Protein Fusion Strategies for Membrane Protein Stabilization and Crystal Structure Determination
Abstract
1. Introduction
2. Using Fusion Protein to Facilitate Membrane Protein Production
3. Fusion Strategies for Membrane Protein Crystallization and Structure Determination
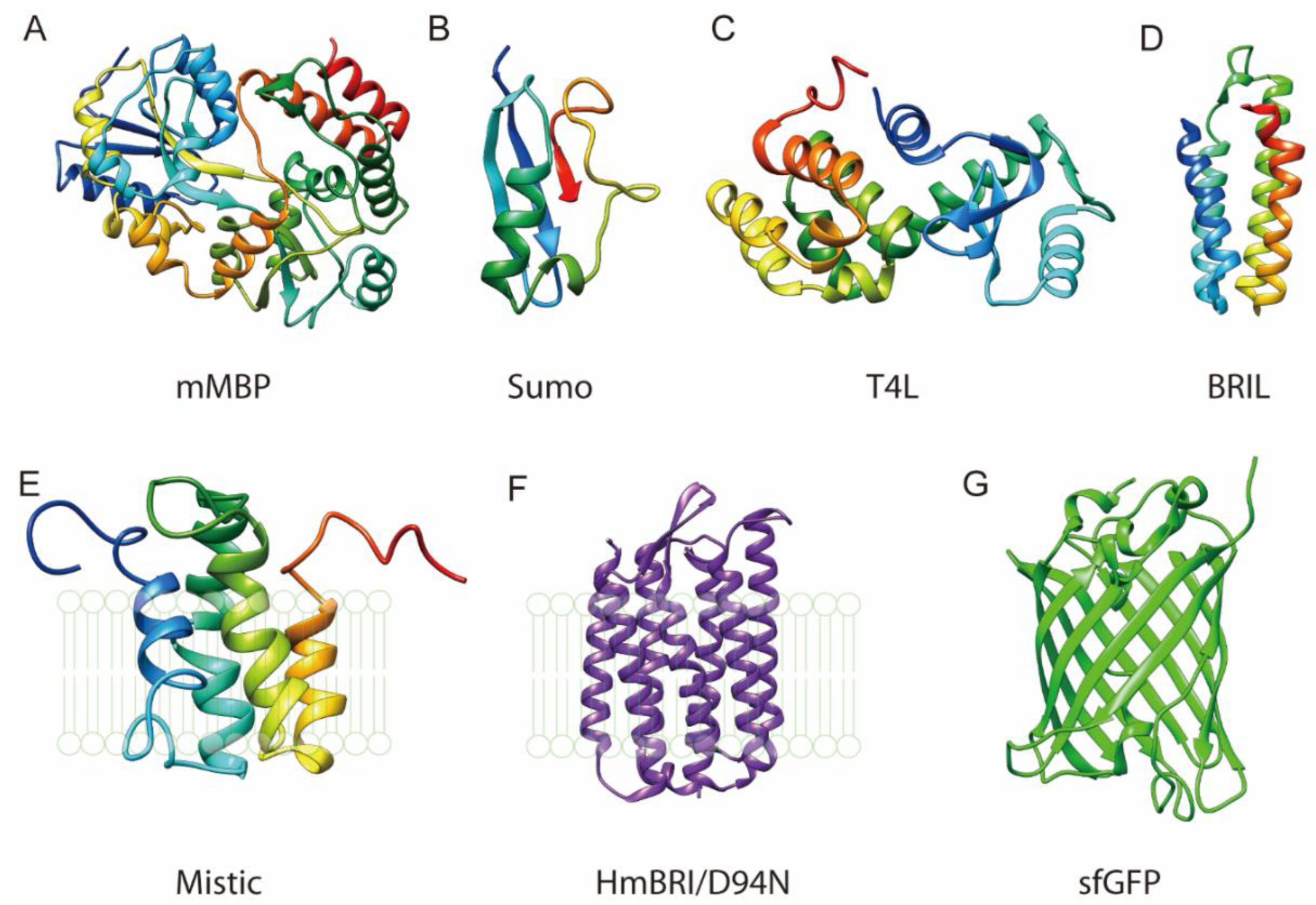
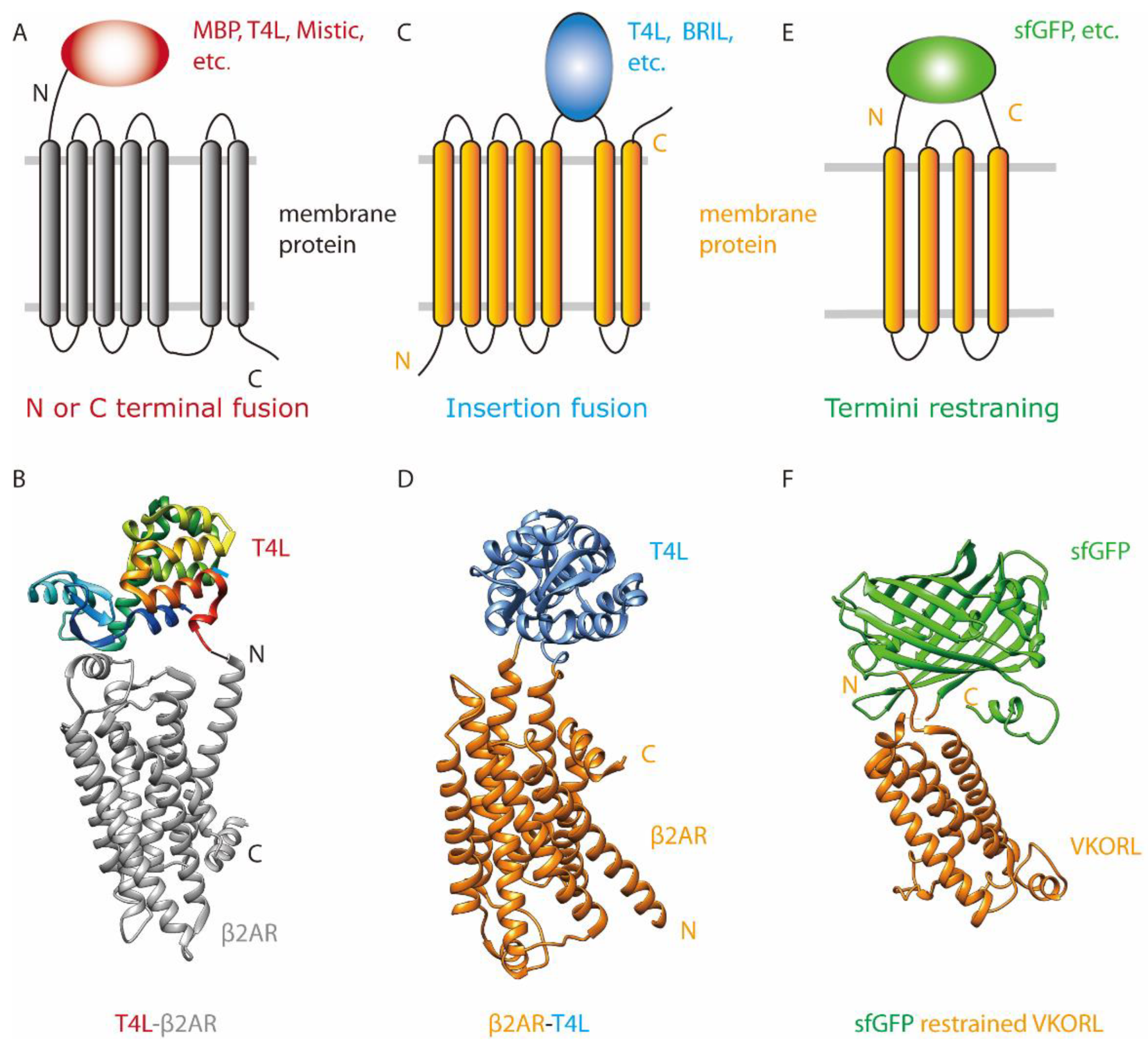
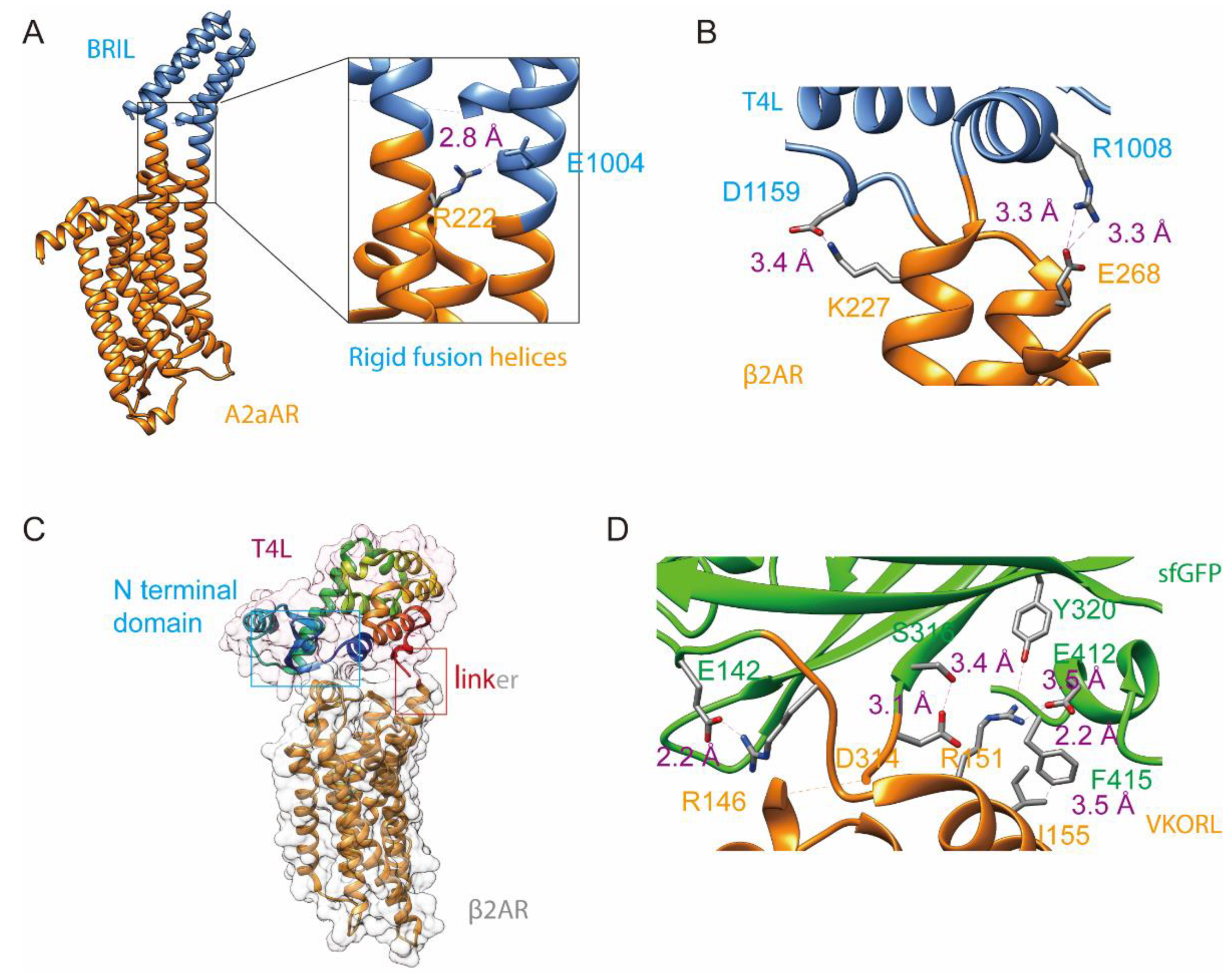
3.1. Fusion to the N Terminus or C Terminus
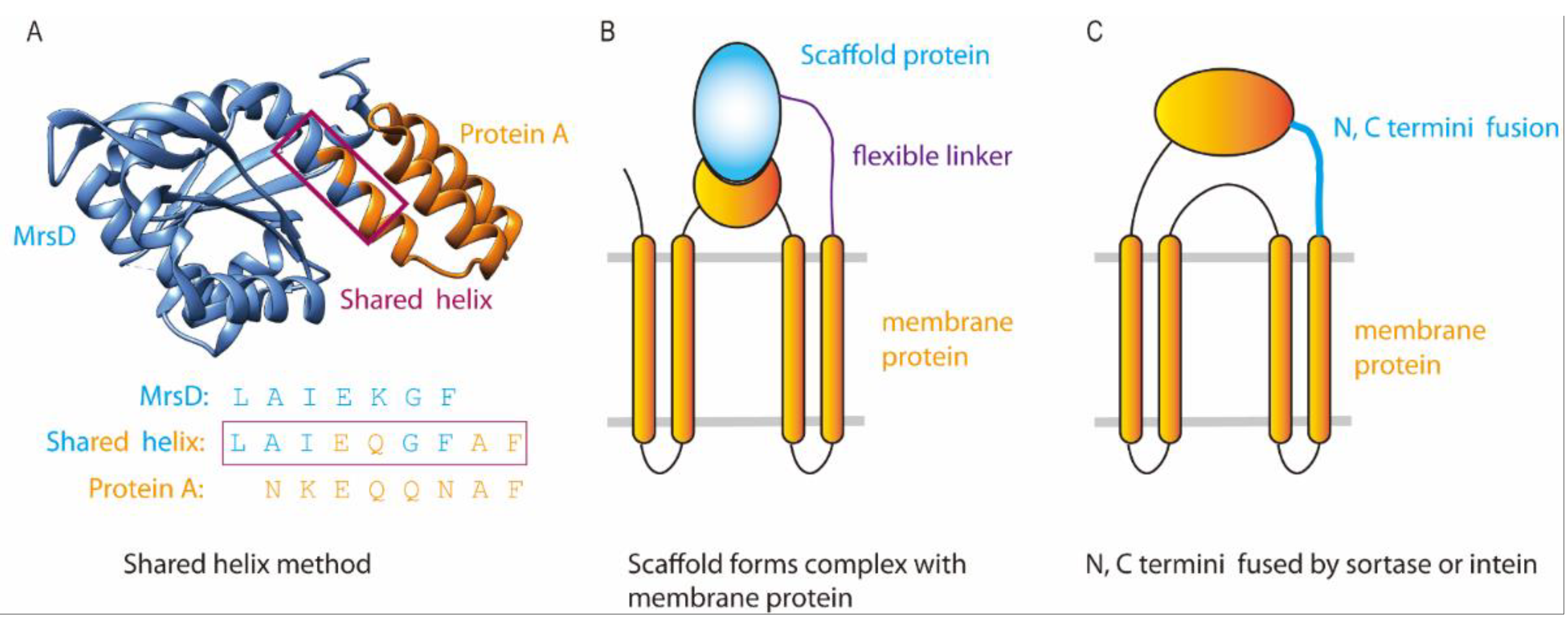
3.2. Insertion Fusion: Scaffold Fusion between Two Transmembrane Helices
3.3. Termini Restraining
4. Selection of Scaffold Proteins
5. Design of Fusion Linkers
6. Crystallization and Phase Determination
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, H.; Flynn, A.D. Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng. 2016, 18, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chen, Y.; Pu, F.; Sun, P.; He, F.; Zhang, L.; Li, Y.; Ma, Z.; Wang, H. Understanding Membrane Protein Drug Targets in Computational Perspective. Curr. Drug Targets 2019, 20, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Basith, S.; Choi, S. Recent Advances in Structure-Based Drug Design Targeting Class A G Protein-Coupled Receptors Utilizing Crystal Structures and Computational Simulations. J. Med. Chem. 2018, 61, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Sowlati-Hashjin, S.; Gandhi, A.; Garton, M. Dawn of a New Era for Membrane Protein Design. BioDes. Res. 2022, 2022, 9791435. [Google Scholar] [CrossRef]
- Ayub, M.; Bayley, H. Engineered transmembrane pores. Curr. Opin. Chem. Biol. 2016, 34, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, C.; Drew, D. Breaking the barriers in membrane protein crystallography. Int. J. Biochem. Cell Biol. 2013, 45, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Kawate, T.; Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 2006, 14, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Hibbs, R.E.; Gouaux, E. A Fluorescence-Detection Size-Exclusion Chromatography-Based Thermostability Assay for Membrane Protein Precrystallization Screening. Structure 2012, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Magnani, F.; Serrano-Vega, M.J.; Shibata, Y.; Abdul-Hussein, S.; Lebon, G.; Miller-Gallacher, J.; Singhal, A.; Strege, A.; Thomas, J.A.; Tate, C.G. A mutagenesis and screening strategy to generate optimally thermostabilized membrane proteins for structural studies. Nat. Protoc. 2016, 11, 1554–1571. [Google Scholar] [CrossRef]
- Waugh, D.S. Crystal structures of MBP fusion proteins. Protein Sci. 2016, 25, 559–571. [Google Scholar] [CrossRef]
- Hu, J.; Qin, H.; Gao, F.P.; Cross, T.A. A systematic assessment of mature MBP in membrane protein production: Overexpression, membrane targeting and purification. Protein Expr. Purif. 2011, 80, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Yeliseev, A.A.; Wong, K.K.; Soubias, O.; Gawrisch, K. Expression of human peripheral cannabinoid receptor for structural studies. Protein Sci. 2005, 14, 2638–2653. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; White, J.F.; Serrano-Vega, M.J.; Magnani, F.; Aloia, A.L.; Grisshammer, R.; Tate, C.G. Thermostabilization of the Neurotensin Receptor NTS1. J. Mol. Biol. 2009, 390, 262–277. [Google Scholar] [CrossRef]
- Schalch, T.; Job, G.; Shanker, S.; Partridge, J.F.; Joshua-Tor, L. The Chp1-Tas3 core is a multifunctional platform critical for gene silencing by RITS. Nat. Struct. Mol. Biol. 2011, 18, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Panavas, T.; Sanders, C.; Butt, T.R. SUMO fusion technology for enhanced protein production in prokaryotic and eukaryotic expression systems. Methods Mol. Biol. 2009, 497, 303–317. [Google Scholar] [PubMed]
- Zuo, X.; Li, S.; Hall, J.; Mattern, M.R.; Tran, H.; Shoo, J.; Tan, R.; Weiss, S.R.; Butt, T.R. Enhanced expression and purification of membrane proteins by SUMO fusion in Escherichia coli. J. Struct. Funct. Genom. 2005, 6, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cherezov, V.; Rosenbaum, D.M.; Hanson, M.A.; Rasmussen, S.G.; Thian, F.S.; Kobilka, T.S.; Choi, H.J.; Kuhn, P.; Weis, W.I.; Kobilka, B.K.; et al. High-resolution crystal structure of an engineered human beta2-adrenergic G protein-coupled receptor. Science 2007, 318, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Chu, R.; Takei, J.; Knowlton, J.R.; Andrykovitch, M.; Pei, W.; Kajava, A.V.; Steinbach, P.J.; Ji, X.; Bai, Y. Redesign of a Four-helix Bundle Protein by Phage Display Coupled with Proteolysis and Structural Characterization by NMR and X-ray Crystallography. J. Mol. Biol. 2002, 323, 253–262. [Google Scholar] [CrossRef]
- Chun, E.; Thompson, A.A.; Liu, W.; Roth, C.B.; Griffith, M.T.; Katritch, V.; Kunken, J.; Xu, F.; Cherezov, V.; Hanson, M.A.; et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 2012, 20, 967–976. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, J.; Kong, W.T. Improved membrane protein expression in Lactococcus lactis by fusion to Mistic. Microbiol.-Sgm. 2013, 159, 1002–1009. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deniaud, A.; Bernaudat, F.; Frelet-Barrand, A.; Juillan-Binard, C.; Vernet, T.; Rolland, N.; Pebay-Peyroula, E. Expression of a chloroplast ATP/ADP transporter in E. coli membranes: Behind the Mistic strategy. Bba-Biomembranes 2011, 1808, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Feng, R.T.; Tong, Q.; Zhang, Y.X.; Xie, X.Q. Mistic and TarCF as fusion protein partners for functional expression of the cannabinoid receptor 2 in Escherichia coli. Protein Expr. Purif. 2012, 83, 128–134. [Google Scholar] [CrossRef]
- Pao, P.J.; Hsu, M.F.; Chiang, M.H.; Chen, C.T.; Lee, C.C.; Wang, A.H. Structural basis of an epitope tagging system derived from Haloarcula marismortui bacteriorhodopsin I D94N and its monoclonal antibody GD-26. FEBS J. 2022, 289, 730–747. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.F.; Yu, T.F.; Chou, C.C.; Fu, H.Y.; Yang, C.S.; Wang, A.H. Using Haloarcula marismortui bacteriorhodopsin as a fusion tag for enhancing and visible expression of integral membrane proteins in Escherichia coli. PLoS ONE 2013, 8, e56363. [Google Scholar] [CrossRef] [PubMed]
- Pedelacq, J.D.; Cabantous, S.; Tran, T.; Terwilliger, T.C.; Waldo, G.S. Engineering and characterization of a superfolder green fluorescent protein. Nat. Biotechnol. 2006, 24, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.S.; Zacharias, D.A.; Tsien, R.Y. Circular permutation and receptor insertion within green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 11241–11246. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Y.; Cui, Z.Q.; Wei, H.P.; Zhang, Z.P.; Zhou, Y.F.; Wang, Y.P.; Zhang, X.E. Split mCherry as a new red bimolecular fluorescence complementation system for visualizing protein-protein interactions in living cells. Biochem. Biophys. Res. Commun. 2008, 367, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Varshavsky, A. The N-end rule pathway and regulation by proteolysis. Protein Sci. 2011, 20, 1298–1345. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Yang, Y.; Li, W. Termini restraining of small membrane proteins enables structure determination at near-atomic resolution. Sci. Adv. 2020, 6, eabe3717. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, S.; Krezel, A.M.; Li, W. Stabilization and structure determination of integral membrane proteins by termini restraining. Nat. Protoc. 2022, 17, 540–565. [Google Scholar] [CrossRef]
- Liu, S.; Li, S.; Shen, G.; Sukumar, N.; Krezel, A.M.; Li, W. Structural basis of antagonizing the vitamin K catalytic cycle for anticoagulation. Science 2021, 371, eabc5667. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Singh, V.K.; Blonde, J.D.; Jia, Z. Crystallization and preliminary X-ray analysis of the GST-fused human Bri3 N-terminal domain. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2005, 61, 62–64. [Google Scholar] [CrossRef]
- Smyth, D.R.; Mrozkiewicz, M.K.; McGrath, W.J.; Listwan, P.; Kobe, B. Crystal structures of fusion proteins with large-affinity tags. Protein Sci. 2003, 12, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Weis, W.I.; Kobilka, B.K. N-terminal T4 lysozyme fusion facilitates crystallization of a G protein coupled receptor. PLoS ONE 2012, 7, e46039. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.G.; DeVree, B.T.; Zou, Y.; Kruse, A.C.; Chung, K.Y.; Kobilka, T.S.; Thian, F.S.; Chae, P.S.; Pardon, E.; Calinski, D.; et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature 2011, 477, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhou, X.E.; Gao, X.; He, Y.; Liu, W.; Ishchenko, A.; Barty, A.; White, T.A.; Yefanov, O.; Han, G.W.; et al. Crystal structure of rhodopsin bound to arrestin by femtosecond X-ray laser. Nature 2015, 523, 561–567. [Google Scholar] [CrossRef]
- Lu, M.; Wu, B. Structural studies of G protein-coupled receptors. IUBMB Life 2016, 68, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, M. A comprehensive review of the lipid cubic phase or in meso method for crystallizing membrane and soluble proteins and complexes. Acta Crystallogr. F Struct. Biol. Commun. 2015, 71, 3–18. [Google Scholar] [CrossRef]
- Thorsen, T.S.; Matt, R.; Weis, W.I.; Kobilka, B.K. Modified T4 Lysozyme Fusion Proteins Facilitate G Protein-Coupled Receptor Crystallogenesis. Structure 2014, 22, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, H.; Asada, H.; Suzuki, M.; Takahashi, Y.; Yasunaga, M.; Suno, C.; Iwata, S.; Saito, J.I. The discovery of a new antibody for BRIL-fused GPCR structure determination. Sci. Rep. 2020, 10, 11669. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Logeman, B.L.; Zhang, X.; Liu, Y.; Thiele, D.J.; Yuan, P. X-ray structures of the high-affinity copper transporter Ctr1. Nat. Commun. 2019, 10, 1386. [Google Scholar] [CrossRef]
- Yin, J.; Mobarec, J.C.; Kolb, P.; Rosenbaum, D.M. Crystal structure of the human OX2 orexin receptor bound to the insomnia drug suvorexant. Nature 2015, 519, 247–250. [Google Scholar] [CrossRef]
- Birch, J.; Axford, D.; Foadi, J.; Meyer, A.; Eckhardt, A.; Thielmann, Y.; Moraes, I. The fine art of integral membrane protein crystallisation. Methods 2018, 147, 150–162. [Google Scholar] [CrossRef]
- Inaba, K.; Murakami, S.; Nakagawa, A.; Iida, H.; Kinjo, M.; Ito, K.; Suzuki, M. Dynamic nature of disulphide bond formation catalysts revealed by crystal structures of DsbB. EMBO J. 2009, 28, 779–791. [Google Scholar] [CrossRef]
- Inaba, K.; Ito, K. Structure and mechanisms of the DsbB-DsbA disulfide bond generation machine. Biochim. Biophys. Acta 2008, 1783, 520–529. [Google Scholar] [CrossRef]
- Phan, J.; Zdanov, A.; Evdokimov, A.G.; Tropea, J.E.; Peters, H.K., 3rd; Kapust, R.B.; Li, M.; Wlodawer, A.; Waugh, D.S. Structural basis for the substrate specificity of tobacco etch virus protease. J. Biol. Chem. 2002, 277, 50564–50572. [Google Scholar] [CrossRef]
- Matthews, D.A.; Dragovich, P.S.; Webber, S.E.; Fuhrman, S.A.; Patick, A.K.; Zalman, L.S.; Hendrickson, T.F.; Love, R.A.; Prins, T.J.; Marakovits, J.T.; et al. Structure-assisted design of mechanism-based irreversible inhibitors of human rhinovirus 3C protease with potent antiviral activity against multiple rhinovirus serotypes. Proc. Natl. Acad. Sci. USA 1999, 96, 11000–11007. [Google Scholar] [CrossRef]
- Kwon, N.Y.; Kim, Y.; Lee, J.O. The application of helix fusion methods in structural biology. Curr. Opin. Struct. Biol. 2020, 60, 110–116. [Google Scholar] [CrossRef]
- Jin, T.; Chuenchor, W.; Jiang, J.; Cheng, J.; Li, Y.; Fang, K.; Huang, M.; Smith, P.; Xiao, T.S. Design of an expression system to enhance MBP-mediated crystallization. Sci. Rep. 2017, 7, 40991. [Google Scholar] [CrossRef]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef]
- Liu, W.; Chun, E.; Thompson, A.A.; Chubukov, P.; Xu, F.; Katritch, V.; Han, G.W.; Roth, C.B.; Heitman, L.H.; AP, I.J.; et al. Structural basis for allosteric regulation of GPCRs by sodium ions. Science 2012, 337, 232–236. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Wacker, D.; Wang, S.; Agegnehu, B.; Liu, J.; Lansu, K.; Tribo, A.R.; Olsen, R.H.J.; Che, T.; Jin, J.; et al. Structural determinants of 5-HT2B receptor activation and biased agonism. Nat. Struct. Mol. Biol. 2018, 25, 787–796. [Google Scholar] [CrossRef]
- White, K.L.; Eddy, M.T.; Gao, Z.G.; Han, G.W.; Lian, T.; Deary, A.; Patel, N.; Jacobson, K.A.; Katritch, V.; Stevens, R.C. Structural Connection between Activation Microswitch and Allosteric Sodium Site in GPCR Signaling. Structure 2018, 26, 259–269.e5. [Google Scholar] [CrossRef]
- Vuckovic, Z.; Gentry, P.R.; Berizzi, A.E.; Hirata, K.; Varghese, S.; Thompson, G.; van der Westhuizen, E.T.; Burger, W.A.C.; Rahmani, R.; Valant, C.; et al. Crystal structure of the M5 muscarinic acetylcholine receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 26001–26007. [Google Scholar] [CrossRef]
- Youn, S.J.; Kwon, N.Y.; Lee, J.H.; Kim, J.H.; Choi, J.; Lee, H.; Lee, J.O. Construction of novel repeat proteins with rigid and predictable structures using a shared helix method. Sci. Rep. 2017, 7, 2595. [Google Scholar] [CrossRef]
- Jeong, W.H.; Lee, H.; Song, D.H.; Eom, J.H.; Kim, S.C.; Lee, H.S.; Lee, H.; Lee, J.O. Connecting two proteins using a fusion alpha helix stabilized by a chemical cross linker. Nat. Commun. 2016, 7, 11031. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Li, W.; Li, F. Cross-crystal averaging with search models to improve molecular replacement phases. Structure 2011, 19, 155–161. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, X.R.; Greenberg, Z.J.; Zhou, F.; He, P.; Fan, L.; Liu, S.; Shen, G.; Egawa, T.; Gross, M.L.; et al. Open conformation of tetraspanins shapes interaction partner networks on cell membranes. EMBO J. 2020, 39, e105246. [Google Scholar] [CrossRef]
- Chen, I.; Dorr, B.M.; Liu, D.R. A general strategy for the evolution of bond-forming enzymes using yeast display. Proc. Natl. Acad. Sci. USA 2011, 108, 11399–11404. [Google Scholar] [CrossRef]
- Pinto, F.; Thornton, E.L.; Wang, B. An expanded library of orthogonal split inteins enables modular multi-peptide assemblies. Nat. Commun. 2020, 11, 1529. [Google Scholar] [CrossRef]
- Conlan, B.F.; Gillon, A.D.; Craik, D.J.; Anderson, M.A. Circular Proteins and Mechanisms of Cyclization. Biopolymers 2010, 94, 573–583. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Li, W. Protein Fusion Strategies for Membrane Protein Stabilization and Crystal Structure Determination. Crystals 2022, 12, 1041. https://doi.org/10.3390/cryst12081041
Liu S, Li W. Protein Fusion Strategies for Membrane Protein Stabilization and Crystal Structure Determination. Crystals. 2022; 12(8):1041. https://doi.org/10.3390/cryst12081041
Chicago/Turabian StyleLiu, Shixuan, and Weikai Li. 2022. "Protein Fusion Strategies for Membrane Protein Stabilization and Crystal Structure Determination" Crystals 12, no. 8: 1041. https://doi.org/10.3390/cryst12081041
APA StyleLiu, S., & Li, W. (2022). Protein Fusion Strategies for Membrane Protein Stabilization and Crystal Structure Determination. Crystals, 12(8), 1041. https://doi.org/10.3390/cryst12081041




