Abstract
Aiming for hydrogen and oxidation easily produced in the melting process of Al-Mg alloys, three processes, including non-vacuum static melt treatment, non-vacuum rotary-injection purification and vacuum-stirring purification, were used to purify the Al-6Mg alloy melt. The hydrogen content and inclusion content were studied by means of the solid-state hydrogen measurement method, reduced pressure test method, FESEM and EDS. The results show that the purification effect of vacuum-stirring purification is better than that of the non-vacuum static melt treatment and non-vacuum rotary-injection purification. The hydrogen content of the melt decreases from 0.48 mL/100 gAl in the non-vacuum static treatment, to 0.32 mL/100 gAl in the non-vacuum rotary-injection purification process and to 0.10 mL/100 gAl in the vacuum-stirring purification process. The inclusion content of the melt decreases from 2.6% in the non-vacuum static treatment to 0.69% in the non-vacuum rotary-injection purification process, and to 0.39%, in the vacuum-stirring purification process.
1. Introduction
With the rapid development of aerospace, rail transit, weapons and equipment, chemical machinery and other industrial fields, the demand for lightweight aluminum alloys is becoming more and more urgent [1]. Under the guidance of the concepts of “carbon peak” and “carbon neutralization”, high-strength aluminum alloy welding has an increasing demand for high-performance aluminum alloy welding wire [2]. As a medium- and high-strength welding wire, ER5356 aluminum alloy welding wire is more and more being used in large welded structural parts [3]. However, as a high-magnesium aluminum alloy, with a magnesium content of 4.5~5.5%, 5356 aluminum alloy is easy to oxidize and can easily absorb hydrogen in the melting process. This makes it very easy to increase the content of pores and inclusions in the 5356 aluminum alloy welding wire material, which leads to a serious reduction in the performance, failure or scrapping of welded parts [4,5,6]. Therefore, it is necessary to purify the 5356 aluminum alloy melt in material preparation [7,8].
There are two types of aluminum alloy purification methods: one is the adsorption purification method, which includes bubble floating, filtration, flux treatment, etc., [9,10], and its purification mechanism is that the hydrogen and oxidation inclusion are adsorbed and removed through the contact between the aluminum melt and flux under the adsorption of the refining agent. The other is the non-adsorption purification method, which includes static treatment, vacuum treatment, ultrasonic purification, electromagnetic purification, etc., and its purification mechanism is that the hydrogen and inclusions can be separated from the melt and removed by changing the equilibrium system of the inclusions, hydrogen and alloy melt, under physical action (vacuum, electromagnetic field, gravity field, etc.) [11,12]. Among these methods, rotary injection, as a relatively mature melt purification process in industrial production, has been widely used in the melt purification of aluminum alloy [13,14,15]. However, for aluminum alloys with high magnesium content, such as the 5356 aluminum alloy, the purification effect of the rotary-injection process is limited, and the hydrogen content of the melt still reaches 0.20–0.30 mL/100 gAl; its purification effect and purification mechanism need to be studied further. As an effective purification method, vacuum purification has also attracted the attention of researchers in recent years, but its purification effect needs to improve and its purification mechanism needs to be studied further [16,17,18].
Based on the vacuum-purification and melt stirring process, which may be one of the most suitable processes for the metallurgy of magnesium and aluminum and their composites [19,20], the aims of this study are to explore the vacuum-stirring purification process, to compare its purification effect with that of the non-vacuum static melt treatment and non-vacuum rotary-injection purification process and to discuss the purification mechanism of the Al-6Mg aluminum melt.
2. Materials and Methods
2.1. Materials
The raw materials including 99.99% high-purity aluminum, pure Mg and Al10Mn, Al10Cr and Al-5Ti-B master alloys are used in the experiment. The composition of the test alloy is shown in Table 1:

Table 1.
The composition of the test alloy in this study.
2.2. Methods and Equipment
The schematic view of the smelting purification process is shown in Figure 1. The experiment procedures are as follows: the configured pure aluminum and Al10Mn, Al10Cr and Al-5Ti-B master alloys are firstly heated in the induction melting furnace, and pure Mg is added to the melt when the melt temperature reaches 720 °C. For the non-vacuum static melt treatment, the melt is held for 20 min and cast into the pyro-HVT reduced pressure test instrument to obtain the testing sample. For the non-vacuum rotary-injection purification, the melt is degassed at a rotating speed of 400 r/min, an argon flow rate of 0.4 m3/h, a degassing time of 30 min, holding for 20 min after degassing and then cast into the pyro-HVT reduced pressure test instrument to obtain the testing sample. For the vacuum-stirring purification, the melt is degassed at a rotating speed of 400 r/min, at a vacuum degree of 6.7 × 10−2 Pa, a degassing time of 30 min, holding for 20 min after degassing and then cast into the pyro-HVT reduced-pressure test instrument to obtain the testing sample. In the experiment, K-type thermocouple is used to monitor the melt temperature and the temperature deviation is controlled at ±1 °C. In order to reduce the influence of impurities induced by melt thermocouples and pouring tools, the thermocouples and slag spoons are sprayed with boron nitride, dried and preheated.
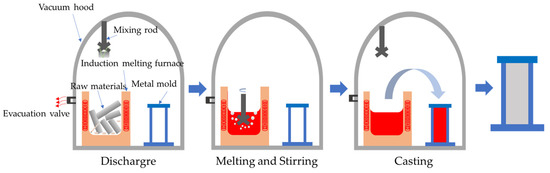
Figure 1.
The schematic view of the purification process of Al-6Mg alloy.
2.3. Testing Methods
The hydrogen content of the melt is qualitatively tested using the pyro-HVT reduced-pressure test instrument. The melt is scooped with a standard sampling spoon and cast into the reduced-pressure test instrument. After the reduced-pressure test instrument is closed, the vacuum is pumped for the reduced-pressure test and the vacuum time is 5 min. The LECO RHEN 602 solid-state hydrogen instrument is used to quantitatively measure hydrogen content in the sample. The microstructure of the aluminum alloy sample is observed by the field emission scanning electron microscope (FSEM) JSM 7900F. Image-Pro Plus software is used to statistically process oxide inclusions in the scanned images; three images are taken from each sample for statistics and the inclusion size and total area occupied in the images are averagely calculated.
3. Results
3.1. Hydrogen Removal Effect of Al-6Mg Alloy Melt
Figure 2 shows the pore sizes and content of the Al-6Mg alloy melt solidified in different purification processes: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification and (c) vacuum-stirring purification. Table 2 shows the ratios of pore areas under different purification processes. In the case of non-vacuum static melt treatment, as shown in Figure 2a, a large number of pores occurs in the sample section, with the largest pore diameter having a range from 0.2 cm to 1.5 cm, and the pore area accounting for 36.05% of the whole sample section, as shown in Table 2. In the case of non-vacuum rotary-injection purification, the number and sizes of pores are significantly reduced, the maximum pore diameter is less than 0.3 cm and the proportion of pore area to the whole sample section is greatly reduced, as shown in Figure 2b; the pore area accounts for 1.22% of the whole sample section, as shown in Table 2. In the case of the vacuum-stirring purification process, the number and sizes of pores are significantly reduced, there are almost no obvious pores, as shown in Figure 2c, and the proportion of pore area is only 0.36%, as shown in Table 2.
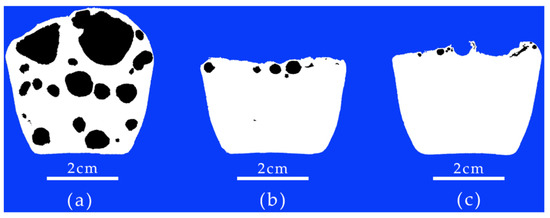
Figure 2.
Pore size and content of Al-6Mg alloy melt solidified under reduced pressure after melt purification: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification, (c) vacuum-stirring purification.

Table 2.
Ratio of pore area under different purification processes.
In order to more accurately measure the hydrogen content of the melt, the solid-state hydrogen instrument was used to test the hydrogen content of the melt purified by three different processes. Table 3 shows the hydrogen content of the Al-6Mg alloy sample under different melt purification conditions. It can be seen from Table 3 that, in the case of non-vacuum static melt treatment, the hydrogen content of the melt reaches up to 0.48 mL/100 gAl and the hydrogen content of the melt is very high. In the case of rotary-injection purification, the hydrogen content decreases to 0.32 mL/100 gAl. In the case of vacuum-stirring purification, the hydrogen content of the melt is significantly reduced to 0.10 mL/100 gAl and the hydrogen content arrives at a very low level.

Table 3.
Hydrogen content of Al-6Mg alloy sample under different melt purification conditions.
3.2. Inclusions Removal Effect of Al-6Mg Alloy Melt
Figure 3 shows the SEM microstructure images of Al-6Mg alloy purified samples under different purification conditions: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification and (c) vacuum-stirring purification. It can be seen from Figure 3 that, in the case of non-vacuum static melt treatment, as shown in Figure 3a, a large number of inclusions occurs in the sample section. In the case of non-vacuum rotary-injection purification, the number and sizes of inclusions are reduced, as shown in Figure 3b. In the case of vacuum-stirring purification process, the number and sizes of inclusions are significantly reduced and the inclusions almost cannot be seen, as shown in Figure 3c.

Figure 3.
SEM microstructure images of Al-6Mg alloy sample after being purified: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification, (c) vacuum-stirring purification.
In order to accurately analyze the inclusion component of Figure 3, EDS was adopted. Figure 4 shows the EDS result of inclusions in the Al-6Mg alloy. It can be seen from Figure 4 that the inclusions in the sample mainly include two kinds of morphologies: one is the black oxide phase containing Al, Mg, O and Ca, which can be judged as Al2O3, MgO and CaO, and the other is the white massive phase, mainly containing Mg, Al, Mn, Cr and Fe, which can be judged as (Fe, Mn)Al6, Al6Mn, Al7Cr and Al3Mg2 [21].
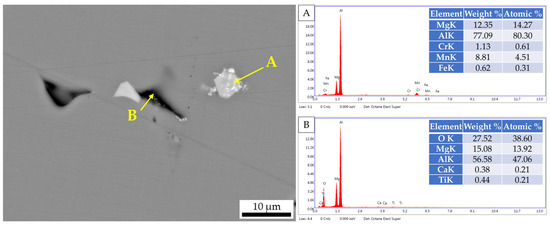
Figure 4.
EDS result of inclusions in Al-6Mg alloy: (A) white inclusion, (B) black inclusion.
In order to further obtain the total amount and size distribution of inclusions, Image-Pro Plus was used to statistically analyze the inclusions in Figure 3. Figure 5 shows the total inclusion content in the three purification processes. Figure 6 shows the inclusions’ size distribution of the Al-6Mg alloy under different conditions.
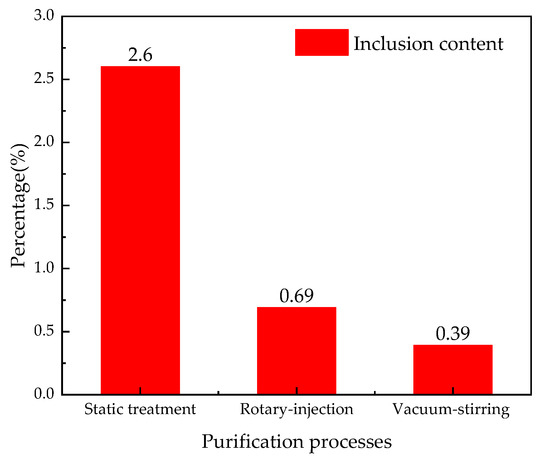
Figure 5.
The total inclusion content in three purification processes.
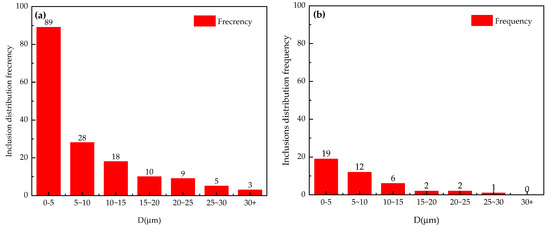
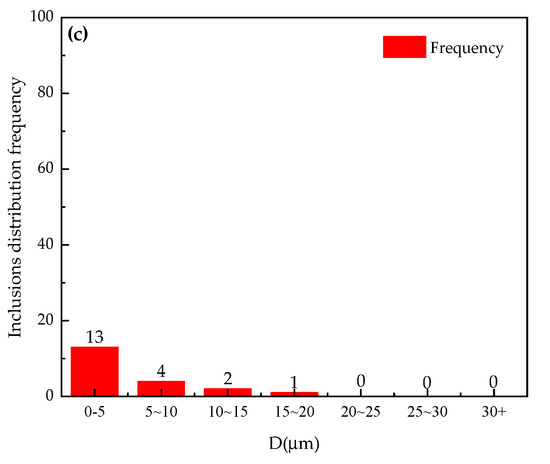
Figure 6.
Size distribution of inclusions of Al-6Mg alloy under different conditions: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification, (c) vacuum-stirring purification.
It can be seen from Figure 5 and Figure 6 that, in the case of non-vacuum static melt treatment, the total inclusion content accounts for 2.6% of the total field of view, the size distribution of inclusion ranges from 0 to 30 μm and the distribution frequency of above 10 μm inclusions exceeds 40, as shown in Figure 6a. In the case of non-vacuum rotary-injection purification, the total inclusion content accounts for 0.69% of the total field of view, the size distribution of inclusion ranges from 0 to 30 μm and the distribution frequency of above 10 μm inclusions decreases to 11, as shown in Figure 6b. In the case of vacuum-stirring purification, the total inclusion content accounts for 0.39% of the total field of view, the size distribution of inclusion ranges from 0 to 15 μm and the distribution frequency of above 10 μm inclusions decreases to 3, as shown in Figure 6c.
4. Discussion
According to the melt purification effects mentioned above, the purification effects of melt under different purification processes are obviously different: the vacuum-stirring purification process is the best, the non-vacuum rotary-injection is inferior and the non-vacuum static melt treatment process is poor.
It is well-known that the Al-6Mg alloy is of high Mg content, which is easy to oxidize and can easily absorb hydrogen, even if there is a thick oxide film on the melt covered with the aluminum alloy (i.e., when the melt was treated in the non-vacuum static melt treatment process, as shown in Figure 7a), so it is difficult to control the content of hydrogen and inclusion.
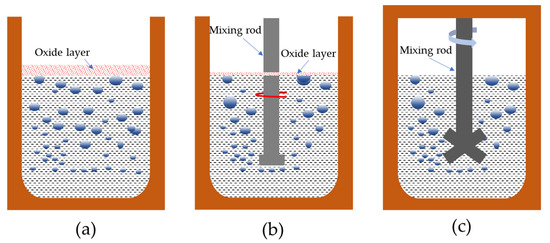
Figure 7.
Different melt treatment processes of Al-6Mg alloy: (a) non-vacuum static melt treatment, (b) non-vacuum rotary-injection purification, (c) vacuum-stirring purification.
Compared to the non-vacuum static melt treatment, in the non-vacuum rotary-injection purification process a steady stream of argon forms tiny bubbles in the melt, increasing the surface area of the bubble in contact with the melt, as shown in Figure 7b. The molecular hydrogen in the melt accumulates to the bubbles due to the local “negative pressure” formed by argon bubbles, and thus, the molecular hydrogen in the melt will be taken out continuously. According to literature [22], the disturbance of the rotor and the injected gas to the melt further reduces the limit solubility of hydrogen in the melt and improves the purity of the final melt.
In addition, during the process of bubbles floating up, due to the existence of surface tension, the bubbles will also absorb small inclusions and float up to the surface of the melt. Although rotary injection can reduce the content of gas and inclusion in the melt to a certain extent, the melt surface is still in contact with the atmosphere, and it easily oxidizes to form a film, as shown in Figure 7b.
In the vacuum-stirring purification process, as shown in Figure 7c, atomic hydrogen is combined to synthesize molecular hydrogen, which then moves to the existing nucleation sites in the melt to form bubbles and then escapes from the melt through the merger, growth and floating of bubbles [16]. On the one hand, the vacuum environment makes the pressure above the melt close to zero, which increases the hydrogen concentration difference between the melt and the outside, consequently improving the mass transfer driving force of dehydrogenation thermodynamics [23,24]. On the other hand, the stirring effect of the melt may reduce the equivalent pressure of the bubbles. Therefore, under the environment of vacuum stirring, the ultimate solubility of hydrogen in the melt can be infinitely close to zero. With the growth and floating of hydrogen bubbles, small inclusions are adsorbed by hydrogen bubbles and discharged to the surface of molten aluminum. As a result, the slag-forming ability of the Al-6Mg alloy melt melted in a vacuum is very low and will not produce oxidation and inclusion.
The hydrogen removal of the melt is also a diffusion process, which takes a long time, and directly determines the final hydrogen content of the melt [25,26]. When the melt is stirred under a vacuum, the mass transfer driving force of hydrogen is greatly increased and the static pressure height of the melt is also significantly reduced, resulting in the transformation of hydrogen in the bottom melt from diffusion precipitation to bubble precipitation, greatly accelerating the hydrogen removal speed.
Therefore, the vacuum-stirring purification process can not only reduce the content of hydrogen and inclusion in the melt, but also minimizes the hydrogen and inclusion in the melt in a short amount of time and improves the purification efficiency of the melt.
5. Conclusions
Al-6Mg alloy was used to study the purification effects of non-vacuum static melt treatment, non-vacuum rotary-injection purification and vacuum-stirring purification processes. The main conclusions are as follows:
- The hydrogen content of the melt decreases from 0.48 mL/100 gAl in the non-vacuum static treatment to 0.32 mL/100 gAl in the non-vacuum rotary-injection purification process and to 0.10 mL/100 gAl in the vacuum-stirring purification process.
- The inclusion content of the melt decreases from 2.6% in the non-vacuum static treatment to 0.69% in the non-vacuum rotary-injection purification process and to 0.39% in the vacuum-stirring purification process.
- The vacuum-stirring purification process increases the mass transfer driving force and diffusion precipitation of hydrogen, significantly reduces the equivalent pressure of the bubbles, greatly accelerates the hydrogen removal speed and significantly improves the purification effect.
Author Contributions
Conceptualization, Y.B. and Z.Z.; methodology, S.L., Y.B. and Z.Z.; software, S.L. and L.J.; validation, S.L., Y.B. and Z.Z.; formal analysis, S.L. and L.J.; investigation, S.L. and Y.B.; resources, Y.B. and Z.Z.; data curation, S.L.; writing—original draft preparation, S.L.; writing—review and editing, S.L., Y.B. and Z.Z.; visualization, S.L. and L.J.; supervision, Y.B. and Z.Z.; project administration, Y.B. and Z.Z.; funding acquisition, Y.B. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge the research platform of the National Engineering & Technology Research Center for Nonferrous Metals Composites, as well as the General Research Institute for Nonferrous Metals.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schlesinger, M.E. Aluminum Recycling; CRC Press: Boca Raton, FL, USA, 2007; pp. 13–15. [Google Scholar]
- Zhang, D.; Wu, A.; Zhao, Y.; Shan, J.; Liu, X. Effects of Al-Mg c Al-Cu wire on the properties of 2219 aluminum alloy TIG-welded joint. Int. J. Mod. Phys. B 2021, 35, 2150139. [Google Scholar] [CrossRef]
- Zuo, W.; Ma, L.; Lu, Y.; Li, S.Y.; Ji, Z.; Ding, M. Effects of Solution Treatment Temperatures on Microstructure and Mechanical Properties of TIG–MIG Hybrid Arc Additive Manufactured 5356 Aluminum Alloy. Met. Mater. Int. 2018, 24, 1346–1358. [Google Scholar] [CrossRef]
- Yang, J.; Bao, S.; Akhtar, S.; Li, Y. Oxide Film Inclusions and Inoculation Particles TiB2 in Aluminum Melt. Metall. Mater. Trans. B 2021, 52, 2497–2508. [Google Scholar] [CrossRef]
- Toda, H.; Hidaka, T.; Kobayashi, M.; Uesugi, K.; Takeuchi, A.; Horikawa, K. Growth behavior of hydrogen micropores in aluminum alloys during high-temperature exposure. Acta Mater. 2009, 57, 2277–2290. [Google Scholar] [CrossRef]
- Young, G.A., Jr.; Scully, J.R. The diffusion and trapping of hydrogen in high purity aluminum. Acta Mater. 1998, 46, 6337–6349. [Google Scholar] [CrossRef]
- Anyalebechi, P.N. Analysis of the effects of alloying elements on hydrogen solubility in liquid aluminum alloys. Scr. Metall. Mater. 1995, 33, 1209–1216. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Shu, D.; Zhang, S.; Sun, B. Removal of Non-Metallic Inclusions from Aluminum by Electroslag Refining. Mater. Trans. 2011, 52, 2266–2269. [Google Scholar] [CrossRef]
- Warke, V.S.; Shankar, S.; Makhlouf, M.M. Mathematical modeling and computer simulation of molten aluminum cleansing by the rotating impeller degasser: Part II. Removal of hydrogen gas and solid particles. J. Mater. Processing Technol. 2005, 168, 119–126. [Google Scholar] [CrossRef]
- Shih, T.S.; Weng, K.Y. Effect of A Degassing Treatment on the Quality of Al-7Si and A356 Melts—Degassing Diffusers. Mater. Trans. 2004, 45, 1852–1858. [Google Scholar] [CrossRef][Green Version]
- Engh, T.A. Principles of Metal Refining; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Birnbaum, H.K.; Buckley, C.; Zeides, F.; Sirois, E.; Rozenak, P.; Spooner, S.; Lin, J.S. Hydrogen in aluminum. J. Alloy. Compd. 1997, 253–254, 260–264. [Google Scholar] [CrossRef]
- Ni, H.; Sun, B.; Jiang, H.; Ding, W. Effects of rotating impeller degassing on microstructure and mechanical properties of the A356 scraps. Mater. Sci. Eng. A 2003, 352, 294–299. [Google Scholar] [CrossRef]
- Walek, J.; Michalek, K.; Tkadleková, M.; Saternus, M. Modelling of Technological Parameters of Aluminium Melt Refining in the Ladle by Blowing of Inert Gas through the Rotating Impeller. Met. Open Access Metall. J. 2021, 11, 284. [Google Scholar] [CrossRef]
- Dsa, B.; Zd, A.; Ao, W.A.; Gga, B.; Ming, W.A. Motion and mass transfer models for single bubble in an aluminum melt under a compound field of ultrasonic and rotating flow. Results Phys. 2020, 19, 103386. [Google Scholar] [CrossRef]
- Popescu, G.; Gheorghe, I.; Dnil, F.; Moldovan, P. Vacuum Degassing of Aluminium Alloys. Mater. Sci. Forum 1996, 217–222, 147–152. [Google Scholar] [CrossRef]
- Voigt, C.; Hubálková, J.; Zienert, T.; Fankhänel, B.; Stelter, M.; Charitos, A.; Aneziris, C.G. Aluminum Melt Filtration with Carbon Bonded Alumina Filters. Materials 2020, 13, 3962. [Google Scholar] [CrossRef]
- Lei, Z.; Ye, P.; Liao, H.; Wang, Q. Degassing of aluminum alloys during re-melting. Mater. Lett. 2012, 66, 328–331. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Mukhopadhyay, N.K. Introduction to magnesium alloy processing technology and development of low-cost stir casting process for magnesium alloy and its composites. J. Magnes. Alloy. 2018, 6, 245–254. [Google Scholar] [CrossRef]
- Hao, Y.; Zhao, H.; Shen, X.; Wang, X.; Zheng, H. Simulation of α-Al grain formation in high vacuum die-casting Al-Si-Mg alloys with multi-component quantitative cellular automaton method. China Foundry 2022, 19, 99–108. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.L.; Lin, Z.Y.; Hu, Z.L.; Zeng, J.M. Quantitative Analysis of Inclusions in Aluminum. Adv. Mater. Res. 2012, 476–478, 453–456. [Google Scholar] [CrossRef]
- Mi, G.; Qi, S.; Liu, X.; Niu, J. Research on water simulation experiment of the rotating impeller degassing process. Mater. Sci. Eng. A 2009, 499, 195–199. [Google Scholar] [CrossRef]
- Qin, Y.; Zhuang, L.; Meng, Z.; Chen, Z.; Tang, X. Calculation of Hydrogen Solubility in Al-Melt under Vacuum Condition. Spec. Cast. Nonferrous Alloy. 2020, 40, 1131–1134. [Google Scholar] [CrossRef]
- Dai, F.R.; Wang, M.; Xie, X.J.; Huang, W.D. Analysis on Thermodynamics and Kinetics of Vacuum Degassing from Aluminum Melt during Adjusted Pressure Casting. Foundry Technol. 2009, 30, 41–43. [Google Scholar]
- Ren, Y.; Ma, W.; Wei, K.; Yu, W.; Dai, Y.; Morita, K. Degassing of aluminum alloys via the electromagnetic directional solidification. Vacuum 2014, 109, 82–85. [Google Scholar] [CrossRef]
- Da, S.; Sun, B.; Ke, L.; Li, T.; Xu, Z.; Zhou, Y. Continuous separation of non-metallic inclusions from aluminum melt using alternating magnetic field. Mater. Lett. 2002, 55, 322–326. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).