Grossular Exsolution at Pyrope Dislocation: New Evidence for the Ultradeep Origin of Dabie Orogenic Peridotite
Abstract
:1. Introduction
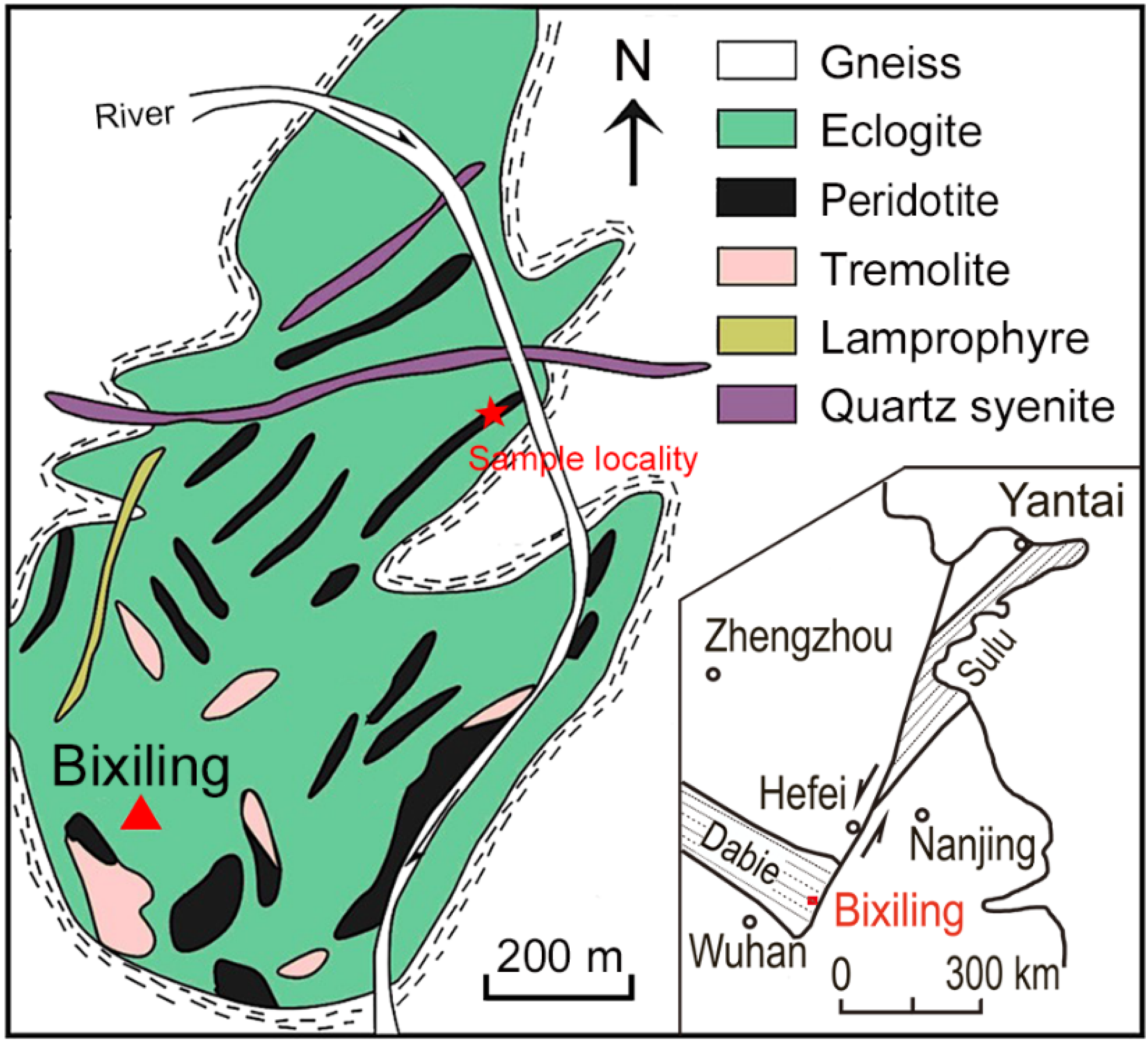
2. Geological Background and Sample Description
3. Methods
4. Results
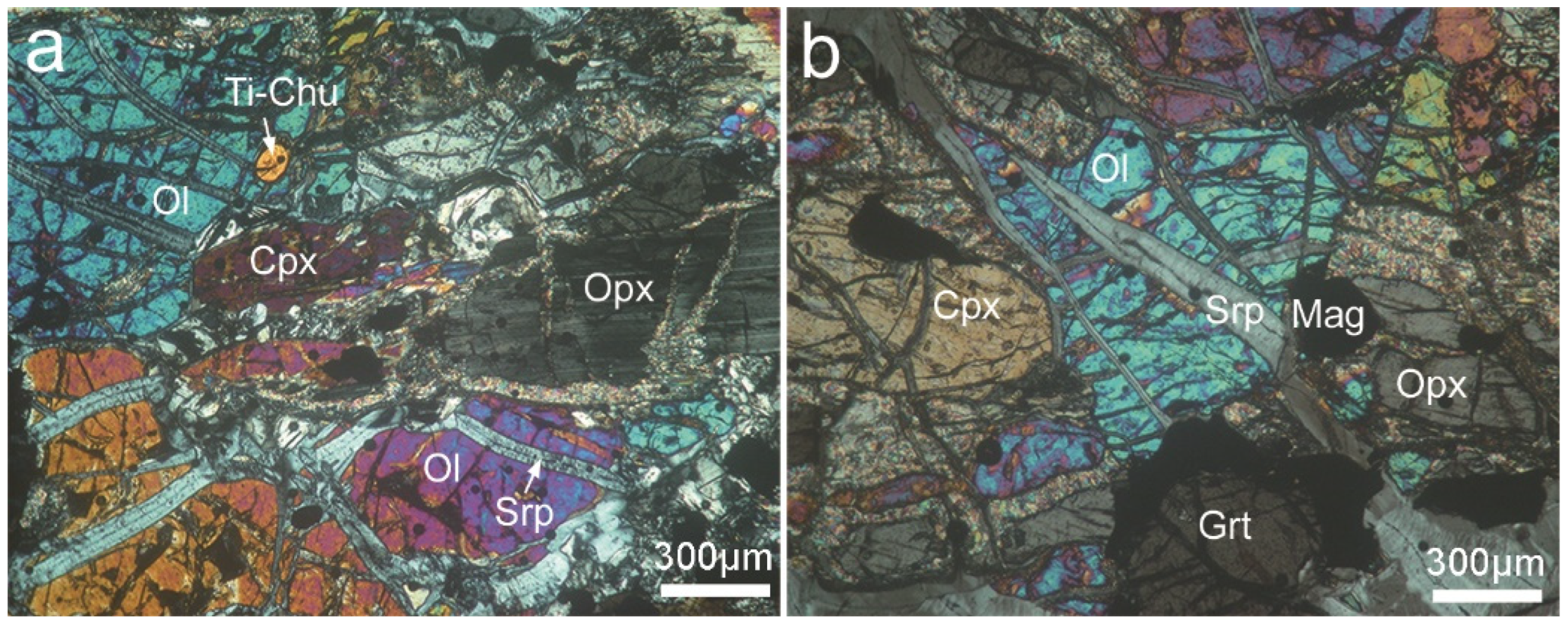
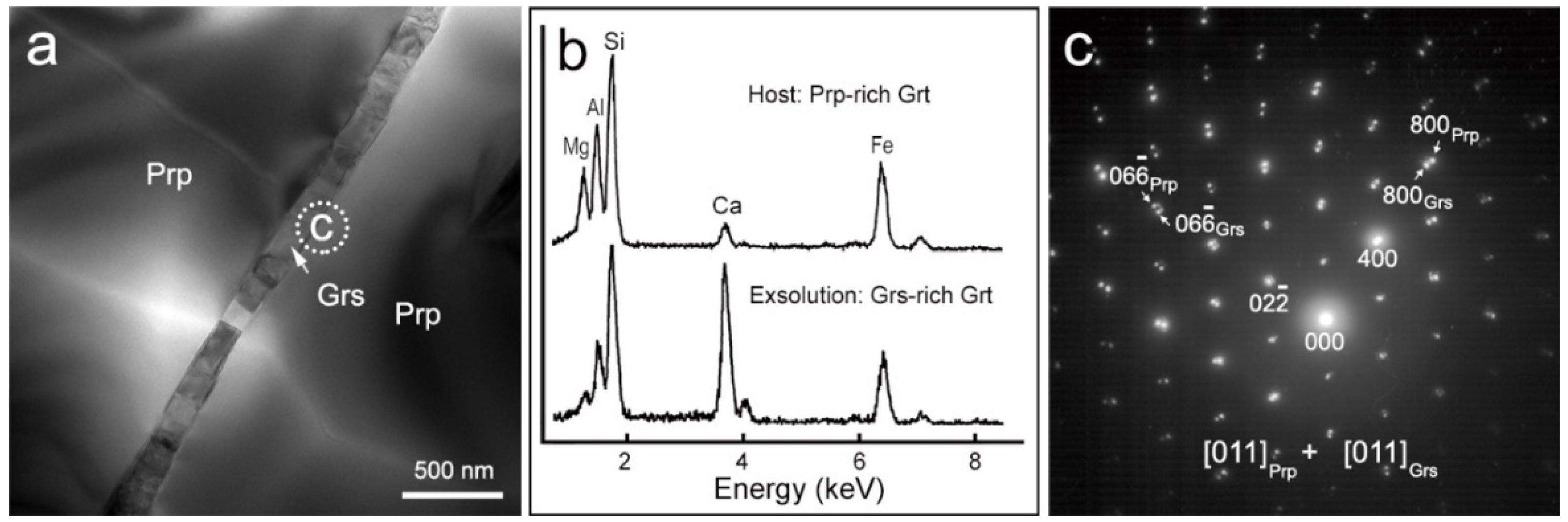
4.1. Microstructures
4.2. Chemical Compositions
5. Discussion
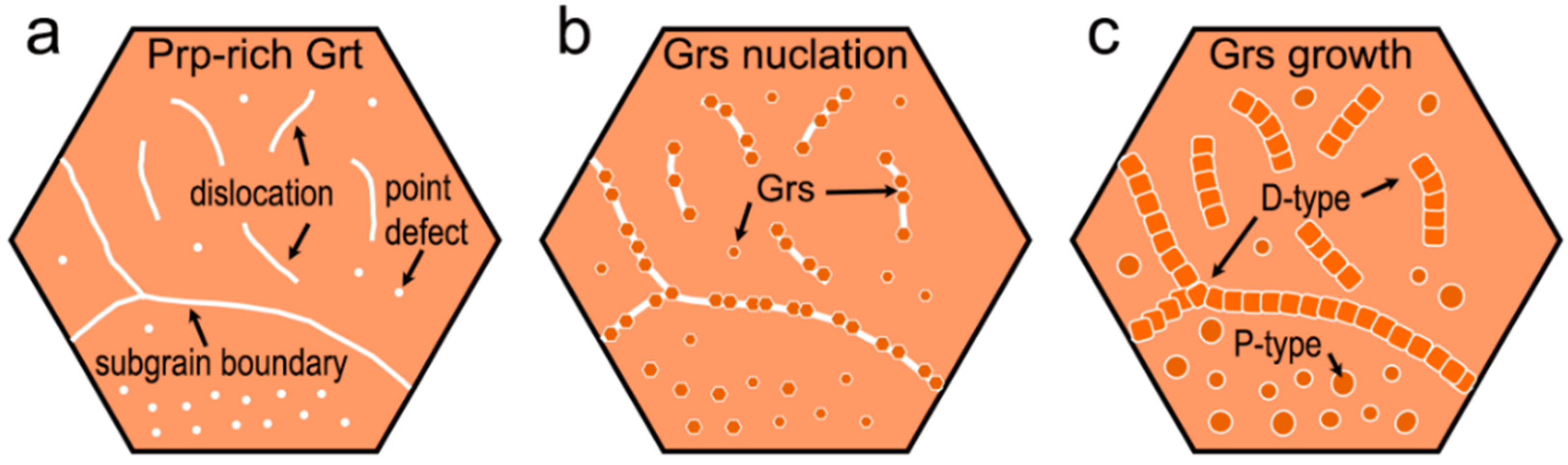
5.1. Exsolution Mechanism
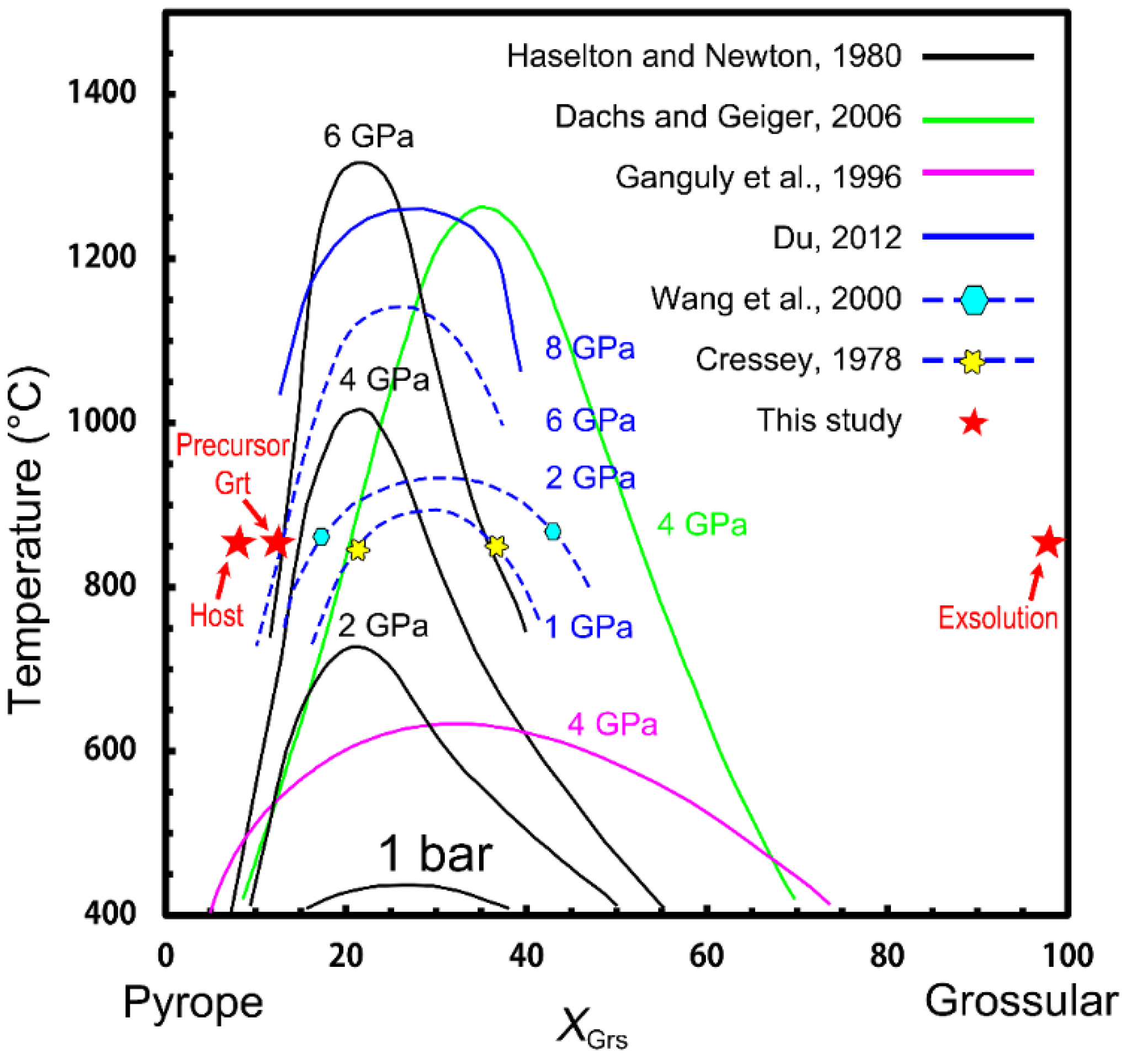
5.2. Constraints on the P–T Conditions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baxter, E.F.; Caddick, M.J.; Ague, J.J. Garnet: Common mineral, uncommonly useful. Elements 2013, 9, 415–419. [Google Scholar] [CrossRef]
- Goncalves, P.; Raimondo, T.; Paquette, J.L.; De Oliveira, J.S.D.S. Garnet as a monitor for melt–rock interaction: Textural, mineralogical, and compositional evidence of partial melting and melt-driven metasomatism. J. Metamorph. Geol. 2021, 39, 617–648. [Google Scholar] [CrossRef]
- Wu, J.; Zhai, M.; Hu, B.; Zhang, H.; Chu, X.; Zhou, N.; Zhang, L.; Zhang, H. Garnet perspectives on the metamorphic history and tectonic significance of Paleoproterozoic high-pressure mafic granulites from the northern Hengshan, North China Craton. Lithos 2021, 394–395, 106139. [Google Scholar] [CrossRef]
- Baxter, E.F.; Scherer, E.E. Garnet geochronology: Timekeeper of tectonometamorphic processes. Elements 2013, 9, 433–438. [Google Scholar] [CrossRef]
- Baxter, E.F.; Caddick, M.J.; Dragovic, B. Garnet: A rock-forming mineral petrochronometer. Rev. Miner. Geochem. 2017, 83, 469–533. [Google Scholar] [CrossRef] [Green Version]
- Beyer, C.; Rosenthal, A.; Myhill, R.; Crichton, W.A.; Yu, T.; Wang, Y.; Frost, D.J. An internally consistent pressure calibration of geobarometers applicable to the Earth’s upper mantle using in situ XRD. Geochim. Cosmochim. Acta 2018, 222, 421–435. [Google Scholar] [CrossRef] [Green Version]
- Caddick, M.J.; Kohn, M.J. Garnet: Witness to the evolution of destructive plate boundaries. Elements 2013, 9, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Du, W. Excess Volume and Exsolution in Pyrope-Grossular Garnet; Columbia University: New York, NY, USA, 2012. [Google Scholar]
- Berman, R.G. Mixing properties of Ca-Mg-Fe-Mn garnets. Am. Miner. 1990, 75, 328–344. [Google Scholar]
- Dapiaggi, M.; Geiger, C.; Artioli, G. Microscopic strain in synthetic pyrope-grossular solid solutions determined by synchrotron X-ray powder diffraction at 5 K: The relationship to enthalpy of mixing behavior. Am. Miner. 2005, 90, 506–509. [Google Scholar] [CrossRef]
- Geiger, C.A. Thermodynamics of (Fe2+, Mn2+, Mg, Ca)3- Al2Si3O12 garnet: A review and analysis. Miner. Pet. 1999, 66, 271–299. [Google Scholar] [CrossRef]
- Geiger, C.A. Silicate garnet: A micro to macroscopic (re)view. Am. Miner. 2008, 93, 360–372. [Google Scholar] [CrossRef]
- Geiger, C.A. A tale of two garnets: The role of solid solution in the development toward a modern mineralogy. Am. Miner. 2016, 101, 1735–1749. [Google Scholar] [CrossRef]
- Wood, B.J. Activity measurements and excess entropy-volume relationships for pyrope-grossular garnets. J. Geol. 1988, 96, 721–729. [Google Scholar] [CrossRef]
- Newton, R.C.; Charlu, T.V.; Kleppa, O.J. Thermochemistry of high pressure garnets and clinopyroxenes in the system CaO-MgO-Al2O3-SiO2. Geochim. Cosmochim. Acta 1977, 41, 369–377. [Google Scholar] [CrossRef]
- Dachs, E.; Geiger, C.A. Heat capacities and entropies of mixing of pyrope-grossular (Mg3Al2Si3O12-Ca3Al2Si3O12) garnet solid solutions: A low-temperature calorimetric and a thermodynamic investigation. Am. Miner. 2006, 91, 894–906. [Google Scholar] [CrossRef]
- Ganguly, J.; Cheng, W.; Tirone, M. Thermodynamics of aluminosilicate garnet solid solution: New experimental data, an optimized model, and thermometric applications. Contrib. Miner. Pet. 1996, 126, 137–151. [Google Scholar] [CrossRef]
- Haselton, H.T.; Newton, R.C. Thermodynamics of pyrope-grossular garnets and their stabilities at high temperatures and high pressures. J. Geophys. Res. Solid Earth 1980, 85, 6973–6982. [Google Scholar] [CrossRef]
- Cressey, G. Exsolution in almandine-pyrope-grossular garnet. Nature 1978, 271, 533–534. [Google Scholar] [CrossRef]
- Wang, L.; Essene, E.J.; Zhang, Y. Direct observation of immiscibility in pyrope-almandine-grossular garnet. Am. Miner. 2000, 85, 41–46. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liou, J.G.; Cong, B.L. Talc-, magnesite- and Ti-clinohumite-bearing ultrahigh-pressure meta-mafic and ultramafic complex in the Dabie mountains, China. J. Pet. 1995, 36, 1011–1037. [Google Scholar] [CrossRef]
- Chen, Y.; Su, B.; Guo, S. The Dabie-Sulu orogenic peridotites: Progress and key issues. Sci. China Earth Sci. 2015, 58, 1679–1699. [Google Scholar] [CrossRef]
- Zhang, R.Y.; Liou, J.G.; Yang, J.S.; Yui, T.F. Petrochemical constraints for dual origin of garnet peridotites from the Dabie-Sulu UHP terrane, eastern-central China. J. Metamorph. Geol. 2000, 18, 149–166. [Google Scholar] [CrossRef]
- Zheng, J.P.; Sun, M.; Griffin, W.L.; Zhou, M.F.; Zhao, G.C.; Robinson, P.; Tang, H.Y.; Zhang, Z.H. Age and geochemistry of contrasting peridotite types in the Dabie UHP belt, eastern China: Petrogenetic and geodynamic implications. Chem. Geol. 2008, 247, 282–304. [Google Scholar] [CrossRef]
- Chavagnac, V.; Jahn, B. Coesite-bearing eclogites from the Bixiling Complex, Dabie Mountains, China: Sm-Nd ages, geochemical characteristics and tectonic implications. Chem. Geol. 1996, 133, 29–51. [Google Scholar] [CrossRef]
- Jin, Z.M.; Jin, S.Y.; Gao, S.; Zhao, W.X. Is the depth where UHP rocks formed in the Dabie Mountains limited to 100–150 km?―Implications from the needle-shaped Ti-Cr-bearing magnetite exsolution in olivine (in Chinese). Chin. Sci. Bull. 1998, 43, 767–771. [Google Scholar]
- Liu, X.; Jin, Z.; Green, H.W. Clinoenstatite exsolution in diopsidic augite of Dabieshan: Garnet peridotite from depth of 300 km. Am. Miner. 2007, 92, 546–552. [Google Scholar] [CrossRef]
- Ganguly, J.; Cheng, W.; O’Neill, H.S.C. Syntheses, volume, and structural changes of garnets in the pyrope-grossular join: Implications for stability and mixing properties. Am. Miner. 1993, 78, 583–593. [Google Scholar]
- Novak, G.A.; Gibbs, G.V. The crystal chemistry of the silicate garnets. Am. Miner. 1971, 56, 791–825. [Google Scholar]
- Locock, A.J. An Excel spreadsheet to recast analyses of garnet into end-member components, and a synopsis of the crystal chemistry of natural silicate garnets. Comput. Geosci. UK 2008, 34, 1769–1780. [Google Scholar] [CrossRef]
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2003. [Google Scholar]
- Dobrzhinetskaya, L.; Green, H.W.; Wang, S. Alpe Arami: A peridotite massif from depths of more than 300 km. Science 1996, 271, 1841–1845. [Google Scholar] [CrossRef]
- Haggerty, S.E.; Sautter, V. Ultradeep (greater than 300 km), ultramafic upper mantle xenoliths. Science 1990, 248, 993–996. [Google Scholar] [CrossRef] [PubMed]
- van Roermund, H.L.M.; Drury, M.R.; Barnhoorn, A.; De Ronde, A. Relict majoritic garnet microstructures from Ultra-deep orogenic peridotites in Western Norway. J. Pet. 2001, 42, 117–130. [Google Scholar] [CrossRef] [Green Version]
- Ye, K.; Cong, B.L.; Ye, D.I. The possible subduction of continental material to depths greater than 200 km. Nature 2000, 407, 734–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, J.X.; Zhang, J.F.; Chen, D.L.; Wang, C.; Yang, W.Q. Exsolution microstructures in ultrahigh-pressure rocks: Progress, controversies and challenges. Chin. Sci. Bull. 2009, 54, 1983–1995. [Google Scholar] [CrossRef] [Green Version]
- Cahn, J.W. Nucleation on dislocations. Acta Metall. 1957, 5, 169–172. [Google Scholar] [CrossRef]
- Gómez-Ramírez, R.; Pound, G.M. Nucleation of a second solid phase along dislocations. Metall. Mater. Trans. B 1973, 4, 1563–1570. [Google Scholar] [CrossRef]
- Hilliard, J.E. Nucleation within crystalline phases. Ind. Eng. Chem. 1966, 58, 18–25. [Google Scholar] [CrossRef]
- Militzer, M.; Sun, W.P.; Jonas, J.J. Modelling the effect of deformation-induced vacancies on segregation and precipitation. Acta Metall. Mater. 1994, 42, 133–141. [Google Scholar] [CrossRef]
- Lee, J.K.; Barnett, D.M.; Aaronson, H.I. The elastic strain energy of coherent ellipsoidal precipitates in anisotropic crystalline solids. Metall. Trans. A 1977, 8, 963–970. [Google Scholar] [CrossRef]
- Axler, J.A.; Ague, J.J. Exsolution of rutile or apatite precipitates surrounding ruptured inclusions in garnet from UHT and UHP rocks. J. Metamorph. Geol. 2015, 33, 829–848. [Google Scholar] [CrossRef]
- Ham, F.S. Stress-assisted precipitation on dislocations. J. Appl. Phys. 1959, 30, 915–926. [Google Scholar] [CrossRef]
- Kohlstedt, D.L.; Vander Sande, J.B. An electron microscopy study of naturally occurring oxidation produced precipitates in iron-bearing olivines. Contrib. Miner. Petr. 1975, 53, 13–24. [Google Scholar] [CrossRef]
- Snow, E.; Yund, R. The effect of ductile deformation on the kinetics and mechanisms of the aragonite-calcite transformation. J. Metamorph. Geol. 1987, 5, 141–153. [Google Scholar] [CrossRef]
- Axler, J.A.; Ague, J.J. Oriented multiphase needles in garnet from ultrahigh-temperature granulites, Connecticut, U.S.A. Am. Miner. 2015, 100, 2254–2271. [Google Scholar] [CrossRef]
- van Roermund, H.L.M.; Drury, M.R.; Barnhoorn, A.; De Ronde, A. Non-silicate inclusions in garnet from an ultra-deep orogenic peridotite. Geol. J. 2000, 35, 209–229. [Google Scholar] [CrossRef]
- van Roermund, H.; Drury, M.R. Ultra-high pressure (P > 6 GPa) garnet peridotites in Western Norway: Exhumation of mantle rocks from > 185 km depth. Terra Nova 1998, 10, 295–301. [Google Scholar] [CrossRef]
- Wang, Z.; Ji, S. Deformation of silicate garnets: Brittle-ductile transition and its geological implications. Can. Miner. 1999, 37, 525–541. [Google Scholar]
- Karato, S.; Wang, Z.; Liu, B.; Fujino, K. Plastic deformation of garnets: Systematics and implications for the rheology of the mantle transition zone. Earth Planet. Sci. Lett. 1995, 130, 13–30. [Google Scholar] [CrossRef]




| Spot | Host | Exsolution | Precursor # | 85 * | 122C * | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prp-1 | Prp-2 | Grs | Prp | g-105 | g-113 | g-C-50 | g-R-51 | g-60 | g-66 | |
| SiO2 | 41.15 | 40.83 | 37.56 | 40.39 | 42.12 | 41.85 | 40.41 | 40.35 | 40.47 | 40.71 |
| TiO2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.01 | 0.03 | 0.05 | 0.05 |
| Al2O3 | 22.17 | 21.88 | 16.24 | 21.62 | 23.23 | 23.47 | 21.17 | 19.71 | 22.24 | 21.95 |
| FeO | 18.23 | 17.15 | 7.79 | 17.25 | 13.57 | 14.17 | 17.53 | 17.84 | 16.48 | 15.87 |
| MnO | 0.75 | 0.68 | 0.42 | 0.90 | 0.28 | 0.27 | 0.69 | 0.78 | 0.83 | 0.95 |
| MgO | 14.89 | 15.61 | 0.30 | 13.83 | 18.37 | 18.03 | 14.70 | 13.72 | 14.82 | 15.03 |
| CaO | 3.06 | 2.61 | 35.68 | 4.94 | 3.45 | 3.64 | 3.69 | 4.50 | 4.40 | 3.78 |
| Cr2O3 | 1.02 | 0.64 | 0.89 | 1.07 | 0.12 | 0.05 | 1.82 | 2.78 | 0.82 | 0.91 |
| Na2O | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 |
| Total | 101.27 | 99.40 | 98.96 | 100.00 | 101.18 | 101.48 | 100.02 | 99.72 | 100.14 | 99.25 |
| XPrp | 53.02 | 54.55 | 0.74 | 50.99 | 64.93 | 63.76 | 54.15 | 47.66 | 54.22 | 54.86 |
| XAlm | 36.64 | 34.99 | 0.00 | 33.93 | 25.65 | 25.72 | 34.51 | 35.99 | 32.81 | 32.81 |
| XSps | 1.55 | 1.42 | 0.00 | 1.89 | 0.56 | 0.54 | 1.44 | 1.65 | 1.73 | 1.99 |
| XGrs | 4.28 | 4.45 | 71.28 | 7.58 | 6.38 | 7.32 | 2.15 | 1.81 | 7.63 | 6.27 |
| XAndr | 0.76 | 0.58 | 25.17 | 2.38 | 1.84 | 1.79 | 2.25 | 1.96 | 1.42 | 0.00 |
| XUvt | 2.95 | 1.87 | 2.81 | 3.14 | 0.34 | 0.14 | 5.33 | 8.24 | 2.39 | 2.67 |
| Binary | Difference in Molar Volume (J/bar) |
|---|---|
| andradite-pyrope * | 1.918 |
| uvarovite-pyrope * | 1.760 |
| andradite-almandine * | 1.710 |
| uvarovite-almandine | 1.553 |
| andradite-spessartine | 1.437 |
| uvarovite-spessartine | 1.280 |
| grossular-pyrope | 1.214 |
| grossular-almandine | 1.006 |
| grossular-spessartine | 0.733 |
| andradite-grossular | 0.704 |
| uvarovite-grossular | 0.547 |
| spessartine-pyrope | 0.480 |
| spessartine-almandine | 0.273 |
| almandine-pyrope | 0.207 |
| andradite-uvarovite | 0.157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Z.; Liu, X.; Jin, Z.; Liu, X. Grossular Exsolution at Pyrope Dislocation: New Evidence for the Ultradeep Origin of Dabie Orogenic Peridotite. Crystals 2022, 12, 647. https://doi.org/10.3390/cryst12050647
Xie Z, Liu X, Jin Z, Liu X. Grossular Exsolution at Pyrope Dislocation: New Evidence for the Ultradeep Origin of Dabie Orogenic Peridotite. Crystals. 2022; 12(5):647. https://doi.org/10.3390/cryst12050647
Chicago/Turabian StyleXie, Zhanjun, Xiangwen Liu, Zhenmin Jin, and Xiaoqing Liu. 2022. "Grossular Exsolution at Pyrope Dislocation: New Evidence for the Ultradeep Origin of Dabie Orogenic Peridotite" Crystals 12, no. 5: 647. https://doi.org/10.3390/cryst12050647
APA StyleXie, Z., Liu, X., Jin, Z., & Liu, X. (2022). Grossular Exsolution at Pyrope Dislocation: New Evidence for the Ultradeep Origin of Dabie Orogenic Peridotite. Crystals, 12(5), 647. https://doi.org/10.3390/cryst12050647






