Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation
Abstract
:1. Introduction
2. Modelling
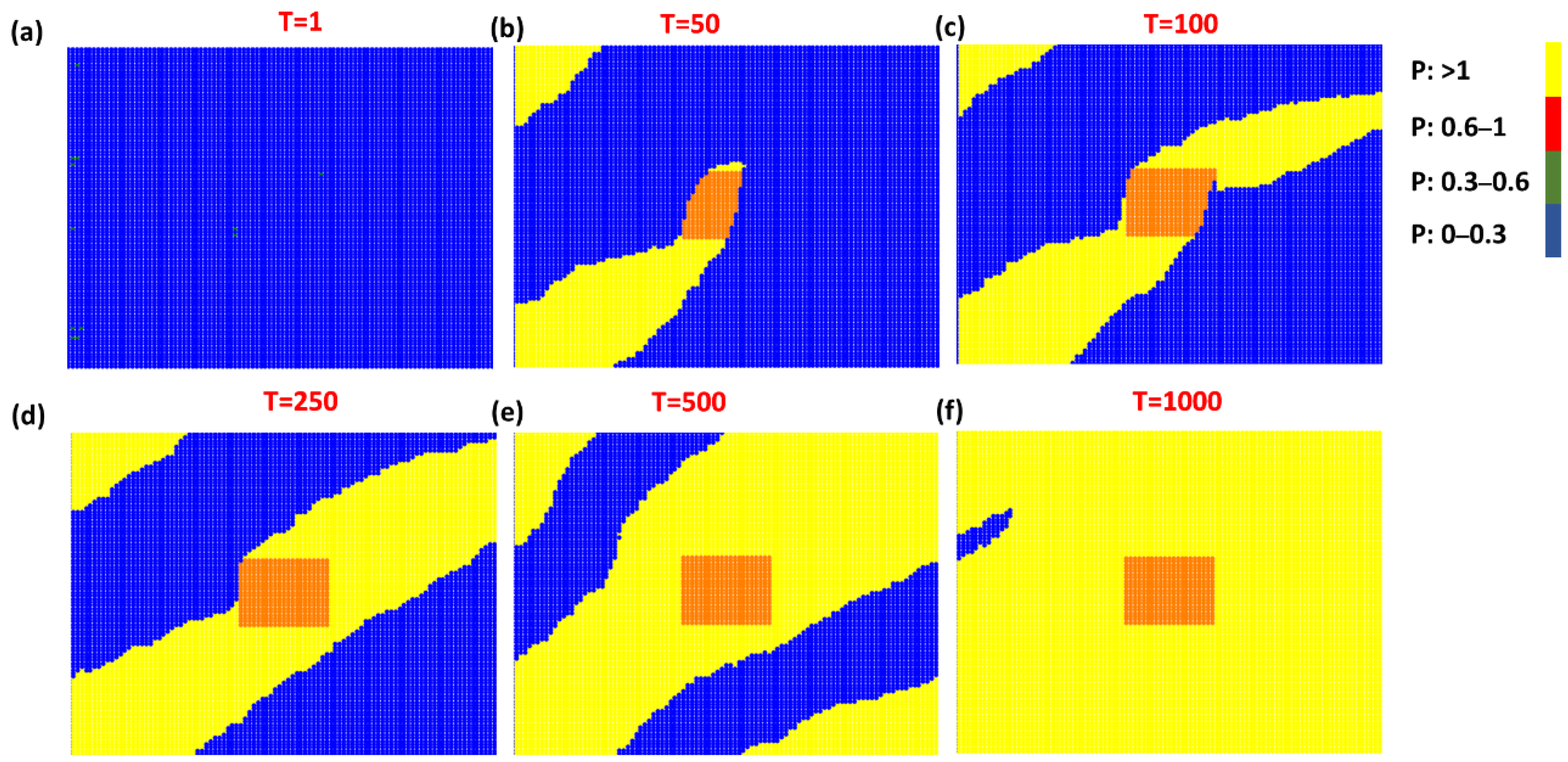
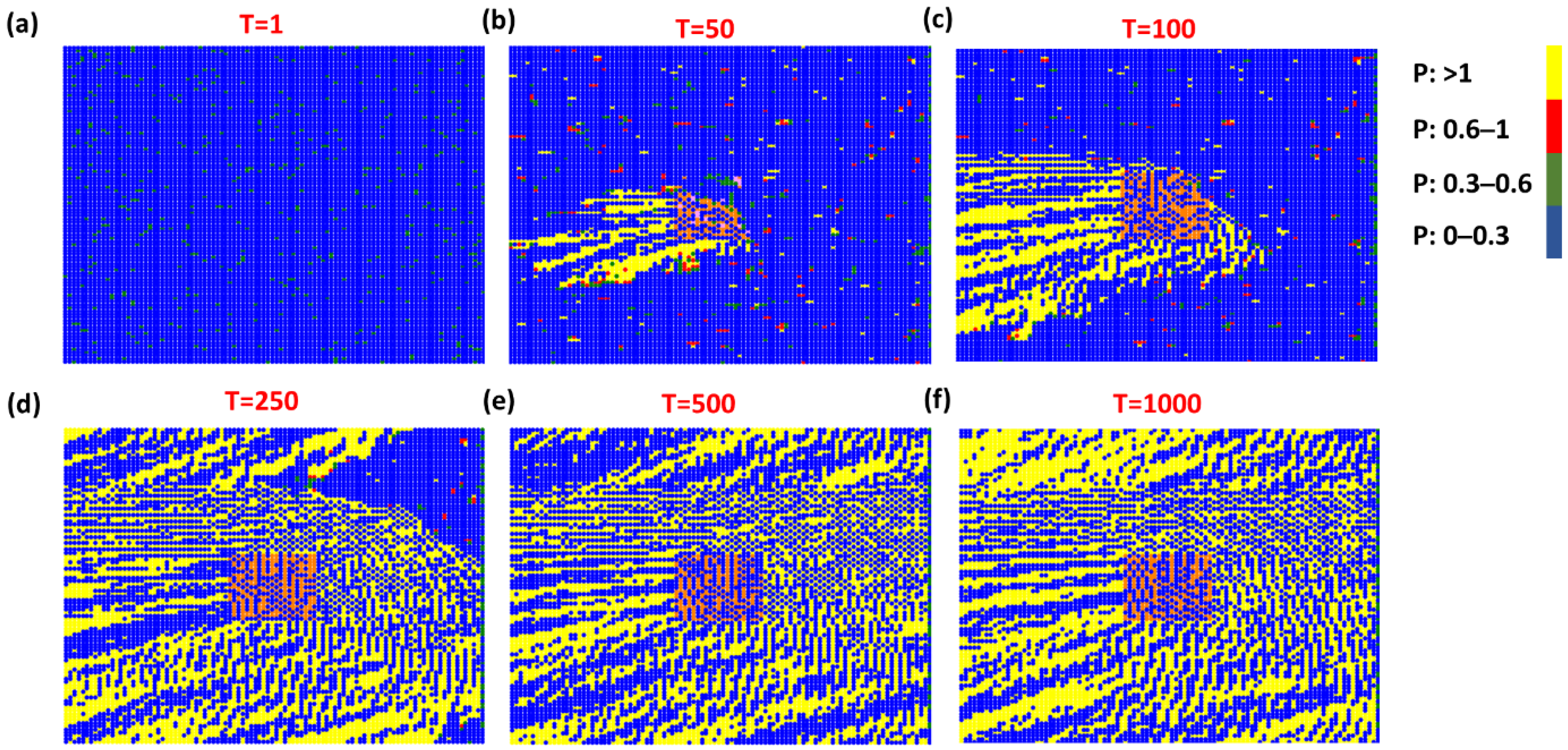
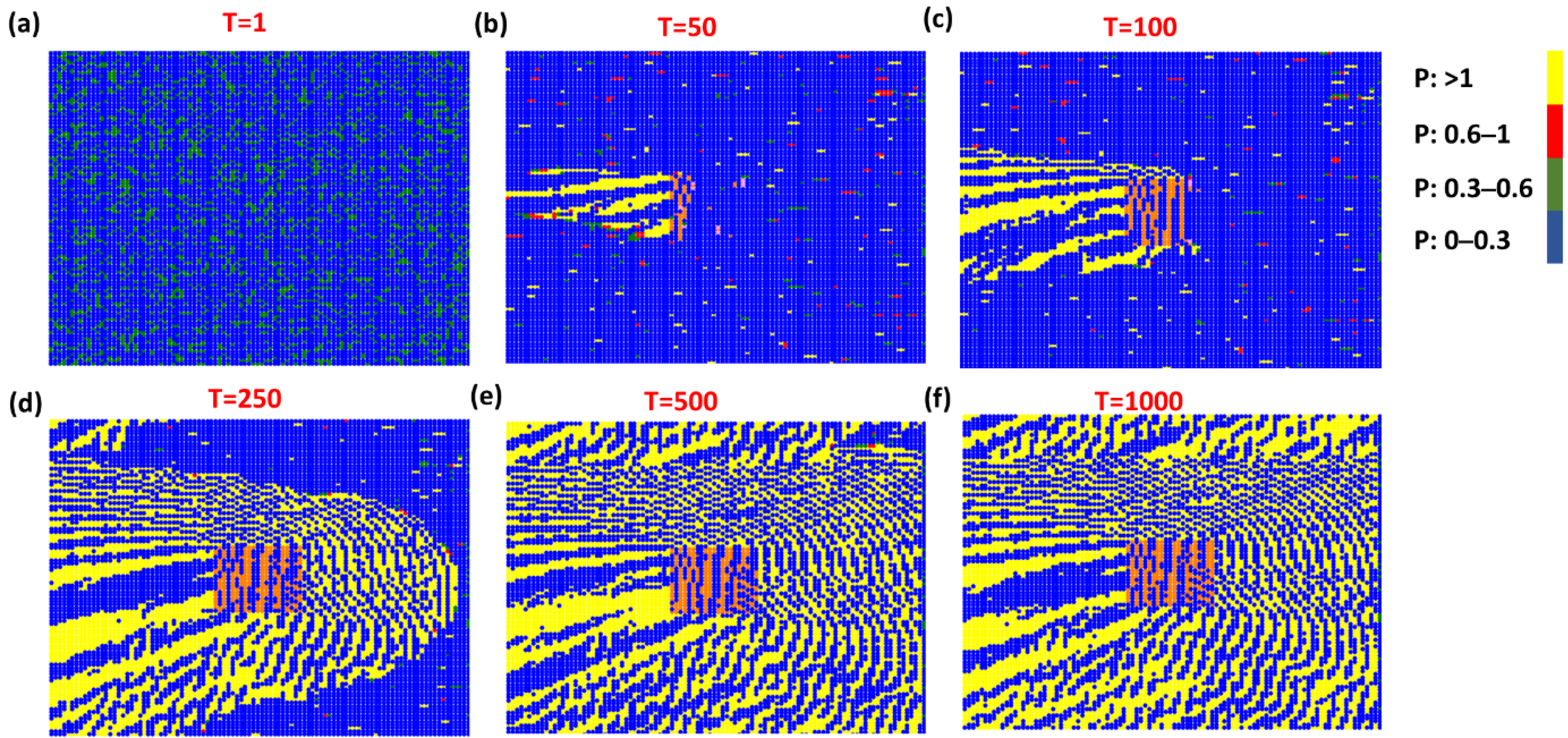
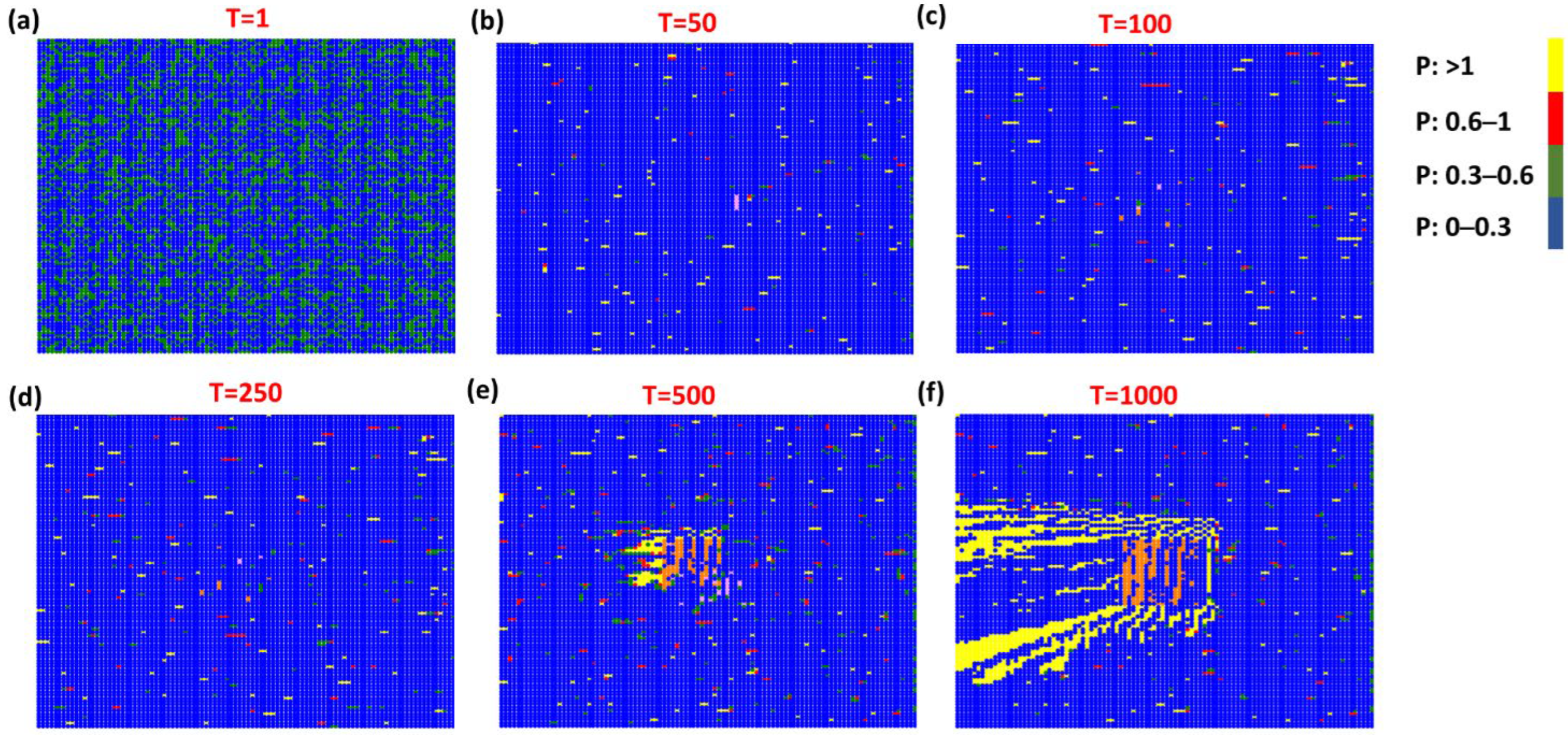
3. Results and Discussion
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharibzadeh, S.; Hossain, I.M.; Fassl, P.; Nejand, B.A.; Abzieher, T.; Schultes, M.; Ahlswede, E.; Jackson, P.; Powalla, M.; Schäfer, S.; et al. 2D/3D Heterostructure for Semitransparent Perovskite Solar Cells with Engineered Bandgap Enables Efficiencies Exceeding 25% in Four-Terminal Tandems with Silicon and CIGS. Adv. Funct. Mater. 2020, 30, 1909919. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, Z.; Fu, C.; Xiao, Q.; Zhang, S.; Zhang, Y.; Song, Y. FAPbI3 Perovskite Solar Cells: From Film Morphology Regulation to Device Optimization. Solar RRL 2022, 6, 2200120. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Li, W.; Li, Y.; Xu, G. Moisture-Stable FAPbI3Perovskite Achieved by Atomic Structure Negotiation. J. Phys. Chem. Lett. 2021, 12, 5332–5338. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.G.; Wang, R.T.; Chen, J.Y.; Xu, G. A Water-Stable Organic-Inorganic Hybrid Perovskite for Solar Cells by Inorganic Passivation. Crystals 2019, 9, 83. [Google Scholar] [CrossRef]
- Kim, M.; Jeong, J.; Lu, H.; Lee, T.K.; Eickemeyer, F.T.; Liu, Y.; Choi, I.W.; Choi, S.J.; Jo, Y.; Kim, H.B.; et al. Conformal Quantum Dot-SnO2 Layers as Electron Transporters for Efficient Perovskite Solar Cells. Science 2022, 375, 302–306. [Google Scholar] [CrossRef]
- Best Research Cell Efficiencies. Available online: https://www.nrel.gov/pv/cell-efficiency.html (accessed on 19 June 2022).
- Song, Z.; Li, C.; Chen, L.; Yan, Y. Perovskite Solar Cells Go Bifacial—Mutual Benefits for Efficiency and Durability. Adv. Mater. 2022, 34, 2106805. [Google Scholar] [CrossRef]
- Girish, K.H.; Vishnumurthy, K.A.; Roopa, T.S. Role of Conducting Polymers in Enhancing the Stability and Performance of Perovskite Solar Cells: A Brief Review. Mater. Today Sustain. 2022, 17, 100090. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Duong, T.; Peng, J.; Wu, Y.; Shen, H.; Walter, D.; Nguyen, H.T.; Mozaffari, N.; Tabi, G.D.; Catchpole, K.R.; et al. Origin of Efficiency and Stability Enhancement in High-Performing Mixed Dimensional 2D-3D Perovskite Solar Cells: A Review. Adv. Funct. Mater. 2022, 32, 2009164. [Google Scholar] [CrossRef]
- Grubišić-Čabo, F.; Nižetić, S.; Čoko, D.; Marinić Kragić, I.; Papadopoulos, A. Experimental Investigation of the Passive Cooled Free-Standing Photovoltaic Panel with Fixed Aluminum Fins on the Backside Surface. J. Clean. Prod. 2018, 176, 119–129. [Google Scholar] [CrossRef]
- Krebs-Moberg, M.; Pitz, M.; Dorsette, T.L.; Gheewala, S.H. Third Generation of Photovoltaic Panels: A Life Cycle Assessment. Renew. Energy 2021, 164, 556–565. [Google Scholar] [CrossRef]
- Liu, D.; Luo, D.; Iqbal, A.N.; Orr, K.W.P.; Doherty, T.A.S.; Lu, Z.H.; Stranks, S.D.; Zhang, W. Strain Analysis and Engineering in Halide Perovskite Photovoltaics. Nat. Mater. 2021, 20, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.T.; Xu, A.F.; Yang, L.W.; Chen, J.Y.; Kitai, A.; Xu, G. Magnetic-Field-Induced Energy Bandgap Reduction of Perovskite KMnF3. J. Mater. Chem. C 2020, 8, 4164–4168. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Jarvis, V.; Britten, J.F.; Xu, G. Pyrrolidinium Lead Iodide from Crystallography: A New Perovskite with Low Bandgap and Good Water Resistance. Chem. Commun. 2019, 55, 3251–3253. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Wang, Z.; Ahn, Y.H.; Azam, M.; Khan, A.A.; Farooq, U.; Zubair, M.; Cao, Y. Recent Defect Passivation Drifts and Role of Additive Engineering in Perovskite Photovoltaics. Nano Energy 2022, 101, 107579. [Google Scholar] [CrossRef]
- Xue, D.J.; Hou, Y.; Liu, S.C.; Wei, M.; Chen, B.; Huang, Z.; Li, Z.; Sun, B.; Proppe, A.H.; Dong, Y.; et al. Regulating Strain in Perovskite Thin Films through Charge-Transport Layers. Nat. Commun. 2020, 11, 1514. [Google Scholar] [CrossRef]
- Chang, X.; Li, W.; Zhu, L.; Liu, H.; Geng, H.; Xiang, S.; Liu, J.; Chen, H. Carbon-Based CsPbBr3 Perovskite Solar Cells: All-Ambient Processes and High Thermal Stability. ACS Appl. Mater. Interfaces 2016, 8, 33649–33655. [Google Scholar] [CrossRef]
- Schlipf, J.; Hu, Y.; Pratap, S.; Bießmann, L.; Hohn, N.; Porcar, L.; Bein, T.; Docampo, P.; Müller-Buschbaum, P. Shedding Light on the Moisture Stability of 3D/2D Hybrid Perovskite Heterojunction Thin Films. ACS Appl. Energy Mater. 2019, 2, 1011–1018. [Google Scholar] [CrossRef]
- Park, N.G.; Grätzel, M.; Miyasaka, T.; Zhu, K.; Emery, K. Towards Stable and Commercially Available Perovskite Solar Cells. Nat. Energy 2016, 1, 16152. [Google Scholar] [CrossRef]
- Hu, H.; Wong, K.K.; Kollek, T.; Hanusch, F.; Polarz, S.; Docampo, P.; Schmidt-Mende, L. Highly Efficient Reproducible Perovskite Solar Cells Prepared by Low-Temperature Processing. Molecules 2016, 21, 542. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Liu, N.; Chen, Q.; Lapierre, R.; Isik Goktas, N.; Xu, G. Pyrrolidinium Containing Perovskites with Thermal Stability and Water Resistance for Photovoltaics. J. Mater. Chem. C 2019, 7, 11104–11108. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Chen, J.Y.; Yang, L.W.; Xu, G.; Jarvis, V.; Britten, J.F. Reversing Organic-Inorganic Hybrid Perovskite Degradation in Water via PH and Hydrogen Bonds. J. Phys. Chem. Lett. 2019, 10, 7245–7250. [Google Scholar] [CrossRef]
- Wang, R.T.; Tai, E.G.; Chen, J.Y.; Xu, G.; LaPierre, R.; Goktas, N.I.; Hu, N. A KMnF3 Perovskite Structure with Improved Stability, Low Bandgap and High Transport Properties. Ceram. Int. 2019, 45, 64–68. [Google Scholar] [CrossRef]
- Leyden, M.R.; Ono, L.K.; Raga, S.R.; Kato, Y.; Wang, S.; Qi, Y. High Performance Perovskite Solar Cells by Hybrid Chemical Vapor Deposition. J. Mater. Chem. A 2014, 2, 18742–18745. [Google Scholar] [CrossRef]
- Mathies, F.; Eggers, H.; Richards, B.S.; Hernandez-Sosa, G.; Lemmer, U.; Paetzold, U.W. Inkjet-Printed Triple Cation Perovskite Solar Cells. ACS Appl. Energy Mater. 2018, 1, 1834–1839. [Google Scholar] [CrossRef]
- Patidar, R.; Burkitt, D.; Hooper, K.; Richards, D.; Watson, T. Slot-Die Coating of Perovskite Solar Cells: An Overview. Mater. Today Commun. 2020, 22, 100808. [Google Scholar] [CrossRef]
- Yang, Z.; Chueh, C.C.; Zuo, F.; Kim, J.H.; Liang, P.W.; Jen, A.K.Y. High-Performance Fully Printable Perovskite Solar Cells via Blade-Coating Technique under the Ambient Condition. Adv. Energy Mater. 2015, 5, 1500328. [Google Scholar] [CrossRef]
- Verma, A.; Martineau, D.; Hack, E.; Makha, M.; Turner, E.; Nüesch, F.; Nüesch, F.; Heier, J. Towards Industrialization of Perovskite Solar Cells Using Slot Die Coating. J. Mater. Chem. C 2020, 8, 6124–6135. [Google Scholar] [CrossRef]
- Pendyala, N.K.; Magdassi, S.; Etgar, L. Fabrication of Perovskite Solar Cells with Digital Control of Transparency by Inkjet Printing. ACS Appl. Mater. Interfaces 2021, 13, 30524–30532. [Google Scholar] [CrossRef]
- Qiu, L.; He, S.; Jiang, Y.; Qi, Y. Metal Halide Perovskite Solar Cells by Modified Chemical Vapor Deposition. J. Mater. Chem. A 2021, 9, 22759–22780. [Google Scholar] [CrossRef]
- Kapoor, V.; Bashir, A.; Haur, L.J.; Bruno, A.; Shukla, S.; Priyadarshi, A.; Mathews, N.; Mhaisalkar, S. Effect of Excess PbI2 in Fully Printable Carbon-Based Perovskite Solar Cells. Energy Technol. 2017, 5, 1880–1886. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Wang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A. Enhanced Performance of CH3NH3PbI3 Perovskite Solar Cells by Excess Halide Modification. Appl. Surf. Sci. 2021, 564, 150464. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Yang, Z.; Xu, Y.; Ding, Y.; Tan, L.; Yi, C.; Zhang, Z.; Meng, K.; Chen, G.; et al. Ligand-Modulated Excess PbI2 Nanosheets for Highly Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, 2000865. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Dong, Q.; Wong, M.K.; Djurišić, A.B.; Ng, A.; Ren, Z.; Shen, Q.; Surya, C.; Chan, W.K.; Wang, J.; et al. Is Excess PbI2 Beneficial for Perovskite Solar Cell Performance? Adv. Energy Mater. 2016, 6, 1502206. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, J.; Luan, J.; Zhang, B.; Tan, Z.; Yao, J.; Dai, S. Achieving Mixed Halide Perovskite via Halogen Exchange during Vapor-Assisted Solution Process for Efficient and Stable Perovskite Solar Cells. Org. Electron. 2017, 50, 33–42. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Xu, Y.; Li, R.; Yu, T.; Lin, Q. Interfacial Engineering of Perovskite Solar Cells with Evaporated PbI2Ultrathin Layers. ACS Appl. Mater. Interfaces 2021, 13, 53282–53288. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; He, T.; Zhang, S.; Lei, X.; Jiang, Y.; Wang, D.; Sun, P.; Zhao, D.; Hsu, H.Y.; Li, X.; et al. Halogen-Halogen Bonds Enable Improved Long-Term Operational Stability of Mixed-Halide Perovskite Photovoltaics. Chem 2021, 7, 3131–3143. [Google Scholar] [CrossRef]
- Abate, A.; Saliba, M.; Hollman, D.J.; Stranks, S.D.; Wojciechowski, K.; Avolio, R.; Grancini, G.; Petrozza, A.; Snaith, H.J. Supramolecular Halogen Bond Passivation of Organic-Inorganic Halide Perovskite Solar Cells. Nano Lett. 2014, 14, 3247–3254. [Google Scholar] [CrossRef]
- Bi, D.; El-Zohry, A.M.; Hagfeldt, A.; Boschloo, G. Unraveling the Effect of PbI2 Concentration on Charge Recombination Kinetics in Perovskite Solar Cells. ACS Photonics 2015, 2, 589–594. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Su, J.; Li, J. First-Principles Study of Molecular Adsorption on Lead Iodide Perovskite Surface: A Case Study of Halogen Bond Passivation for Solar Cell Application. J. Phys. Chem. C 2016, 120, 23536–23541. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, J.; McKechnie, S. Interactions between Molecules and Perovskites in Halide Perovskite Solar Cells. Sol. Energy Mater. Sol. Cells 2018, 175, 1–19. [Google Scholar] [CrossRef]
- Xu, K.J.; Wang, R.T.; Xu, A.F.; Chen, J.Y.; Xu, G. Hysteresis and Instability Predicted in Moisture Degradation of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 48882–48889. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Q.; Xiao, Y.; Zhang, X.; Sun, J.; Han, C.B.; Gao, H.; Zhang, Y.; Lu, Y.; Yan, H. Mechanism of PbI2 in Situ Passivated Perovskite Films for Enhancing the Performance of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 44101–44108. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Yao, X.; Hou, F.; Guo, S.; Li, Y.; Wei, C.; Ding, Y.; Li, Y.; Zhao, Y.; Zhang, X. Unraveling the Passivation Process of PbI2 to Enhance the Efficiency of Planar Perovskite Solar Cells. J. Phys. Chem. C 2018, 122, 21269–21276. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xu, F.; Wang, Q.; Tai, L.; Xu, G. Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation. Crystals 2022, 12, 1073. https://doi.org/10.3390/cryst12081073
Wang T, Xu F, Wang Q, Tai L, Xu G. Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation. Crystals. 2022; 12(8):1073. https://doi.org/10.3390/cryst12081073
Chicago/Turabian StyleWang, Taoran, Fan Xu, Qi Wang, Lu Tai, and Gu Xu. 2022. "Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation" Crystals 12, no. 8: 1073. https://doi.org/10.3390/cryst12081073
APA StyleWang, T., Xu, F., Wang, Q., Tai, L., & Xu, G. (2022). Improved Perovskite Structural Stability by Halogen Bond from Excessive Lead Iodide via Numerical Simulation. Crystals, 12(8), 1073. https://doi.org/10.3390/cryst12081073









