Bias-Voltage Dependence of Tunneling Decay Coefficient and Barrier Height in Arylalkane Molecular Junctions with Graphene Contacts as a Protecting Interlayer
Abstract
:1. Introduction
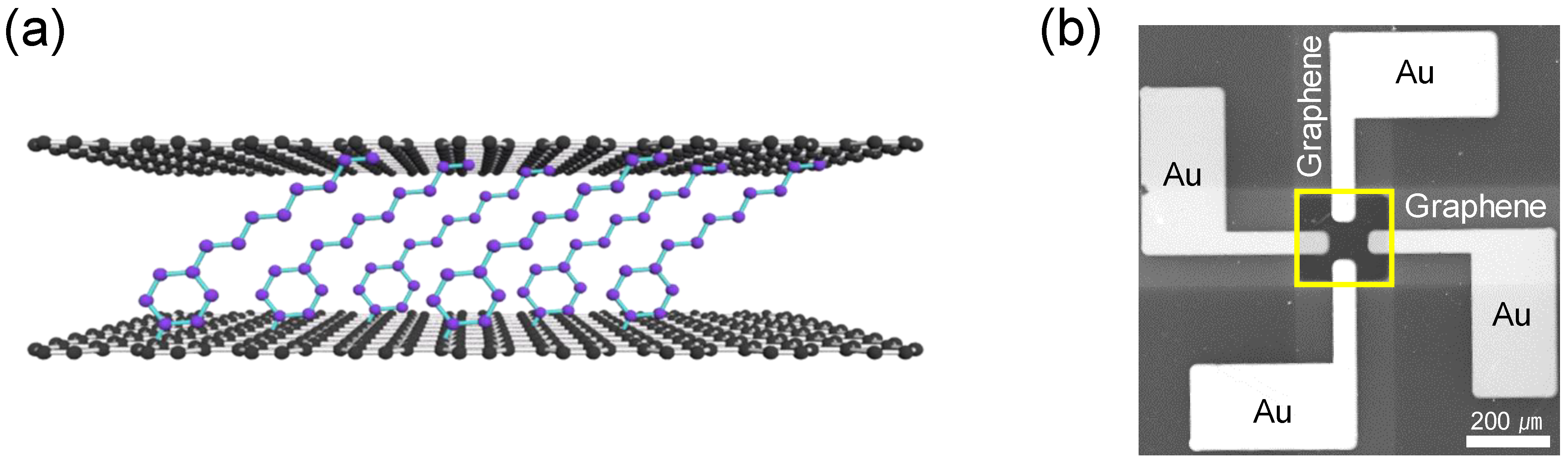
2. Experimental Details
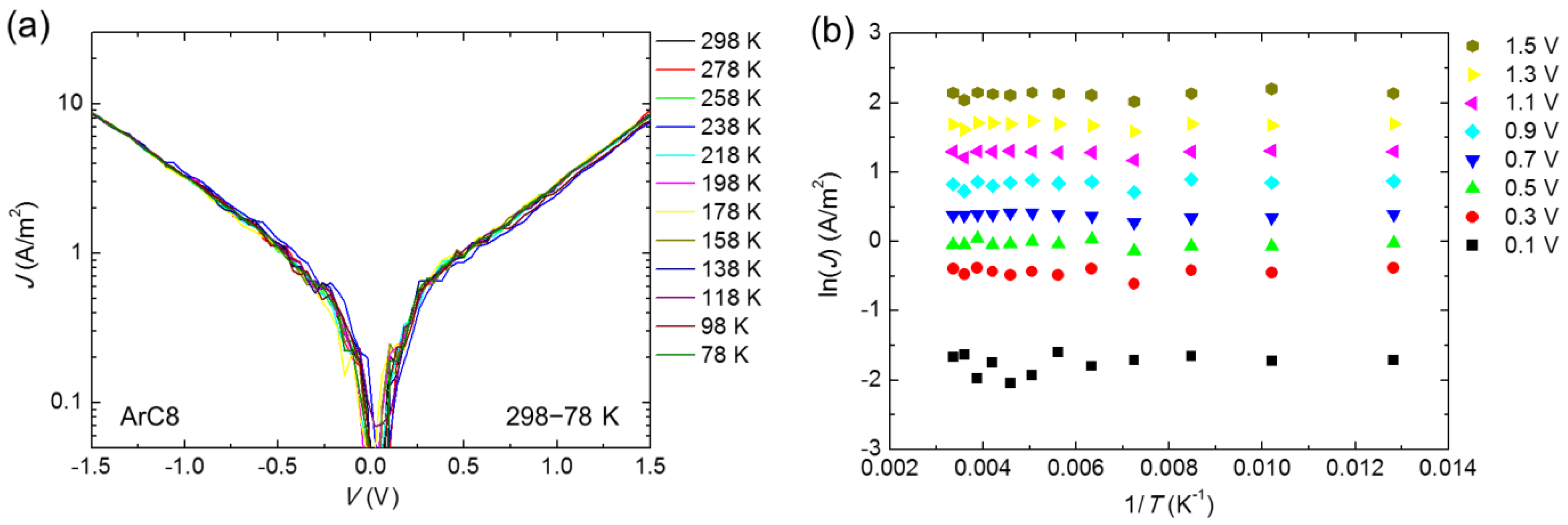
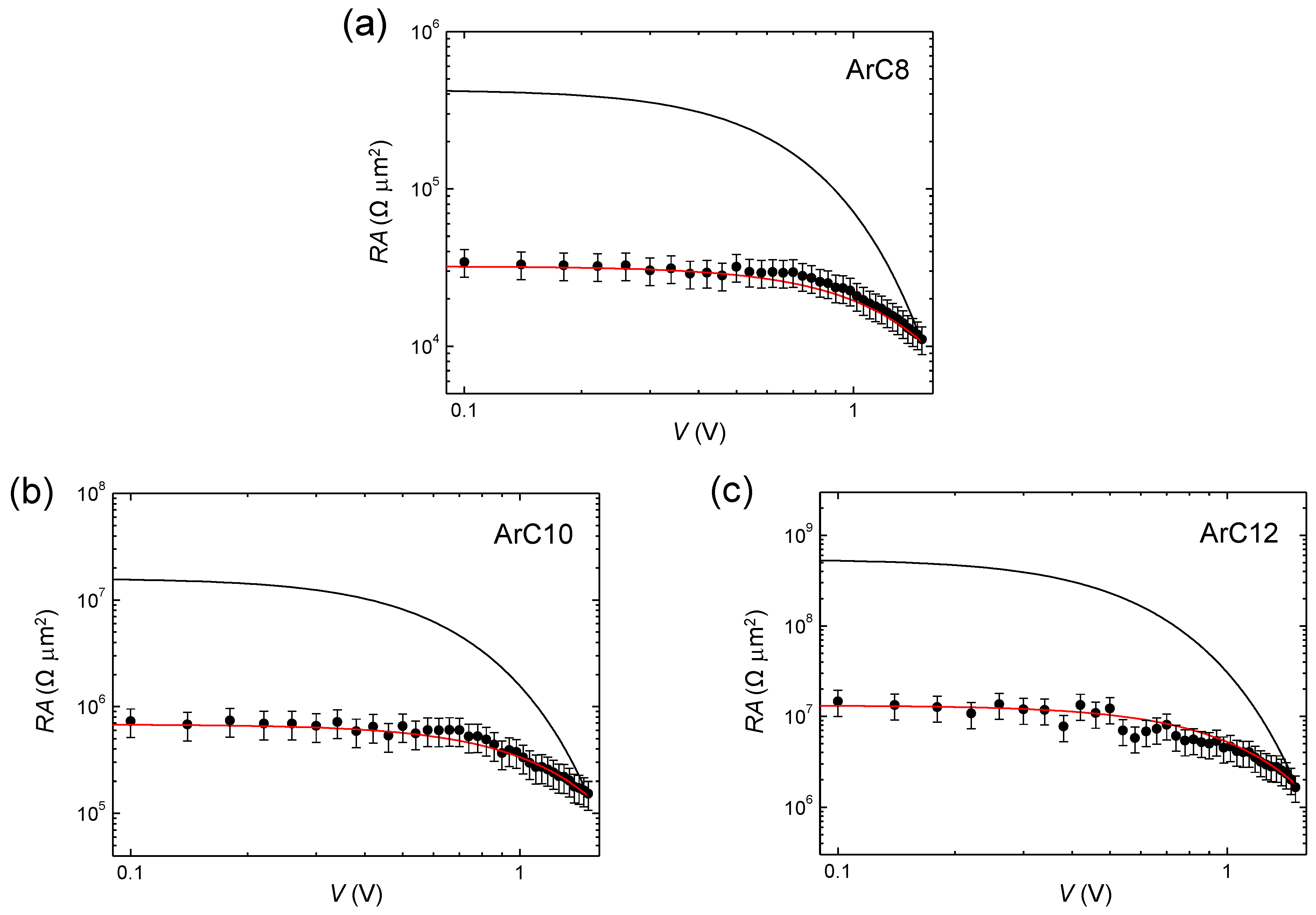
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Stoddart, J.F. From molecular to supramolecular electronics. Nat. Rev. Mater. 2018, 6, 804–828. [Google Scholar] [CrossRef]
- Bryce, M.R. A review of functional linear carbon chains (oligoynes, polyynes, cumulenes) and their applications as molecular wires in molecular electronics and optoelectronics. J. Mater. Chem. C 2021, 9, 10524–10546. [Google Scholar] [CrossRef]
- Gupta, R.; Jash, P.; Sachan, P.; Bayat, A.; Singh, V.; Mondal, P.C. Electrochemical Potential-Driven High-Throughput Molecular Electronic and Spintronic Devices: From Molecules to Applications. Angew. Chem. Int. Ed. 2021, 60, 26904–26921. [Google Scholar] [CrossRef]
- Xin, N.; Guan, J.; Zhou, C.; Chen, X.; Gu, C.; Li, Y.; Ratner, M.A.; Nitzan, A.; Stoddart, J.F.; Guo, X. Concepts in the design and engineering of single-molecule electronic devices. Nat. Rev. Phys. 2019, 1, 211–230. [Google Scholar] [CrossRef]
- Fu, H.; Zhu, X.; Li, P.; Li, M.; Yang, L.; Jia, C.; Guo, X. Recent progress in single-molecule transistors: Their designs, mechanisms and applications. J. Mater. Chem. C 2022, 10, 2375–2389. [Google Scholar] [CrossRef]
- Caneva, S.; Gehring, P.; García-Suárez, V.M.; García-Fuente, A.; Stefani, D.; Olavarria-Contreras, I.J.; Ferrer, J.; Dekker, C.; Van Der Zant, H.S.J. Mechanically controlled quantum interference in graphene break junctions. Nat. Nanotechnol. 2018, 13, 1126–1131. [Google Scholar] [CrossRef]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorenskaia, E.; Turner, K.L.; Martín, S.; Cea, P.; Low, P.J. Fabrication of metallic and non-metallic top electrodes for large-area molecular junctions. Nanoscale 2021, 13, 9055–9074. [Google Scholar] [CrossRef] [PubMed]
- Martín-Barreiro, A.; Soto, R.; Chiodini, S.; García-Serrano, A.; Martín, S.; Herrer, L.; Pérez-Murano, F.; Low, P.J.; Serrano, J.L.; Marcos, S.; et al. Uncapped Gold Nanoparticles for the Metallization of Organic Monolayers. Adv. Mater. Interfaces 2021, 8, 2100876. [Google Scholar] [CrossRef]
- Puebla-Hellmann, G.; Venkatesan, K.; Mayor, M.; Lörtscher, E. Metallic nanoparticle contacts for high-yield, ambient-stable molecular-monolayer devices. Nature 2018, 559, 232–235. [Google Scholar] [CrossRef]
- Akkerman, H.B.; Blom, P.W.M.; de Leeuw, D.M.; de Boer, B. Towards molecular electronics with large-area molecular junctions. Nature 2006, 441, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, Y.-L.; Lang, D.V.; Rogers, A.J.A.; Hsu, J.W.P. Electrical Contacts to Molecular Layers by Nanotransfer Printing. Nano Lett. 2003, 3, 913–917. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, D.; Kwon, H.; Hwang, W.-T.; Jang, Y.; Min, M.; Char, K.; Xiang, D.; Jeong, H.; Lee, T. Statistical investigation of the length-dependent deviations in the electrical characteristics of molecular electronic junctions fabricated using the direct metal transfer method. J. Phys. Condens. Matter 2016, 28, 94003. [Google Scholar] [CrossRef] [PubMed]
- Neuhausen, A.B.; Hosseini, A.; Sulpizio, J.A.; Chidsey, C.E.D.; Goldhaber-Gordon, D. Molecular Junctions of Self-Assembled Monolayers with Conducting Polymer Contacts. ACS Nano 2012, 6, 9920–9931. [Google Scholar] [CrossRef]
- Wang, G.; Kim, Y.; Choe, M.; Kim, T.-W.; Lee, T. A New Approach for Molecular Electronic Junctions with a Multilayer Graphene Electrode. Adv. Mater. 2011, 23, 755–760. [Google Scholar] [CrossRef]
- Seo, S.; Min, M.; Lee, S.M.; Lee, H. Photo-switchable molecular monolayer anchored between highly transparent and flexible graphene electrodes. Nat. Commun. 2013, 4, 1920. [Google Scholar] [CrossRef] [Green Version]
- Kühnel, M.; Overgaard, M.H.; Hels, M.C.; Cui, A.; Vosch, T.; Nygård, J.; Li, T.; Laursen, B.W.; Nørgaard, K. High-Quality Reduced Graphene Oxide Electrodes for Sub-Kelvin Studies of Molecular Monolayer Junctions. J. Phys. Chem. C 2018, 122, 25102–25109. [Google Scholar] [CrossRef]
- Simmons, J.G. Generalized Formula for the Electric Tunnel Effect between Similar Electrodes Separated by a Thin Insulating Film. J. Appl. Phys. 1963, 34, 1793–1803. [Google Scholar] [CrossRef] [Green Version]
- Akkerman, H.B.; Naber, R.C.G.; Jongbloed, B.; van Hal, P.A.; Blom, P.W.M.; de Leeuw, D.M.; de Boer, B. Electron tunneling through alkanedithiol self-assembled monolayers in large-area molecular junctions. Proc. Natl. Acad. Sci. USA 2007, 104, 11161–11166. [Google Scholar] [CrossRef] [Green Version]
- Huisman, E.H.; Guédon, C.M.; van Wees, B.J.; van der Molen, S.J. Interpretation of Transition Voltage Spectroscopy. Nano Lett. 2009, 9, 3909–3913. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Bâldea, I.; Smith, C.E.; Wu, Y.; Frisbie, C.D. Experimental and Theoretical Analysis of Nanotransport in Oligophenylene Dithiol Junctions as a Function of Molecular Length and Contact Work Function. ACS Nano 2015, 9, 8022–8036. [Google Scholar] [CrossRef]
- Wang, W.; Lee, T.; Reed, M.A. Mechanism of electron conduction in self-assembled alkanethiol monolayer devices. Phys. Rev. B 2003, 68, 035416. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Haag, R.; Chabinyc, M.L.; Ismagilov, R.F.; Cohen, A.E.; Terfort, A.; Rampi, M.A.; Whitesides, G.M. Electron Transport through Thin Organic Films in Metal−Insulator−Metal Junctions Based on Self-Assembled Monolayers. J. Am. Chem. Soc. 2001, 123, 5075–5085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engelkes, V.B.; Beebe, A.J.M.; Frisbie, C.D. Length-Dependent Transport in Molecular Junctions Based on SAMs of Alkanethiols and Alkanedithiols: Effect of Metal Work Function and Applied Bias on Tunneling Efficiency and Contact Resistance. J. Am. Chem. Soc. 2004, 126, 14287–14296. [Google Scholar] [CrossRef]
- Liang, X.; Sperling, B.A.; Calizo, I.; Cheng, G.; Hacker, C.; Zhang, Q.; Obeng, Y.; Yan, K.; Peng, H.; Li, Q.; et al. Toward Clean and Crackless Transfer of Graphene. ACS Nano 2011, 5, 9144–9153. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.M.; Rosei, F. Molecular Self-Assembly on Graphene. Small 2014, 10, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.B.; Seol, G.; Guo, J. Modeling of a vertical tunneling graphene heterojunction field-effect transistor. Appl. Phys. Lett. 2012, 101, 033503. [Google Scholar] [CrossRef] [Green Version]
- Fallahazad, B.; Lee, K.; Kang, S.; Xue, J.; Larentis, S.; Corbet, C.; Kim, K.; Movva, H.C.P.; Taniguchi, T.; Watanabe, K.; et al. Gate-Tunable Resonant Tunneling in Double Bilayer Graphene Heterostructures. Nano Lett. 2015, 15, 428–433. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Nawarat, P.; Beach, K.; Meunier, V.; Terrones, H.; Wang, G.-C.; Lewis, K.M. Voltage-Dependent Barrier Height of Electron Transport through Iron Porphyrin Molecular Junctions. J. Phys. Chem. C 2021, 125, 7350–7357. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Im, K.; Seo, D.-H.; Song, H. Bias-Voltage Dependence of Tunneling Decay Coefficient and Barrier Height in Arylalkane Molecular Junctions with Graphene Contacts as a Protecting Interlayer. Crystals 2022, 12, 767. https://doi.org/10.3390/cryst12060767
Im K, Seo D-H, Song H. Bias-Voltage Dependence of Tunneling Decay Coefficient and Barrier Height in Arylalkane Molecular Junctions with Graphene Contacts as a Protecting Interlayer. Crystals. 2022; 12(6):767. https://doi.org/10.3390/cryst12060767
Chicago/Turabian StyleIm, Kyungjin, Dong-Hyoup Seo, and Hyunwook Song. 2022. "Bias-Voltage Dependence of Tunneling Decay Coefficient and Barrier Height in Arylalkane Molecular Junctions with Graphene Contacts as a Protecting Interlayer" Crystals 12, no. 6: 767. https://doi.org/10.3390/cryst12060767
APA StyleIm, K., Seo, D.-H., & Song, H. (2022). Bias-Voltage Dependence of Tunneling Decay Coefficient and Barrier Height in Arylalkane Molecular Junctions with Graphene Contacts as a Protecting Interlayer. Crystals, 12(6), 767. https://doi.org/10.3390/cryst12060767




