Crystallization Kinetics of the Fe68Nb6B23Mo3 Glassy Ribbons Studied by Differential Scanning Calorimetry
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
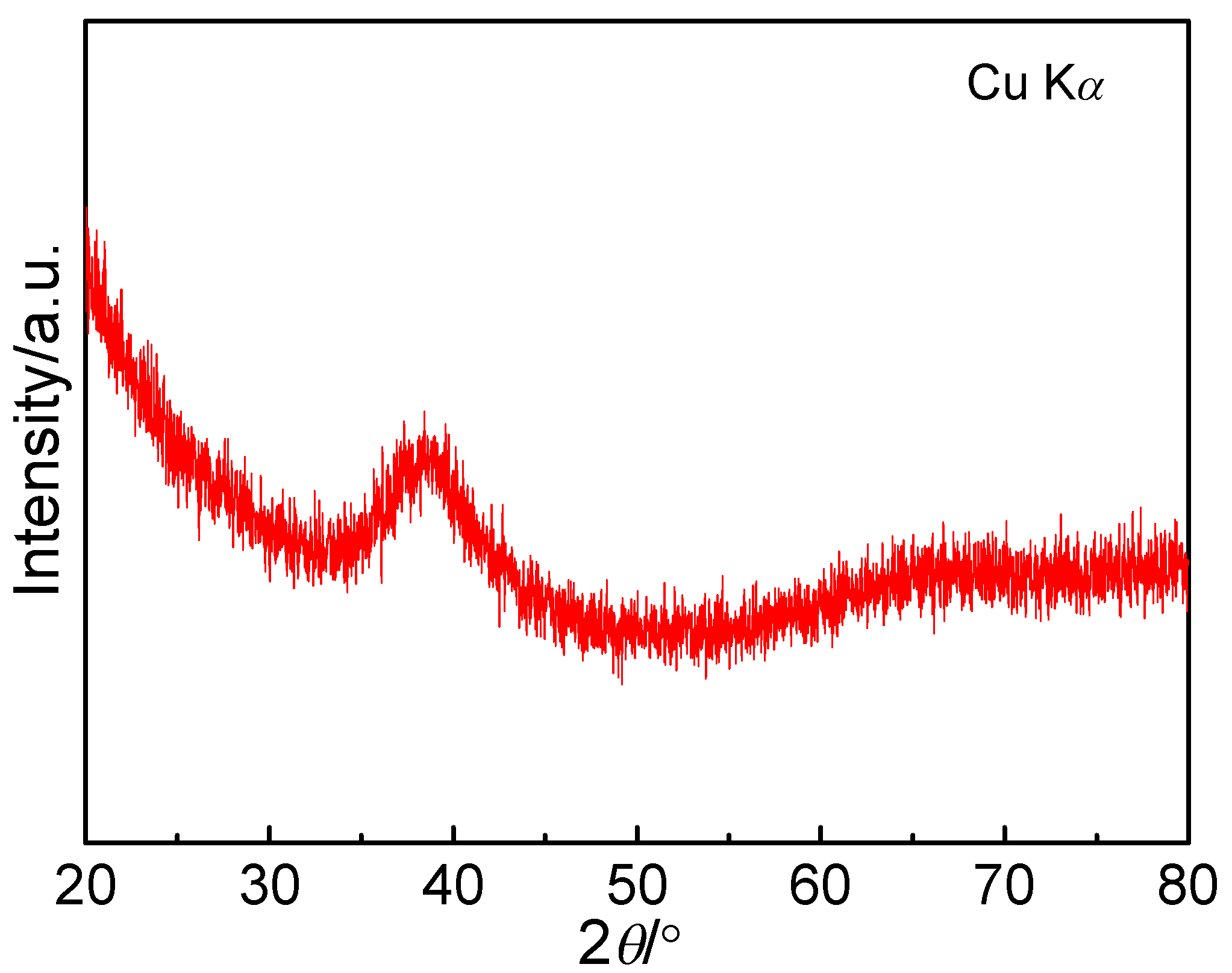
3.1. Structure Analysis and Non-Isothermal Crystallization Behavior of the Fe68Nb6B23Mo3 Glassy Alloys
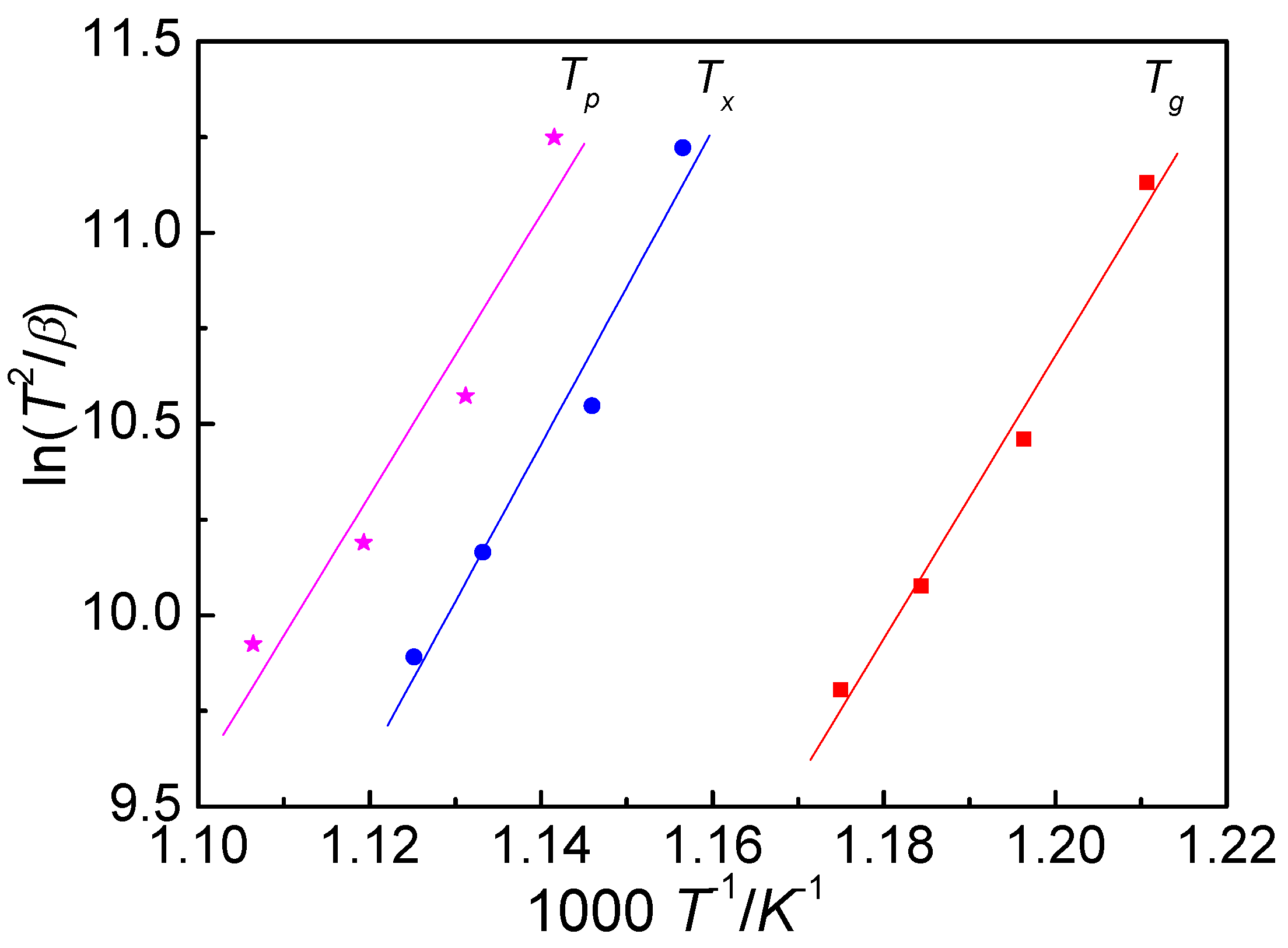
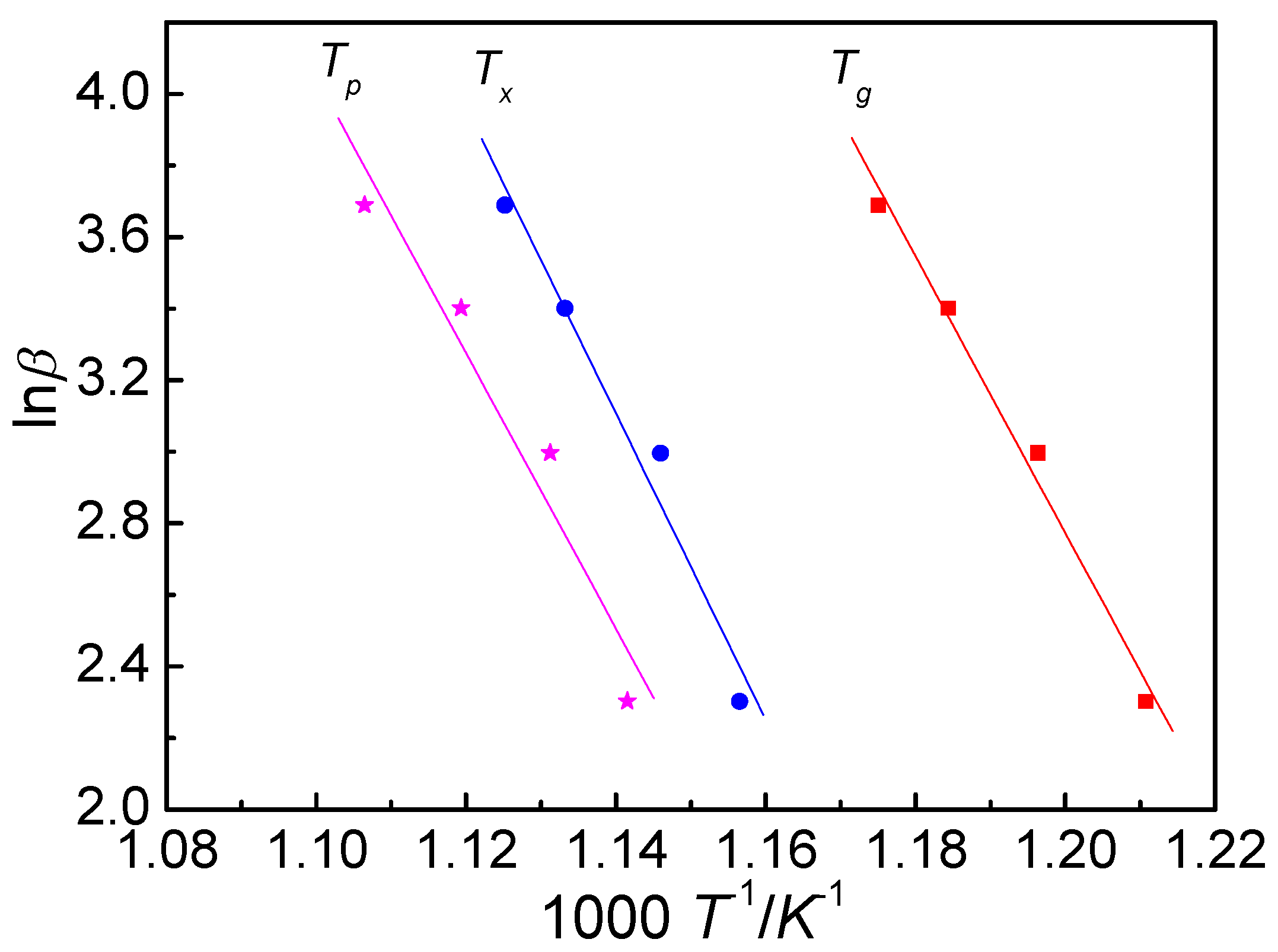
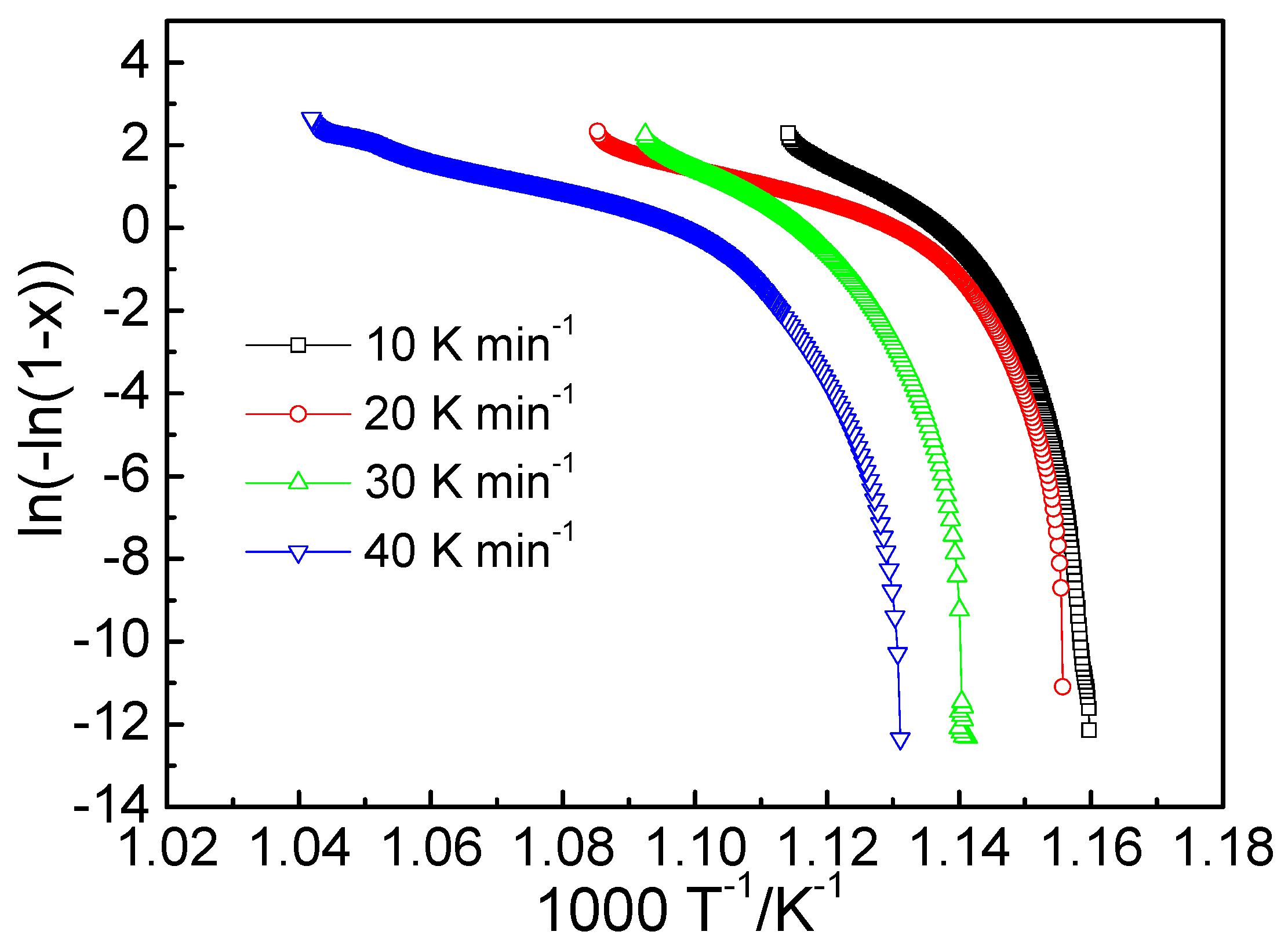
3.2. Activation Energy
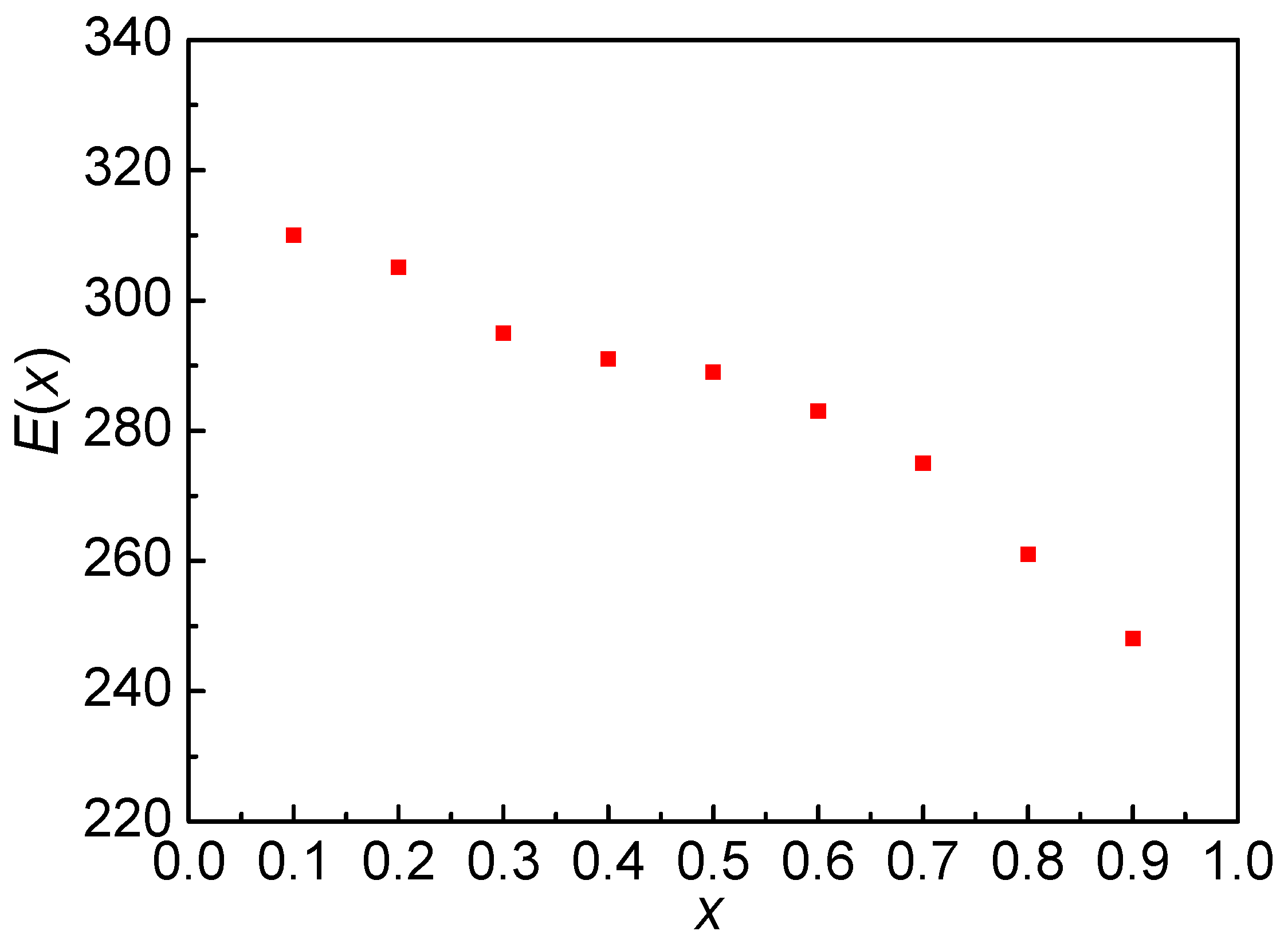
3.3. Local Activation Energy E(x)
3.4. Local Avrami Exponent n(x)
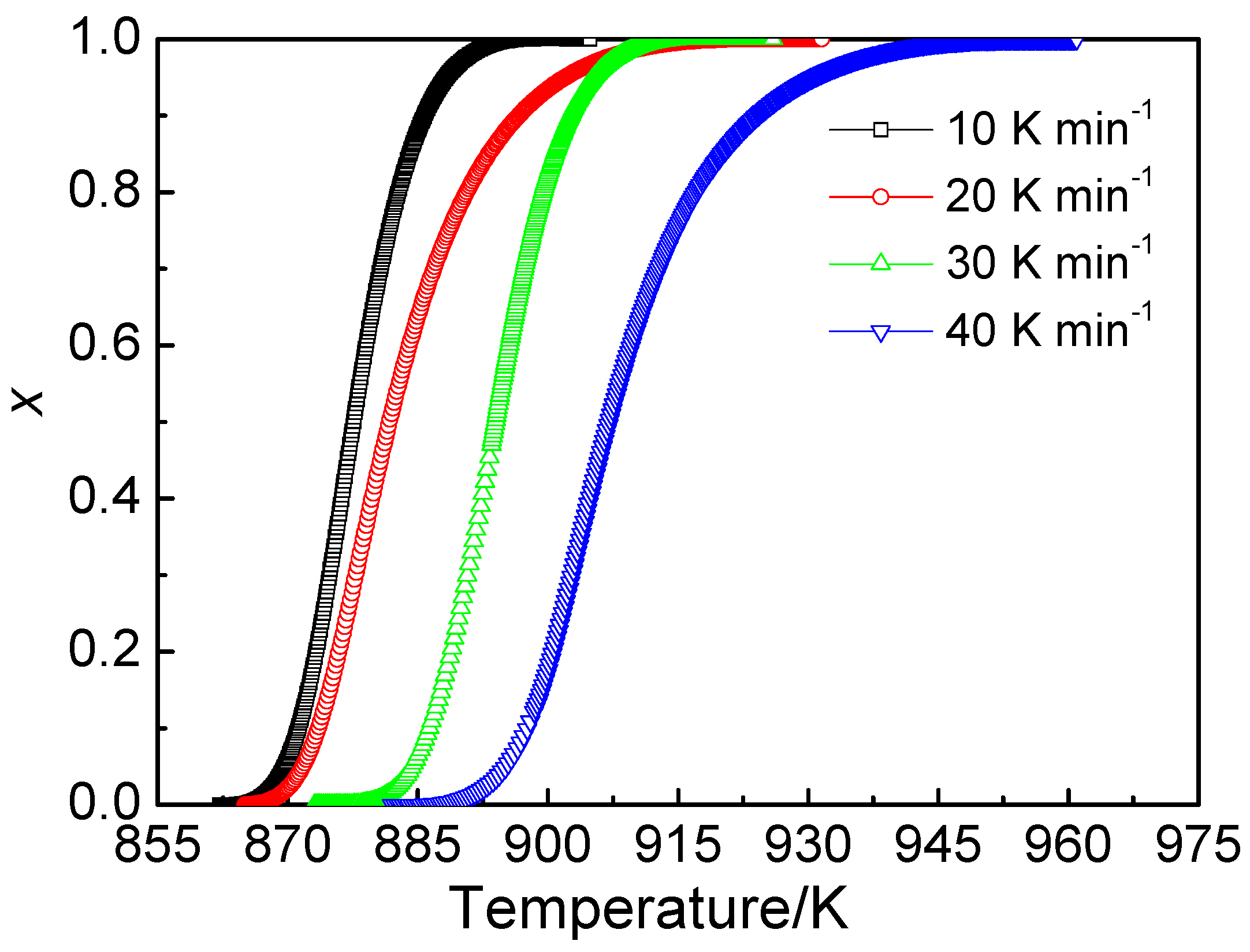
3.5. Dependence of Glass Transition and Crystallization Event on Heating Rates
4. Conclusions
- Both the glass transition process and crystallization process display an obvious kinetic effect. The activation energy was calculated by using the Kissinger equation and the Ozawa equation. The values of Eg, Ex and Ep, calculated by Kissinger equation, are 308 ± 4, 342 ± 5 and 310 ± 7 kJ mol−1, respectively, and they are 322 ± 3, 356 ± 5 and 325 ± 7 kJ mol−1 calculated by the Ozawa equation, respectively.
- With the increase of crystallization volume fraction x, the Avrami exponent n(x) first decreases and then increases. The 2.5 < n(x) < 4.0 in the initial stage of 0 < x < 0.25 stands for the growth from small dimensions with an increasing nucleation rate. With the increase of x in the range 0.25–0.71, n(x) decreases from 2.5 to 1.5, indicating that it is controlled by the growth of small particles with decreasing nucleation rate at this stage. The value of n(x) decreases from 1.5 to 1.0 with x ranging from 0.71 to 0.97, suggesting that it is controlled by the growth of particles with appreciable initial volume.
- The fitting curves, using Lasocka’s equation, clearly indicate that the course of the crystallization of Fe68Nb6B23Mo3 is most susceptible to the heating rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inoue, A.; Takeuchi, A. Recent development and application products of bulk glassy alloys. Acta Mater. 2011, 59, 2243–2267. [Google Scholar] [CrossRef]
- Liu, T.; Lai, L.; Xiao, S.; Tang, M.; Zhang, H.; Guo, S. Ternary Fe–W–B bulk metallic glasses with ultrahigh thermal stabilities. Mater. Sci. Eng. A 2021, 826, 142034. [Google Scholar] [CrossRef]
- Li, M.; Guan, H.; Yang, S.; Ma, X.; Li, Q. Minor Cr alloyed Fe–Co–Ni–P–B high entropy bulk metallic glass with excellent mechanical properties. Mater. Sci. Eng. A 2021, 805, 140542. [Google Scholar] [CrossRef]
- Li, H.X.; Lu, Z.C.; Wang, S.L.; Wu, Y.; Lu, Z.P. Fe-based bulk metallic glasses: Glass formation, fabrication, properties and applications. Prog. Mater. Sci. 2019, 103, 235–318. [Google Scholar] [CrossRef]
- Shen, B.L.; Chang, C.T.; Inoue, A. Superhigh strength and good soft-magnetic properties of (Fe, Co)-B-Si-Nb bulk glassy alloys with high glass-forming ability. Appl. Phys. Lett. 2004, 85, 4911–4913. [Google Scholar] [CrossRef]
- Stoica, M.; Hajlaoui, K.; Lemoulec, A.; Yavari, A.R. New ternary Fe-based bulk metallic glass with high boron content. Philos. Mag. Lett. 2006, 86, 267–275. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, K.; Li, Q. Comparative study of non-isothermal crystallization kinetics between Fe80P13C7 bulk metallic glass and melt-spun glassy ribbon. J. Alloys Compd. 2012, 540, 6–15. [Google Scholar] [CrossRef]
- Stoica, M.; Kumar, S.; Roth, S.; Ram, S.; Eckert, J.; Vaughan, G.; Yavari, A.R. Crystallization kinetics and magnetic properties of Fe66Nb4B30 bulk metallic glass. J. Alloys Compd. 2009, 483, 632–637. [Google Scholar] [CrossRef]
- Zhuang, Y.X.; Duan, T.F.; Shi, H.Y. Calorimetric study of non-isothermal crystallization kinetics of Zr60Cu20Al10Ni10 bulk metallic glass. J. Alloys Compd. 2011, 509, 9019–9025. [Google Scholar] [CrossRef]
- Lu, W.; Yan, B.; Huang, W.-H. Complex primary crystallization kinetics of amorphous Finemet alloy. J. Non-Cryst. Solids 2005, 351, 3320–3324. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Chan, K.C. Crystallization kinetics of the Zr55.9Cu18.6Ta8Al7.5Ni10 bulk metallic glass matrix composite under isothermal conditions. J. Alloys Compd. 2006, 419, 71–75. [Google Scholar] [CrossRef]
- Hu, X.; Qiao, J.; Pelletier, J.M.; Yao, Y. Evaluation of thermal stability and isochronal crystallization kinetics in the Ti40Zr25Ni8Cu9Be18 bulk metallic glass. J. Non-Cryst. Solids. 2016, 432, 254–264. [Google Scholar] [CrossRef]
- Prajapati, S.R.; Kasyap, S.; Patel, A.T.; Pratap, A. Non-isothermal crystallization kinetics of Zr52Cu18Ni14Al10Ti6 metallic glass. J. Therm. Anal. Calorim. 2016, 124, 21–33. [Google Scholar] [CrossRef]
- Gong, P.; Zhao, S.F.; Wang, X.; Yao, K.F. Non-isothermal crystallization kinetics and glass-forming ability of Ti41Zr25Be28Fe6 bulk metallic glass investigated by differential scanning calorimetry. Appl. Phys. A 2015, 120, 145–153. [Google Scholar] [CrossRef]
- Zhu, M.; Fa, Y.; Jian, Z.; Yao, L.; Jin, C.; Nan, R.; Chang, F. Non-isothermal crystallization kinetics and soft magnetic properties of the Fe67Nb5B28 metallic glasses. J. Therm. Anal. Calorim. 2018, 132, 173–180. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetic analysis of derivative curves in thermal analysis. J. Therm. Anal. 1970, 2, 301–324. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Zhu, M.; Li, J.J.; Yao, L.J.; Jian, Z.Y.; Chang, F.E.; Yang, G.C. Non-isothermal crystallization kinetics and fragility of (Cu46Zr47Al7)97Ti3 bulk metallic glass investigated by differential scanning calorimetry. Thermochim. Acta 2013, 565, 132–136. [Google Scholar] [CrossRef]
- Christian, J.W. The Theory of Transformations in Metals and Alloys; Elsevier Science Ltd.: Oxford, UK, 2002; p. 546. [Google Scholar]
- Sun, N.X.; Liu, X.D.; Lu, K. An explanation to the anomalous avrami exponent. Scr. Mater. 1996, 34, 1201–1207. [Google Scholar] [CrossRef]
- Lasocka, M. The effect of scanning rate on glass transition temperature of splat-cooled Te85Ge15. Mater. Sci. Eng. 1975, 23, 173–177. [Google Scholar] [CrossRef]




| Heating Rate (K min−1) | Tg (K) | Tx (K) | Tp (K) | ΔTx (K) |
|---|---|---|---|---|
| 10 | 826 | 864 | 876 | 38 |
| 20 | 836 | 873 | 888 | 37 |
| 30 | 844 | 882 | 893 | 38 |
| 40 | 851 | 888 | 904 | 37 |
| Activation Energy (kJ mol−1) | |||
|---|---|---|---|
| Equation | Eg | Ex | Ep |
| Kissinger | 308 ± 4 | 342 ± 5 | 310 ± 7 |
| Ozawa | 322 ± 3 | 356 ± 5 | 325 ± 7 |
| Tg | Tx | Tp | |
|---|---|---|---|
| A | 783.77 | 823.27 | 829.51 |
| B | 17.93 | 17.38 | 19.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhu, M.; Du, Y.; Yao, L.; Jian, Z. Crystallization Kinetics of the Fe68Nb6B23Mo3 Glassy Ribbons Studied by Differential Scanning Calorimetry. Crystals 2022, 12, 852. https://doi.org/10.3390/cryst12060852
Liu Y, Zhu M, Du Y, Yao L, Jian Z. Crystallization Kinetics of the Fe68Nb6B23Mo3 Glassy Ribbons Studied by Differential Scanning Calorimetry. Crystals. 2022; 12(6):852. https://doi.org/10.3390/cryst12060852
Chicago/Turabian StyleLiu, Yongqin, Man Zhu, Yuanyuan Du, Lijuan Yao, and Zengyun Jian. 2022. "Crystallization Kinetics of the Fe68Nb6B23Mo3 Glassy Ribbons Studied by Differential Scanning Calorimetry" Crystals 12, no. 6: 852. https://doi.org/10.3390/cryst12060852
APA StyleLiu, Y., Zhu, M., Du, Y., Yao, L., & Jian, Z. (2022). Crystallization Kinetics of the Fe68Nb6B23Mo3 Glassy Ribbons Studied by Differential Scanning Calorimetry. Crystals, 12(6), 852. https://doi.org/10.3390/cryst12060852





