Thermodynamic Characterization of a Highly Transparent Microfluidic Chip with Multiple On-Chip Temperature Control Units
Abstract
:1. Introduction
2. Materials and Methods
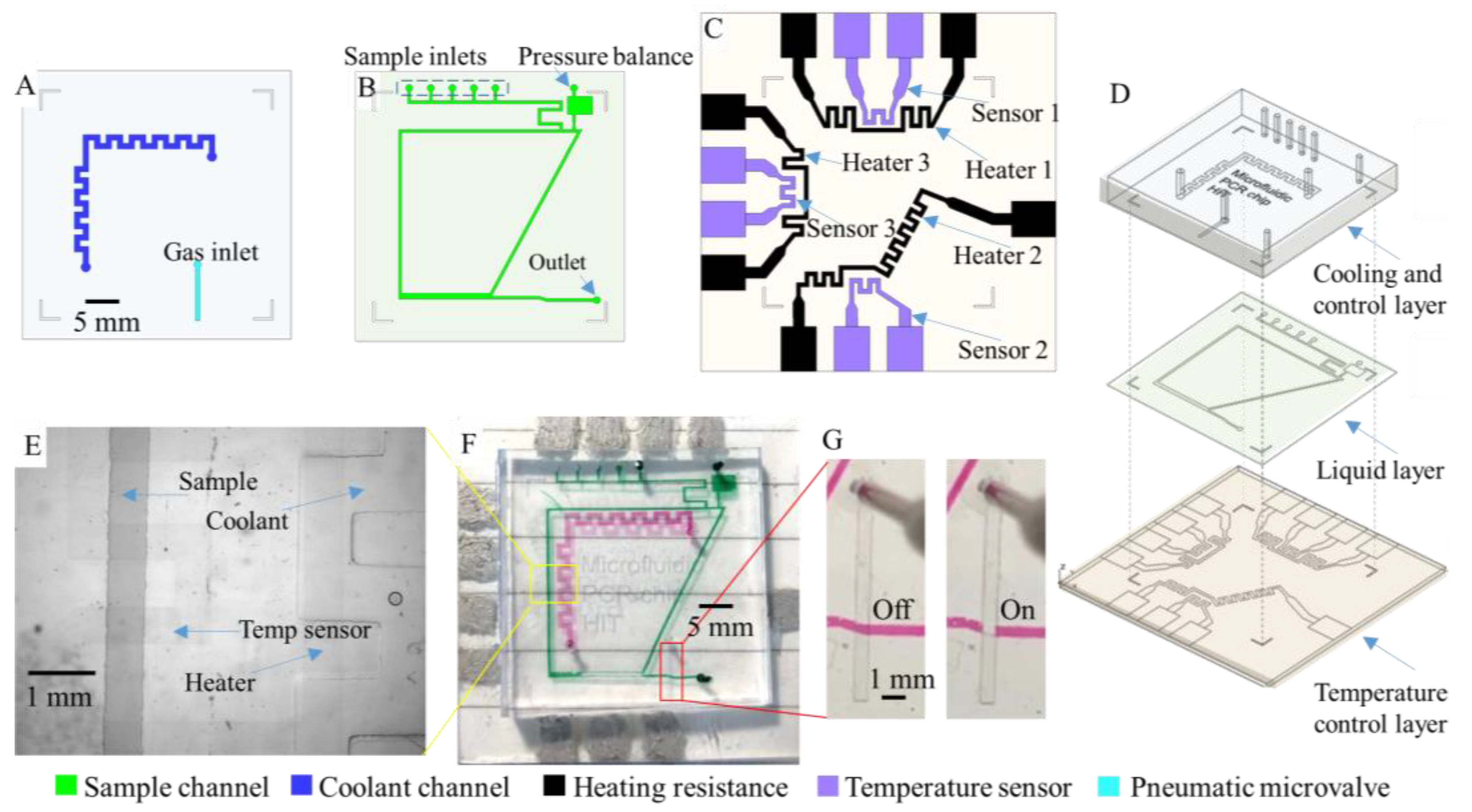
2.1. Microdevice Fabrication
2.2. Microfluidic Control System
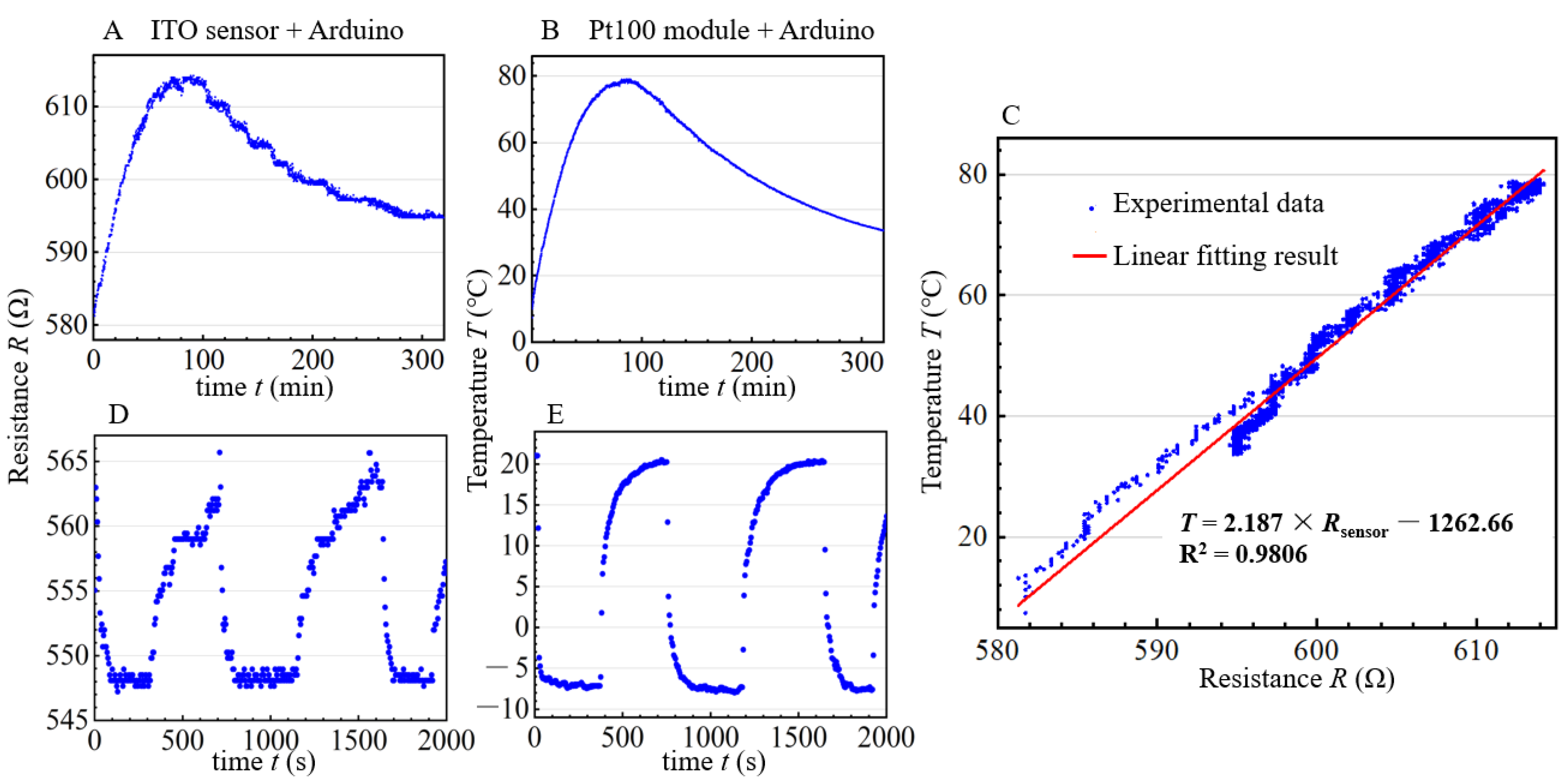
2.3. Calibration and Performance Test for the On–Chip Sensor
2.4. Experimental Setup for On-Chip Thermodynamic Characterization
3. Results
3.1. Assembling of the Multilayer Microfluidic Chip
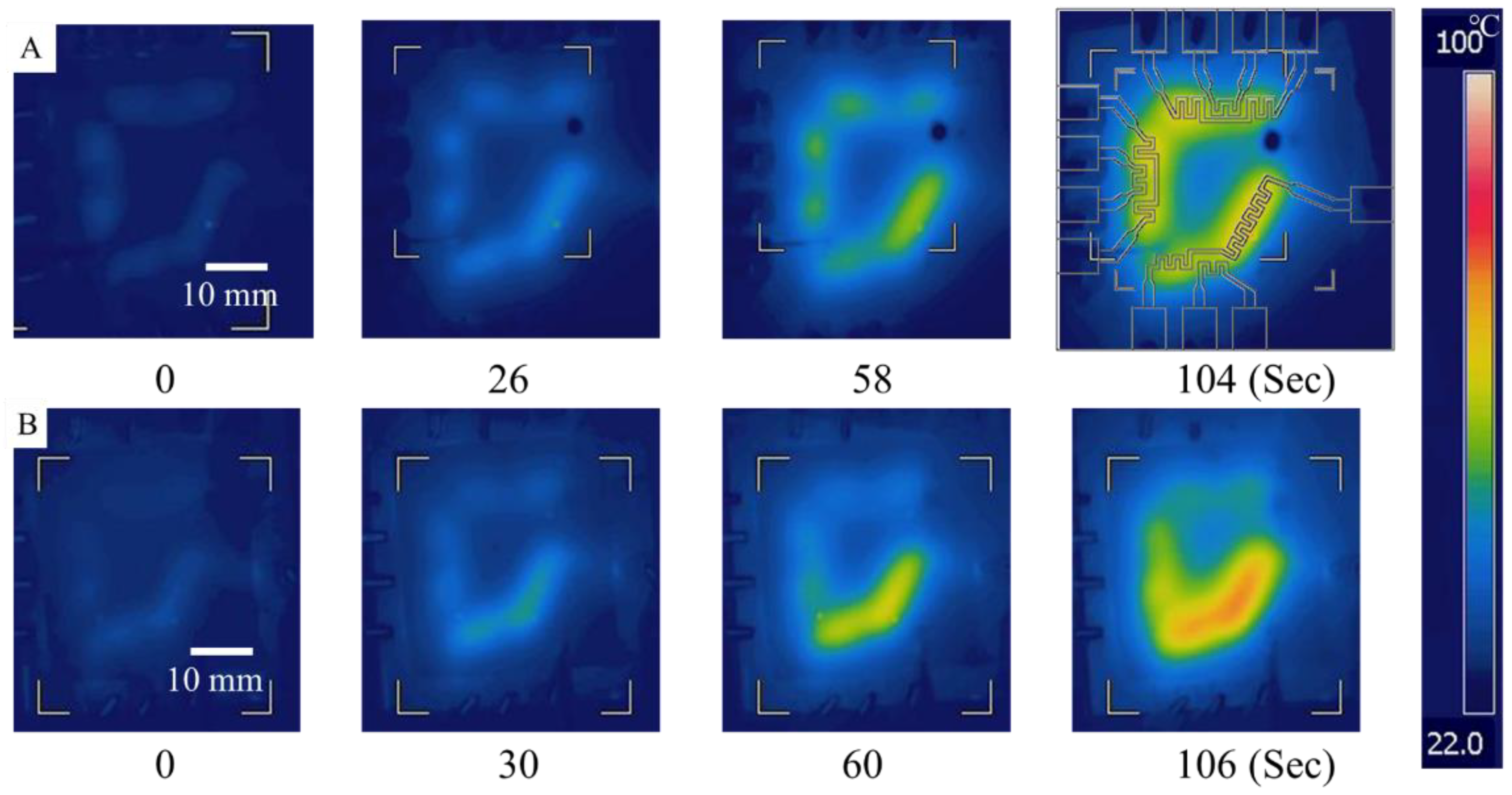
3.2. Characterization of the On–Chip Temperature Control System
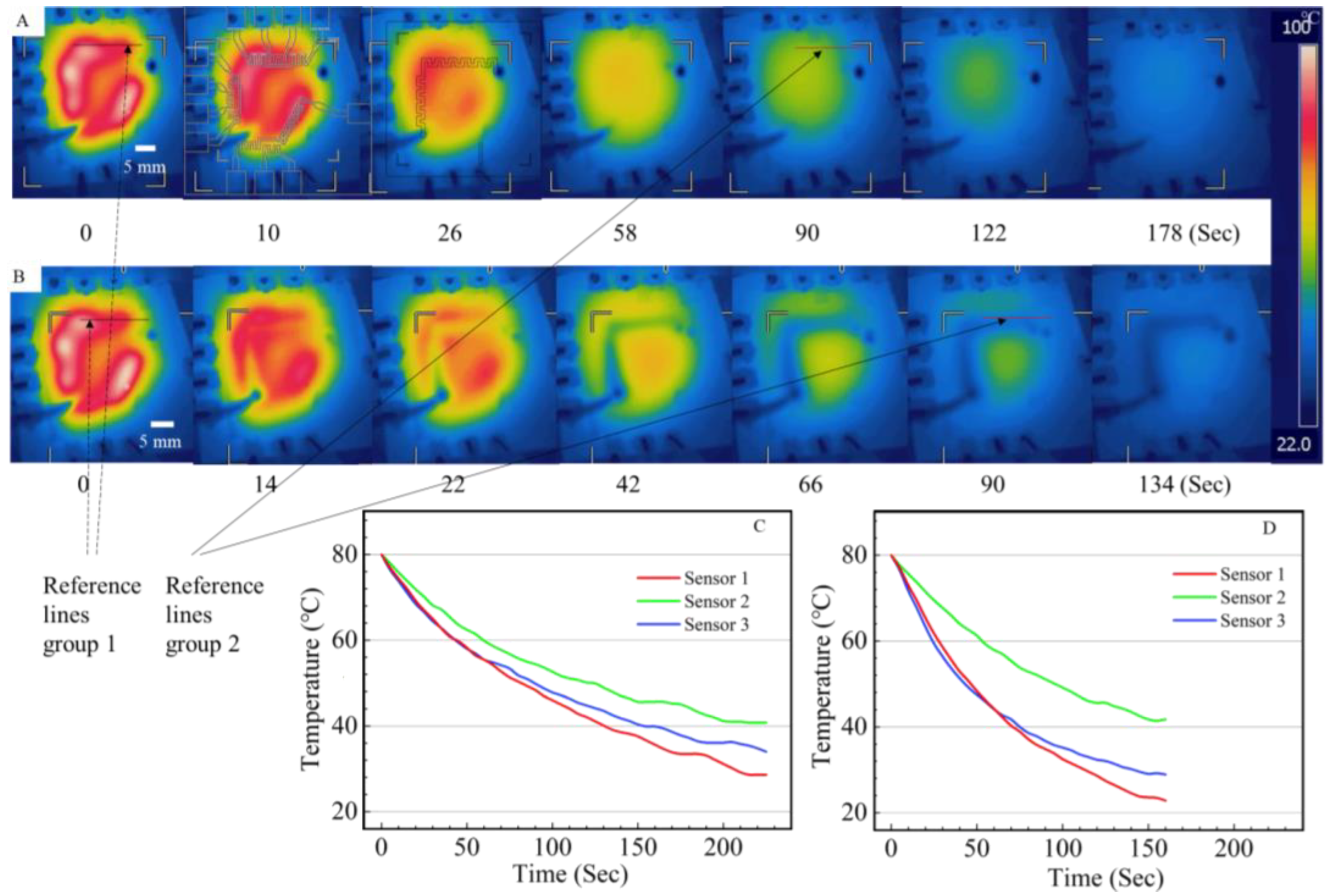
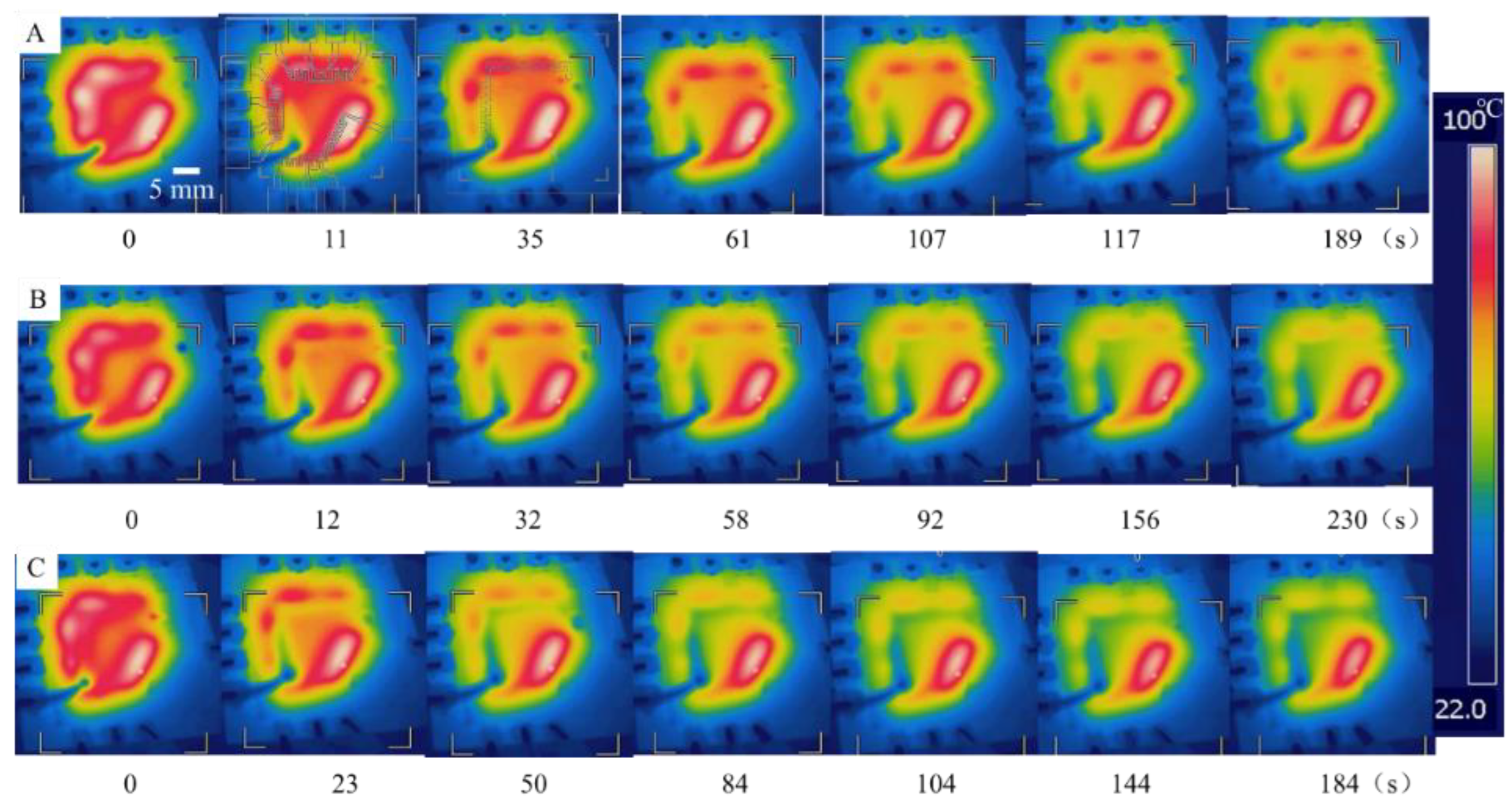
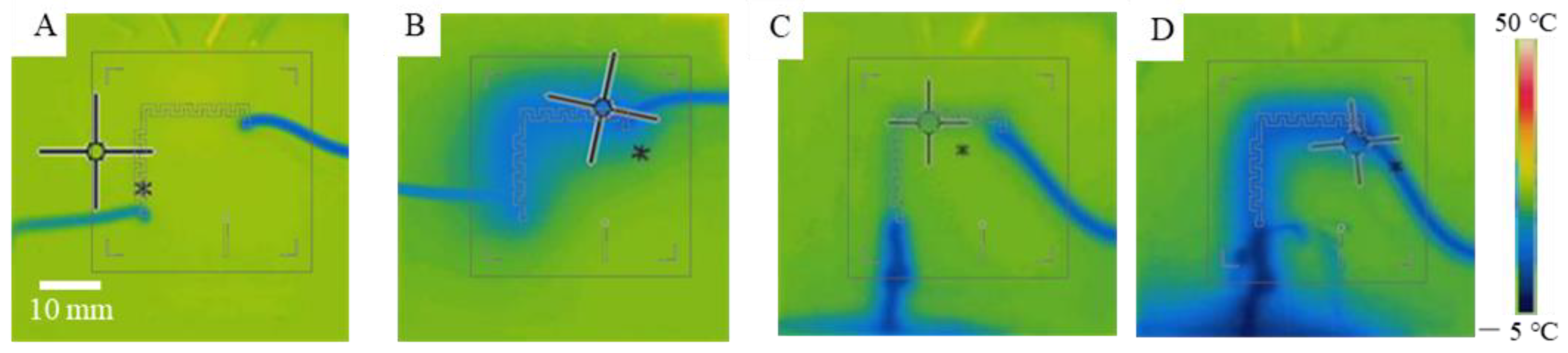
3.3. Verification of the On-Chip Heating and Cooling
3.4. Theoretical Analysis and Characterization of On-Chip Cooling
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, P.K.; Patel, A.; Kaffenes, A.; Hord, C.; Kesterson, D.; Prakash, S. Microfluidic approaches and methods enabling extracellular vesicle isolation for cancer diagnostics. Micromachines 2022, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Y.; Qin, Y.; Du, X.; Wang, Q.; Lyu, J. A novel microfluidic device that integrates nucleic acid extraction, amplification, and detection to identify an EGFR mutation in lung cancer tissues. RSC Adv. 2016, 6, 13399–13406. [Google Scholar] [CrossRef]
- Whitesides, G.M.; McDonald, J.C. Poly(dimethyl siloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar]
- Liu, J.X.; Fu, H.; Yang, T.H.; Li, S.J. Automatic sequential fluid handling with multilayer microfluidic sample isolated pumping. Biomicrofluidics 2015, 9, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Maimouni, I.; Cejas, C.M.; Cossy, J.; Tabeling, P.; Russo, M. Microfluidics mediated production of foams for biomedical applications. Micromachines 2020, 11, 83. [Google Scholar] [CrossRef]
- Lin, L.; Chung, C.K. PDMS microfabrication and design for microfluidics and sustainable energy application. Micromachines 2021, 12, 1350. [Google Scholar] [CrossRef]
- Tomar, S.; Lasne, C.; Barraud, S.; Ernst, T.; Guiducci, C. Integration of ultra-low volume pneumatic microfluidics with a three-dimensional electrode network for on-chip biochemical sensing. Micromachines 2021, 12, 762. [Google Scholar] [CrossRef]
- Liu, J.; Enzelberger, M.; Quake, S.R. A nanoliter rotary device for polymerase chain reaction. Electrophoresis 2002, 23, 1531–1536. [Google Scholar] [CrossRef]
- Kaprou, G.D.; Papadopoulos, V.; Loukas, C.-M.; Kokkoris, G.; Tserepi, A. Towards PCB-based miniaturized thermocyclers for DNA amplification. Micromachines 2020, 11, 258. [Google Scholar] [CrossRef]
- Yang, T.; Peng, J.; Fang, C.; Li, S.; Gao, D. Numerical modeling of temperature-dependent cell membrane permeability to water based on a microfluidic system with dynamic temperature control. SLAS Technol. 2021, 34, 50–60. [Google Scholar] [CrossRef]
- Li, Y.; Wei, S.; Zheng, T. Measurement of the thermal effect of standing surface acoustic waves in microchannel by fluoresence intensity. Micromachines 2021, 12, 934. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Xie, D.; Mbogba, M.K.; Chen, Z.; Tian, C.; Xu, L.; Zhao, G. A microfluidic platform with cell-scale precise temperature control for simultaneous investigation of the osmotic responses of multiple oocytes. Lab Chip 2019, 19, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.L.; Liao, H.Y.; Liu, H.M.; Lu, Y.W.; Yeh, P.K.; Chang, J.Y.; Fan, S.K. Digital microfluidic qPCR cartridge for SARS-CoV-2 detection. Micromachines 2022, 13, 196. [Google Scholar] [CrossRef]
- Veltkamp, H.-W.; Monteiro, F.A.; Sanders, R.; Wiegerink, R.; Lötters, J. Disposable DNA amplification chips with integrated low-cost heaters. Micromachines 2020, 11, 238. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.U.; De Mello, A.J.; Manz, A. Chemical amplification: Continuous-flow PCR on a chip. Science 1998, 280, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press, Inc.: New York, NY, USA, 1990; pp. 314–322. [Google Scholar]
- Mauk, M.G.; Liu, C.; Sadik, M.M.; Bau, H.H. Microfluidic devices for nucleic acid (NA) isolation, isothermal NA amplification, and real-time detection. In Mobile Health Technologies; Herold, K.E., Rasooly, A., Eds.; Humana Press: New York, NY, USA, 2015; pp. 15–40. [Google Scholar]
- Crews, N.; Wittwer, C.; Gale, B. Continuous-flow thermal gradient PCR. Biomed. Microdevices 2008, 10, 187–195. [Google Scholar] [CrossRef]
- Peng, J.; Fang, C.; Ren, S.; Pan, J.; Jia, Y.; Shu, Z.; Gao, D. Development of a microfluidic device with precise on-chip temperature control by integrated cooling and heating components for single cell-based analysis. Int. J. Heat Mass Transf. 2019, 130, 660–667. [Google Scholar] [CrossRef]
- Koziej, D.; Floryan, C.; Sperling, R.A.; Ehrlicher, A.J.; Issadore, D.; Westervelt, R.; Weitz, D.A. Microwave dielectric heating of non-aqueous droplets in a microfluidic device for nanoparticle synthesis. Nanoscale 2013, 5, 5468–5475. [Google Scholar] [CrossRef]
- Natrajan, V.K.; Christensen, K.T. A two-color fluorescent thermometry technique for microfluidic systems. Bull. Am. Phys. Soc. 2008, 53, 15. [Google Scholar]
- Hoang, V.N.; Kaigala, G.V.; Backhouse, C.J. Dynamic temperature measurement in microfluidic devices using thermochromic liquid crystals. Lab Chip 2004, 8, 484–487. [Google Scholar] [CrossRef]
- Fang, C.; Ji, F.; Shu, Z.; Gao, D. Determination of the temperature-dependent cell membrane permeabilities using microfluidics with integrated flow and temperature control. Lab Chip 2017, 17, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Stan, C.A.; Schneider, G.F.; Shevkoplyas, S.S.; Hashimoto, M.; Ibanescu, M.; Wiley, B.J.; Whitesides, G.M. A microfluidic apparatus for the study of ice nucleation in supercooled water drops. Lab Chip 2009, 9, 2293–2305. [Google Scholar] [CrossRef] [PubMed]
- Park, N.; Kim, S.; Hahn, J.H. Cylindrical compact thermal-cycling device for continuous-flow polymerase chain reaction. Anal. Chem. 2003, 75, 6029–6033. [Google Scholar] [CrossRef] [PubMed]
- Maltezos, G.; Gomez, A.; Zhong, J.; Gomez, F.A.; Scherer, A. Microfluidic polymerase chain reaction. Appl. Phys. Lett. 2008, 93, 243901. [Google Scholar] [CrossRef]
- Maltezos, G.; Johnston, M.; Taganov, K.; Srichantaratsamee, C.; Gorman, J.; Baltimore, D.; Chantratita, W.; Scherer, A. Exploring the limits of ultrafast polymerase chain reaction using liquid for thermal heat exchange: A proof of principle. Appl. Phys. Lett. 2010, 97, 264101. [Google Scholar] [CrossRef]
- Mahjoob, S.; Vafai, K.; Beer, N.R. Rapid microfluidic thermal cycler for polymerase chain reaction nucleic acid amplification. Int. J. Heat Mass Transf. 2008, 51, 2109–2122. [Google Scholar] [CrossRef]
- Krishnan, M.; Ugaz, V.M.; Burns, M.A. PCR in a Rayleigh-Bénard convection cell. Science 2002, 298, 793. [Google Scholar] [CrossRef]
- Sun, K.; Yamaguchi, A.; Ishida, Y.; Matsuo, S.; Misawa, H. A heater-integrated transparent microchannel chip for continuous-flow PCR. Sens. Actuator B Chem. 2002, 84, 283–289. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Tian, X.X.; Qu, B.Y.; Chen, K.; Fang, F. Self-heating and sensing static chip polymerase chain reaction (PCR) of transparent electro-conductive glass substrate (ITO). Chem. J. Chin. Univ. 2007, 12, 43–47. [Google Scholar]
- Zeng, W.; Fu, H. Quantitative measurements of the somatic cell count of fat-free milk based on droplet microfluidics. J. Mater. Chem. C 2020, 8, 13770–13776. [Google Scholar] [CrossRef]
- Snodgrass, R.; Gardner, A.; Jiang, L.; Fu, C.; Cesarman, E.; Erickson, D. KS-detect—Validation of solar thermal PCR for the diagnosis of Kaposi’s sarcoma using pseudo-biopsy samples. PLoS ONE 2016, 11, e0147636. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Nguyen, N.T.; Kwok, Y.C. High-throughput polymerase chain reaction in parallel circular loops using magnetic actuation. Anal. Chem. 2008, 80, 6127–6130. [Google Scholar] [CrossRef] [PubMed]
- Yobas, L.; Feng Cheow, L.; Tang, K.C.; Yong, S.E.; Kye-Zheng Ong, E.; Wong, L.; Cheng-Yong Teo, W.; Ji, H.; Rafeah, S.; Yu, C. A self-contained fully-enclosed microfluidic cartridge for ab on a chip. Biomed. Microdevices 2009, 11, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Design Instruction for Mini Refrigeration Equipment; China Machine Press: Beijing, China, 1998; pp. 20–21. [Google Scholar]


| Flow Rate of Coolant (m/s) | Water in 20 °C (k = 0.599 W/(m·K)) | 30%CaCl2 in 0 °C (k = 0.528 W/(m·K)) | ||||
|---|---|---|---|---|---|---|
| Re | Nu | h (W/(m2·K)) | Re | Nu | h (W/(m2·K)) | |
| 0.001 | 0.035 | 0.238 | 3.569 | 0.008 | 0.183 | 2.422 |
| 0.002 | 0.070 | 0.337 | 5.048 | 0.016 | 0.259 | 3.425 |
| 0.005 | 0.175 | 0.533 | 7.981 | 0.040 | 0.410 | 5.416 |
| 0.01 | 0.340 | 0.740 | 11.096 | 0.080 | 0.580 | 7.659 |
| 0.05 | 1.757 | 1.685 | 25.241 | 0.401 | 1.299 | 17.149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Wang, J.; Lv, S.; Li, S.; Luo, G. Thermodynamic Characterization of a Highly Transparent Microfluidic Chip with Multiple On-Chip Temperature Control Units. Crystals 2022, 12, 856. https://doi.org/10.3390/cryst12060856
Yang T, Wang J, Lv S, Li S, Luo G. Thermodynamic Characterization of a Highly Transparent Microfluidic Chip with Multiple On-Chip Temperature Control Units. Crystals. 2022; 12(6):856. https://doi.org/10.3390/cryst12060856
Chicago/Turabian StyleYang, Tianhang, Jinxian Wang, Sining Lv, Songjing Li, and Gangyin Luo. 2022. "Thermodynamic Characterization of a Highly Transparent Microfluidic Chip with Multiple On-Chip Temperature Control Units" Crystals 12, no. 6: 856. https://doi.org/10.3390/cryst12060856
APA StyleYang, T., Wang, J., Lv, S., Li, S., & Luo, G. (2022). Thermodynamic Characterization of a Highly Transparent Microfluidic Chip with Multiple On-Chip Temperature Control Units. Crystals, 12(6), 856. https://doi.org/10.3390/cryst12060856







