In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Na0.91MnO2 Hexagonal Tablets
2.3. Instrumentation and Sample Analysis
2.4. Electrochemical Measurements
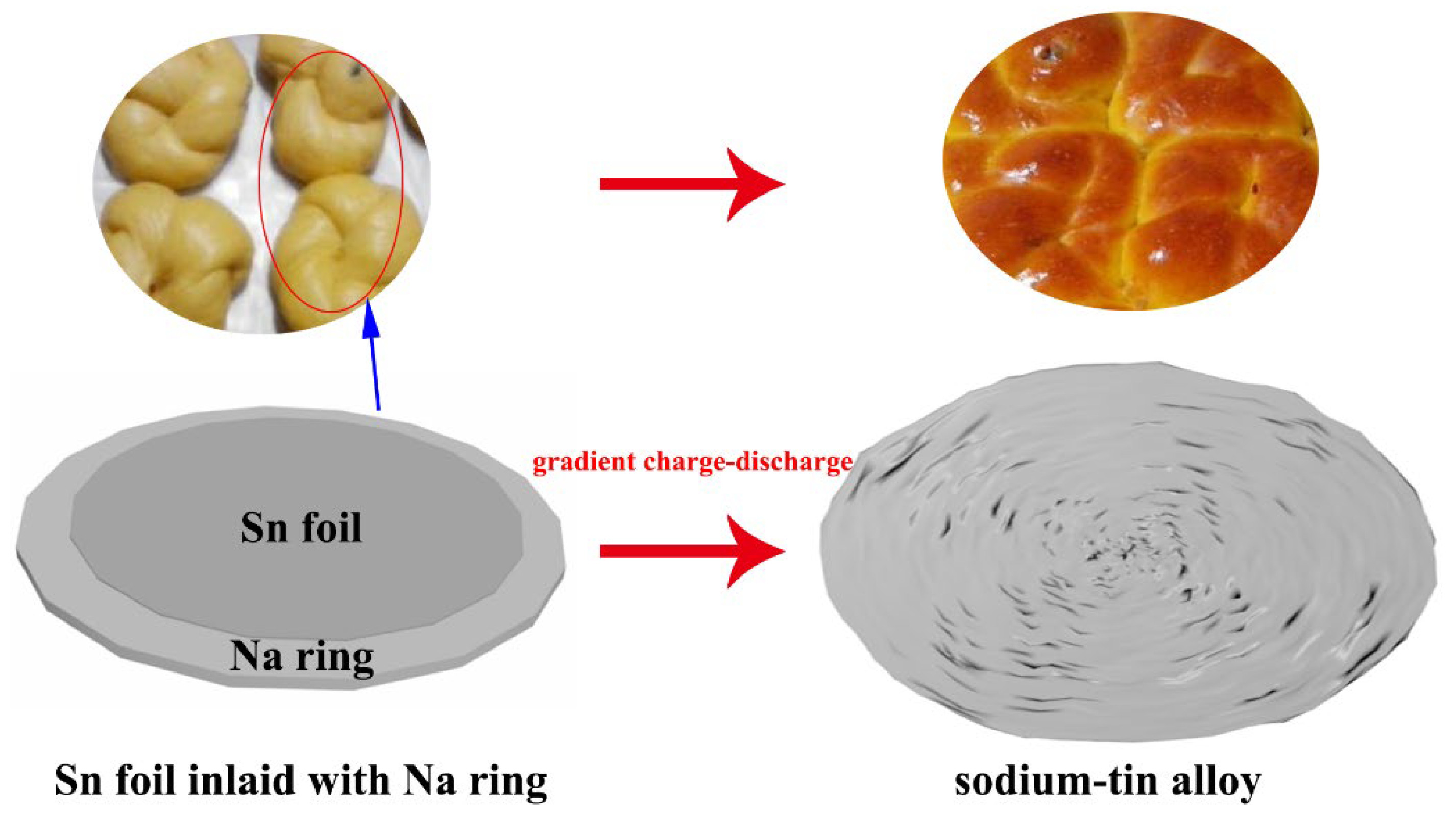
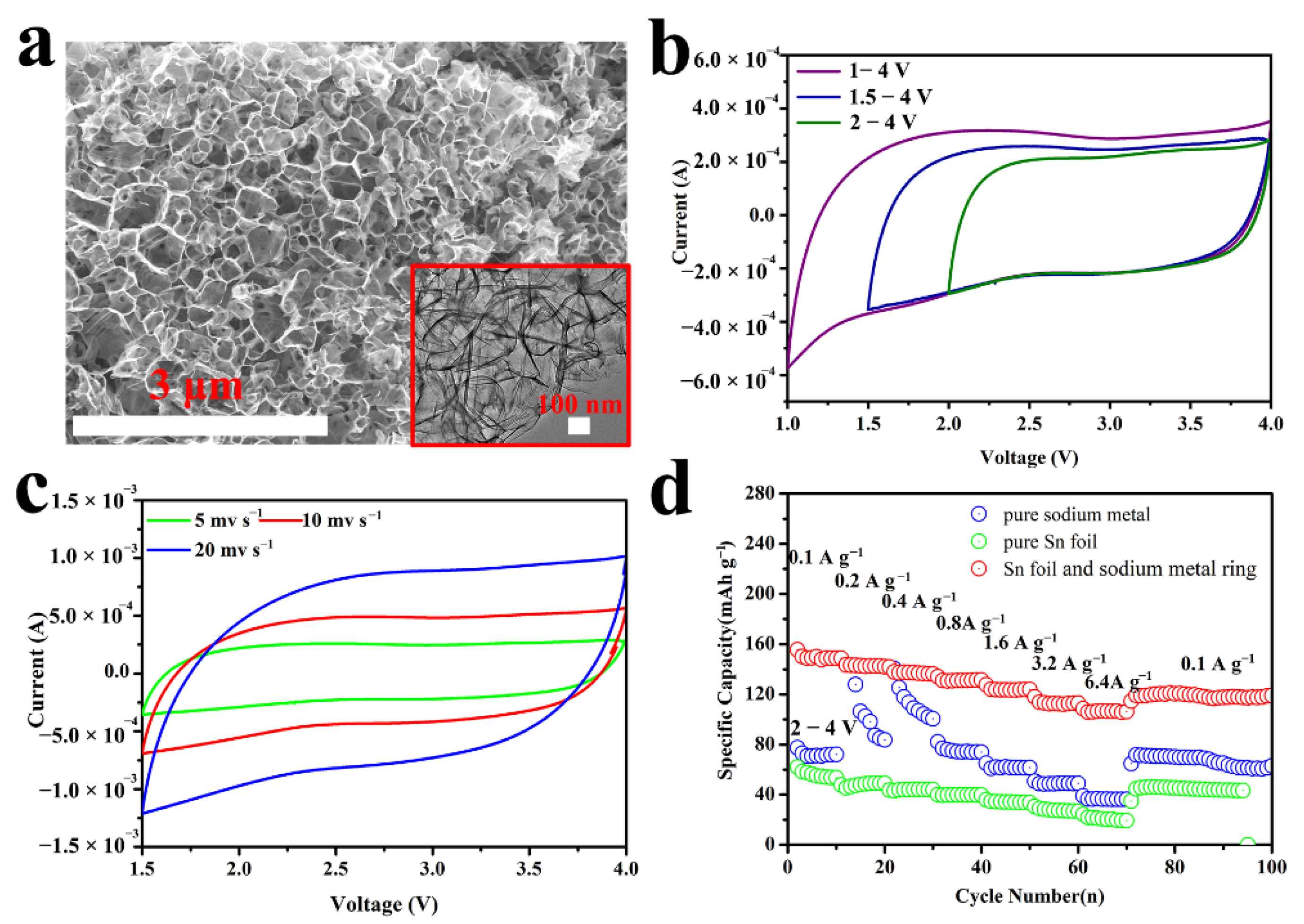
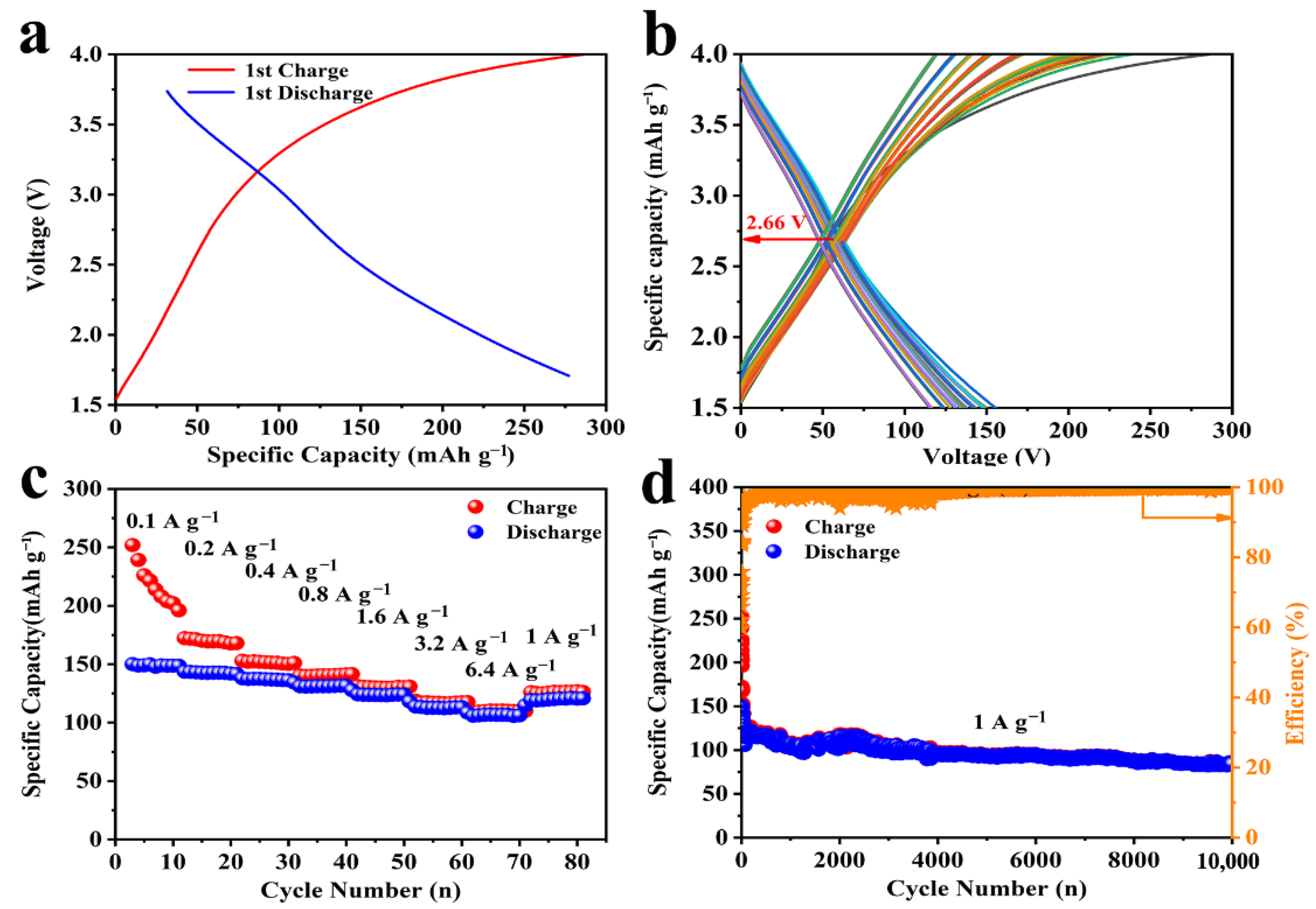
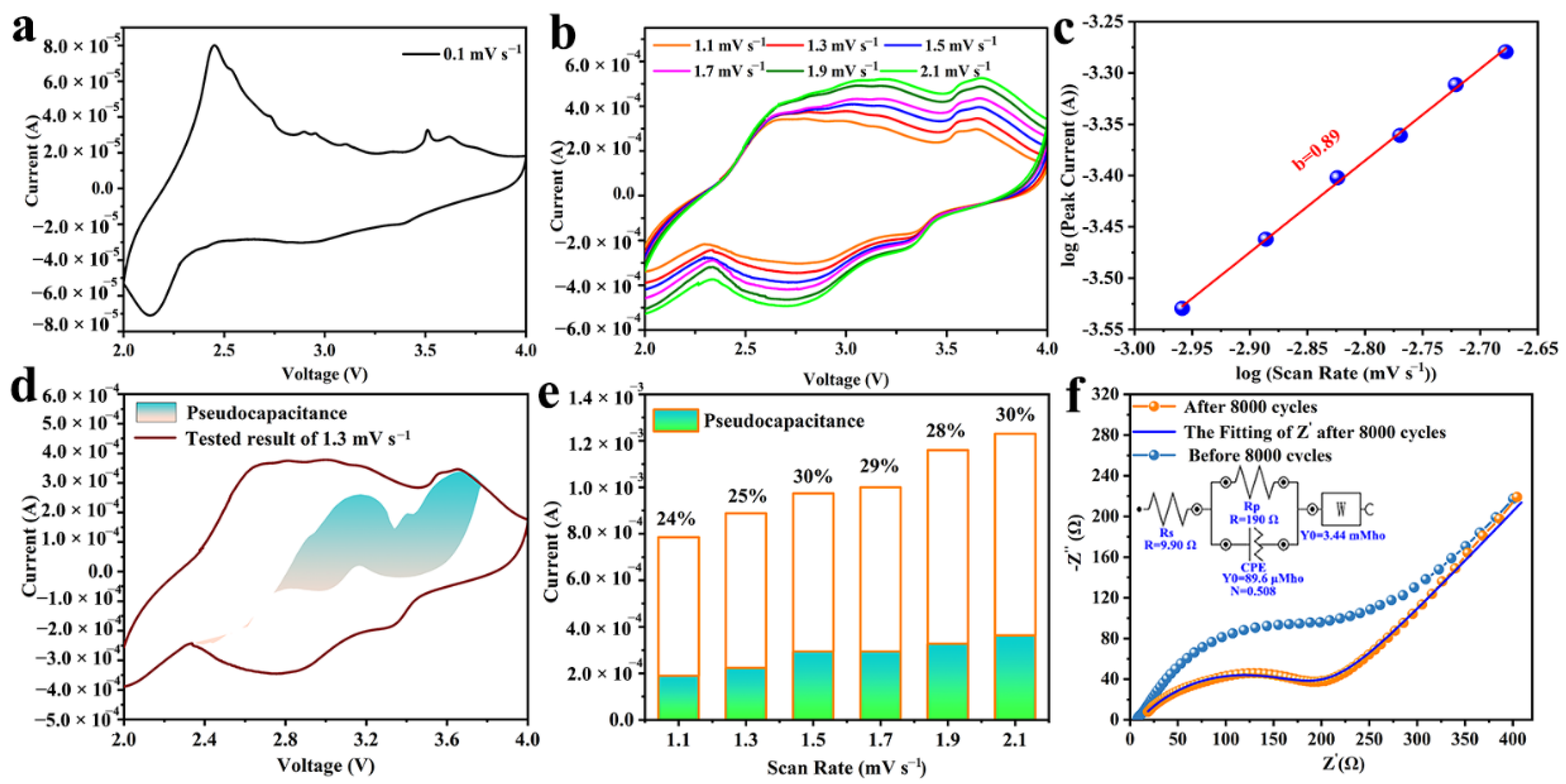
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, M.; Wang, Z.; Ni, J.; Li, L. Dual-doped hematite nanorod arrays on carbon cloth as a robust and flexible sodium anode. Adv. Funct. Mater. 2020, 30, 1910043. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research development on sodium-ion batteries. Chem. Rev. 2014, 114, 11636–11682. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, M.; Feng, X. Carbon materials for ion-intercalation involved rechargeable battery technologies. Chem. Soc. Rev. 2021, 50, 2388–2443. [Google Scholar] [CrossRef] [PubMed]
- Usiskin, R.; Lu, Y.; Popovic, J.; Law, M.; Balaya, P.; Hu, Y.S.; Maier, J. Fundamentals, status and promise of sodium-based batteries. Na. Rev. Mater. 2021, 6, 1020–1035. [Google Scholar] [CrossRef]
- Cao, Y.; Xiao, L.; Sushko, M.L.; Wang, W.; Schwenzer, B.; Xiao, J.; Nie, Z.; Saraf, L.V.; Yang, Z.; Liu, J. Sodium ion insertion in hollow carbon nanowires for battery applications. Nano Lett. 2012, 12, 3783–3787. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Park, K.; Lu, W.; Wang, C.; Xue, W.; Yang, F.; Zhou, J.; Suo, L.; Lin, T. Nitrogen-doped carbon for sodium-ion battery anode by self-etching and graphitization of bimetallic MOF-based composite. Chem 2017, 3, 152–163. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Kang, F.; Tarascon, J.M.; Kim, J.K. Recent advances in electrospun carbon nanofibers and their application in electrochemical energy storage. Prog. Mater. Sci. 2016, 76, 319–380. [Google Scholar] [CrossRef]
- Wenzel, S.; Hara, T.; Janek, J.; Adelhelm, P. Room-temperature sodium-ion batteries: Improving the rate capability of carbon anode materials by templating strategies. Energy Env. Sci. 2011, 4, 3342–3345. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Ding, J.; Mitlin, D. Tin and tin compounds for sodium ion battery anodes: Phase transformations and performance. Acc. Chem. Res. 2015, 48, 1657–1665. [Google Scholar] [CrossRef]
- Jing, W.T.; Yang, C.C.; Jiang, Q. Recent progress on metallic Sn- and Sb-based anodes for sodium-ion batteries. J. Mater. Chem. A 2020, 8, 2913–2933. [Google Scholar] [CrossRef]
- Kim, C.; Kim, H.; Sadan, M.K.; Jeon, M.; Cho, G.; Ahn, J.; Kim, K.; Cho, K.; Ahn, H. Development and evaluation of Sn foil anode for sodium-ion batteries. Small 2021, 17, 2102618. [Google Scholar] [CrossRef]
- Song, M.; Wang, C.; Du, D.; Li, F.; Chen, J. A high-energy-density sodium-ion full battery based on tin anode. Sci. China Chem. 2019, 62, 616–621. [Google Scholar] [CrossRef]
- Chen, L.; He, X.; Chen, H.; Huang, S.; Wei, M. N-Doped carbon encapsulating Bi nanoparticles derived from metal-organic frameworks for high-performance sodium-ion batteries. J. Mater. Chem. A 2021, 9, 22048–22055. [Google Scholar] [CrossRef]
- Cheng, D.; Yang, L.; Hu, R.; Liu, J.; Che, R.; Cui, J.; Wu, Y.; Chen, W.; Huang, J.; Zhu, M. Sn-C and Se-C Co-bonding SnSe/few-layered graphene micro-nano structure: Route to a densely compacted and durable anode for lithium/sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2019, 11, 36685–36696. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, J.; Li, R.; Xu, Z.; Cao, L.; Ouyang, H.; Li, J.; Qi, H.; Wang, C. Enhanced cycling performances of hollow Sn compared to solid Sn in Na-ion battery. Electrochim. Acta 2015, 180, 227–233. [Google Scholar] [CrossRef]
- Lin, Y.M.; Abel, P.R.; Gupta, A.; Goodenough, J.B.; Heller, A.; Mullins, C.B. Sn-Cu nanocomposite anodes for rechargeable sodium-ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 8273–8277. [Google Scholar] [CrossRef]
- Liu, J.; Yu, L.; Wu, C.; Wen, Y.; Yin, K.; Chiang, F.K.; Hu, R.; Liu, J.; Sun, L.; Gu, L. New nanoconfined galvanic replacement synthesis of hollow Sb@C yolk-shell spheres constituting a stable anode for high-rate Li/Na-ion batteries. Nano Lett. 2017, 17, 2034–2042. [Google Scholar] [CrossRef]
- Liu, S.; Luo, Z.; Guo, J.; Pan, A.; Cai, Z.; Liang, S. Bismuth nanosheets grown on carbon fiber cloth as advanced binder-free anode for sodium-ion batteries. Electrochem. Commun. 2017, 81, 10–13. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, P.; Lei, K.; Zhao, Q.; Tao, Z.; Chen, J. Selenium phosphide (Se4P4) as a new and promising anode material for sodium-ion batteries. Adv. Energy Mater. 2017, 7, 1601973. [Google Scholar] [CrossRef]
- Luo, B.; Qiu, T.; Ye, D.; Wang, L.; Zhi, L. Tin nanoparticles encapsulated in graphene backboned carbonaceous foams as high-performance anodes for lithium-ion and sodium-ion storage. Nano Energy 2016, 22, 232–240. [Google Scholar] [CrossRef]
- Mao, J.; Fan, X.; Luo, C.; Wang, C. Building self-healing alloy architecture for stable sodium-ion battery anodes: A case study of tin anode materials. ACS Appl. Mater. Interfaces 2016, 8, 7. [Google Scholar] [CrossRef]
- Nagulapati, V.M.; Lee, J.H.; Kim, H.S.; Oh, J.; Kim, I.T.; Hur, J.; Lee, S.G. Novel hybrid binder mixture tailored to enhance the electrochemical performance of SbTe bi-metallic anode for sodium ion batteries. J. Electroanal. Chem. 2020, 865, 114160. [Google Scholar] [CrossRef]
- Oh, J.A.S.; Sun, J.; Goh, M.; Chua, B.; Zeng, K.; Lu, L. A robust solid-solid interface using sodium-tin alloy modified metallic sodium anode paving way for all-solid-state battery. Adv. Energy Mater. 2021, 11, 2101228. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Y.; Wu, L.; Cao, Y.; Ai, X.; Yang, H. High capacity Na-storage and superior cyclability of nanocomposite Sb/C anode for Na-ion batteries. Chem. Commun. Camb 2012, 48, 7070–7072. [Google Scholar] [CrossRef]
- Sheng, M.; Zhang, F.; Ji, B.; Tong, X.; Tang, Y. A novel tin-graphite dual-ion battery based on sodium-ion electrolyte with high energy density. Adv. Energy Mater. 2017, 7, 1601963. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, Y.; Zhu, Y.; Culver, J.N.; Wang, C. Tin-coated viral nanoforests as sodium-ion battery anodes. ACS Nano 2013, 7, 3627–3634. [Google Scholar] [CrossRef]
- Yu, H.; Seomoon, K.; Kim, J.; Kim, J.-K. Low-cost and highly safe solid-phase sodium ion battery with a Sn-C nanocomposite anode. J. Ind. Eng. Chem. 2021, 100, 112–118. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.X.; Guo, Y.G. High-Capacity Te Anode Confined in Microporous Carbon for Long-Life Na-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 27838–27844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, A.; Ding, L.; Zhou, Z.; Wang, Y.; Niu, S.; Liang, S.; Cao, G. Nitrogen-Doped Yolk-Shell-Structured CoSe/C Dodecahedra for High-Performance Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 3624–3633. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Chen, Y.; Weadock, N.; Wan, J.; Vaaland, O.; Han, X.; Li, T.; Hu, L. Tin anode for sodium-ion batteries using natural wood fiber as a mechanical buffer and electrolyte reservoir. Nano Lett. 2013, 13, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Brezesinski, T.; Wang, J.; Tolbert, S.H.; Dunn, B. Ordered mesoporous alpha-MoO3 with iso-oriented nanocrystalline walls for thin-film pseudocapacitors. Nat. Mater. 2010, 9, 146–151. [Google Scholar] [CrossRef]
- Brezesinski, K.; Wang, J.; Haetge, J.; Reitz, C.; Steinmler, S.O.; Tolbert, S.H.; Smarsly, B.M.; Dunn, B.; Brezesinski, T. Pseudocapacitive Contributions to Charge Storage in Highly Ordered Mesoporous Group V Transition Metal Oxides with Iso-Oriented Layered Nanocrystalline Domains. J. Am. Chem. Soc. 2010, 132, 6982–6990. [Google Scholar] [CrossRef]
- Pu, X.; Zhao, D.; Fu, C.; Chen, Z.; Cao, S.; Wang, C.; Cao, Y. Understanding and Calibration of Charge Storage Mechanism in Cyclic Voltammetry Curves. Angew. Chem. Inter. Ed. 2021, 133, 21480–21488. [Google Scholar] [CrossRef]
- Chen, M.; Chen, L.; Hu, Z.; Liu, Q.; Zhang, B.; Hu, Y.; Gu, Q.; Wang, J.L.; Wang, L.Z.; Guo, X. Carbon-coated Na3.32Fe2.34(P2O7)2 cathode material for high-rate and long-life sodium-ion batteries. Adv. Mater. 2017, 29, 1605535. [Google Scholar] [CrossRef]
- Wu, L.; Ou Yang, J.; Guo, S.; Yao, L.; Li, H.; Zhang, S.; Yue, H.; Cai, K.; Zhang, C.; Yang, C. Pseudocapacitive trimetal Fe0.8CoMnO4 nanoparticles@carbon nanofibers as high-performance sodium storage anode with self-supported mechanism. Adv. Funct. Mater. 2020, 30, 2001718. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, L.; Guo, S.; Liang, W.; Song, L.; Song, B.; Zhang, Q.; Wu, L. In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics. Crystals 2022, 12, 575. https://doi.org/10.3390/cryst12050575
Niu L, Guo S, Liang W, Song L, Song B, Zhang Q, Wu L. In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics. Crystals. 2022; 12(5):575. https://doi.org/10.3390/cryst12050575
Chicago/Turabian StyleNiu, Liangfeng, Shoujie Guo, Wei Liang, Limin Song, Burong Song, Qianlong Zhang, and Lijun Wu. 2022. "In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics" Crystals 12, no. 5: 575. https://doi.org/10.3390/cryst12050575
APA StyleNiu, L., Guo, S., Liang, W., Song, L., Song, B., Zhang, Q., & Wu, L. (2022). In Situ Electrochemical Derivation of Sodium-Tin Alloy as Sodium-Ion Energy Storage Devices Anode with Overall Electrochemical Characteristics. Crystals, 12(5), 575. https://doi.org/10.3390/cryst12050575




