Abstract
Sulphonamide motif is found extensively in numerous chemotherapeutic drug candidates, it acts by stopping the production of folate inside the bacterial cell. Current research has established the synthesis and characterization of new bioprecursor prodrugs of sulfadiazine. The first prodrug, 3, was synthesized via the coupling of diazonium salt of sulfadiazine with ethyl acetoacetate in AcONa at 0 °C. The second prodrug, sulfadiazine-pyrazole, 5, was furnished via cyclocondensation of the hydrazono derivative, 3, and 2-pyridyl hydrazine, 4. The generated data from the X-ray analysis is interpreted and refined to obtain the crystal structure of the target compound, 5. Density functional theory (DFT) method was used to calculate the optimized geometrical parameters, electronic state (HOMO–LUMO), and the electronic properties. Moreover, Hirshfeld analysis revealed that the most important contributions to the crystal packing of the prodrug 5 are H···H, O···H and H···C contacts.
1. Introduction
Sulfonamides inhibit bacterial folate biosynthesis and have been extensively used as broad-spectrum antimicrobials for decades, making use of the different metabolic pathways of microbial and human cells. Nonetheless, bacteria invariably develop resistance to any introduced therapy and only drug combinations, currently used in clinic, can effectively combat the multidrug-resistant (MDR) [1]. Inherent and developed resistance modes of bacteria to antibiotics are problems in the design of new drugs. In the era of bacterial resistance, a prodrug strategy can be employed that requires bacterium-specific enzymes to release the active drug at the infection site. Therefore, targeting the prodrugs to a specific enzyme has potential as a selective drug delivery system in microbial chemotherapy [2,3]. Additionally, prodrugs may enhance the pharmacological activity or pharmacokinetic properties of a parent drug molecule.
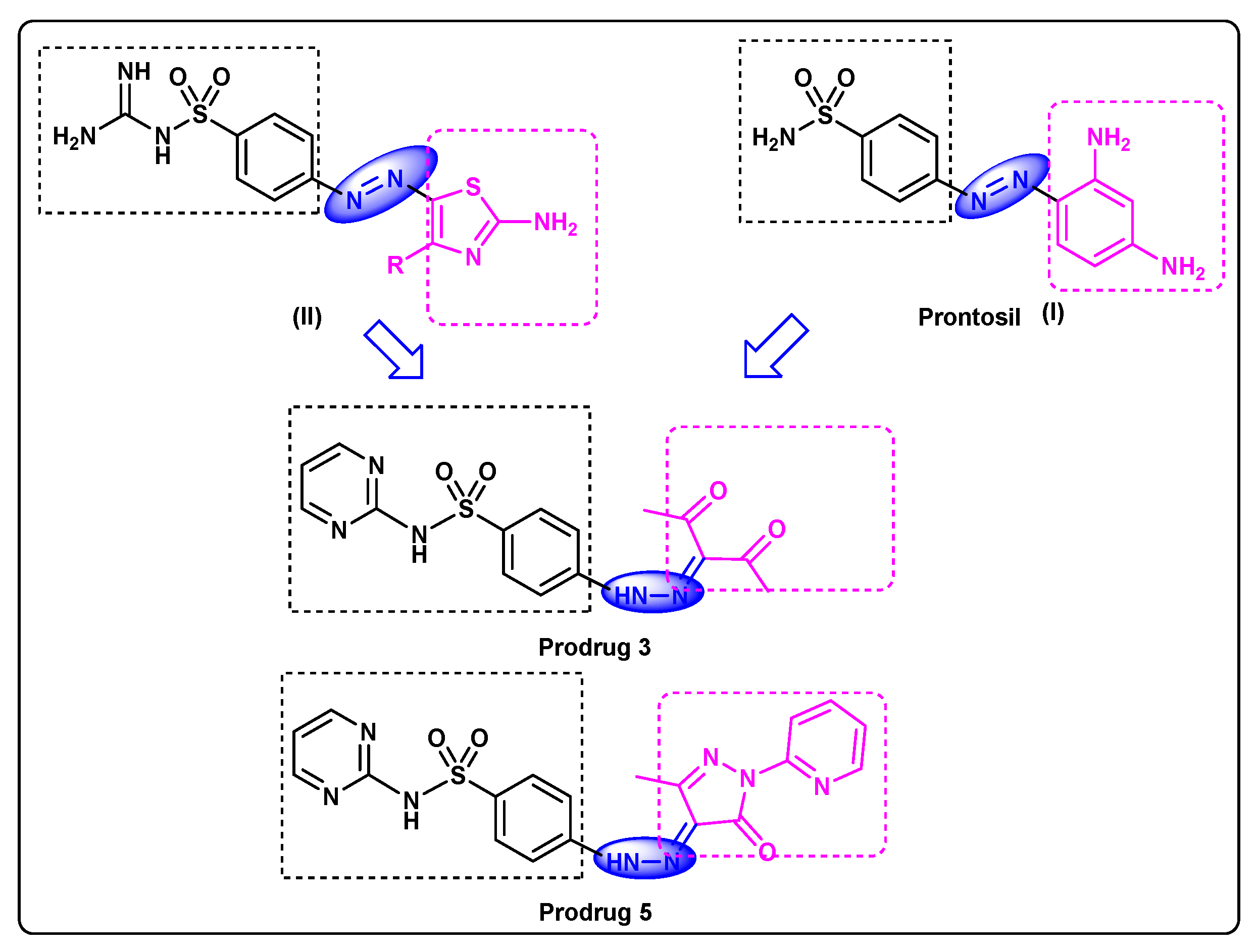
The azo group/sulphonamide hybrid structure was the first well-organized chemotherapeutic agent that could be applied efficiently for the treatment of infections caused by bacteria in humans. Prontosil (I, Figure 1) was recognized as a bioprecursor to the active compound, sulfanilamide, possibly metabolized by azoreductases released either in the liver or by gut microbiota [4,5]. Azoreductases are flavoenzymes that have been distinguished in a range of prokaryotes and eukaryotes [6]. Gaffer et al. [7] explored the synthetic methodology of new amino-thiazolylazo-sulphonamide (II). The synthesized dyes were investigated for their anti-bacterial and anti-fungal activities against Gram-positive and Gram-negative bacteria, as well as a fungi (C. albicans).
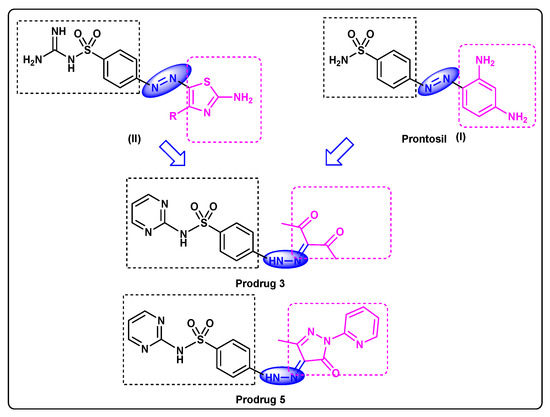
Figure 1.
Feature similarities between leads I, II and target compound.
Sulfadiazine (2-sulfanilamidopyrimidine) is used to a large extent for the elimination of bacteria that cause urinary tract infections. It is also used in combined therapy with pyrimethamine and folinic acid for the treatment of some parasitic diseases such as malaria and toxoplasmosis. Silver sulfadiazine is an efficient prescription for burn and wounds treatment [1]; in addition, it has been prescribed for treating bacterial infections, e.g., otitis media, encephalitis, and severe meningococcal meningitis, besides its role as a prophylactic treatment for rheumatic fever. Sulfadiazine targets the dihydropetroate synthase (DHPS), producing a bacteriostatic effect, with a wide spectrum against most Gram-positive and many Gram-negative organisms [1,8].
As a continuation to our research on sulfa drugs [9,10,11], our current research focus is on sulfadiazine [12], a model scaffold to discover new sulfadiazine bioprecursors. Herein, novel models have been designed which feature similarities between our target compounds and lead compounds, as clarified in Figure 1. They are synthesized and characterized by microanalytical analyses, 1HNMR and 13CNMR. Particularly, sulfadiazine-pyrazole prodrug 5 is further investigated via X-ray single crystal diffraction which provides the screening, testing, and complete data collection. Furthermore, density functional methods (DFT) will be applied to achieve a valuable understanding of the electronic and molecular properties of the target 5.
2. Materials and Instrumentations
2.1. Materials and Equipments
All materials and instruments are given in Supplementary materials.
2.2. Synthesis of (E)-4-(2-(3-Methyl-5-oxo-1-(pyridin-2-yl)-1H-pyrazol-4(5H)-ylidene) hydrazinyl)-N-(pyrimidin-2-yl)benzenesulfonamide (5)
Synthesis of compound 3 was performed according to the method reported in the literature as described in Supplementary materials [12]. Then, a solution of the hydrazino 3 (1.0 mmol) in absolute EtOH (10 mL), 2-hydrazinopyridine 4 (1.2 mmol) was added, and the mixture was then allowed to reflux for 8 h, before the formed precipitate was collected by filtration, recrystallized from EtOH to create pale yellow crystals, (270 mg, 62%), m.p = 292–294 °C 1HNMR (500 MHz, DMSO-d6) δH: 13.03 (bs, 1H, N-H), 11.81(bs, 1H, N-H), 8.47 (d, J = 4.5 Hz, 2H, Ar-H), 8.41 (d, J = 3.0 Hz, 1H, Ar-H), 7.98 (d, J = 9.0 Hz, 2H, Ar-H), 7.87 (t, J = 8.5 Hz, 1H, Ar-H), 7.78 (d, J = 8.0 Hz, 1H, Ar-H), 7.70 (d, J = 9.0 Hz, 2H, Ar-H), 7.22 (t, J = 7.5 Hz, 1H, Ar-H), 7.01 (t, J = 6.5 Hz, 1H, Ar-H), 2.23 (s, 3H, CH3); 13C NMR (125 MHz, DMSO-d6) δC: 158.9, 157.3, 156.9, 149.8, 149.4, 148.9, 145.4, 138.9, 136.5, 129.9, 121.7, 116.3, 114.5 (Ar-C), 12.2 (CH3); Anal. Calcd. for C19H16N8O3S: C, 52.29; H, 3.70; N, 25.67; Found C, 52.47; H, 3.91; N, 25.42.
2.3. X-ray Structure Analyses
The accomplished utilizing of 5 was determined using the method described in Supplementary data [13,14,15,16]. The crystal data are given in Table 1. Analysis of the crystal packing was accomplished utilizing using Crystal Explorer 17.5 program [17].

Table 1.
Data of the Crystal 5.
2.4. Computational Methods
3. Results and Discussion
3.1. Chemistry
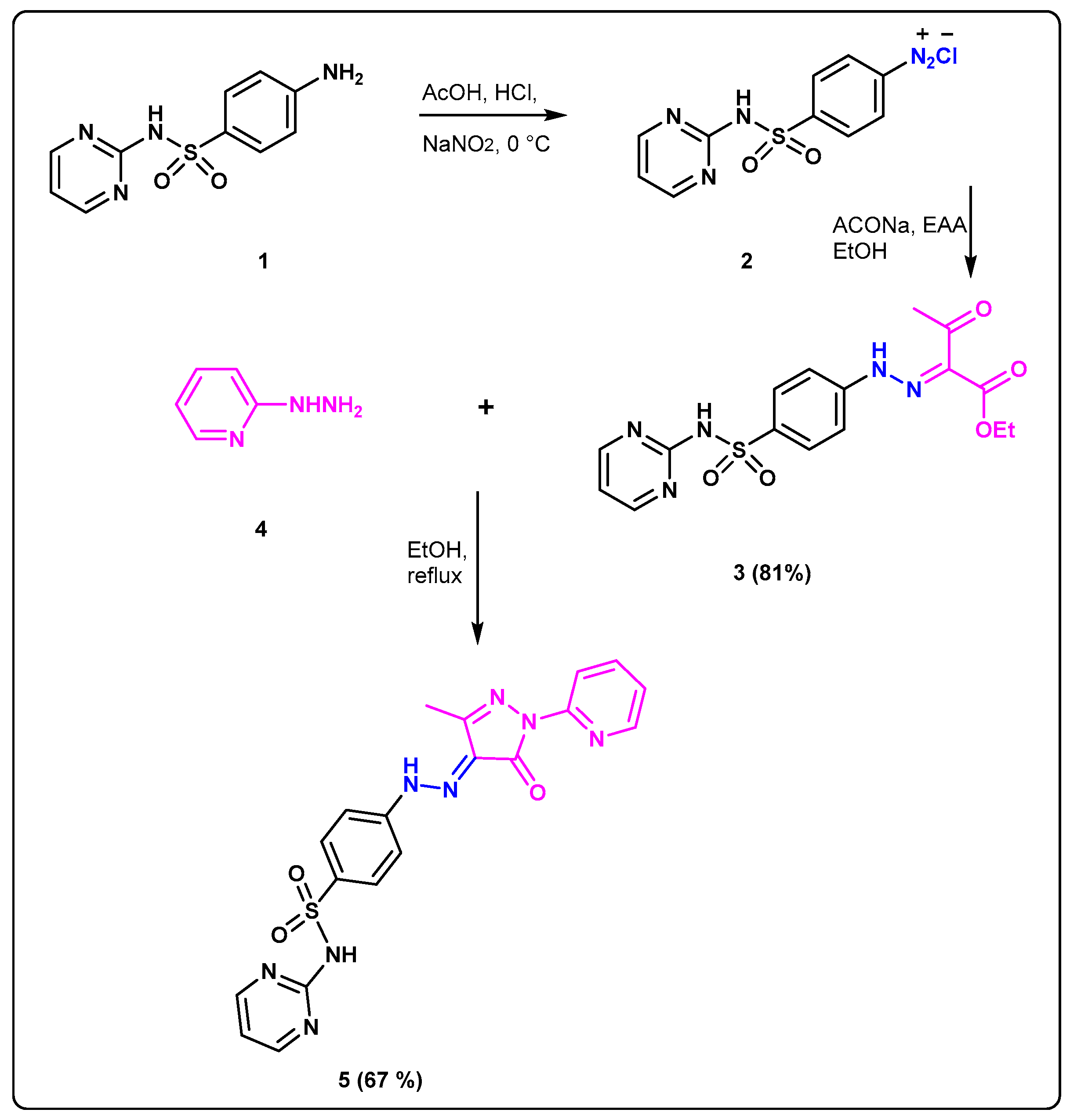
The facile synthetic protocol was performed for preparation of new sulfadiazine prodrug 5 (Scheme 1). Reaction of the sulfadiazine 1 with the solution of NaNO2 to accomplish the diazotization step at 0 °C at pH = 2–3 afforded the corresponding diazonium salt 2 as a clear solution. The coupling of 2 with the previously prepared carbanion salt of ethylacetoacetate at 0 °C gave the desired hydrazono-ethylacetoacetate 3. Confirmation of the correct structure 3 was accomplished by the spectroscopic analysis where 1HNMR provided two separate peaks at δH: 11.72 and 11.53 ppm correspond to two N-H protons, as well as two non-homotopic protons signals at 4.26 and 1.22 ppm representing two types of ethyl protons. Furthermore, the singlet signal at δH: 2.38 revealed to CH3CO; 13CNMR spectra of 3 showed two different types of C=O at δC: 194.5 and 162.7 beside two different signals at δC 25.7, 14.1 ppm revealed to carbons of two non-homotopic CH3. Cyclocondensation of the prodrug 3 with 2-hydrazinopyridine 4 was accomplished by heating in absolute EtOH affording the desired sulfadiazinepyrazolo prodrugs derivative 5. Confirmation of the correct structure of the sulfonamide 5 was accomplished by the spectroscopic analysis where 1HNMR gave characteristic signals at δH: 13.03 and 11.81 ppm revealed to two different sets of NH protons beside one signals in the aliphatic region at δH: 2.23 ppm representing to CH3 also 13C NMR of 5 showed 3 types of carbonyl carbons at δC: 158.9, as well one signal in aliphatic region at δC 12.2 ppm corresponded to CH3 carbons.
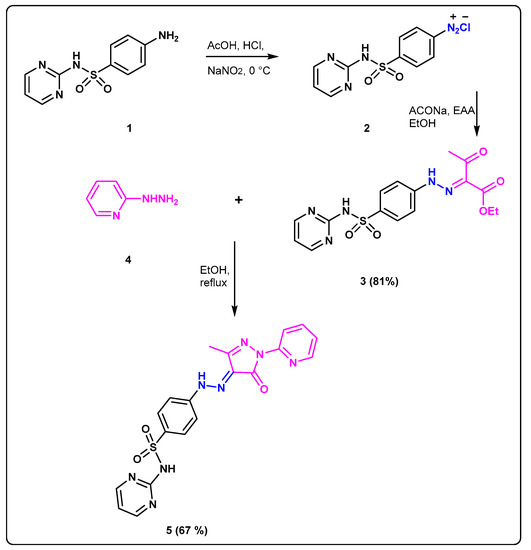
Scheme 1.
Synthesis of the target prodrugs 3 and 5.
3.2. The Description of X-ray Structure of 5
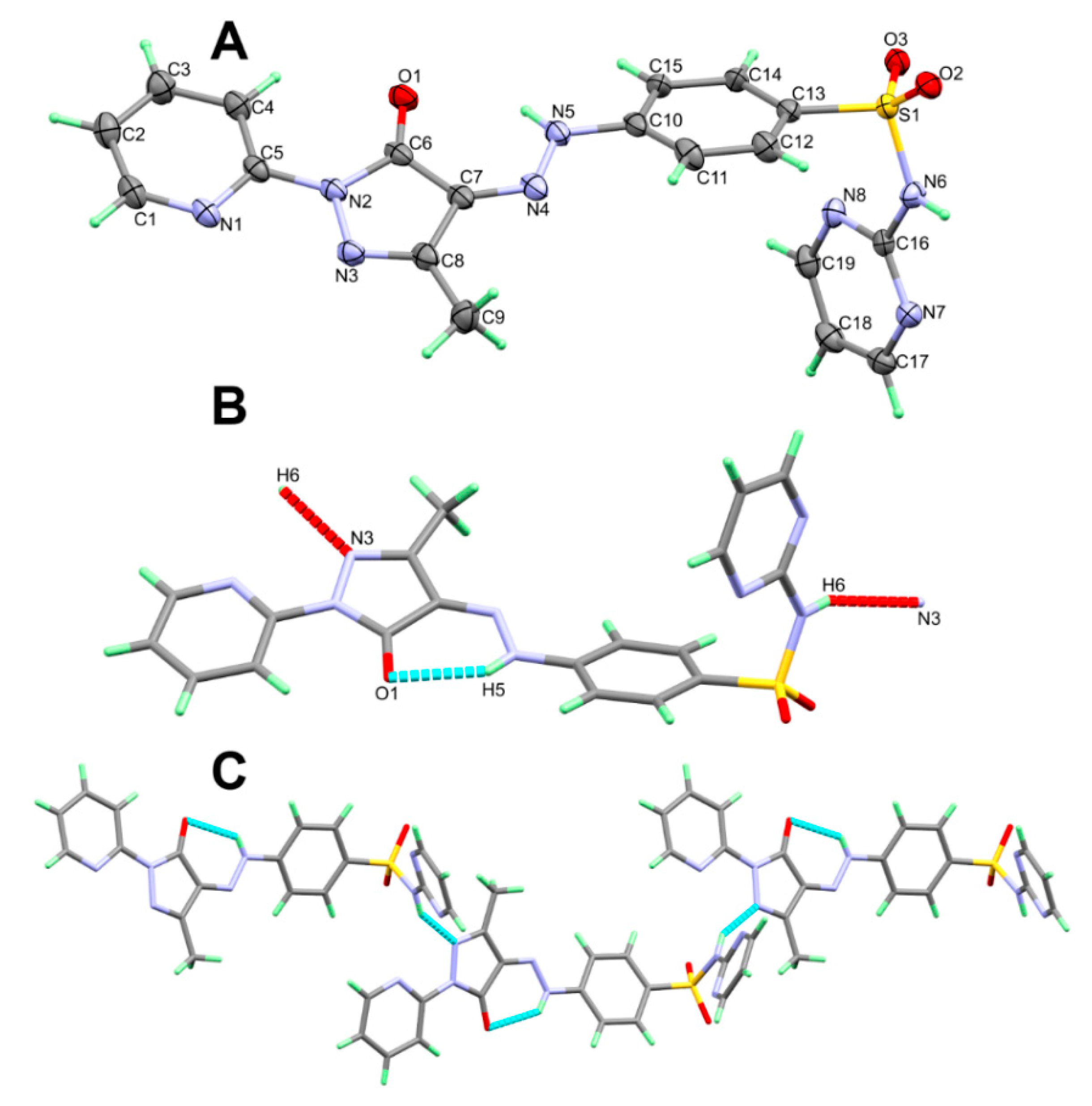
The X-ray structure of 5 shown in Figure 2A agreed very well with its spectral data. It crystallized in the monoclinic C2/c space group. There are eight molecules per unit cell and one molecular unit as an asymmetric formula. The unit cell parameters are a = 21.8909(2) Å, b = 11.45860(10) Å, c = 15.77880(10) Å, β = 99.0430(10)° and V = 3908.74(6) Å3. Selected geometric parameters are depicted in Table 2. The pyridyl and the five membered rings are connected to one another by C(5)-N(2) bond where both rings are twisted from one another by only 6.45°. On the other hand, the phenyl moiety is found twisted further from the five membered ring mean plane. The twist angle in this case is 23.30°.
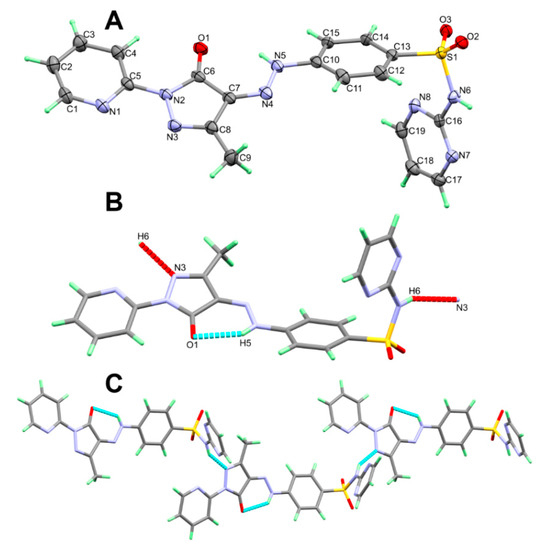
Figure 2.
The asymmetric formula (A), H-bond contacts (B) and packing (C) of 5.

Table 2.
Selected geometric parameters [Å and °] for 5.
The molecular structure of 5 is stabilized by the N(5)-H(5)···O(1) intramolecular H-bond while the crystal is packed in by the N(6)-H(6)···N(3) intermolecular H-bond (Figure 2B). The donor–acceptor distance is 2.793(2) and 2.932(2) Å, respectively (Table 3). The packing of these molecular units showed a 1D hydrogen bonding polymer extended along the a-direction (Figure 2C).

Table 3.
Hydrogen bonds for prodrug 5 [Å and °].
3.3. Hirshfeld Surface Analysis
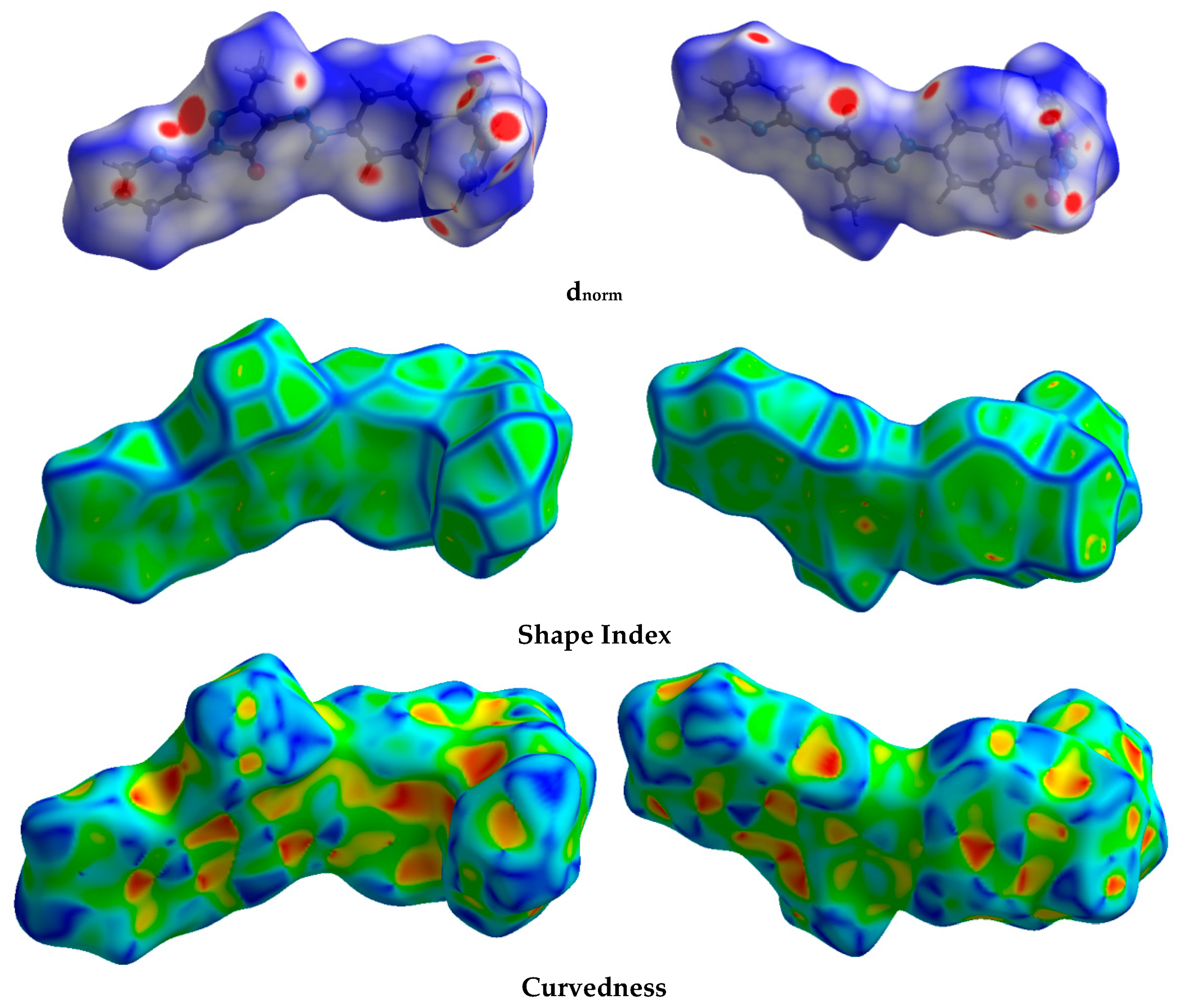
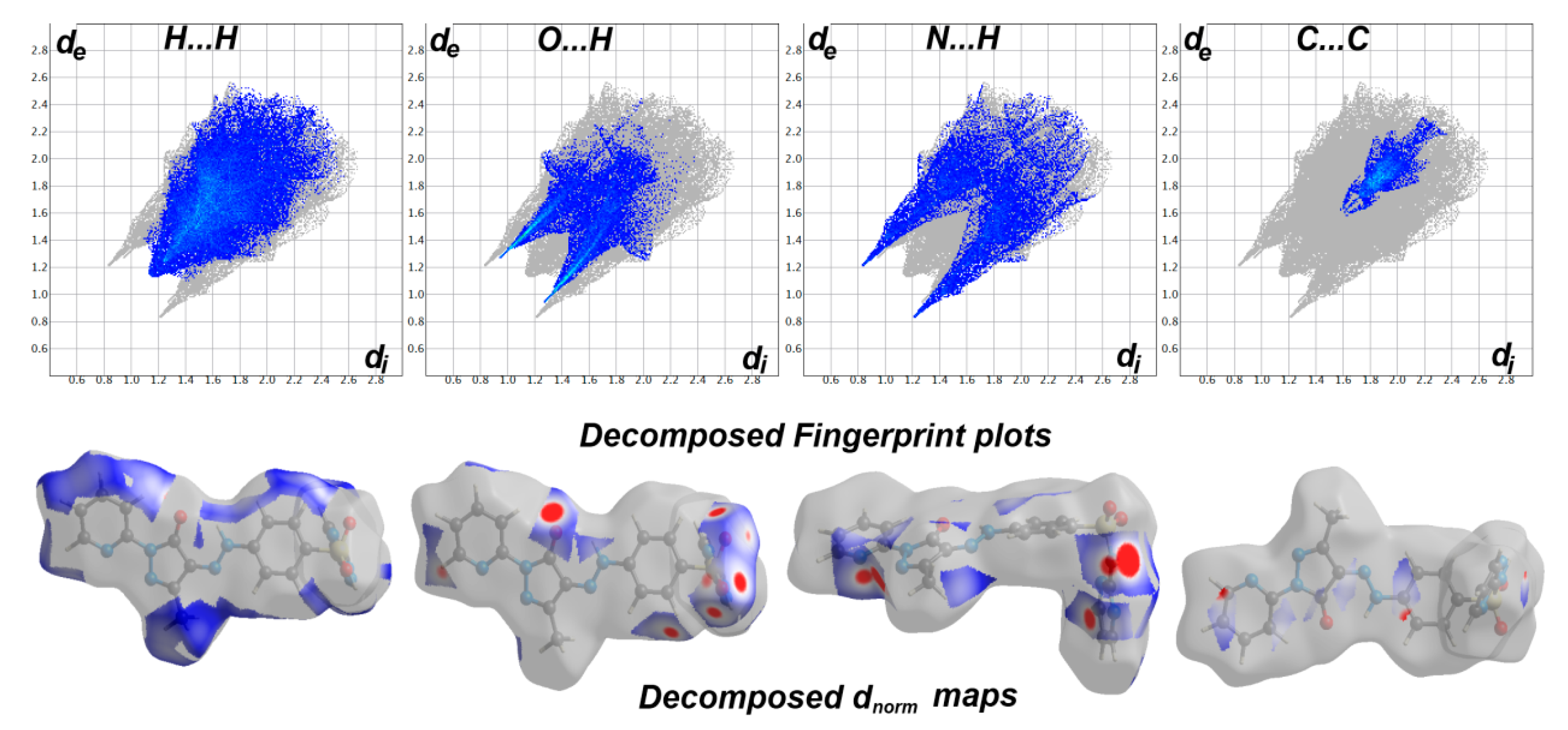
The Hirshfeld calculation is a simple and accurate tool for the finding the different atom–atom contacts in the crystal structure. Hence, decomposition of the different intermolecular contacts in the crystal structure of 5 was performed using Hirshfeld calculations. The resulting Hirshfeld maps are presented in Figure 3. There are different levels of intermolecular contacts as indicated from the presence of red, white, and blue regions in the dnorm map.
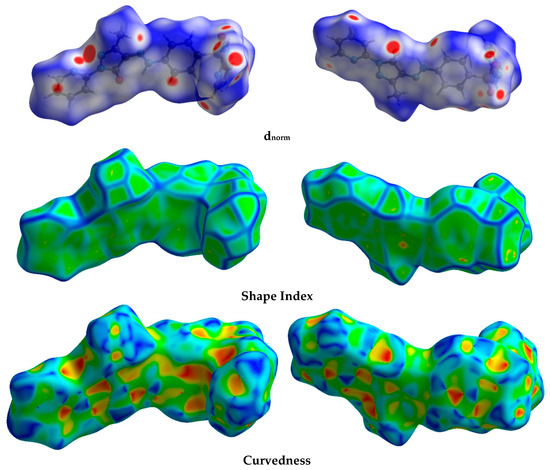
Figure 3.
Hirshfeld surfaces of prodrug 5.
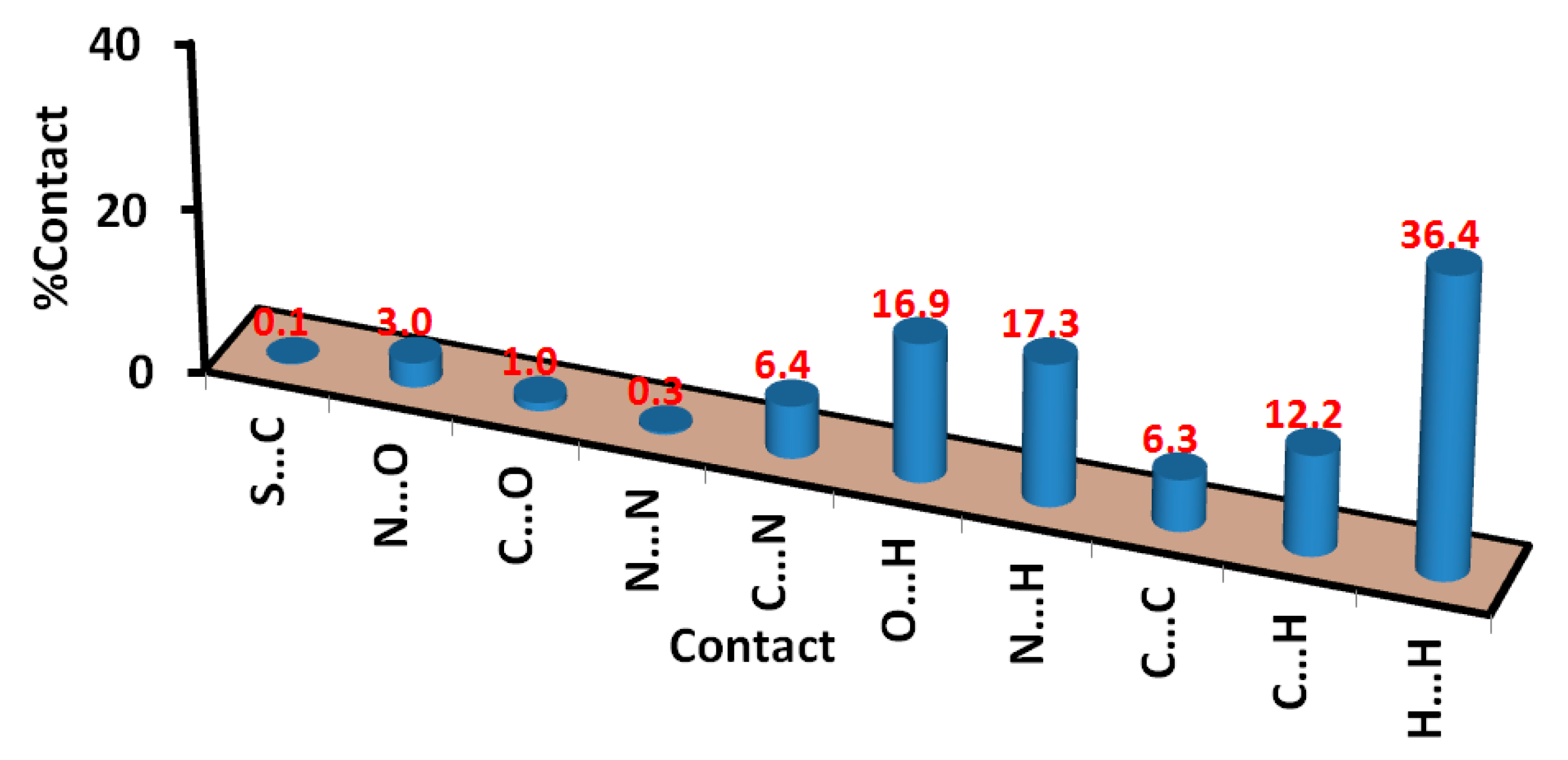
Analysis of these interactions using fingerprint plot is given in Figure 4. The fingerprint area gave the percentages of all intermolecular interactions in the crystal structure of 5. Presentation for these interactions and the percentages for all contacts in 5 is shown in Figure 5. The percentages of the H···H, H···C, N···H, and O···H interactions are 36.4, 12.2, 17.3, and 16.9%, respectively. It is worth noting that the N···H and O···H contacts have small interaction distances (Table 4). In addition, the presence of π–π stacking interactions is revealed by the presence of short C1···C15 contact (3.211 Å) and the presence of red/blue triangles in the shape index map and flat green area in curvedness (Figure 3).
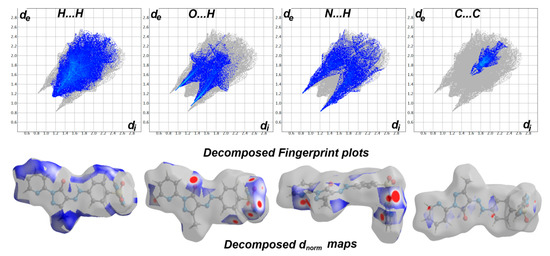
Figure 4.
Fingerprint plots and decomposed dnorm maps in 5.
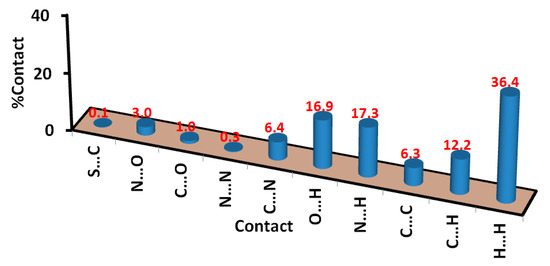
Figure 5.
Intermolecular contacts and their percentages in prodrug 5.

Table 4.
Short contacts in 5.
3.4. DFT Studies
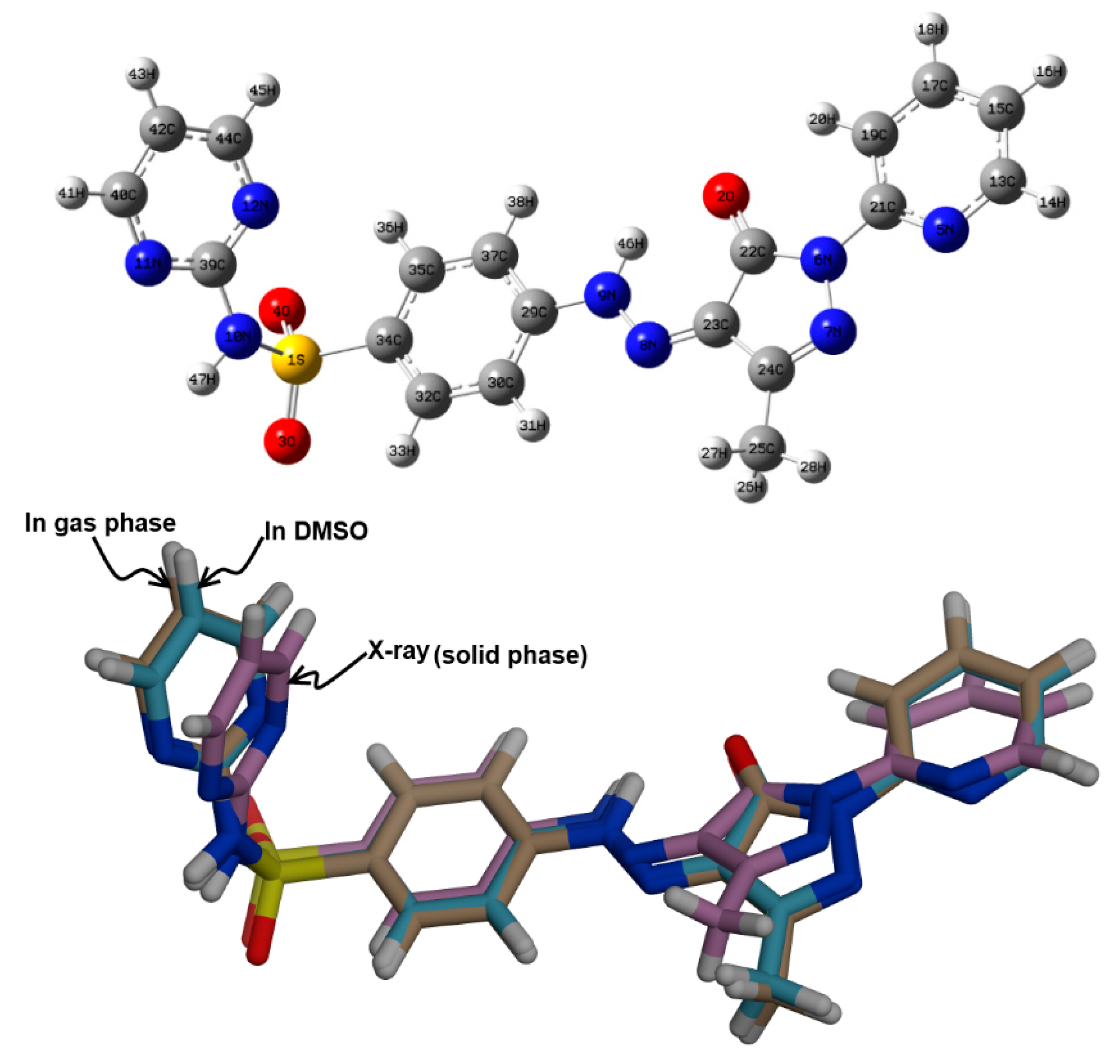
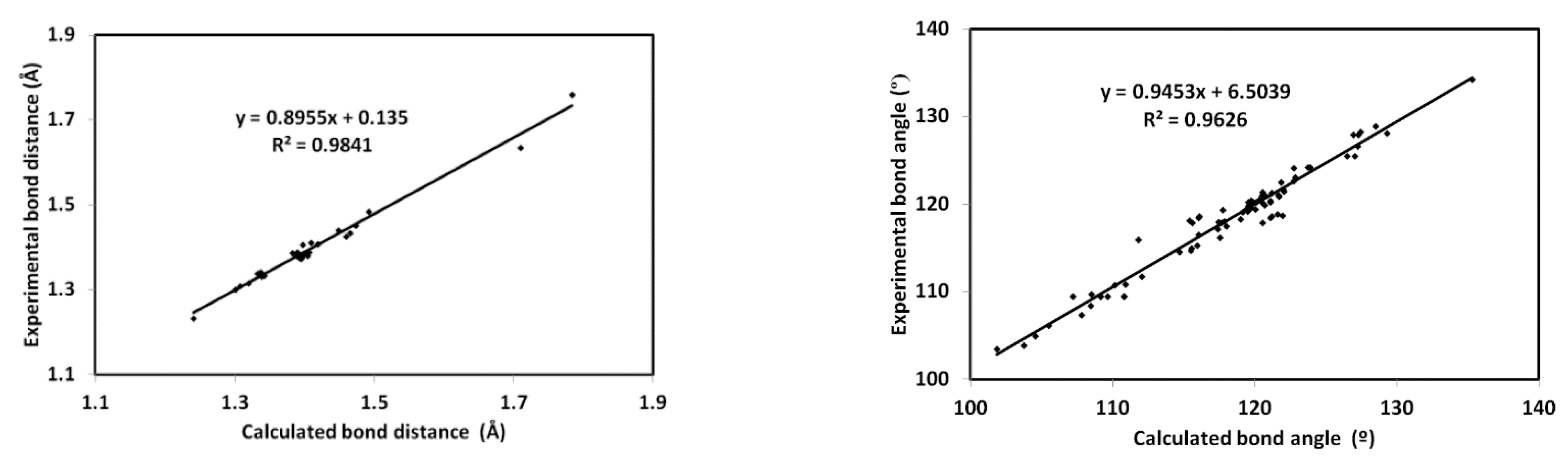
The minimum energy structure of 5 is shown in Figure 6. Its overlay with the X-ray geometry is shown in the same figure. Generally, there is structural matching between both structures and also good correlations between the optimized and X-ray geometric parameters (Figure 7) which reveal these observations very well (Table S1, Supporting information).
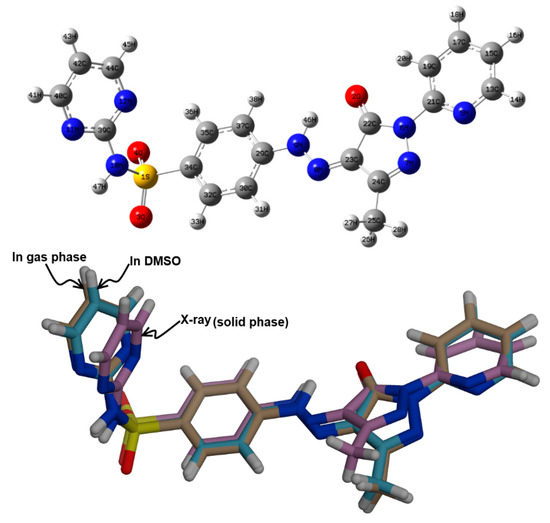
Figure 6.
The minimum energy structure (upper) compared with the X-ray structure (lower) of 5.
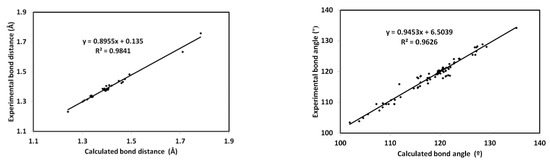
Figure 7.
Correlations between the optimized and X-ray geometric parameters.
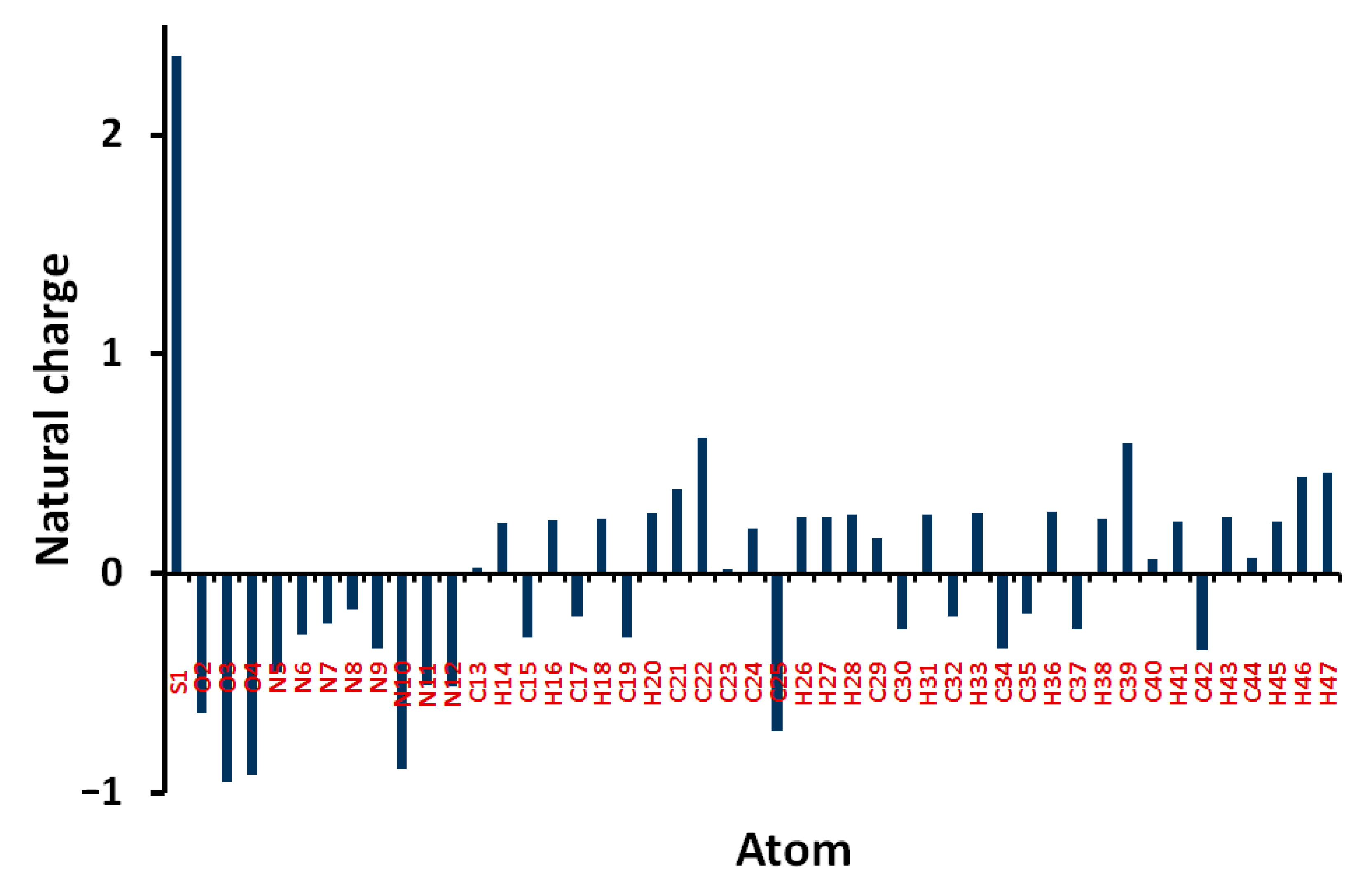
Natural charges of 5 shown in Figure 8 indicate that the O, N, and most of C-atoms are electronegative. The O-sites have charges ranging from −0.6363 to −0.9475 e where the most negative oxygen sites are those for the sulphonyl group. The corresponding S-atom is the most electropositive (2.3582 e). Regarding carbon atoms, C39 (0.5956 e) which is bonded to three nitrogen atoms and the carbonyl carbon (0.6173 e) is the most electropositive carbon site. In contrast, all the H-atoms are charged positively where the H46 (0.4409 e) and H47 (0.4617 e) are the most positive hydrogen sites. Presentation of electrostatic potential (MEP) is shown in Figure 9. The molecule has a net dipole moment of 4.9143 D.
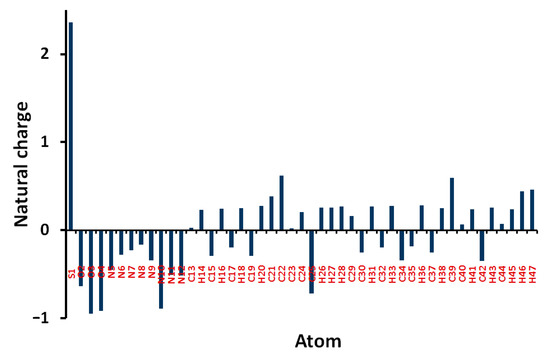
Figure 8.
Atomic charges in 5.
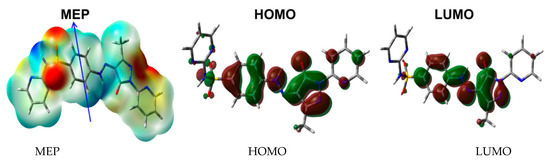
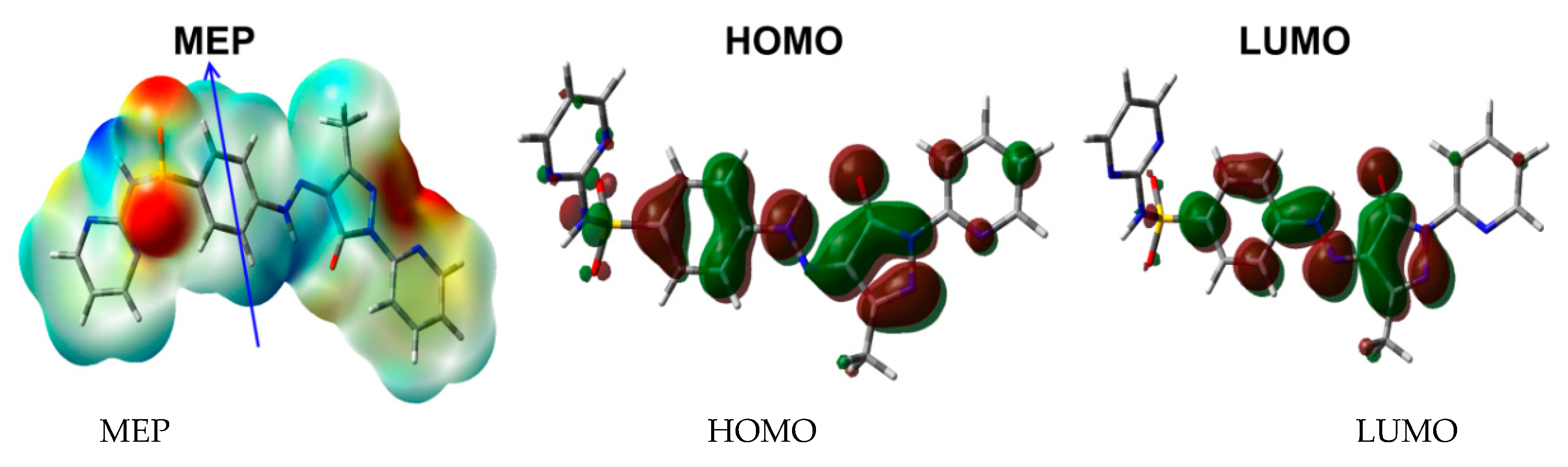
Figure 9.
The MEP, HOMO, and LUMO of 5.
Among electronic parameters which play an important role are the HOMO and LUMO levels. These molecular orbitals are shown in Figure 9. It is clear that both are distributed over the π-system of the molecule. Hence, the HOMO→LUMO excitation is mainly a π–π* transition. Their energies are calculated to be −6.1158 and −2.7671 eV, respectively, and the HOMO–LUMO gap is 3.3487 eV. As a result, the reactivity indices [21,22,23,24,25,26,27] such as ionization potential (I), electron affinity (A), hardness (η), electrophilicity index (ω) and chemical potential (μ) are calculated to be 6.1158, 2.7671, 3.3487, 2.9455 and −4.4415 eV, respectively.
3.5. NBO Analysis
The delocalization of the electron processes has a greatly effective role in the molecule stability [28,29,30]. There are many π→π*, n→σ*, σ→σ* and n→π* electron delocalization processes which stabilize the structure (Table 5). The stabilization energies (E(2)) of the σ→σ* and π→π* processes are in the range of 4.25–4.69 and 6.18–35.16 kcal/mol. The BD(2)N11-C40→BD*(2) N12-C39 (35.16 kcal/mol) and BD(2)C42-C44→BD*(2)N11-C40 (34.43 kcal/mol) are the strongest electron delocalization processes. In addition, the LP(2)O2→BD*(1)N6-C22 (26.42 kcal/mol) and LP(1)N6→BD*(2)N5-C21 (38.97 kcal/mol) are the strongest n→σ* and n→π* electron delocalization processes, respectively.

Table 5.
Electron delocalization processes in 5 a.
4. Conclusions
The novel bioprecursor prodrugs of sulfadiazine have been synthesized and evidenced through the elemental and spectral analyses “FT-IR, 1HNMR, 13CNMR, and MS“. More structural elucidations of the prodrug 5 were determined via X-ray and its supramolecular structure aspects were analyzed using Hirshfeld calculations. Additionally, the natural charge distribution, dipole moment, HOMO, LUMO, and MEP map of 5 were analyzed based on B3LYP/6-31G (d,p) calculations. Its structural aspects were investigated using DFT and NBO calculations.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst12081016/s1, NMR spectrum; Table S1: The calculated geometric parameters of 5 a. Table S2: The calculated natural charges of 5 a.
Author Contributions
Conceptualization, M.S.A. (Mohammed Salah Ayoup), A.B. and S.M.S.; methodology, M.S.A. (Mohammed Salah Ayoup) and A.B.; software, S.M.S. and M.H.; validation, M.S.A. (Mezna Saleh Altowyan) and M.M.F.I.; formal analysis, M.S.A. (Mohammed Salah Ayoup), M.M.F.I. and M.H.; investigation, M.S.A. (Mohammed Salah Ayoup); resources, M.S.A. (Mezna Saleh Altowyan) and A.B.; data curation, A.B. and S.M.S.; writing—original draft preparation, M.S.A. (Mohammed Salah Ayoup), A.B. and S.M.S.; writing—review and editing, A.B. and S.M.S.; visualization, A.B. and M.S.A. (Mohammed Salah Ayoup); supervision, A.B. and M.S.A. (Mezna Saleh Altowyan); project administration, M.S.A. (Mezna Saleh Altowyan); funding acquisition, M.S.A. (Mezna Saleh Altowyan). All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R86), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R86), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Villa, D.F.; Aguilar, M.R.; Rojo, L. Folic Acid Antagonists: Antimicrobial and Immunomodulating Mechanisms and Applications. Int. J. Mol. Sci. 2019, 20, 4996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshti, S.; Patil, P.A.; Patil, C.B.; Patil, A.S. Synthesis and Characterization of Prodrugs of Sulfonamides as an Azo Derivatives of Carvacrol. Der. Pharma. Chem. 2018, 10, 1–15. [Google Scholar]
- Pradere, U.; Garnier-Amblard, E.C.; Coats, S.J.; Amblard, F.; Schinazi, R.F. Synthesis of Nucleoside Phosphate and Phosphonate Prodrugs. Chem. Rev. 2014, 114, 9154–9218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.V.; Wuest, W.M. Signed, Sealed, Delivered: Conjugate and Prodrug Strategies as Targeted Delivery Vectors for Antibiotics. ACS Infect Dis. 2019, 14, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Gingell, R.; Bridges, J.W.; Williams, R.T. The Role of the Gut Flora in the Metabolism of Prontosil and Neoprontosil in the Rat. Xenobiotica 1971, 1, 143–156. [Google Scholar] [CrossRef]
- Ryan, A. Azoreductases in drug metabolism. Br. J. Pharmacol. 2017, 174, 2161–2173. [Google Scholar] [CrossRef] [Green Version]
- Gaffer, H.E. Antimicrobial sulphonamide azo dyes. Coloration Technol. 2019, 135, 484–500. [Google Scholar] [CrossRef]
- Gündüz, M.G.; Tahir, M.N.; Armakovic, S.; Koçak, C.O.; Armakovic, S.J. Design, synthesis and computational analysis of novel acridine-(sulfadiazine/sulfathiazole) hybrids as antibacterial agents. J. Mol. Struct. 2019, 1186, 39–49. [Google Scholar] [CrossRef]
- Ayoup, M.S.; Soliman, S.M.; Haukka, M.; Harras, M.F.; Menofy, N.G.E.; Ismail, M.M.F. Prodrugs of sulfacetamide: Synthesis, X-ray structure, Hirshfeld analysis, antibacterial assessment, and docking studies. J. Mol. Struct. 2022, 1251, 132017. [Google Scholar] [CrossRef]
- Ismail, M.M.F.; Ghorab, M.M.; Noaman, E.; Ammar, Y.A.; Heiba, H.I.; Sayed, M.Y. Novel synthesis of pyrrolo[2,3-d]pyrimidines bearing sulfonamide moieties as potential antitumor and radioprotective agents. Arzneim.-Forsch./Drug Res. 2006, 56, 301–308. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Noaman, E.; Ismail, M.M.F.; Heiba, H.I.; Ammar, Y.A.; Sayed, M.Y. Novel Antitumor And Radioprotective Sulfonamides Containing Pyrrolo[2,3-D]Pyrimidines. Arzneimittelforschung 2006, 56, 405–413. [Google Scholar]
- El-Sayed, H.A.; Moustafa, A.H.; Fadda, A.A.; Abd El-Rahman, K.E. Pyrazole and Nicotinonitrile Derivatives Synthesized from Sulfa Drugs, and Their Antibacterial Activity. Russ. J. Gen. Chem. 2019, 89, 339–347. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Agilent Technologies Inc.: Oxfordshire, UK, 2018. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Crystal Explorer17; University of Western Australia: Crawley, WA, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 20 May 2017).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- GaussView; Version 4.1; Dennington, R., II; Keith, T.; Millam, J. (Eds.) Semichem Inc.: Shawnee Mission, KS, USA, 2007. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, Æ. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino) methyl] phenol. Spectrochim. Acta 2011, 78, 160–167. [Google Scholar] [CrossRef]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Parr, R.G.; Szentpaly, L.V.; Liu, S. Electrophilicity index. J. Am. Chem. Soc. 1999, 121, 1922–1924. [Google Scholar] [CrossRef]
- Singh, R.N.; Kumar, A.; Tiwari, R.K.; Rawat, P.; Gupta, V.P. A combined experimental and quantum chemical (DFT and AIM) study on molecular structure, spectroscopic properties, NBO and multiple interaction analysis in a novel ethyl 4-[2-(carbamoyl) hydrazinylidene]-3, 5-dimethyl-1H-pyrrole-2-carboxylate and its dimer. J. Mol. Strut. 2013, 1035, 427–440. [Google Scholar] [CrossRef]
- Joe, I.H.; Kostova, I.; Ravikumar, C.; Amalanathan, M.; Cîntǎ Pînzaru, S. Theoretical and vibrational spectral investigation of sodium salt of acenocoumarol. J. Raman Spectrosc. 2009, 40, 1033–1038. [Google Scholar] [CrossRef]
- Sebastian, S.; Sundaraganesan, N. The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 941–952. [Google Scholar] [CrossRef] [PubMed]
- Amer, A.; Ayoup, M.S.; Khattab, S.N.; Yassen, S.H.; Langer, V.; Senior, S.; El Massry, A. A regio- and stereo-controlled approach to triazoloquinoxalinyl C-nucleosides. Carbohydr. Res. 2010, 345, 2474–2484. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).