 33, 345701. [Google Scholar] [CrossRef]
- Mohammadi, R.; Alamgholiloo, H.; Gholipour, B.; Rostamnia, S.; Khaksar, S.; Farajzadeh, M.; Shokouhimehr, M. Visible-light-driven photocatalytic activity of ZnO/g-C3N4 heterojunction for the green synthesis of biologically interest small molecules of thiazolidinones. J. Photochem. Photobiol. A Chem. 2020, 402, 112786. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.; Assadi, M.H. MOF-derived nanocrystalline ZnO with controlled orientation and photocatalytic activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef]
- Sushma, C.; Girish Kumar, S. Advancements in the zinc oxide nanomaterials for efficient photocatalysis. Chem. Pap. 2017, 71, 2023–2042. [Google Scholar] [CrossRef]
- Jyothi, N.S.; Ravichandran, K. Optimum pH for effective dye degradation: Mo, Mn, Co and Cu doped ZnO photocatalysts in thin film form. Ceram. Int. 2020, 46, 23289–23292. [Google Scholar] [CrossRef]
- Xiu, X.; Pang, Z.; Lv, M.; Dai, Y.; Ye, L.; Han, S. Transparent conducting molybdenum-doped zinc oxide films deposited by RF magnetron sputtering. Appl. Surf. Sci. 2007, 253, 3345–3348. [Google Scholar] [CrossRef]
- Zhang, D.H.; Yang, T.L.; Ma, J.; Wang, Q.P.; Gao, R.W.; Ma, H.L. Preparation of transparent conducting ZnO: Al films on polymer substrates by rf magnetron sputtering. Appl. Surf. Sci. 2000, 158, 43. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, X.L.; Chen, H.X.; Shen, J.; Jiang, Y.M.; Zhang, Z.J.; Hua, Z.Y. A new transparent conductive thin film In2O3: Mo. Thin Solid Film. 2001, 394, 219. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffraction; Addison Wesley: Reading, MA, USA, 1956. [Google Scholar]
- Umar, K.; Aris, A.; Parveen, T.; Jaafar, J.; Majid, Z.A.; Reddy, A.V.; Talib, J. Synthesis, characterization of Mo and Mn doped ZnO and their photocatalytic activity for the decolorization of two different chromophoric dyes. Appl. Catal. A Gen. 2015, 505, 507–514. [Google Scholar] [CrossRef]
- Prashanth, G.K.; Prashanth, P.A.; Bora, U.; Gadewar, M.; Nagabhushana, B.M.; Ananda, S.; Krishnaiah, G.M.; Sathyananda, H.M. In vitro antibacterial and cytotoxicity studies of ZnO nanopowders prepared by combustion assisted facile green synthesis. Karbala Int. J. Mod. Sci. 2015, 1, 67–77. [Google Scholar]
- Nehru, L.C.; Swaminathan, V.; Sanjeeviraja, C. Rapid synthesis of nanocrystalline ZnO by a microwave-assisted combustion method. Powder Technol. 2012, 226, 29–33. [Google Scholar] [CrossRef]
- Naik, E.I.; Naik, H.S.B.; Swamy, B.E.K.; Viswanath, R.; Suresh Gowda, I.K.; Prabhakara, M.C.; Chetankumar, K. Influence of Cu doping on ZnO nanoparticles for improved structural, optical, electrochemical properties and their applications in efficient detection of latent fingerprints. Chem. Data Collect. 2021, 33, 100671. [Google Scholar] [CrossRef]
- Swapna, R.; Santhosh Kumar, M.C. Growth and characterization of molybdenum doped ZnO thin films by spray pyrolysis. J. Phys. Chem. Solids 2013, 74, 418–425. [Google Scholar] [CrossRef]
- Kaleemulla, S.; Rao, N.M.; Joshi, M.G.; Reddy, A.S.; Uthanna, S.; Reddy, P.S. Electrical and optical properties of In2O3: Mo thin films prepared at various Mo-doping levels. J. Alloys. Compd. 2010, 504, 351. [Google Scholar] [CrossRef]
- Pascariu, P.; Homocianu, M.; Olaru, N.; Airinei, A.; Ionescu, O. New Electrospun ZnO:MoO3 Nanostructures: Preparation, Characterization and Electrocatalytic Performance. Nanomaterials 2020, 10, 1476. [Google Scholar] [CrossRef]
- Rao, T.P.; Santhoshkumar, M.C.; Safarulla, A.; Ganesan, V.; Barman, S.R.; Sanjeeviraja, C. Physical properties of ZnO thin films deposited at various substrate temperatures using spray pyrolysis. Phys. B Condensed Matter 2010, 405, 2226. [Google Scholar]
- Aydın, C.; Abd El-Sadek, M.S.; Zheng, K.; Yahia, I.S.; Yakuphanoglu, F. Synthesis, diffused reflectance and electrical properties of nanocrystalline Fe-doped ZnO via sol-gel calcination technique. Opt. Laser Technol. 2013, 48, 447–452. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Orooji, Y. Sonocatalytic activity of biochar-supported ZnO nanorods in degradation of gemifloxacin: Synergy study, effect of parameters and phytotoxicity evaluation. Ultrason. Sonochem. 2019, 55, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, M.; Arora, A.K.; Bendre, B.S.; Mahamuni, S.J. Optical Phonon Confinement in Zinc oxide nanoparticles. Appl. Phys. 2000, 87, 2445. [Google Scholar] [CrossRef]
- Pavithra, N.S.; Lingaraju, K.; Raghu, G.K.; Nagaraju, G. Citrus maxima (Pomelo) juice mediated eco-friendly synthesis of ZnO nanoparticles: Applications to electrocatalytic, electrochemical sensor and antibacterial activities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 185, 11–19. [Google Scholar] [CrossRef]
- Sánchez Zeferino, R.; Barboza Flores, M.; Pal, U. Photoluminescence and Raman Scattering in Ag-doped ZnO Nanoparticles. J. Appl. Phys. 2011, 109, 014308. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, B.P.; Dhar, S.; Gondorf, A.; Spasova, M. Effect of surface groups on the luminescence property of ZnO nanoparticles synthesized by sol-gel route. Surf. Sci. 2012, 606, L13–L17. [Google Scholar] [CrossRef]
- Muravitskaya, A.; Rumyantseva, A.; Kostcheev, S.; Dzhagan, V.; Stroyuk, O.; Adam, P.-M. Enhanced Raman scattering of ZnO nanocrystals in the vicinity of gold and silver nanostructured surfaces. Opt. Express 2016, 24, A168–A173. [Google Scholar] [CrossRef]
- Yu, C.; Yang, K.; Shu, Q.; Yu, J.C.; Cao, F.; Li, X.; Zhou, X. Preparation, characterization, and electrocatalytic performance of Mo-doped ZnO photocatalysts. Sci. China Chem. 2012, 55, 9. [Google Scholar] [CrossRef]
- Chen, Y.P.; Lu, C.; Xu, L.; Ma, Y.; Hou, W.H.; Zhu, J.J. Single-crystallineorthorhombic molybdenum oxide nanobelts: Synthesis and electrocatalytic properties. CrystEngComm 2010, 12, 3740–3747. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.G.; User, K.B.; Williams, K.B. Photoluminescence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi 2006, 3, 3577. [Google Scholar] [CrossRef]
- Hufschmidt, D.; Liu, L.; Seizer, V.; Bahnemann, D. Electrocatalytic water treatment: Fundamental knowledge required for its practical application. Water Sci. Technol. 2004, 49, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Anbazhagan, A.; Dineshbabu, N.; Ravidhas, C. Influence of Mo doping on transparent conducting properties of ZnO films prepared by a simplified spray technique. J. Mater. Sci. Mater. Electron. 2015, 26, 7649–7654. [Google Scholar] [CrossRef]
- Hussien, M.S.A. Facile Synthesis of Nanostructured Mn-doped Ag3PO4 for Visible Photodegradation of Emerging Pharmaceutical Contaminants: Streptomycin Photodegradation. J. Inorg. Organomet. Polym. Mater. 2021, 31, 945–959. [Google Scholar] [CrossRef]
- AnjuChanu, L.; Joychandra Singh, W.; Jugeshwar Singh, K.; Nomita Devi, K. Effect of operational parameters on the electrocatalytic degradation of Methylene blue dye solution using Manganese doped ZnO nanoparticles. Results Phys. 2019, 12, 1230–1237. [Google Scholar] [CrossRef]
- Shkir, M.; Yahia, I.S.; Al-Qahtani, A.M.A. Bulk monocrystal growth, optical, dielectric, third-order nonlinear, thermal, and mechanical studies on HCl added l-alanine: An organic NLO material. Mater. Chem. Phys. 2016, 184, 12–22. [Google Scholar] [CrossRef]
- Jeseentharani, V.; Dayalan, A.; Nagaraja, K.S. Co-precipitation synthesis, humidity sensing and photoluminescence properties of nanocrystalline Co2+ substituted zinc (II) molybdate (Zn1−x Cox MoO4; x = 0, 0.3, 0.5, 0.7, 1). Solid-State Sci. 2017, 67, 46–58. [Google Scholar] [CrossRef]
- Srinivas, K.; Venugopal Reddy, P. Synthesis, Structural, and Magnetic Properties of Nanocrystalline Ti 0.95Co0.05O2-Diluted Magnetic Semiconductors. J. Supercond. Nov. Magn. 2014, 27, 2521–2538. [Google Scholar] [CrossRef]
- Shejwal, N.N.; Anis, M.; Hussaini, S.S.; Shirsat, M.D. Investigation on structural, UV- visible, SHG efficiency, dielectric, mechanical and thermal behavior of L-cystine doped zinc thiourea sulphate crystal for NLO device applications. Int. J. Mod. Phys. B 2016, 30, 1650159. [Google Scholar] [CrossRef]
- Štengl, V.; Bakardjieva, S. Molybdenum-Doped Anatase and Its Extraordinary Electrocatalytic Activity in the Degradation of Orange II in the UV and vis Regions. J. Phys. Chem. C 2010, 1145, 19308–19317. [Google Scholar] [CrossRef]
- Al Farsi, B.; Souier, T.M.; Al Marzouqi, F.; Al Maashani, M.; Bououdina, M.; Widatallah, H.M.; Al-Abri, M. Structural and optical properties of visible active electrocatalytic Al-doped ZnO nanostructured thin films prepared by dip coating. Opt. Mater. 2021, 113, 110868. [Google Scholar] [CrossRef]
- Sagadevan, S.; Vennila, S.; Lett, J.A.; Marlinda, A.R.; Hamizi, N.A.B.; Johan, M.R. Tailoring the structural, morphological, optical, thermal, and dielectric characteristics of ZnO nanoparticles using starch as a capping agent. Results Phys. 2019, 15, 102543. [Google Scholar] [CrossRef]
- Putri, N.A.; Fauzia, V.; Iwan, S.; Roza, L.; Ali, A.; Umar, A.A.; Budi, S. Mn-doping-induced electrocatalytic activity enhancement of ZnO nanorods prepared on glass substrates. Appl. Surf. Sci. 2018, 439, 285–297. [Google Scholar] [CrossRef]
- Labchir, N.; Amaterz, E.; Hannover, A.; Ait hssi, A.; Vincent, D.; Ihlal, A.; Sajieddine, M. Highly efficient nanostructured CoFe2O4 thin-film electrodes for electrochemical degradation of rhodamine B. Water Environ. Res. 2020, 92, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Baddour, A.; Bessegato, G.G.; Rguiti, M.M.; El Ibrahimi, B.; Bazzi, L.; Hilali, M.; Zanoni, M.V.B. Electrochemical decolorization of Rhodamine B dye: Influence of anode material, chloride concentration and current density. J. Environ. Chem. Eng. 2018, 6, 2041–2047. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhu, Y. Synergetic degradation of rhodamine B at a porous ZnWO4 film electrode by combined electro-oxidation and photocatalysis. Environ. Sci. Technol. 2006, 40, 3367–3372. [Google Scholar]
- Nareejun, W.; Poncho, W. Novel electrocatalytic/solar cell improvement for organic dye degradation based on simple dip-coating WO3/BiVO4 photoanode electrode. Sol. Energy Mater. Sol. Cells 2020, 212, 110556. [Google Scholar] [CrossRef]
- Pedanekar, R.S.; Madake, S.B.; Narewadikar, N.A.; Mohite, S.V.; Patil, A.R.; Kumbhar, S.M.; Rajpure, K.Y. Electrocatalytic degradation of Rhodamine B by spray deposited Bi2WO6 photoelectrode under solar radiation. Mater. Res. Bull. 2022, 147, 111639. [Google Scholar] [CrossRef]
- Chennai, A.; Naciri, Y.; Taoufyq, A.; Bakiz, B.; Bazzi, L.; Guinneton, F.; Villain, S.; Gavarri, J.R.; Benlhachemi, A. Electrodeposited zinc phosphate hydrate electrodes for electrocatalytic applications. J. Appl. Electrochem. 2019, 42, 163–177. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, N.; Luo, M.; Fan, L.; Zhao, K.; Qu, J.; Guan, J.; Yuan, X. Enhancement mechanism of fiddlehead-shaped TiO2-BiVO4 type II heterojunction in SPEC towards RhB degradation and detoxification. Appl. Surf. Sci. 2019, 463, 234–243. [Google Scholar] [CrossRef]
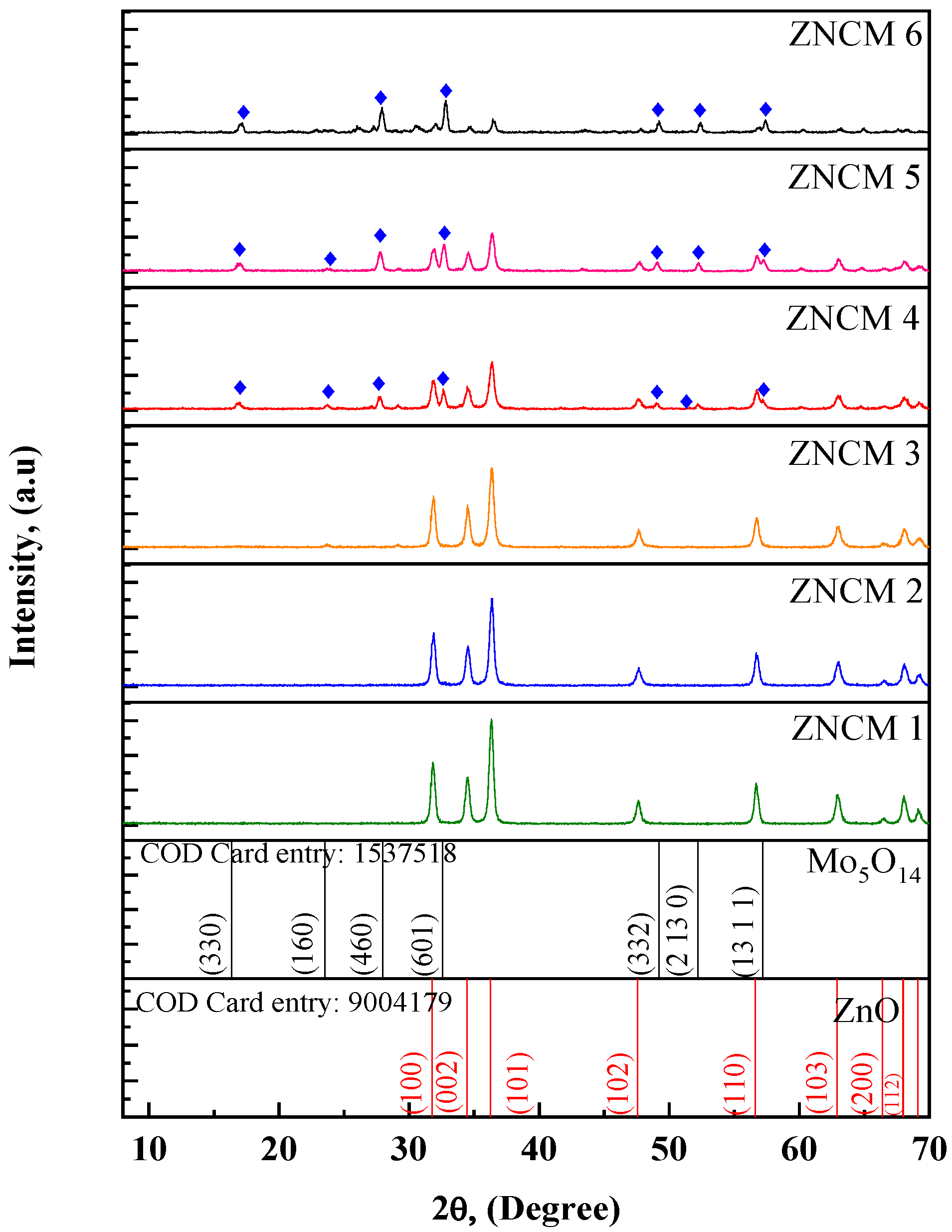

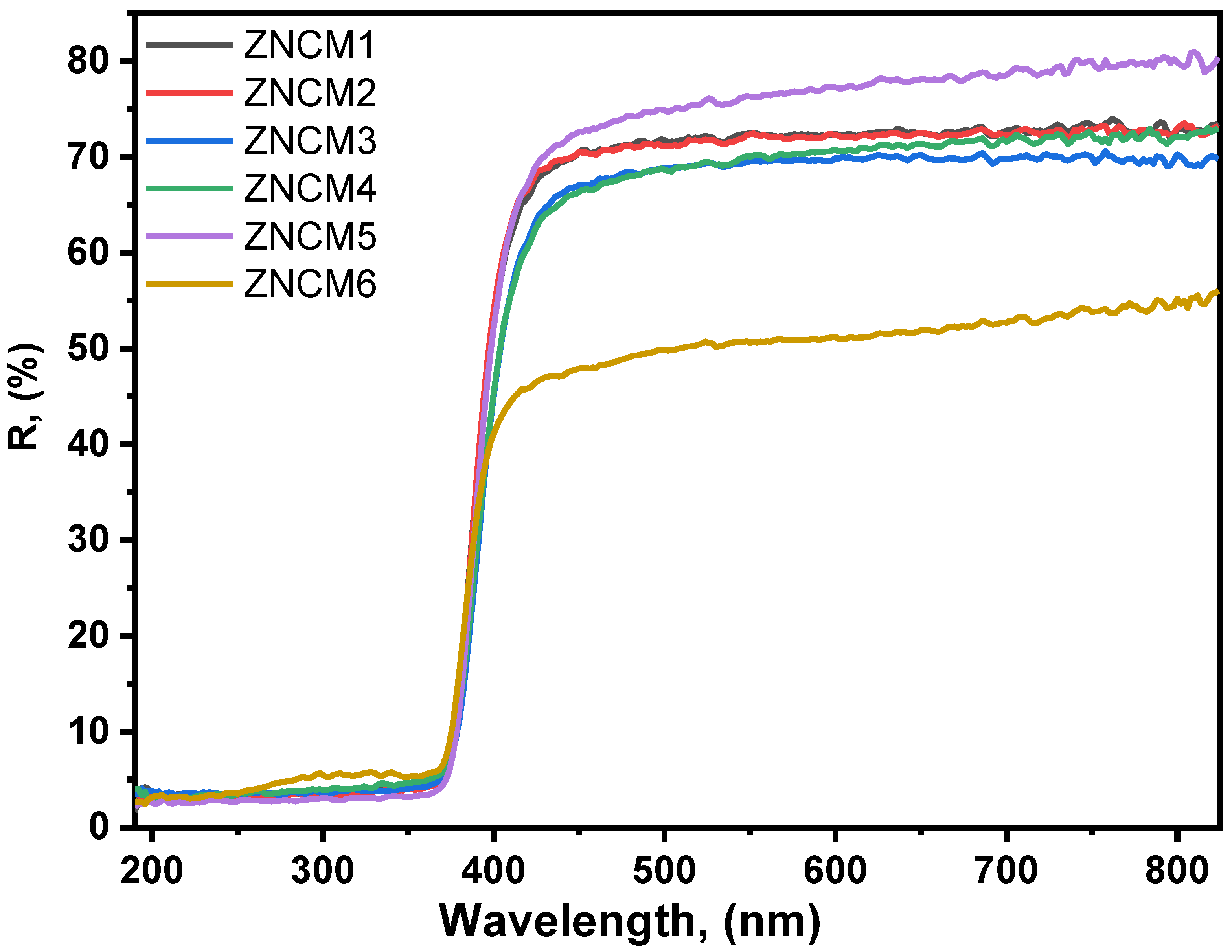

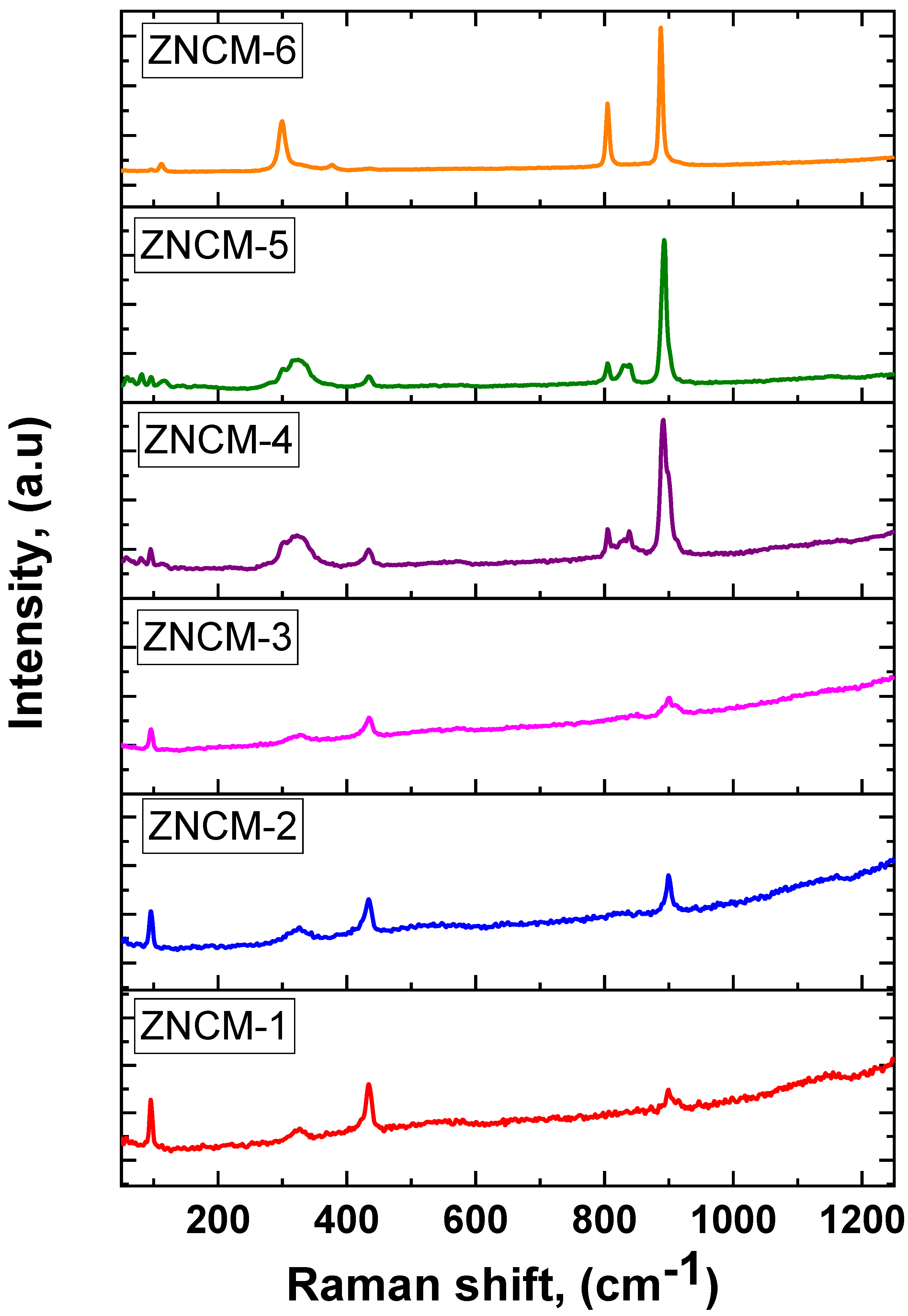
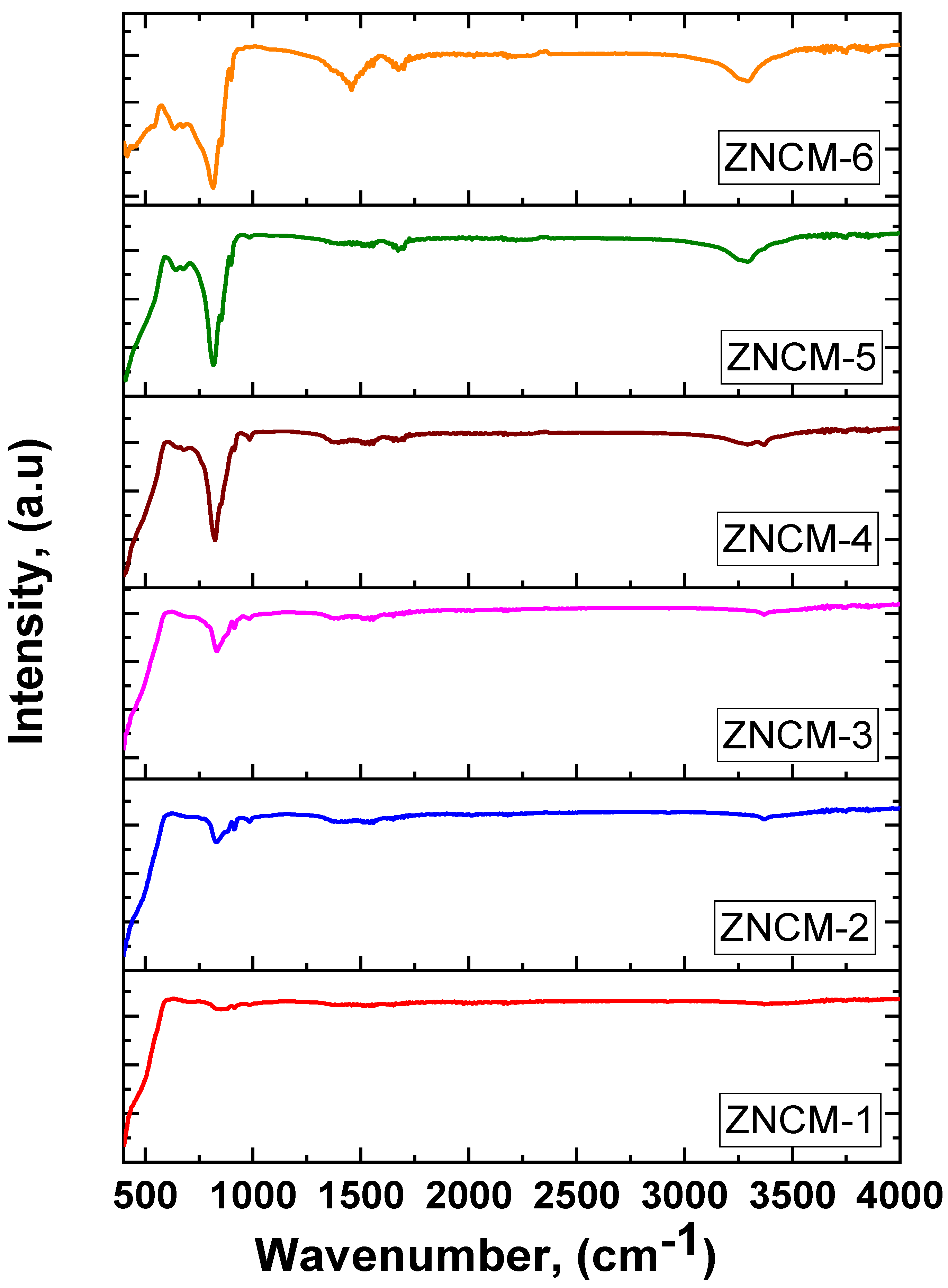



| Sample | 2θ | FWHM | d-Spacing | a, (nm) | c, (nm) | D, (nm) | |||
|---|---|---|---|---|---|---|---|---|---|
| ZNCM-1 (0 wt% Mo) | ZnO | 36.316 (101) | 0.3738 | 2.47177 | 3.2445 | 5.1979 | 23.37 | 5 | 1.831 |
| ZNCM-2 (0.001 wt% Mo) | ZnO | 36.338 (101) | 0.3789 | 2.47035 | 3.2425 | 5.1956 | 23.06 | 5 | 1.881 |
| ZNCM-3 (0.01 wt% Mo) | ZnO | 36.262 (101) | 0.4800 | 2.47531 | 3.2494 | 5.2038 | 18.2 | 6.4 | 3.019 |
| ZNCM-4 (0.1 wt% Mo) | ZnO | 36.303 (101) | 0.4800 | 2.47263 | 3.24523 | 5.2017 | 18.2 | 6.4 | 3.019 |
| Mo5O14 | 32.484 (601) | 0.5200 | 2.75408 | 23.0115 | 3.9571 | 16.63 | 7.8 | 3.616 | |
| ZNCM-5 (0.5 wt% Mo) | ZnO | 31.773 (100) | 0.4399 | 2.81406 | 3.2494 | 5.2038 | 19.62 | 6.7 | 2.597 |
| Mo5O14 | 32.570 (601) | 0.4144 | 2.7470 | 22.8809 | 3.9334 | 20.87 | 6.2 | 2.296 | |
| ZNCM-6 (1.0 wt% Mo) | ZnO | 31.945 (100) | 0.5836 | 2.79931 | 3.2323 | 5.1795 | 14.8 | 8.9 | 4.565 |
| Mo5O14 | 32.708 (601) | 0.3600 | 2.7357 | 23.0176 | 3.9108 | 24.03 | 5.4 | 1.732 |
| Samples | Synthesis Method | Dye Concentration | K, (min−1) | % Removal | Refs. |
|---|---|---|---|---|---|
| ZNCM-1 (0 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.2671 | 97% after 15 min | Present work |
| ZNCM-2 (0.001 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.29064 | 98% after 15 min | |
| ZNCM-3 (0.01 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.40605 | 100 % after 7 min | |
| ZNCM-4 (0.1 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.31455 | 98 % after 15 min | |
| ZNCM-5 (0.5 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.32801 | 97% after 15 min | |
| ZNCM-6 (1.0 wt% Mo) | Combustion method | Rhodamine B (50 mgL−1) | 0.21218 | 96% after 15 min | |
| CoFe2O4 | Electrodeposition | Rhodamine B (10 mgL−1) | 99% after 3 min | [65] | |
| Ti/RuO2-IrO2 | DSA (De Nora Company) | Rhodamine B | 100% after 90 min | [66] | |
| ZnWO4 | Dip-coating | Rhodamine B (5 mgL−1) | 0.0023 | after 60 min | [67] |
| Ca10(PO4)6(OH)2 | Electrodeposition | Rhodamine B (5 mgL−1) | 0.0399 | 98 % after 105 min | [68] |
| Zn3(PO4)2·4H2O | Electrodeposition | Rhodamine B (30 mgL−1) | 90 % after 10 min | [69] | |
| TiO2-NTs/Ce-PbO2 | Electrodeposition | Rhodamine B (30 mgL−1) | 90 % after 10 min | [70] | |
| BiVO4/TiO2 | Sol-gel and | Rhodamine B (10 mgL−1) | 93.9 % after 5 h | [71] | |
| hydrothermal |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ganesh, V.; Hussien, M.S.A.; Shaik, U.P.; Ade, R.; Mohammed, M.I.; AlAbdulaal, T.H.; Zahran, H.Y.; Yahia, I.S.; Abdel-wahab, M.S. Impact of Mo-Doping on the Structural, Optical, and Electrocatalytic Degradation of ZnO Nanoparticles: Novel Approach. Crystals 2022, 12, 1239. https://doi.org/10.3390/cryst12091239
Ganesh V, Hussien MSA, Shaik UP, Ade R, Mohammed MI, AlAbdulaal TH, Zahran HY, Yahia IS, Abdel-wahab MS. Impact of Mo-Doping on the Structural, Optical, and Electrocatalytic Degradation of ZnO Nanoparticles: Novel Approach. Crystals. 2022; 12(9):1239. https://doi.org/10.3390/cryst12091239
Chicago/Turabian StyleGanesh, Vanga, Mai S. A. Hussien, Ummar Pasha Shaik, Ramesh Ade, Mervat I. Mohammed, Thekrayat H. AlAbdulaal, Heba Y. Zahran, Ibrahim S. Yahia, and Mohamed Sh. Abdel-wahab. 2022. "Impact of Mo-Doping on the Structural, Optical, and Electrocatalytic Degradation of ZnO Nanoparticles: Novel Approach" Crystals 12, no. 9: 1239. https://doi.org/10.3390/cryst12091239
APA StyleGanesh, V., Hussien, M. S. A., Shaik, U. P., Ade, R., Mohammed, M. I., AlAbdulaal, T. H., Zahran, H. Y., Yahia, I. S., & Abdel-wahab, M. S. (2022). Impact of Mo-Doping on the Structural, Optical, and Electrocatalytic Degradation of ZnO Nanoparticles: Novel Approach. Crystals, 12(9), 1239. https://doi.org/10.3390/cryst12091239







