Electron Transport Layer-Free Ruddlesden–Popper Two-Dimensional Perovskite Solar Cells Enabled by Tuning the Work Function of Fluorine-Doped Tin Oxide Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Device Fabrication
2.2. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.M.; Teuscher, J.; Miyasaka, T.; Murakami, T.N.; Snaith, H.J. Efficient Hybrid Solar Cells Based on Meso-Superstructured Organometal Halide Perovskites. Science 2012, 338, 643–647. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide–based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low Trap-State Density and Long Carrier Diffusion in Organolead Trihalide Perovskite Single Crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- NREL Best Research-Cell Efficiency Chart (Photovoltaic Research). Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 30 June 2022).
- Hu, Y.; Qiu, T.; Bai, F.; Miao, X.; Zhang, S. Enhancing Moisture-Tolerance and Photovoltaic Performances of FAPbI3 by Bismuth Incorporation. J. Mater. Chem. A 2017, 5, 25258–25265. [Google Scholar] [CrossRef]
- Christians, J.A.; Miranda Herrera, P.A.; Kamat, P.V. Transformation of the Excited State and Photovoltaic Efficiency of CH3NH3PbI3 Perovskite upon Controlled Exposure to Humidified Air. J. Am. Chem. Soc. 2015, 137, 1530–1538. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.; Nie, W.; Blancon, J.-C.; Stoumpos, C.C.; Asadpour, R.; Harutyunyan, B.; Neukirch, A.J.; Verduzco, R.; Crochet, J.J.; Tretiak, S.; et al. High-Efficiency Two-Dimensional Ruddlesden–Popper Perovskite Solar Cells. Nature 2016, 536, 312–316. [Google Scholar] [CrossRef]
- Cao, D.H.; Stoumpos, C.C.; Farha, O.K.; Hupp, J.T.; Kanatzidis, M.G. 2D Homologous Perovskites as Light-Absorbing Materials for Solar Cell Applications. J. Am. Chem. Soc. 2015, 137, 7843–7850. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, K.-G.; Lee, T.-W. Planar Heterojunction Organometal Halide Perovskite Solar Cells: Roles of Interfacial Layers. Energy Environ. Sci. 2016, 9, 12–30. [Google Scholar] [CrossRef]
- Wang, P.-C.; Govindan, V.; Chiang, C.-H.; Wu, C.-G. Room-Temperature-Processed Fullerene/TiO2 Nanocomposite Electron Transporting Layer for High-Efficiency Rigid and Flexible Planar Perovskite Solar Cells. Solar RRL 2020, 4, 2000247. [Google Scholar] [CrossRef]
- Kogo, A.; Sanehira, Y.; Ikegami, M.; Miyasaka, T. Brookite TiO2 as a Low-Temperature Solution-Processed Mesoporous Layer for Hybrid Perovskite Solar Cells. J. Mater. Chem. A 2015, 3, 20952–20957. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming Ultraviolet Light Instability of Sensitized TiO2 with Meso-Superstructured Organometal Tri-Halide Perovskite Solar Cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced Electron Extraction Using SnO2 for High-Efficiency Planar-Structure HC(NH2)2PbI3-based Perovskite Solar Cells. Nat. Energy 2017, 2, 16177. [Google Scholar] [CrossRef]
- Luo, J.; Wang, Y.; Zhang, Q. Progress in Perovskite Solar Cells based on ZnO nanostructures. Sol. Energy 2018, 163, 289–306. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transport Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, K.; Leijtens, T.; Siprova, S.; Schlueter, C.; Hörantner, M.T.; Wang, J.T.-W.; Li, C.-Z.; Jen, A.K.-Y.; Lee, T.-L.; Snaith, H.J. C60 as an Efficient n-Type Compact Layer in Perovskite Solar Cells. J. Phys. Chem. Lett. 2015, 6, 2399–2405. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, J.; Liu, K.; Zheng, G.; Zhao, G.; Li, L.; Meng, Y.; Guo, T.; Zhou, H.; Zhan, X. A Low Temperature Processed Fused-Ring Electron Transport Material for Efficient Planar Perovskite Solar Cells. J. Mater. Chem. A 2017, 5, 24820–24825. [Google Scholar] [CrossRef]
- Huang, L.; Hu, Z.; Xu, J.; Sun, X.; Du, Y.; Ni, J.; Cai, H.; Li, J.; Zhang, J. Efficient Electron-Transport Layer-Free Planar Perovskite Solar Cells via Recycling the FTO/Glass Substrates from Degraded Devices. Sol. Energy Mater. Sol. Cells 2016, 152, 118–124. [Google Scholar] [CrossRef]
- Huang, S.; Dong, Q.; Lu, Y.; Duan, L.; Zhang, D. Outstanding Performance of Electron-Transport-Layer-Free Perovskite Solar Cells Using a Novel Small-Molecule Interlayer Modified FTO Substrate. Chem. Eng. J. 2021, 422, 130001. [Google Scholar] [CrossRef]
- Wu, W.-Q.; Liao, J.-F.; Zhong, J.-X.; Xu, Y.-F.; Wang, L.; Huang, J. Suppressing Interfacial Charge Recombination in Electron-Transport-Layer-Free Perovskite Solar Cells to Give an Efficiency Exceeding 21%. Angew. Chem. Int. Ed. 2020, 59, 20980–20987. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, T.; Wang, Y.; Liu, D.; Xu, H.; Chen, L.; Li, Y.; Wu, J.; Chen, Z.D.; Li, S. Enhanced Thermal Stability of Electron Transport Layer-Free Perovskite Solar Cells via Interface Strain Releasing. J. Power Sources 2019, 439, 227091. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Liu, Z.; Lee, E.-C. A Transparent Poly(3,4-Ethylenedioxylenethiophene):Poly(Styrene Sulfonate) Cathode for Low Temperature Processed, Metal-Oxide Free Perovskite Solar Cells. J. Mater. Chem. A 2017, 5, 6974–6980. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Lee, E.-C. High-Performance Inverted Perovskite Solar Cells Using Doped Poly(triarylamine) as the Hole Transport Layers. ACS Appl. Energy Mater. 2019, 2, 1932–1942. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Xu, C.; Xie, X. Electron-Transport-Layer-Free Two-Dimensional Perovskite Solar Cells based on a Flexible Poly(3,4-ethylenedioxythiophene):Poly(styrenesulfonate) Cathode. Sustain. Energy Fuels 2021, 5, 2595–2601. [Google Scholar]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low-Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Sun, Q.; Lee, E.-C. Morphology Control of SnO2 Layer by Solvent Engineering for Efficient Perovskite Solar Cells. Sol. Energy 2021, 214, 280–287. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, X.; Liu, B.; Munir, R.; Zhu, X.; Yang, D.; Li, J.; Liu, Y.; Smilgies, D.; Li, R.; et al. Stable High Efficiency Two-Dimensional Perovskite Solar Cells via Cesium Doping. Energy Environ. Sci. 2017, 10, 2095–2102. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Xie, X. Improving the Performance of Inverted Two-Dimensional Perovskite Solar Cells by Adding an Anti-Solvent into the Perovskite Precursor. J. Mater. Chem. C 2020, 8, 11882–11889. [Google Scholar]
- He, T.; Li, S.; Jiang, Y.; Qin, C.; Cui, M.; Qiao, L.; Xu, H.; Yang, J.; Long, R.; Wang, H.; et al. Reduced-Dimensional Perovskite Photovoltaics with Homogeneous Energy Landscape. Nat. Commun. 2020, 11, 1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Wang, L.; Xie, X.; Xu, C.; Tang, J.; Li, W. High-Performance Ruddlesden-Popper Two-Dimensional Perovskite Solar Cells via Solution Processed Inorganic Charge Transport Layers. Phys. Chem. Chem. Phys. 2022, 24, 15912–15919. [Google Scholar] [CrossRef]
- Xia, F.; Wu, Q.; Zhou, P.; Li, Y.; Chen, X.; Liu, Q.; Zhu, J.; Dai, S.; Lu, Y.; Yang, S. Efficiency Enhancement of Inverted Structure Perovskite Solar Cells via Oleamide Doping of PCBM Electron Transport Layer. ACS Appl. Mater. Interfaces 2015, 7, 13659–13665. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, Z.; Lee, E.-C. Stability and Efficiency Improved Perovskite Solar Cells through Tuning the Hydrophobicity of the Hole Transport Layer with an Organic Semiconductor. J. Mater. Chem. C 2021, 9, 679–686. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; Hames, B.C.; Mora-Seró, I.; Barea, E.M. Conjugated Organic Cations to Improve the Optoelectronic Properties of 2D/3D Perovskites. ACS Energy Lett. 2017, 2, 1969–1970. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; Sanchez-Diaz, J.; Echeverria-Arrondo, C.; Masi, S.; Esparza, D.; Barea, E.M.; Mora-Seró, I. Widening the 2D/3D Perovskites Family for Efficient and Thermal-Resistant Solar Cells by the Use of Secondary Ammonium Cations. ACS Energy Lett. 2020, 5, 1013–1021. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, X.; Yang, R.; Yang, Z.; Yu, W.; Wang, X.; Li, C.; Liu, S.; Chang, R.P.H. Surface Optimization to Eliminate Hysteresis for Record Efficiency Planar Perovskite Solar Cells. Energy Environ. Sci. 2016, 9, 3071–3078. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, X.; Liu, G.; Lee, E.-C. High-Performance Metal-Oxide-Free Perovskite Solar Cells based on Organic Electron Transport Layer and Cathode. Org. Electron. 2019, 64, 195–201. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, L.; Xu, C.; Xie, X.; Zhang, Y. Hole-Tranport-Underlayer-Induced Crystallization Management of Two-Dimensional Perovskite for High-Performance Inverted Solar Cells. ACS Appl. Energy Mater. 2021, 4, 10574–10583. [Google Scholar] [CrossRef]
- Wang, R.; Mujahid, M.; Duan, Y.; Wang, Z.-K.; Xue, J.; Yang, Y. A Review of Perovskite Solar Cell Stability. Adv. Funct. Mater. 2019, 29, 1808843. [Google Scholar] [CrossRef]
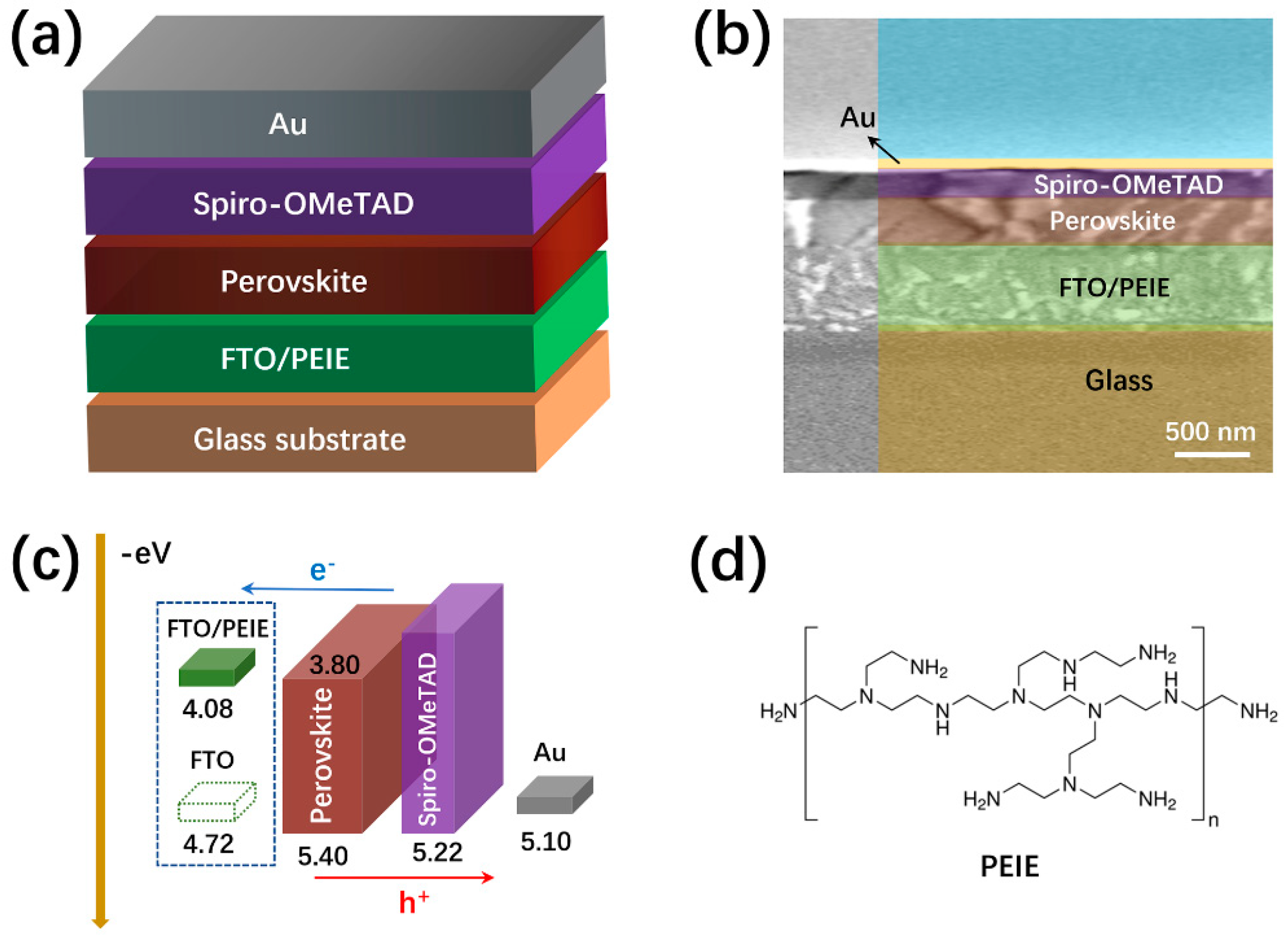
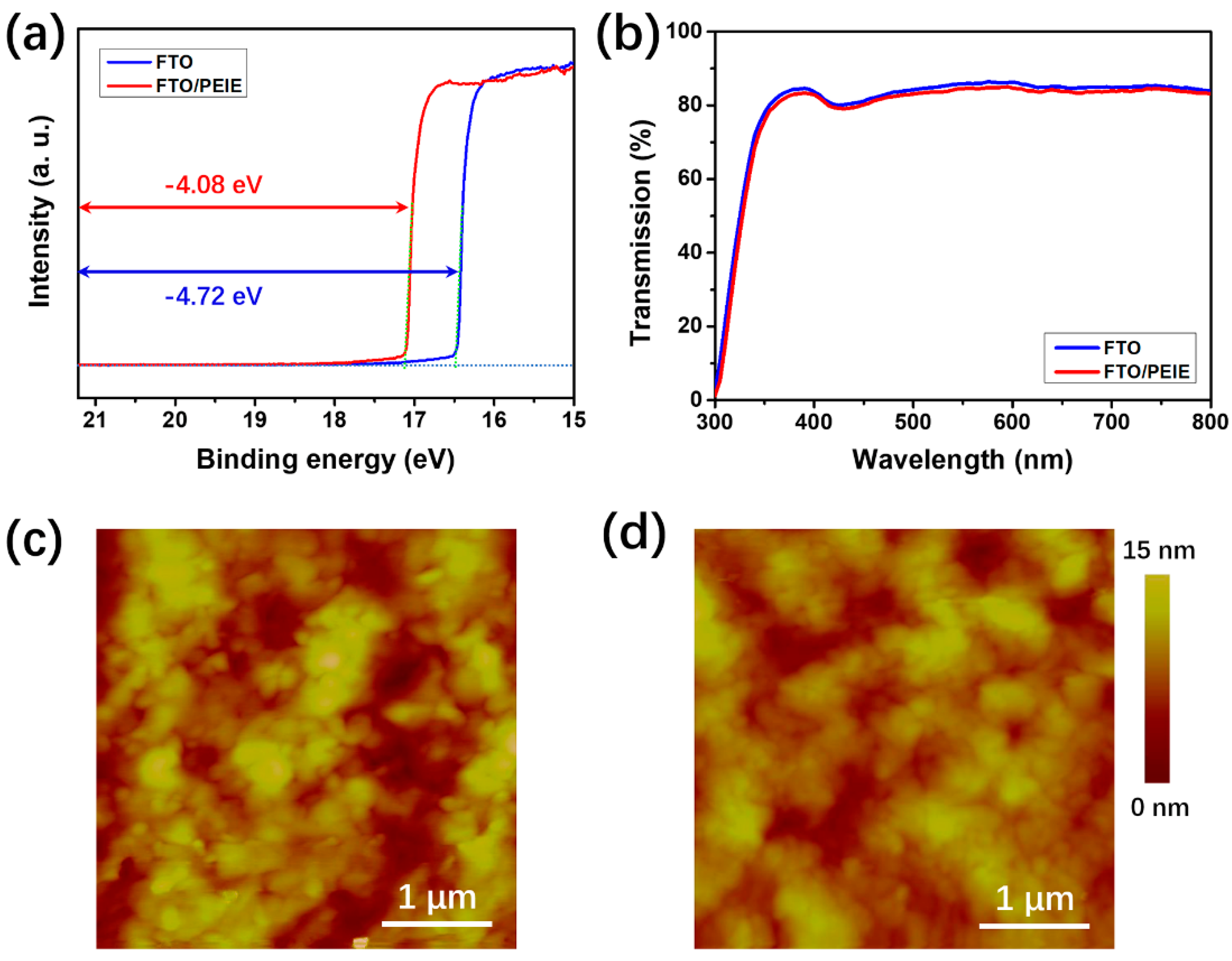
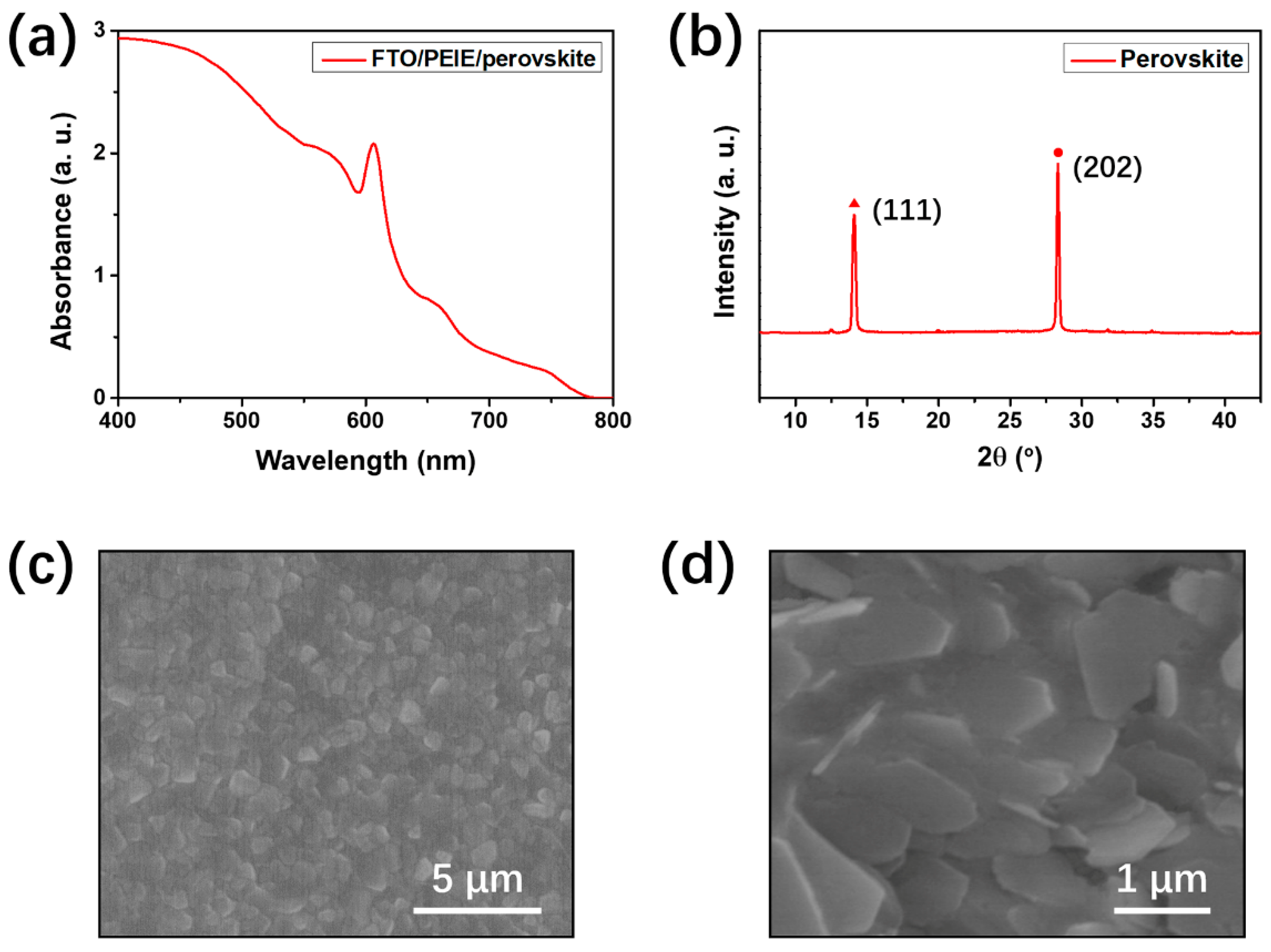
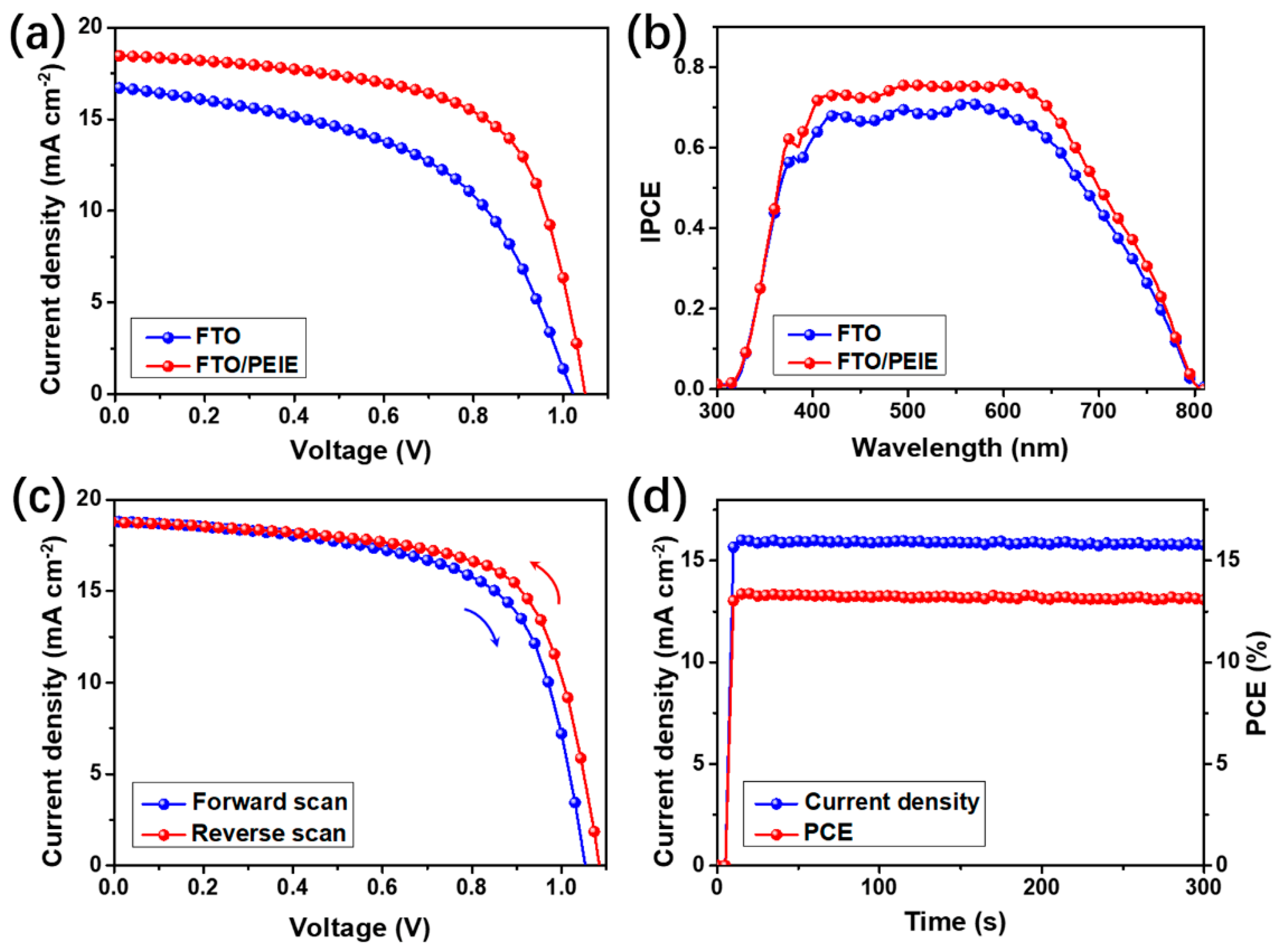
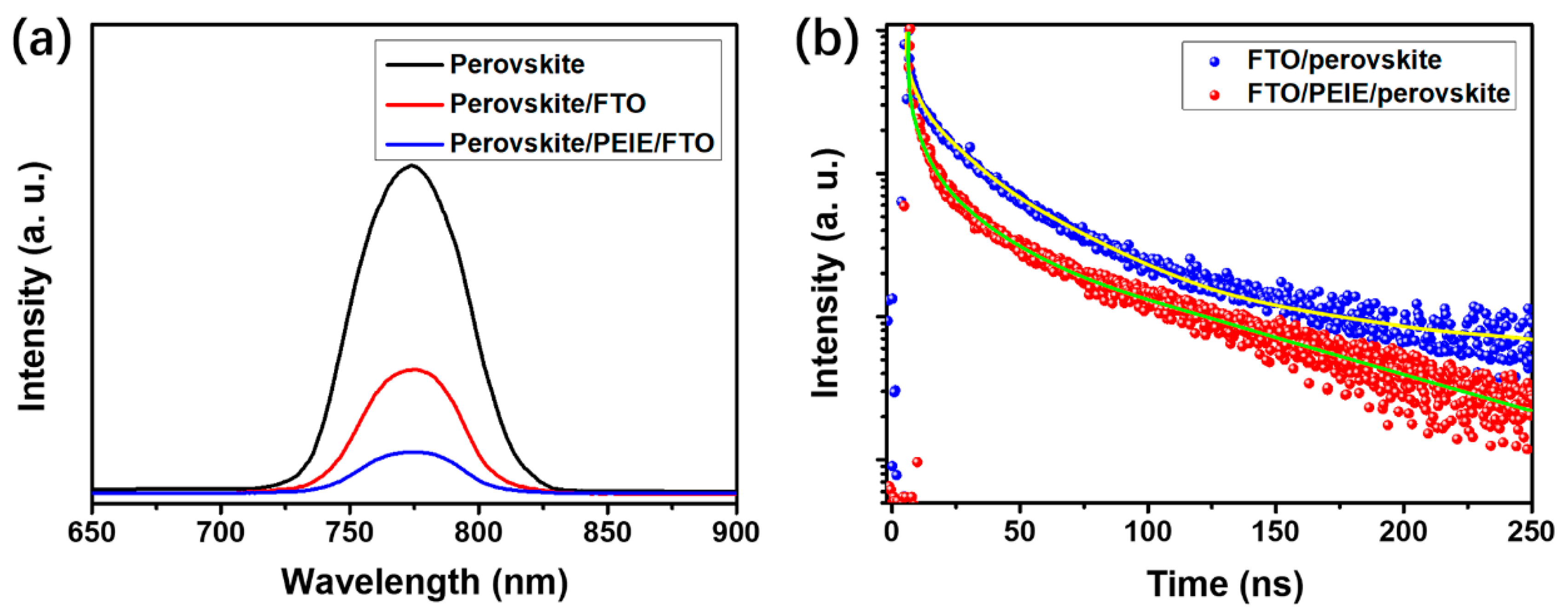
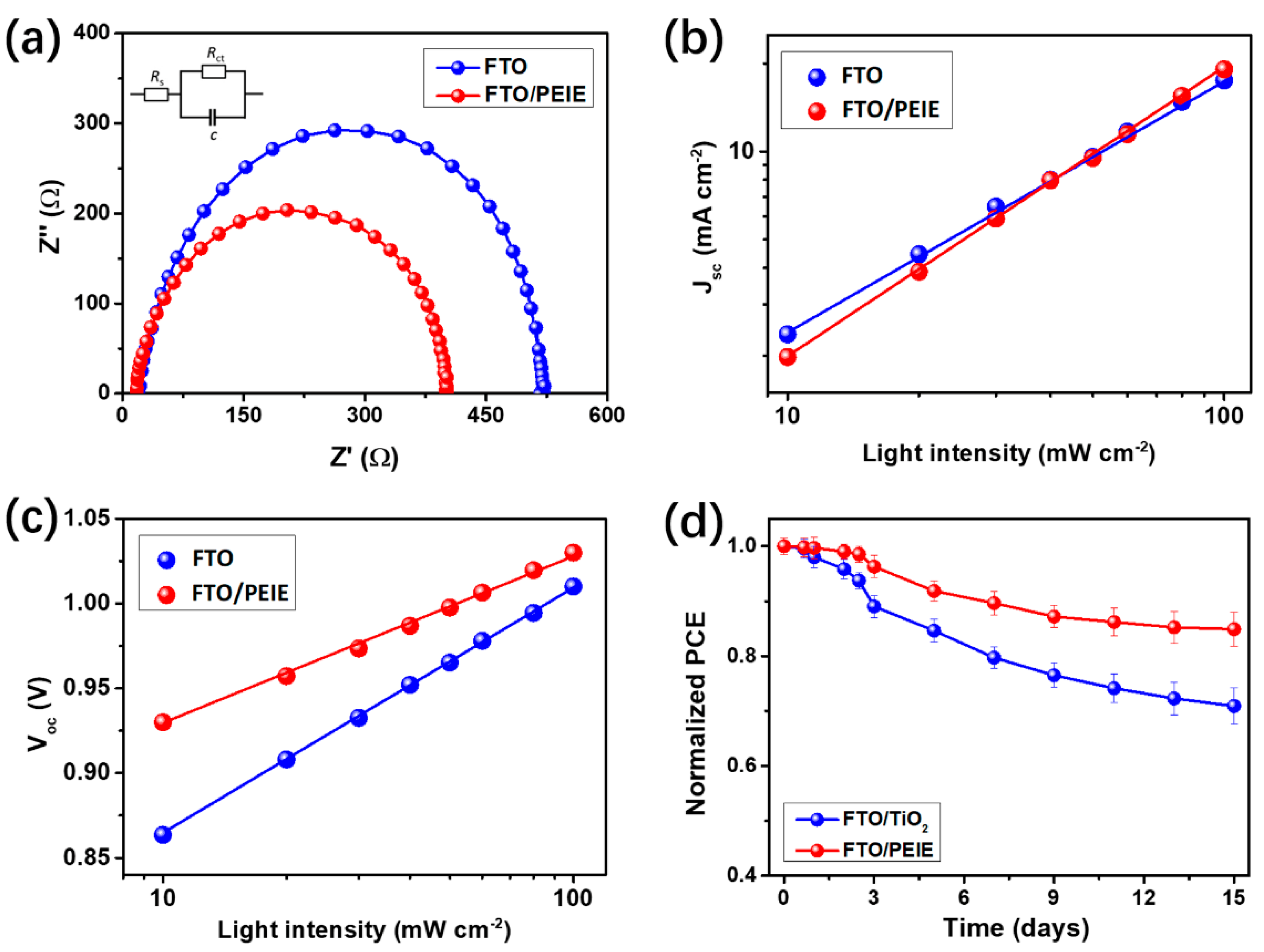
| Device Configuration | Voc (V) | Jsc (mA cm−2) | FF (%) | Average PCE (%) | Best PCE (%) |
|---|---|---|---|---|---|
| FTO/perovskite | 1.01 ± 0.01 | 16.7 ± 0.3 | 57.2 ± 1.3 | 9.6 ± 0.3 | 9.9 |
| FTO/PEIE/perovskite | 1.03 ± 0.01 | 18.5 ± 0.2 | 66.8 ± 1.2 | 12.7 ± 0.3 | 13.0 |
| FTO/TiO2/perovskite | 1.03 ± 0.01 | 19.4 ± 0.3 | 67.5 ± 1.3 | 13.5 ± 0.3 | 13.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, N.; Zhou, H.; Wang, L.; Liu, Z. Electron Transport Layer-Free Ruddlesden–Popper Two-Dimensional Perovskite Solar Cells Enabled by Tuning the Work Function of Fluorine-Doped Tin Oxide Electrodes. Crystals 2022, 12, 1090. https://doi.org/10.3390/cryst12081090
Dong N, Zhou H, Wang L, Liu Z. Electron Transport Layer-Free Ruddlesden–Popper Two-Dimensional Perovskite Solar Cells Enabled by Tuning the Work Function of Fluorine-Doped Tin Oxide Electrodes. Crystals. 2022; 12(8):1090. https://doi.org/10.3390/cryst12081090
Chicago/Turabian StyleDong, Ningfei, Haosu Zhou, Lei Wang, and Zhihai Liu. 2022. "Electron Transport Layer-Free Ruddlesden–Popper Two-Dimensional Perovskite Solar Cells Enabled by Tuning the Work Function of Fluorine-Doped Tin Oxide Electrodes" Crystals 12, no. 8: 1090. https://doi.org/10.3390/cryst12081090
APA StyleDong, N., Zhou, H., Wang, L., & Liu, Z. (2022). Electron Transport Layer-Free Ruddlesden–Popper Two-Dimensional Perovskite Solar Cells Enabled by Tuning the Work Function of Fluorine-Doped Tin Oxide Electrodes. Crystals, 12(8), 1090. https://doi.org/10.3390/cryst12081090





