To Be or Not to Be a (4,4) Net: Reactions of 4′-{4-(N,N-Diethylaminophenyl)}- and 4′-{4-(N,N-Diphenylaminophenyl)}-3,2′:6′,3″- and 4,2′:6′,4″-Terpyridines with Cobalt(II) Thiocyanate
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Compound 1
2.3. Compound 2
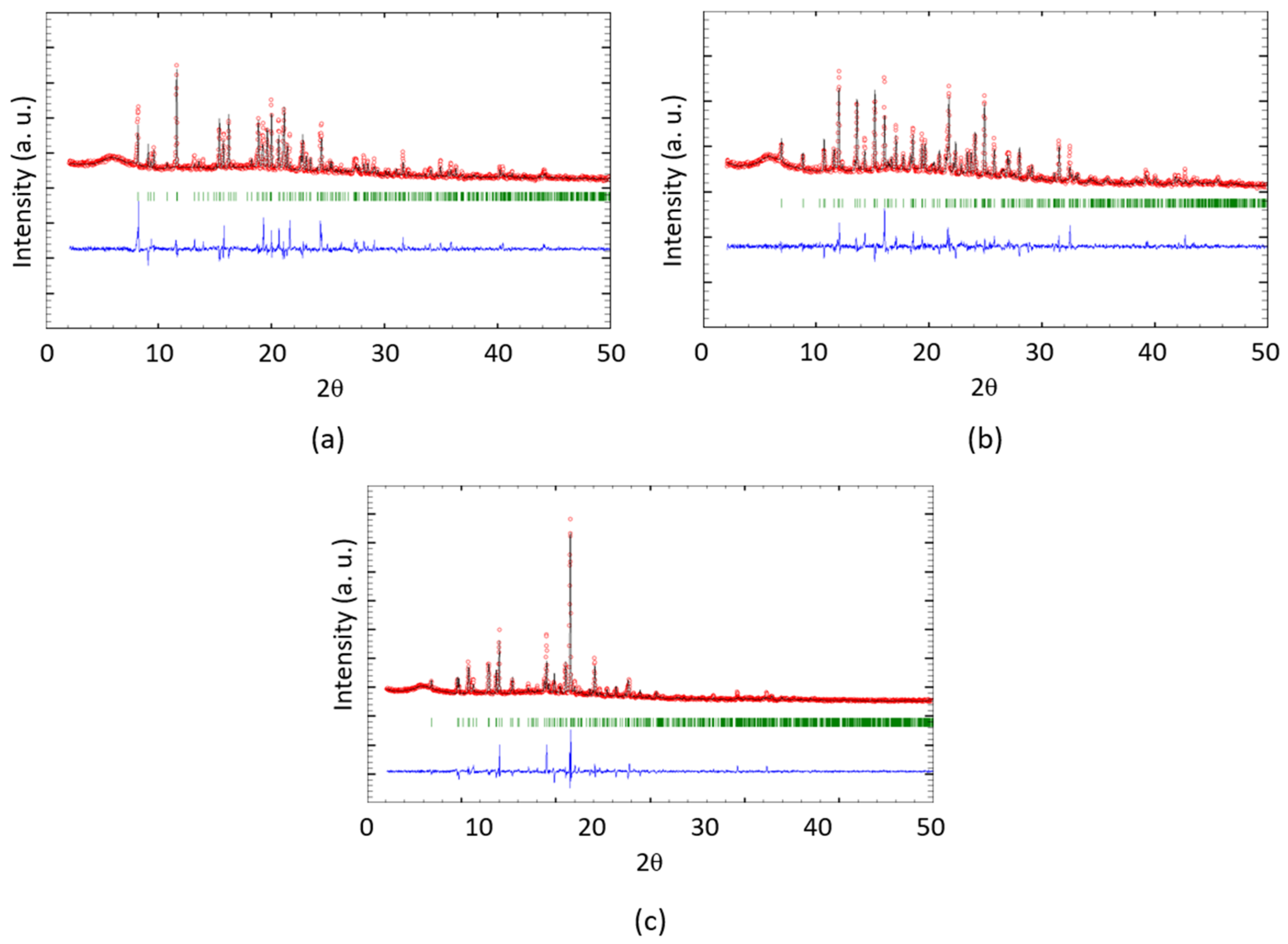
2.4. Crystallography
2.5. Crystal Growth of the Coordination Complexes
2.6. Compound 2
2.7. [Co(NCS)2(1)]n·0.8nCHCl3
2.8. [Co(NCS)2(2)2(MeOH)2]·3CHCl3
2.9. [Co(NCS)2(3)]n·2nCHCl3
2.10. [Co(NCS)2(4)]n
3. Results and Discussion
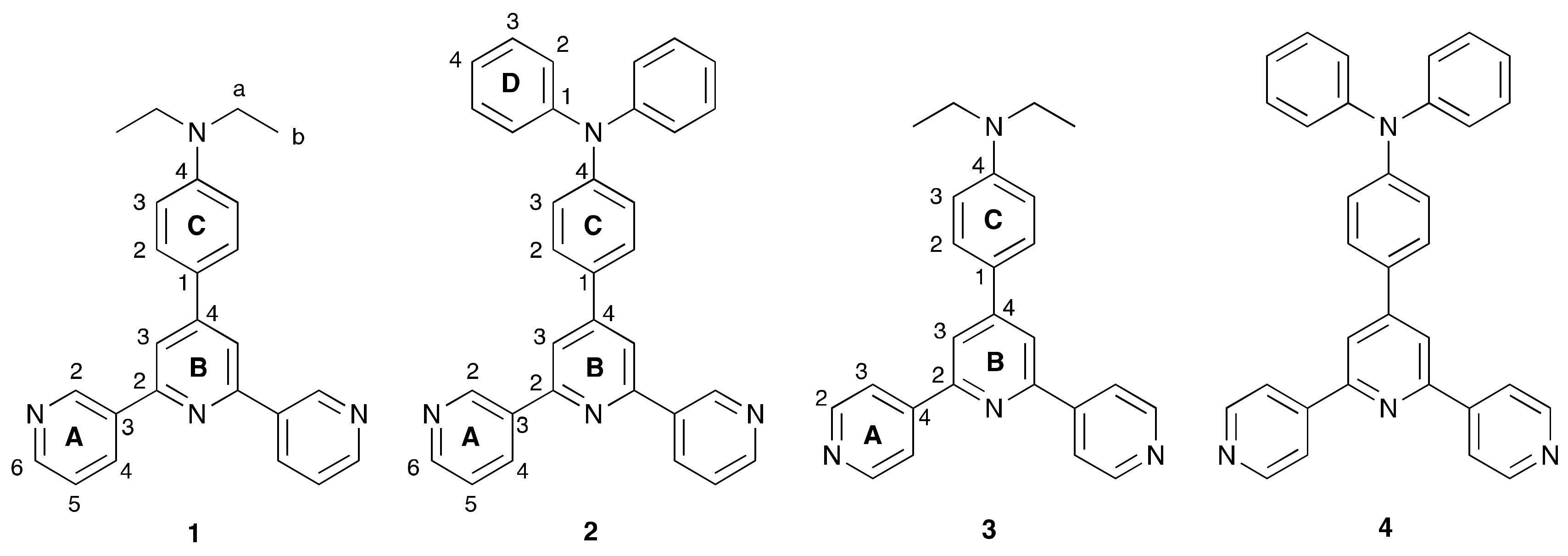
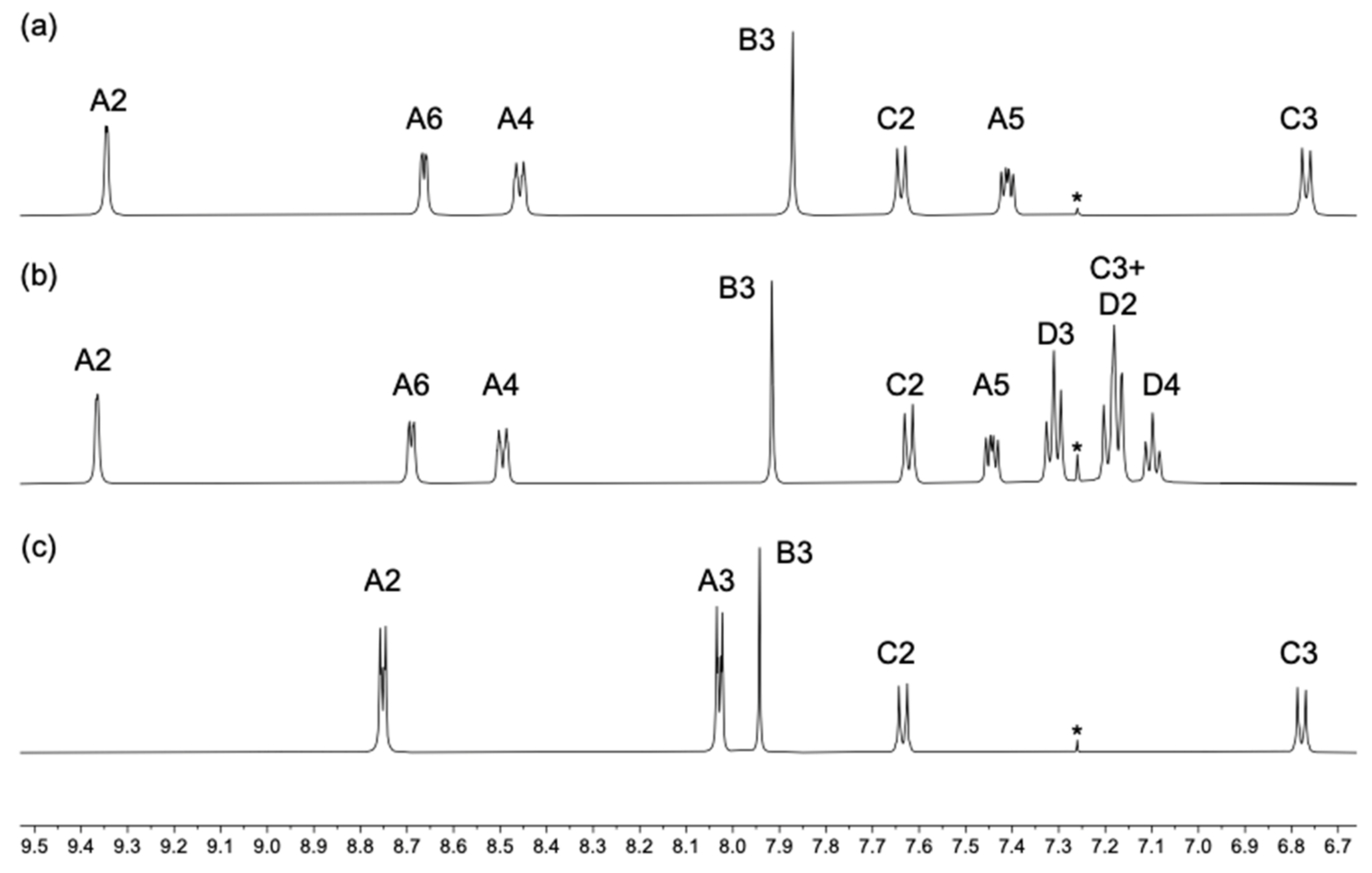
3.1. Ligand Synthesis and Characterization
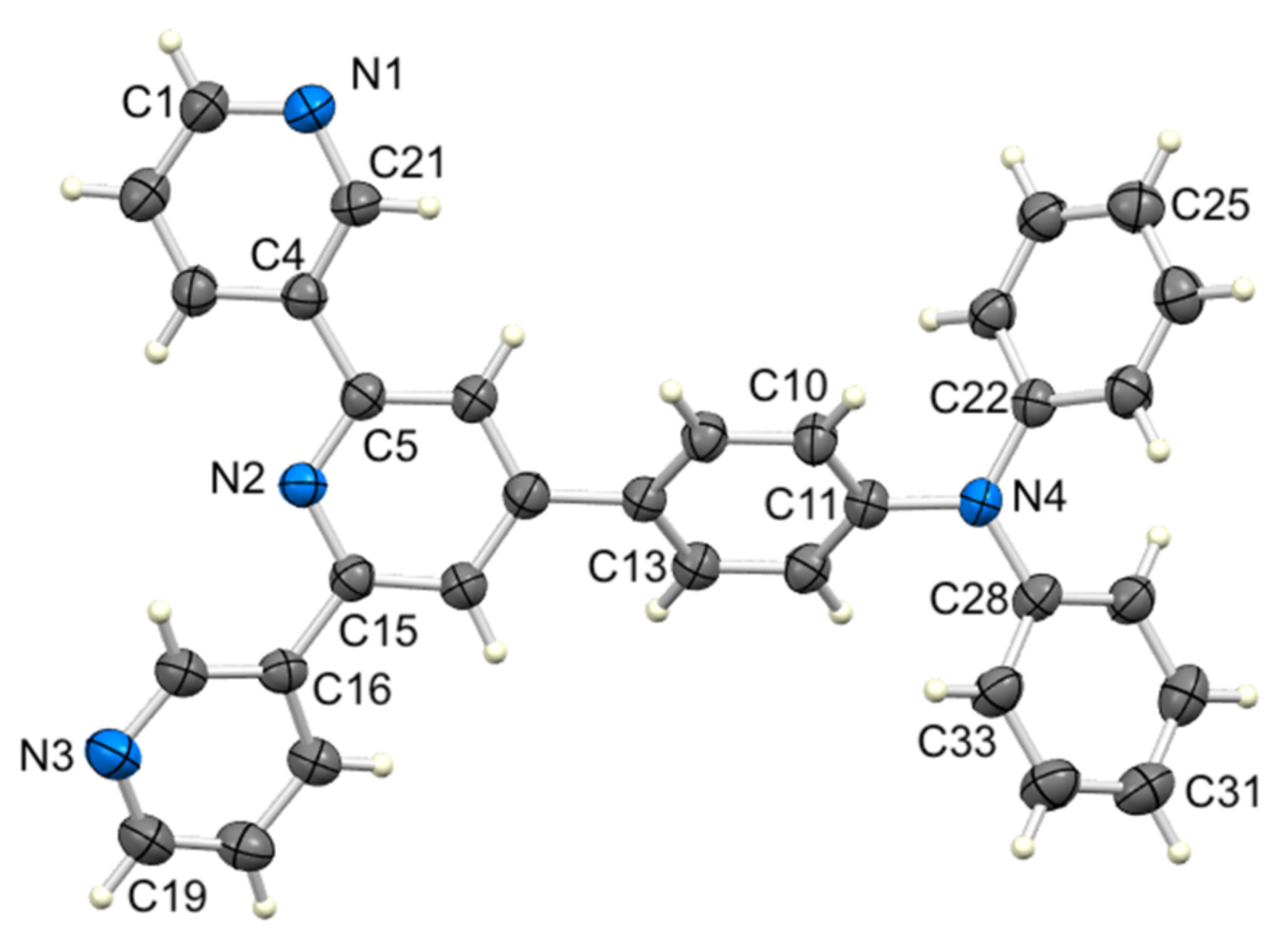
3.2. Single Crystal Structure of Compound 2
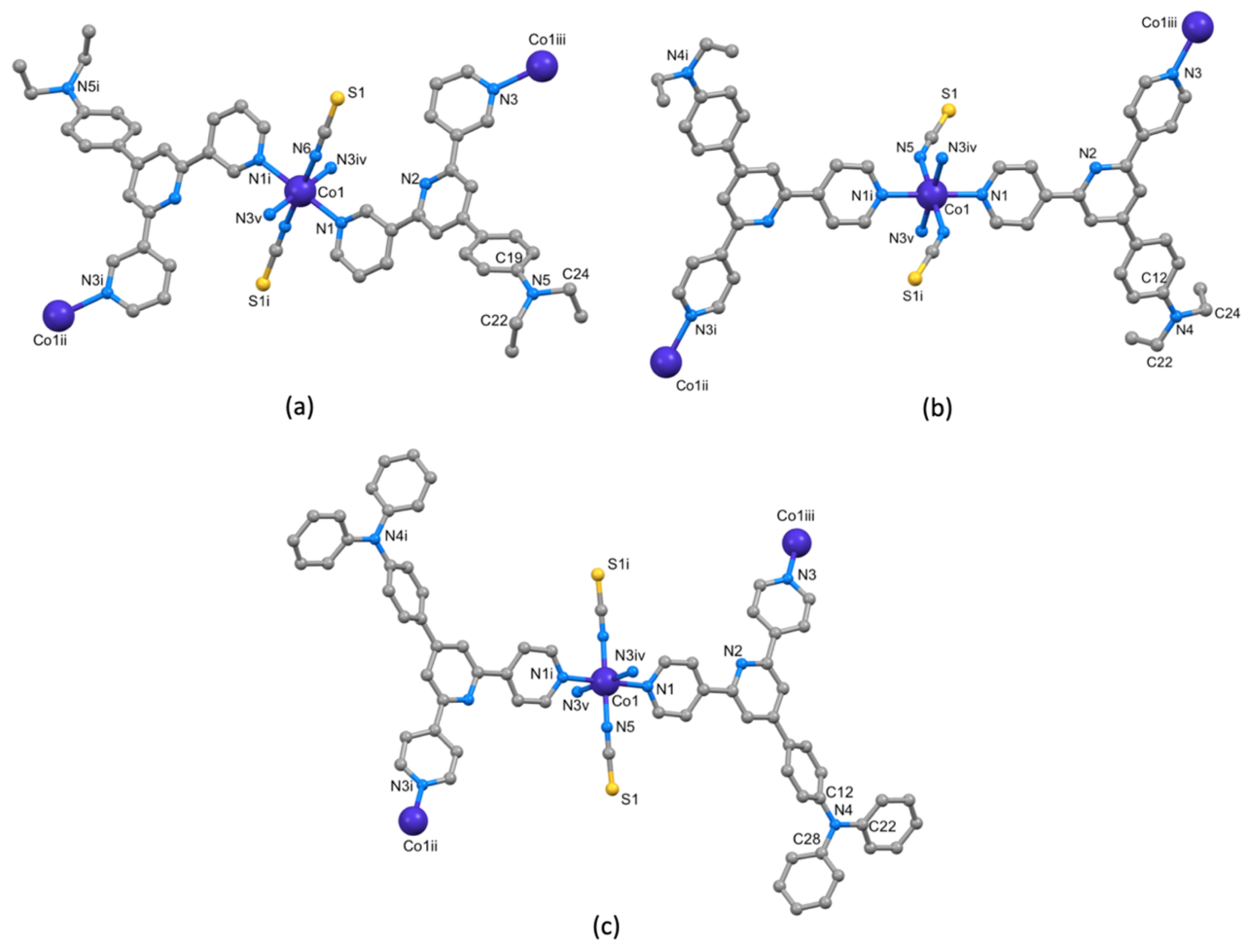
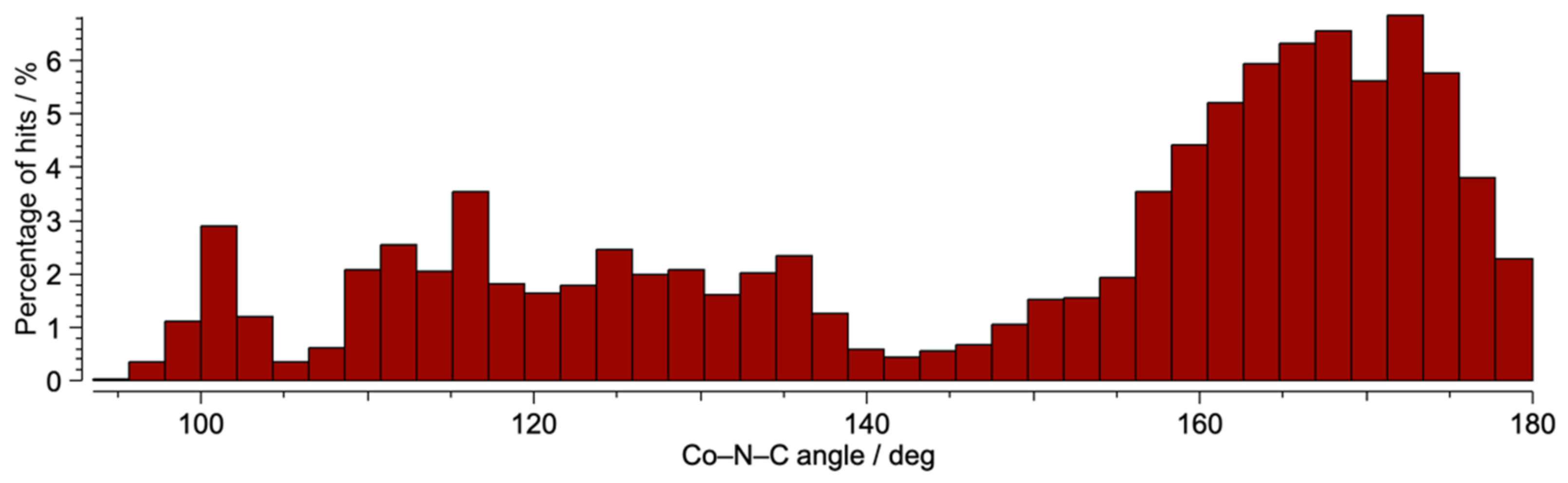
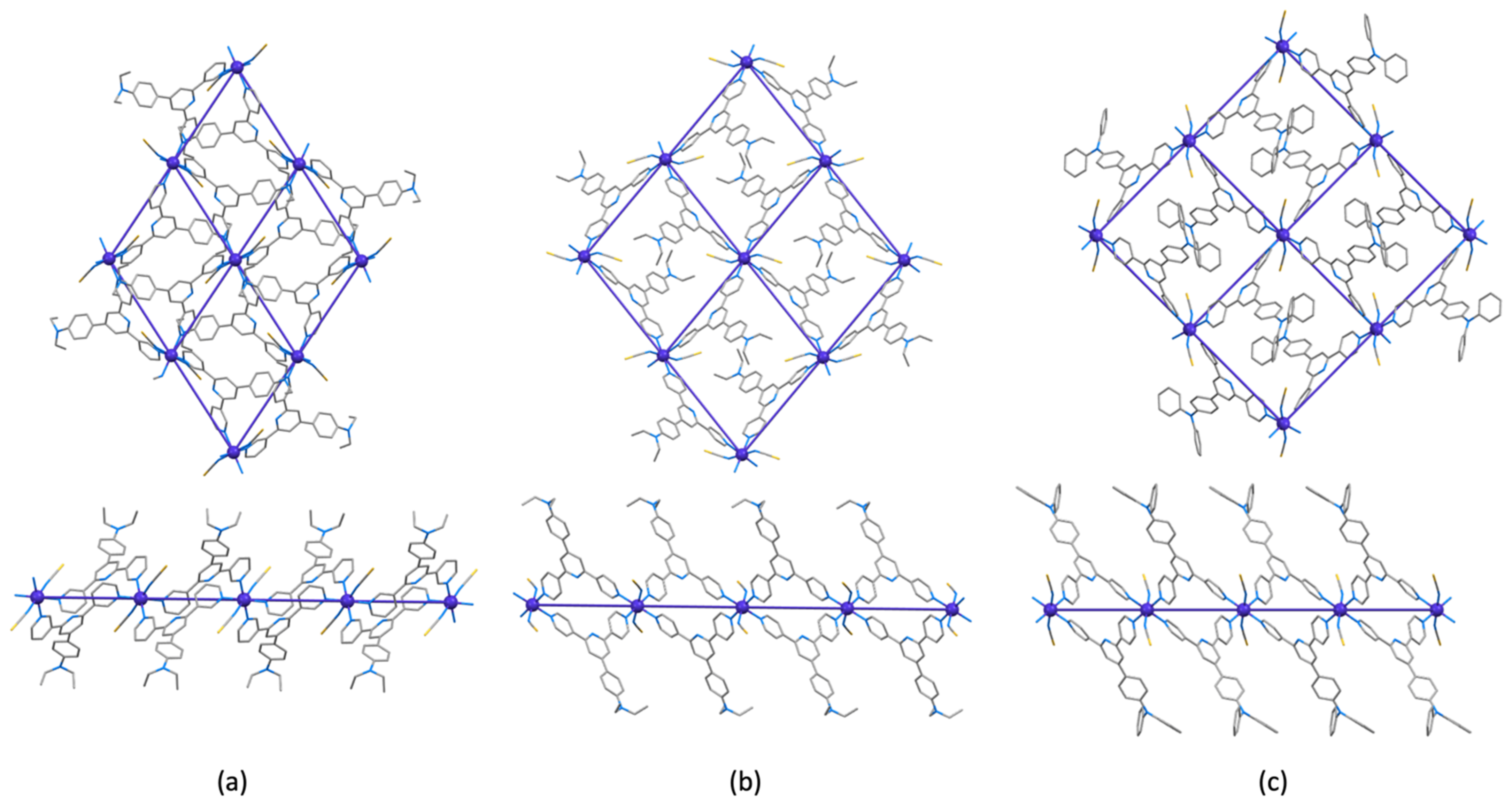
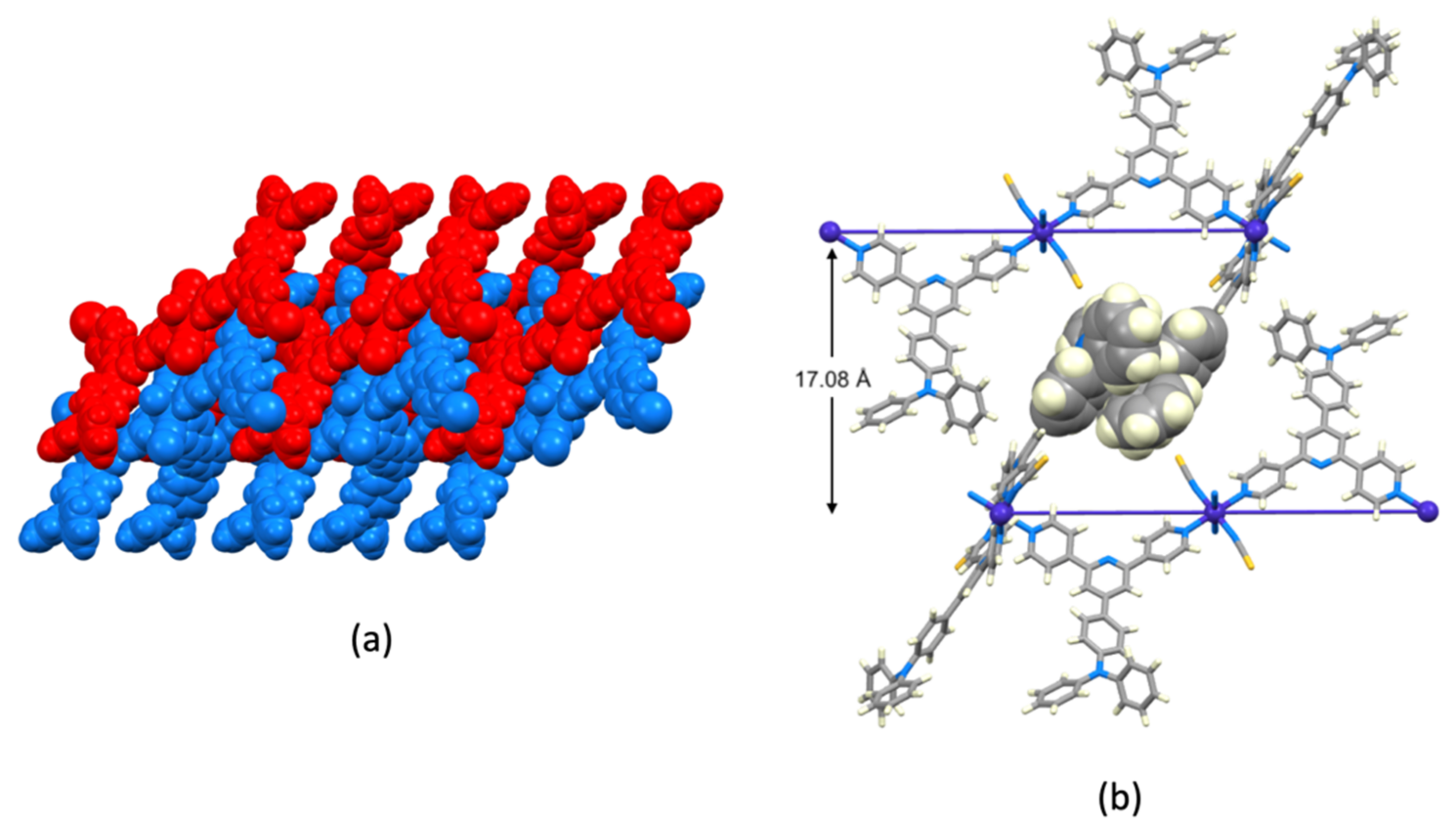
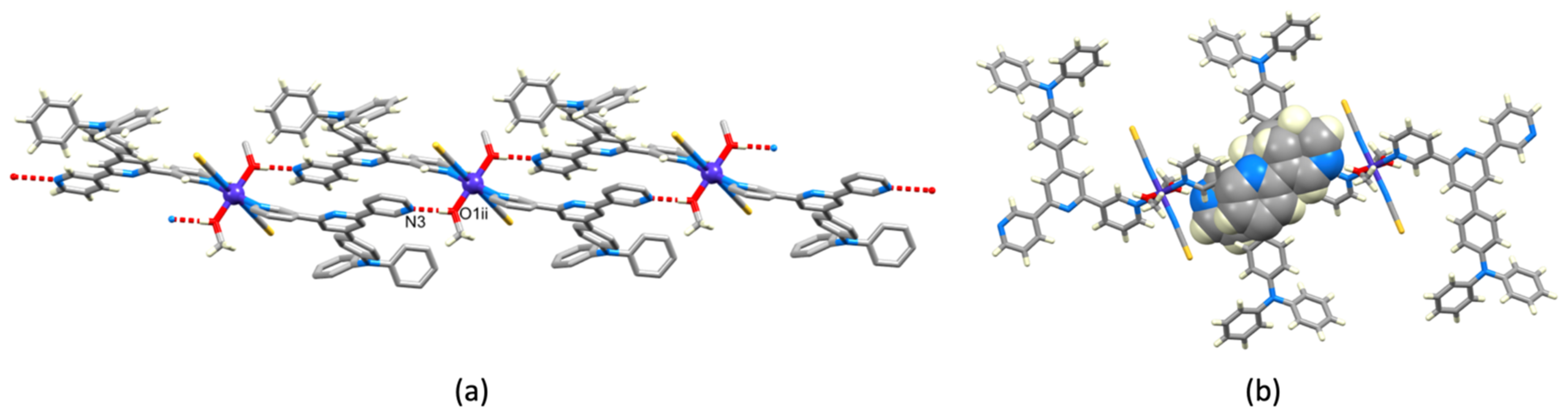
3.3. Crystal Structures of [Co(NCS)2(1)]n·0.8nCHCl3, [Co(NCS)2(3)]n·2nCHCl3 and [Co(NCS)2(4)]n
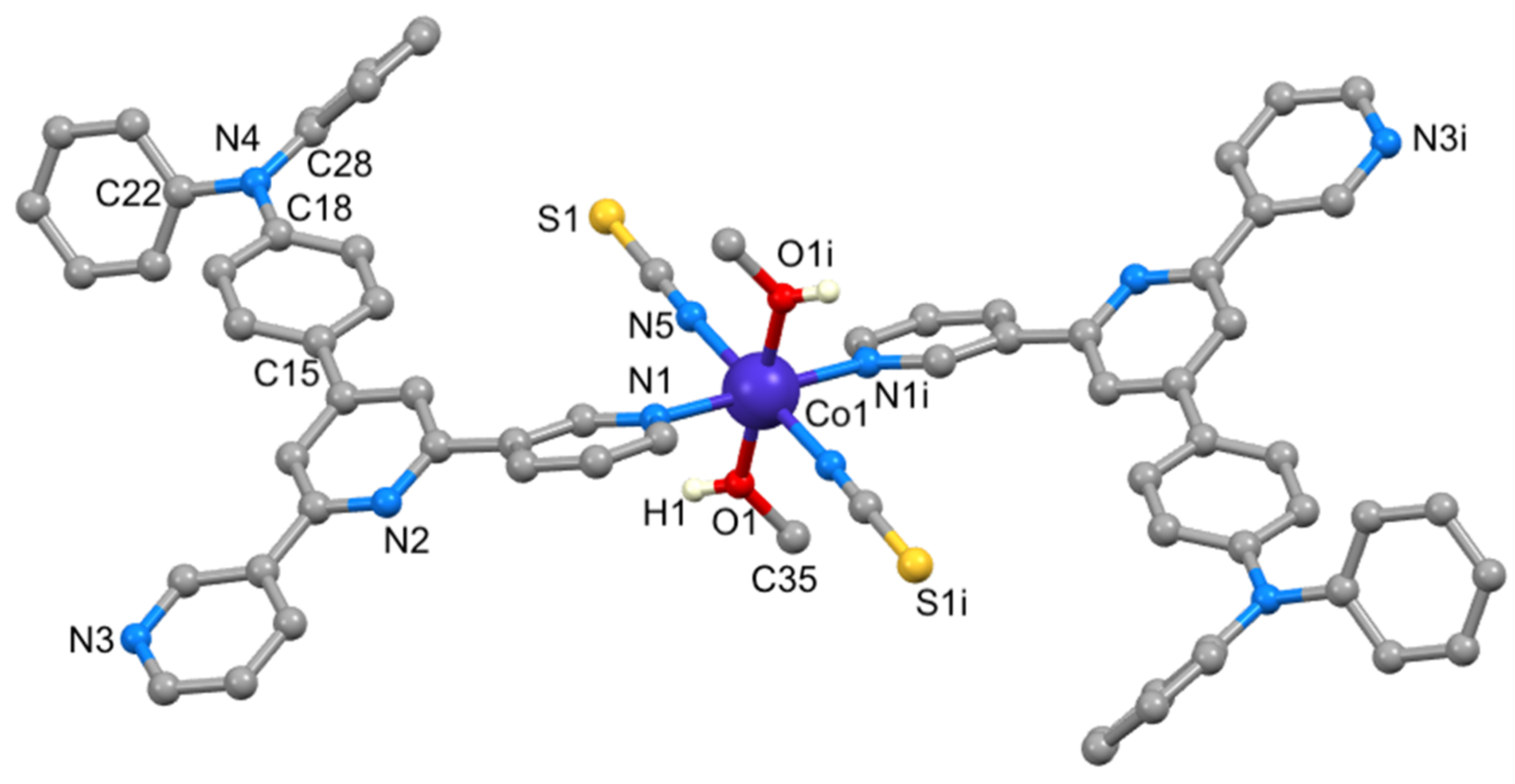
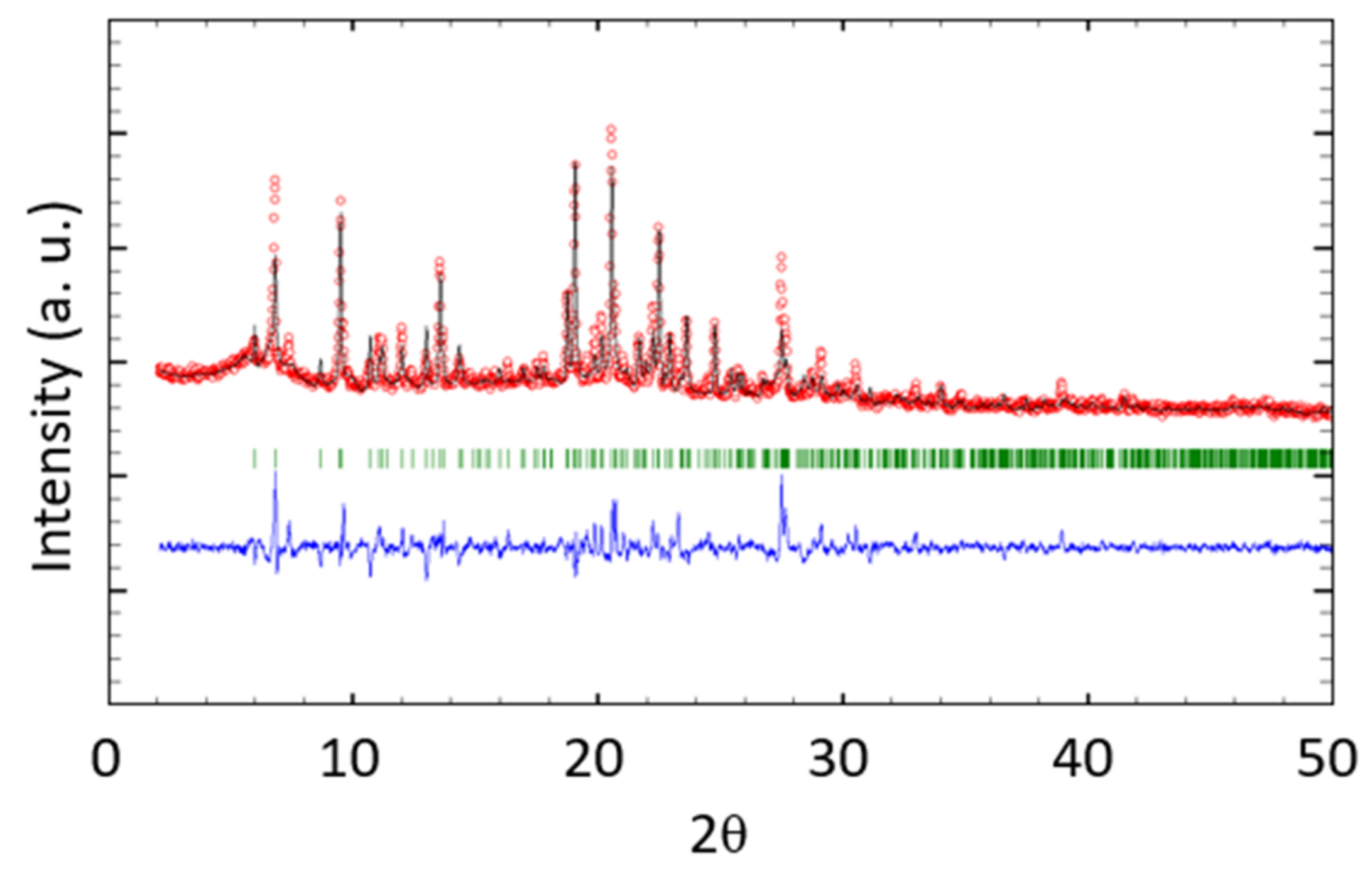
3.4. Crystal Structure of [Co(NCS)2(2)2(MeOH)2]·3CHCl3
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Housecroft, C.E. 4,2′:6′,4′′-Terpyridines: Diverging and diverse building blocks in coordination polymers and metallomacrocycles. Dalton Trans. 2014, 43, 6594–6604. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E. Divergent 4,2′:6′,4′′- and 3,2′:6′,3′′-terpyridines as linkers in 2- and 3-dimensional architectures. CrystEngComm 2015, 17, 7461–7468. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Constable, E.C. The terpyridine isomer game: From chelate to coordination network building block. Chem. Commun. 2020, 56, 10786–10794. [Google Scholar] [CrossRef]
- Housecroft, C.; Constable, E. Isomers of Terpyridine as Ligands in Coordination Polymers and Networks Containing Zinc(II) and Cadmium(II). Molecules 2021, 26, 3110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, H. Photophysical investigaions and the bioimaings of α-, β-, γ-pyridine-based terpyridine derivatives. J. Mol. Struct. 2018, 1157, 457–462. [Google Scholar] [CrossRef]
- Zhang, J.; Abudoureheman, M.; Lian, Z.; Liu, J.; Wu, Q.; Xuan, X. Controllable Synthesis of Centrosymmetric/Noncentrosymmetric Phases for the Family of Halogen-Based Photonic Coordination Polymers to Enhance the Phase-Matching Second-Harmonic-Generation Response. Inorg. Chem. 2022, 61, 3716–3722. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Zhu, Y.; Hu, L.; Xiong, Q.; Zhao, M.; Zhang, M.; Tian, X. A series of terpyridine containing flexible amino diethylacetate derivatives with large two-photon action cross-sections for effective mitochondrial imaging in living liver cancerous cells. Spectrochim. Acta Part A 2018, 188, 633–639. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Cheng, J.; Zhang, G. Iron(ii) coordination polymer catalysed hydroboration of ketones. Dalton Trans. 2018, 47, 9579–9584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jia, Y.-X.; Chen, W.; Lo, W.-F.; Brathwaite, N.; Golen, J.A.; Rheingold, A.L. Diverse zinc(ii) coordination assemblies built on divergent 4,2′:6′,4′′-terpyridine derivatives: Syntheses, structures and catalytic properties. RSC Adv. 2015, 5, 15870–15879. [Google Scholar] [CrossRef]
- Zhang, G.; Tan, J.; Phoenix, T.; Manke, D.R.; Golen, J.A.; Rheingold, A.L. Amalgamating 4′-substituted 4,2′:6′,4′′-terpyridine ligands with double-helical chains or ladder-like networks. RSC Adv. 2016, 6, 9270–9277. [Google Scholar] [CrossRef]
- Sotnik, S.A.; Polunin, R.A.; Kiskin, M.A.; Kirillov, A.M.; Dorofeeva, V.N.; Gavrilenko, K.S.; Eremenko, I.L.; Novotortsev, V.M.; Kolotilov, S.V. Heterometallic Coordination Polymers Assembled from Trigonal Trinuclear Fe2Ni-Pivalate Blocks and Polypyridine Spacers: Topological Diversity, Sorption, and Catalytic Properties. Inorg. Chem. 2015, 54, 5169–5181. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Wang, X.; Hu, H.-M.; Shen, S.-S.; An, R.; Xue, G. Syntheses, structures and luminescent properties of two new zinc coordination polymers based on 4′-(4-aminephenyl)-4,2′:6′,4″-terpyridine. Inorg. Chem. Commun. 2014, 48, 26–29. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.Z.; Yang, C.; Liu, E.; Golen, J.A.; Zhang, G. One-dimensional copper(II) coordination polymers built on 4′-substituted 4,2′:6′,4″- and 3,2′:6′,3″-terpyridines: Syntheses, structures and catalytic properties. Polyhedron 2016, 105, 115–122. [Google Scholar] [CrossRef]
- Constable, E.C.; Zhang, G.; Housecroft, C.E.; Neuburger, M.; Zampese, J.A. Sheet, ladder or chain? Small substituents in 4′-phenyl-4,2′:6′,4″-terpyridines control dimensionality in cadmium(ii) coordination polymers. CrystEngComm 2010, 12, 3733–3739. [Google Scholar] [CrossRef]
- Su, J.; Zhang, J.; Tian, X.; Zhao, M.; Song, T.; Yu, J.; Cui, Y.; Qian, G.; Zhong, H.; Luo, L.; et al. A series of multifunctional coordination polymers based on terpyridine and zinc halide: Second-harmonic generation and two-photon absorption properties and intracellular imaging. J. Mater. Chem. B 2017, 5, 5458–5463. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Neuburger, M.; Vujovic, S.; Zampese, J.A.; Zhang, G. Cobalt(ii) coordination polymers with 4′-substituted 4,2′:6′,4′′- and 3,2′:6′,3′′-terpyridines: Engineering a switch from planar to undulating chains and sheets. CrystEngComm 2012, 14, 3554–3563. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Directing 2D-Coordination Networks: Combined Effects of a Conformationally Flexible 3,2′:6′,3″-Terpyridine and Chain Length Variation in 4′-(4-n-Alkyloxyphenyl) Substituents. Molecules 2020, 25, 1663. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Adapting (4,4) Networks through Substituent Effects and Conformationally Flexible 3,2′:6′,3″-Terpyridines. Molecules 2021, 26, 6337. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Straight Versus Branched Chain Substituents in 4′-(Butoxyphenyl)-3,2′:6′,3″-terpyridines: Effects on (4,4) Coordination Network Assemblies. Polymers 2020, 12, 1823. [Google Scholar] [CrossRef]
- Rocco, D.; Novak, S.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. Coordination networks assembled from Co(NCS)2 and 4′-[4-(naphthalen-1-yl)phenyl]-3,2′:6′,3″-terpyridine: Role of lattice solvents. Polyhedron 2021, 208, 115445. [Google Scholar] [CrossRef]
- Rocco, D.; Prescimone, A.; Klein, Y.M.; Gawryluk, D.J.; Constable, E.C.; Housecroft, C.E. Competition in Coordination Assemblies: 1D-Coordination Polymer or 2D-Nets Based on Co(NCS)2 and 4′-(4-methoxyphenyl)-3,2′:6′,3″-terpyridine. Polymers 2019, 11, 1224. [Google Scholar] [CrossRef] [PubMed]
- Palatinus, L.; Chapuis, G. SUPERFLIP— A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Palatinus, L.; Prathapa, S.J.; van Smaalen, S. EDMA: A computer program for topological analysis of discrete electron densities. J. Appl. Crystallogr. 2012, 45, 575–580. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2010, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C27, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Le Bail, A.; Duroy, H.; Fourquet, J. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-cell refinement from powder diffraction scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condens. Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodríguez-Carvajal, J. WinPLOTR: A Windows tool for powder diffraction patterns analysis Materials Science Forum. In Proceedings of the Seventh European Powder Diffraction Conference (EPDIC 7), Barcelona, Spain, 20–23 May 2000; pp. 118–123. [Google Scholar]
- Hanan, G.S.; Wang, J. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2′′-Terpyridines. Synlett 2005, 251–1254. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. CrystEngComm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. Sect. B Struct. Sci. 2002, 58, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Dance, I.; Scudder, M. Supramolecular Motifs: Concerted Multiple Phenyl Embraces between Ph4P+Cations Are Attractive and Ubiquitous. Chem. Eur. J. 1996, 2, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Dance, I.; Scudder, M. The sextuple phenyl embrace, a ubiquitous concerted supramolecular motif. J. Chem. Soc. Chem. Commun. 1995, 1039–1040. [Google Scholar] [CrossRef]
- Suckert, S.; Jess, I.; Näther, C. Synthesis, Structures, and Thermal Properties of Cobalt(II) Thiocyanate Coordination Compounds with 2,5-Dimethylpyrazine. Z. Anorg. Allg. Chem. 2017, 643, 721–728. [Google Scholar] [CrossRef]
- Klein, Y.M.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. 4,2′:6′,4”- and 3,2′:6′,3″-Terpyridines: The Conflict between Well-Defined Vectorial Properties and Serendipity in the Assembly of 1D-, 2D- and 3D-Architectures. Materials 2017, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.M.; Lee, A.I.S.; Chung, Y.K. Self-Assemblies of New Rigid Angular Ligands and Metal Centers toward the Rational Construction and Modification of Novel Coordination Polymer Networks. Inorg. Chem. 2003, 42, 8838–8846. [Google Scholar] [CrossRef] [PubMed]
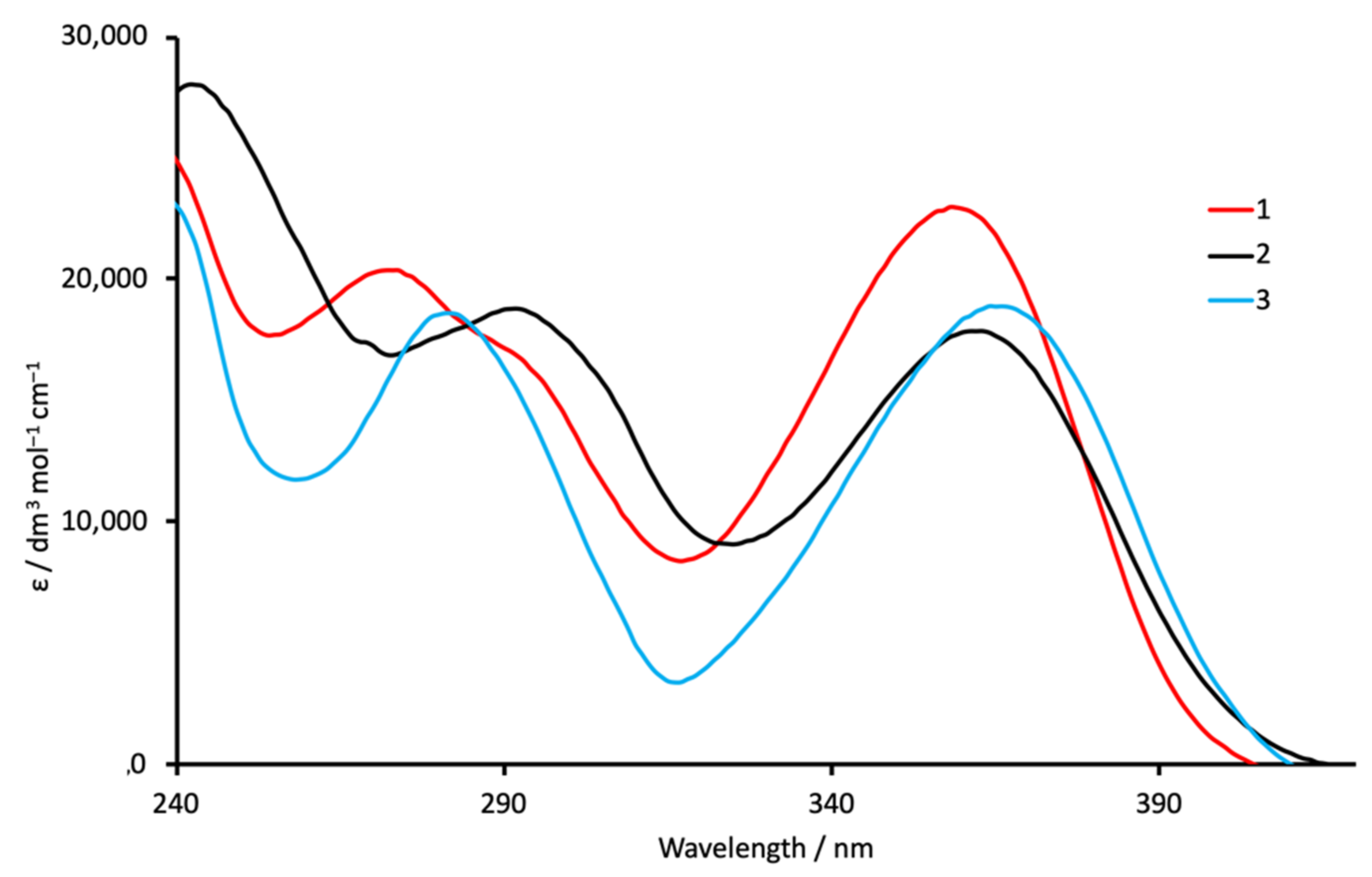
| Compound | λmax/nm (ε/dm3 mol−1 cm−1) |
|---|---|
| 1 | 273 (20,390), 293 sh (16,600), 358 (23,000) |
| 2 | 242 (28,020), 292 (18,780), 363 (17,850) |
| 3 | 281 (18,590), 366 (18,870) |
| Compound | Co–NNCS/Å | Co–Ntpy/Å | Co–NNCS–CNCS/° | Range of N–Co–N a/° |
|---|---|---|---|---|
| [Co(NCS)2(1)]n·0.8nCHCl3 | 2.078(4) | 2.211(4), 2.205(4) | 164.9(4) | 89.07(15)–90.93(15) |
| [Co(NCS)2(3)]n·2nCHCl3 | 2.085(4) | 2.194(4), 2.230(4) | 143.3(5) | 86.32(15)–93.68(15) |
| [Co(NCS)2(4)]n | 2.076(8) | 2.186(7), 2.202(7) | 163.7(7) | 86.4(3)–93.6(3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocco, D.; Nikoletić, A.; Prescimone, A.; Constable, E.C.; Housecroft, C.E. To Be or Not to Be a (4,4) Net: Reactions of 4′-{4-(N,N-Diethylaminophenyl)}- and 4′-{4-(N,N-Diphenylaminophenyl)}-3,2′:6′,3″- and 4,2′:6′,4″-Terpyridines with Cobalt(II) Thiocyanate. Crystals 2022, 12, 1136. https://doi.org/10.3390/cryst12081136
Rocco D, Nikoletić A, Prescimone A, Constable EC, Housecroft CE. To Be or Not to Be a (4,4) Net: Reactions of 4′-{4-(N,N-Diethylaminophenyl)}- and 4′-{4-(N,N-Diphenylaminophenyl)}-3,2′:6′,3″- and 4,2′:6′,4″-Terpyridines with Cobalt(II) Thiocyanate. Crystals. 2022; 12(8):1136. https://doi.org/10.3390/cryst12081136
Chicago/Turabian StyleRocco, Dalila, Anamarija Nikoletić, Alessandro Prescimone, Edwin C. Constable, and Catherine E. Housecroft. 2022. "To Be or Not to Be a (4,4) Net: Reactions of 4′-{4-(N,N-Diethylaminophenyl)}- and 4′-{4-(N,N-Diphenylaminophenyl)}-3,2′:6′,3″- and 4,2′:6′,4″-Terpyridines with Cobalt(II) Thiocyanate" Crystals 12, no. 8: 1136. https://doi.org/10.3390/cryst12081136
APA StyleRocco, D., Nikoletić, A., Prescimone, A., Constable, E. C., & Housecroft, C. E. (2022). To Be or Not to Be a (4,4) Net: Reactions of 4′-{4-(N,N-Diethylaminophenyl)}- and 4′-{4-(N,N-Diphenylaminophenyl)}-3,2′:6′,3″- and 4,2′:6′,4″-Terpyridines with Cobalt(II) Thiocyanate. Crystals, 12(8), 1136. https://doi.org/10.3390/cryst12081136