Purification of AZ80 and Degassing of AZ91 Alloy by Ultrasonic Treatment
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
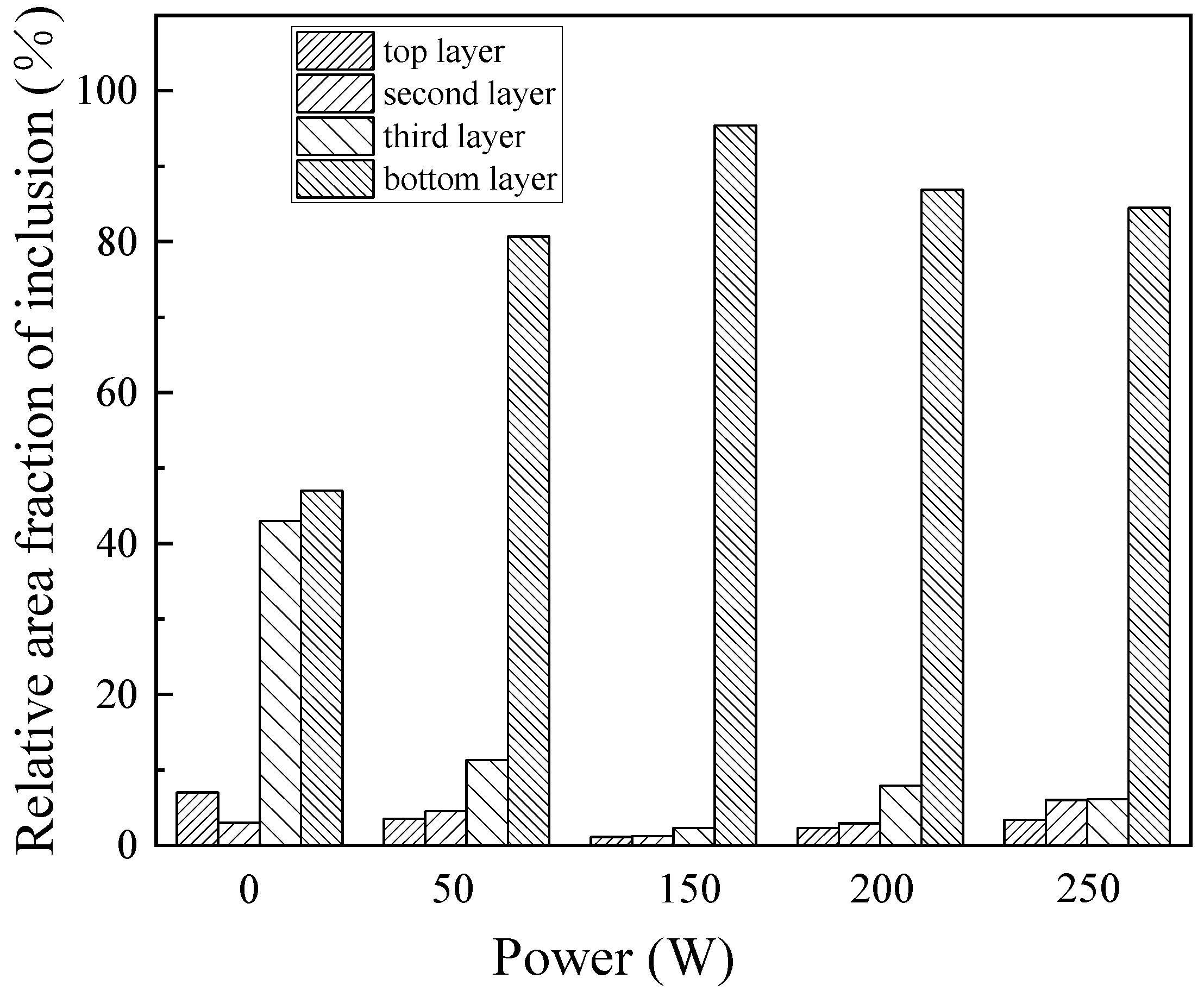
3.1. Effect of Ultrasonic Power on Purification of Ingot
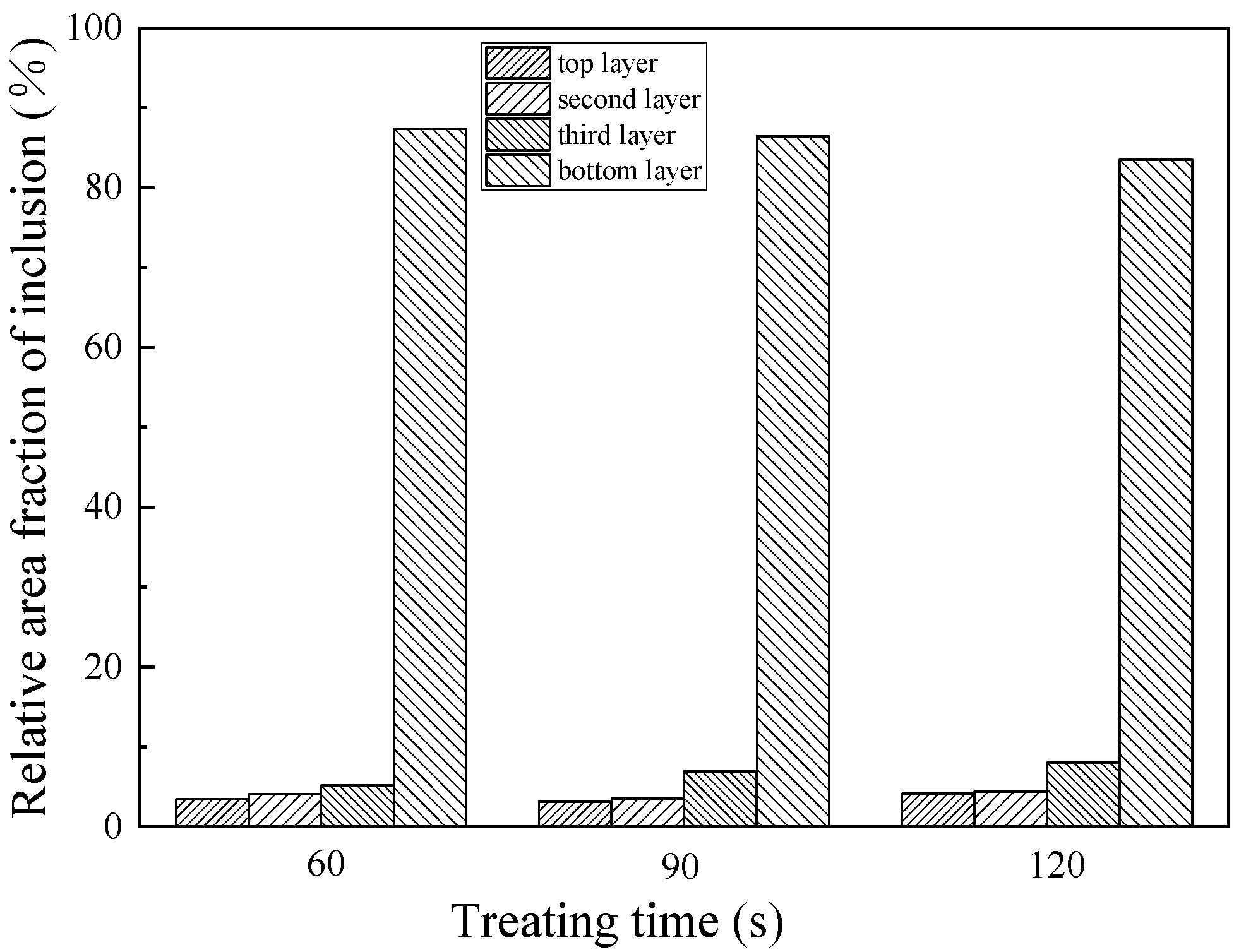
3.2. Effect of Ultrasonic Treatment Duration on Purification of Ingot
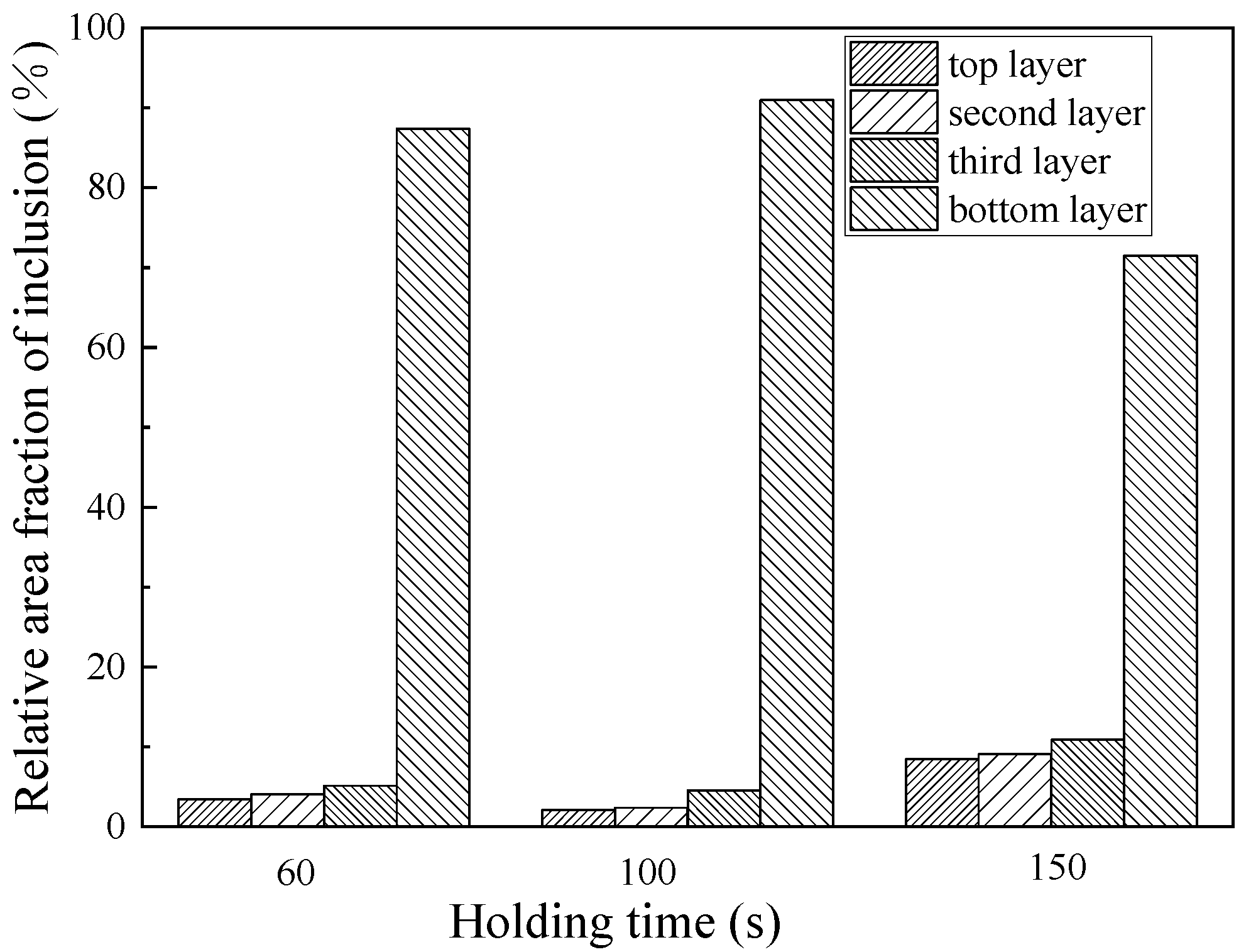
3.3. Effect of Holding Time on Purification of Ingot
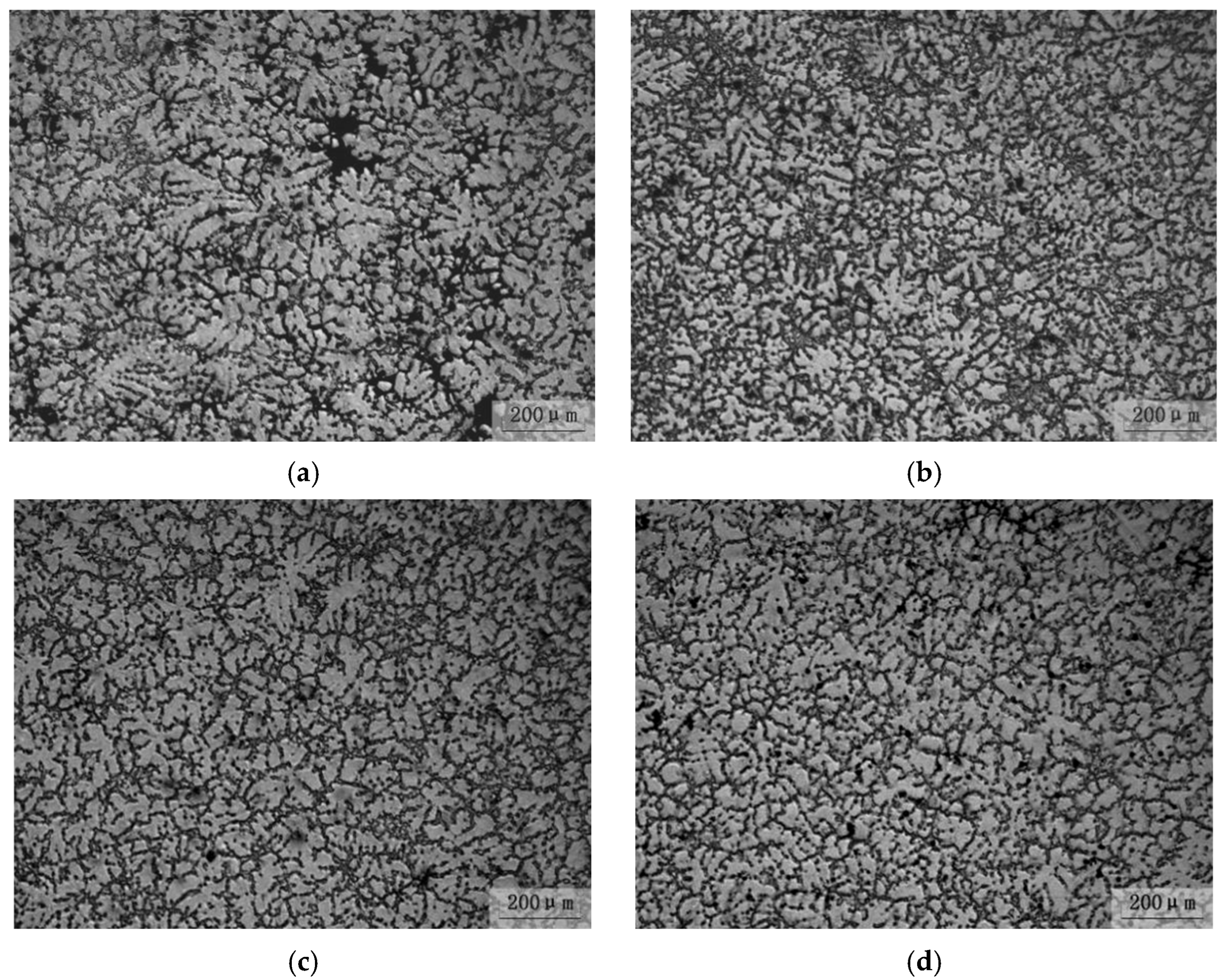
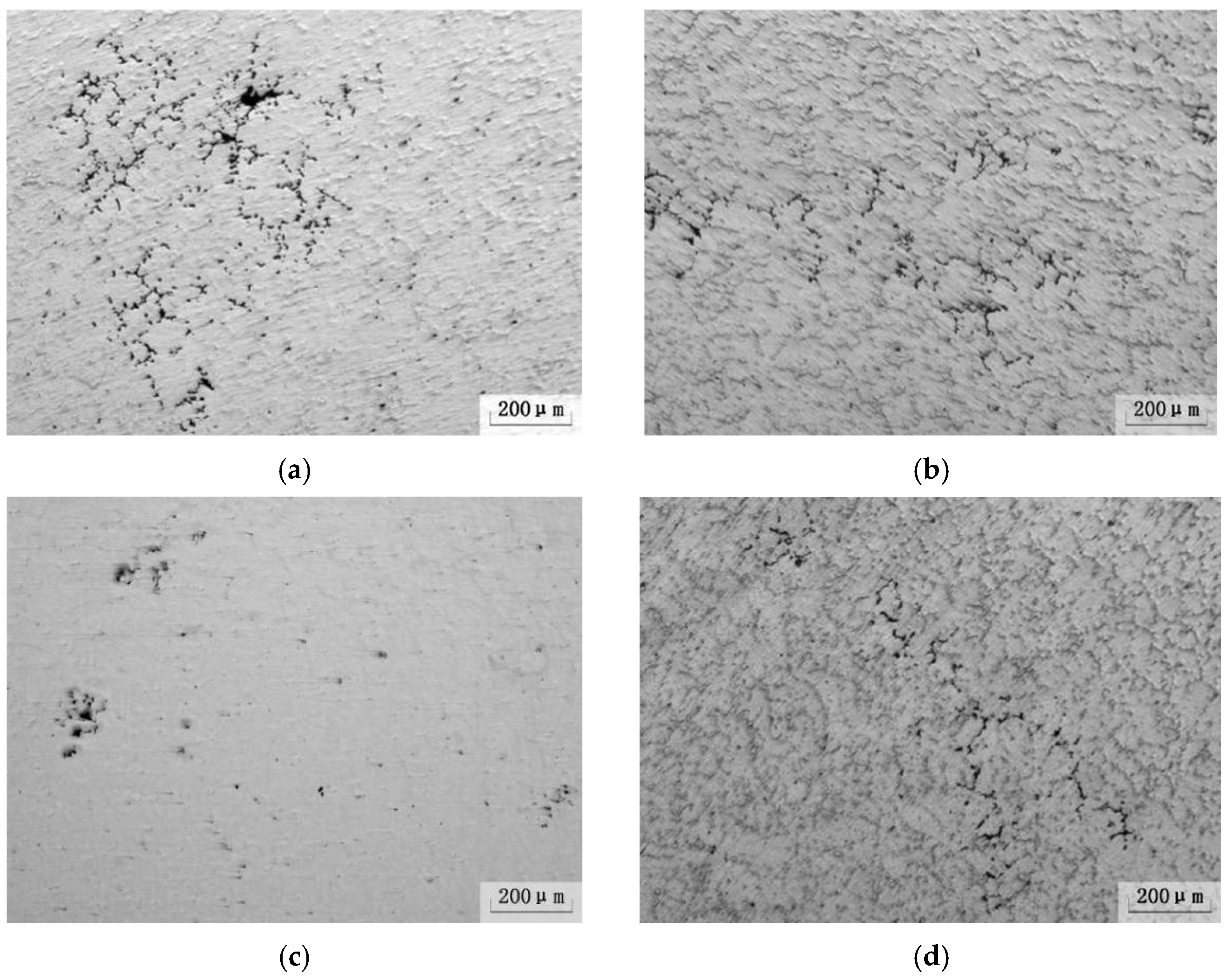
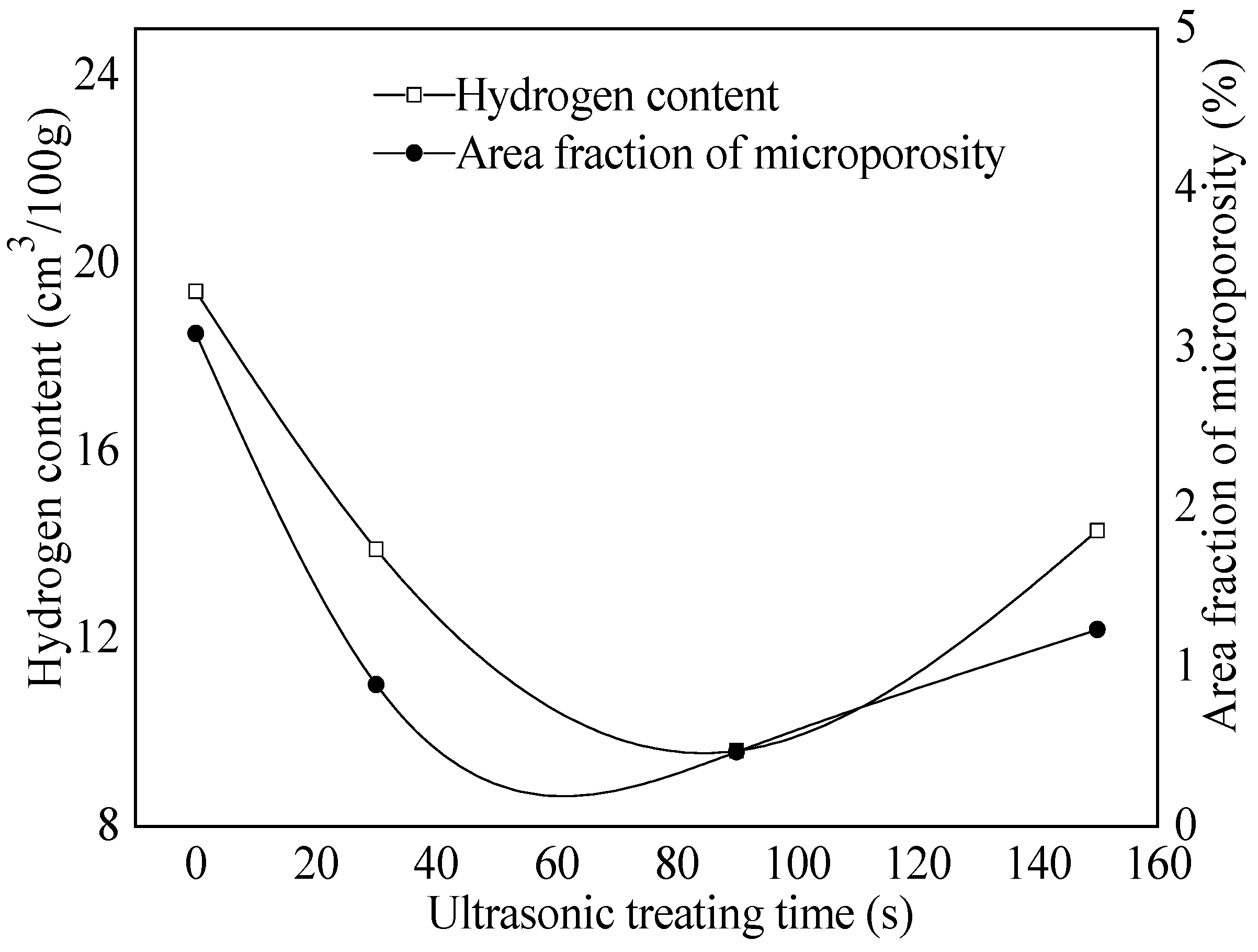
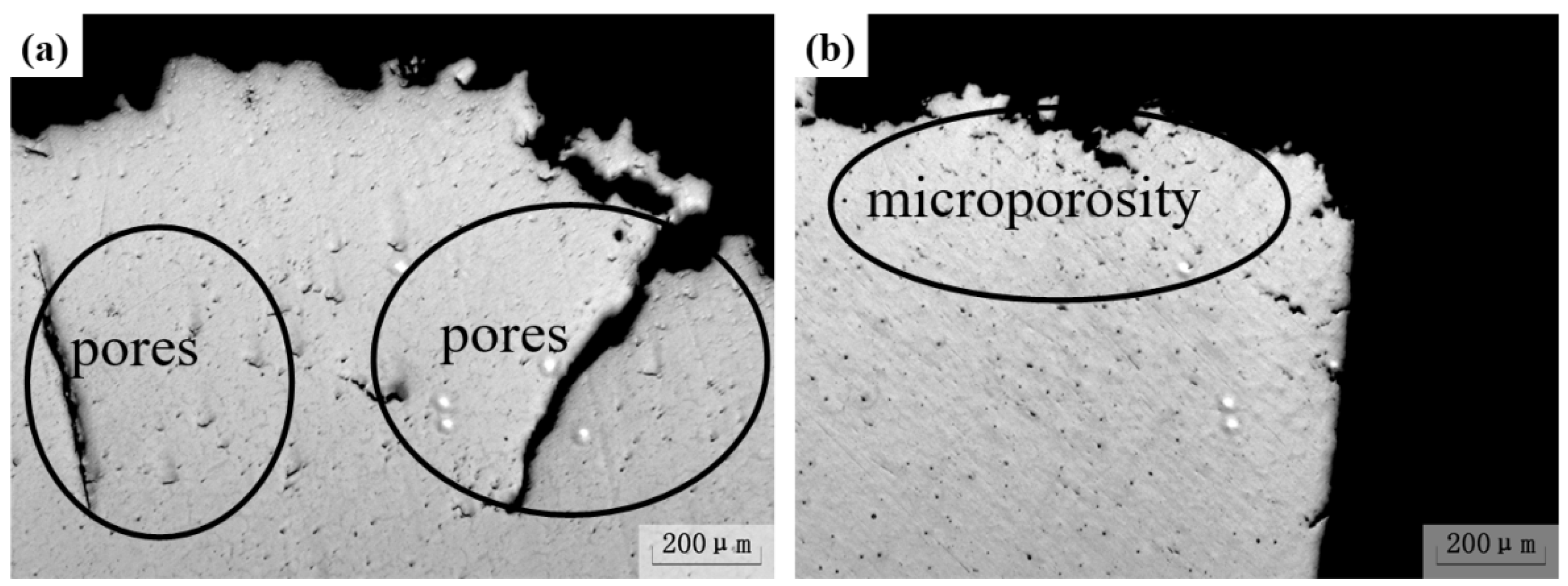
3.4. Effect of Ultrasonic Degassing on AZ91 Alloy
4. Conclusions
- (1)
- The rapid separation of inclusions in magnesium alloy melt can be realized by ultrasonic treatment, which proves that purifying metal melt by ultrasonic field is entirely feasible.
- (2)
- The purification of ultrasonic melt is related to ultrasonic power, duration and standing time. The best purification effect is obtained by 80 W for 60 s at 650 °C, and the standing time is 100 s.
- (3)
- The hydrogen content in magnesium alloy melt can be reduced by ultrasonic treatment technology. When the ultrasonic power was 150 W for 90 s, the hydrogen content and degassing efficiency are 9.6 cm3/100 g and 50.5%, respectively. The corresponding mechanical properties are Rm = 194 MPa, R0.2 = 133 MPa and A = 4.8%, respectively. The “area” and “shell” effects of the resonant has an efficient degassing result, and the big pores, because of high hydrogen content, are the main reason for fracture by ultrasonic treatment in AZ91 alloys.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, X.; Du, W.; Wang, Z. Simultaneously improved mechanical and thermal properties of Mg-Zn-Zr alloy reinforced by ultra-low content of graphene nanoplatelets. Appl. Surf. Sci. 2021, 536, 147791. [Google Scholar] [CrossRef]
- Prasada, S.V.S.; Prasada, S.B.; Vermab, K.; Mishrac, R.K.; Kumard, V.; Singh, S. The role and significance of Magnesium in modern day research-A review. J. Magnes. Alloy. 2021, 10, 1–61. [Google Scholar] [CrossRef]
- Li, K.; Chen, Z.; Chen, T. Hot deformation and dynamic recrystallization behaviors of Mg-Gd-Zn alloy with LPSO phases. J. Alloy. Compd. 2019, 792, 894–906. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Liu, J.; Dong, M.L. Microstructure, Mechanical and Thermal Properties of Mg-0.5Ca-xZr Alloys. Crystals 2022, 12, 209. [Google Scholar] [CrossRef]
- Chang, L.L.; Tang, H.; Guo, J. Strengthening effect of nano and micro-sized precipitates in the hot-extruded Mg-5Sn-3Zn alloys with Ca addition. J. Alloy. Compd. 2017, 703, 552–559. [Google Scholar] [CrossRef]
- Rashed, H.M.M.A.; Mesbah, M.B. Effects of Sn addition on dynamic grain growth in a binary Mg-1Ca alloy. Mater. Res. Express 2021, 8, 1–10. [Google Scholar] [CrossRef]
- Yaghoobi, M.; Voyiadjis, G.Z.; Sundararaghavan, V. Crystal Plasticity Simulation of Magnesium and Its Alloys: A Review of Recent Advances. Crystals 2021, 11, 435. [Google Scholar] [CrossRef]
- Somekawa, H.; Yi, J.; Singh, A.; Tsuchiya, K. Microstructural evolution via purity grade of magnesium produced by high pressure torsion. Mater. Sci. Eng. A 2021, 823, 141735. [Google Scholar] [CrossRef]
- Yang, B.; Liang, D.; Xiong, N.; Tian, Y.; Xu, B.; Dai, Y. Effect of crystallization on purity of volatile metallic magnesium prepared from a one-step multi-region condensation process under vacuum condition. J. Magnes. Alloy. 2021, 2213–9567. [Google Scholar] [CrossRef]
- Hu, Z.C.; Zhang, E.L.; Zeng, S.Y. Degassing of magnesium alloy by rotating impeller degasser: Part 1-Mathematical modelling. Mater. Sci. Technol. 2008, 24, 1304–1308. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, G.J.; Hu, Z.C. Degassing of magnesium alloy by rotating impeller degasser Part 2-effect on microstructure and mechanical properties. Mater. Sci. Technol. 2010, 26, 1253–1258. [Google Scholar] [CrossRef]
- Cao, H.X.; Huang, M.T.; Wang, C.C. Research status and prospects of melt refining and purification technology of magnesium alloys. J. Magnes. Alloy. 2019, 7, 370–380. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.S.; Tong, W.H. Effect of purification treatment on corrosion resistance of Mg-Gd-Y-Zr alloy. Trans. Nonferrous Met. Soc. China 2011, 21, 945–954. [Google Scholar] [CrossRef]
- Kolin, A. An Electromagnetokinetic phenomenon involving migration of neutral particles. Science 1953, 117, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Li, S.T.; Zhang, Y.H.; Huang, H.W. Black phosphorus-based heterostructures for photocatalysis and photoelectrochemical water splitting. J. Energy Chem. 2022, 67, 745–779. [Google Scholar] [CrossRef]
- Chen, X.J.; Huang, G.H.; An, C.J. Superwetting polyethersulfone membrane functionalized with ZrO2 nanoparticles for polycyclic aromatic hydrocarbon removal. J. Mater. Sci. Amp Technol. 2022, 98, 14–25. [Google Scholar] [CrossRef]
- Qian, W.Q.; Xu, S.W.; Zhang, X.M. Di erences and Similarities of Photocatalysis and Electrocatalysis in Two-Dimensional Nanomaterials: Strategies, Traps, Applications and Challenges. Nano-Micro Lett. 2021, 13, 220–257. [Google Scholar] [CrossRef]
- Ding, W.; Yang, F.; Bonk, A.; Bauer, T. Molten chloride salts for high-temperature thermal energy storage: Continuous electrolytic salt purification with two Mg-electrodes and alternating voltage for corrosion control. Sol. Energy Mater. Sol. Cells 2021, 223, 110979. [Google Scholar] [CrossRef]
- Pan, F.S.; Chen, X.H.; Yan, T.; Liu, T.; Mao, J.; Luo, W.; Wang, Q.; Peng, J.; Tang, A.; Jiang, B. A novel approach to melt purification of magnesium alloys. J. Magnes. Alloy. 2016, 4, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Shao, Z.W.; Le, Q.C.; Cui, J.Z. Numerical simulation of standing waves for ultrasonic purification of magnesium alloy melt. Trans. Nonferrous Met. Soc. China 2010, 20, 382–387. [Google Scholar] [CrossRef]
- Liu, N.; Li, B.; He, Z.X. Recent advances and perspectives on vanadium-and manganese-based cathode materials for aqueous zinc ion batteries. J. Energy Chem. 2021, 59, 134–159. [Google Scholar] [CrossRef]
- Meidani, A.R.N.; Hasan, M. A study of hydrogen bubble growth during ultrasonic degassing of Al-Cu alloy melts. J. Mater. Process. Technol. 2004, 147, 311–320. [Google Scholar] [CrossRef]
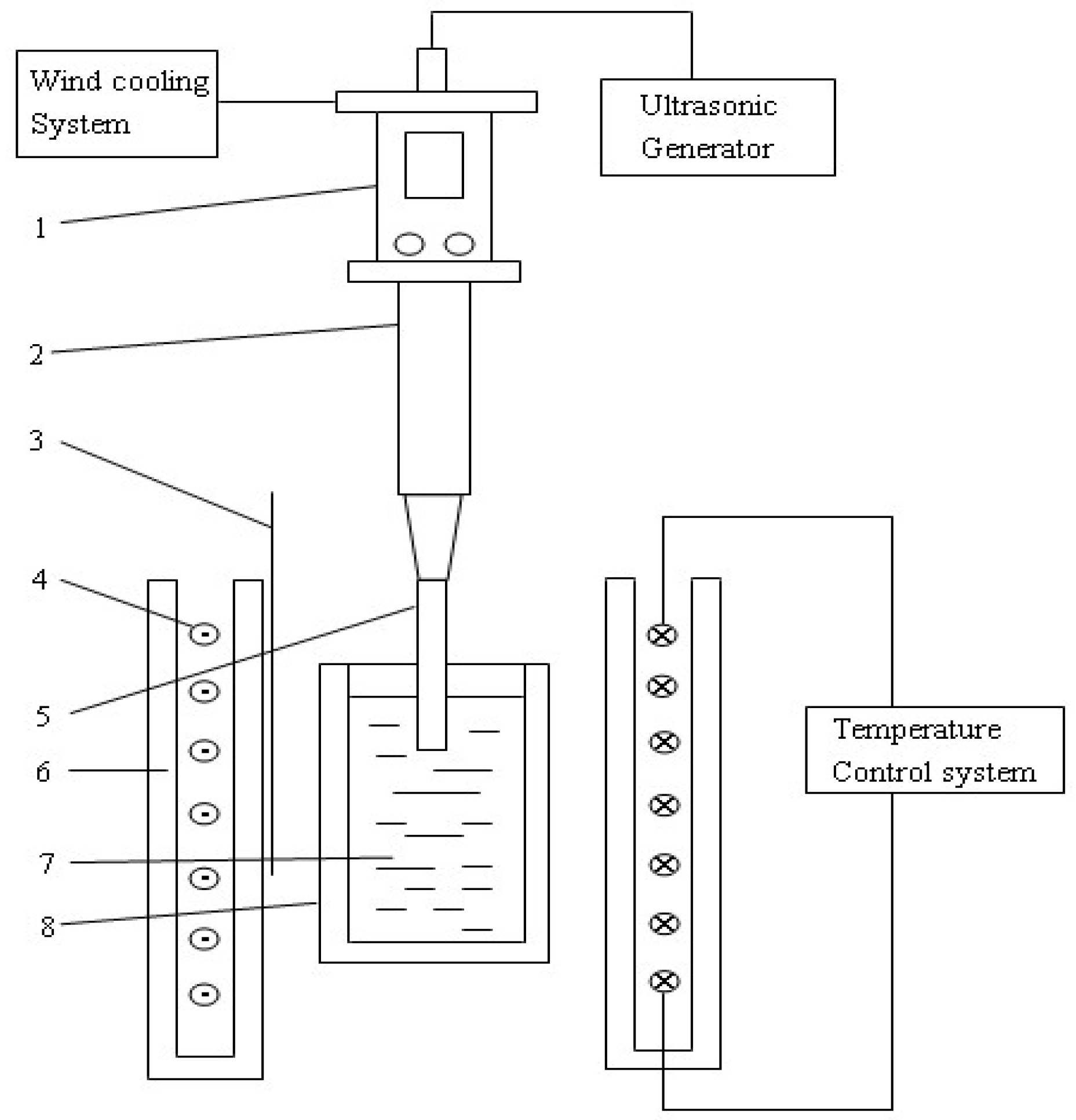
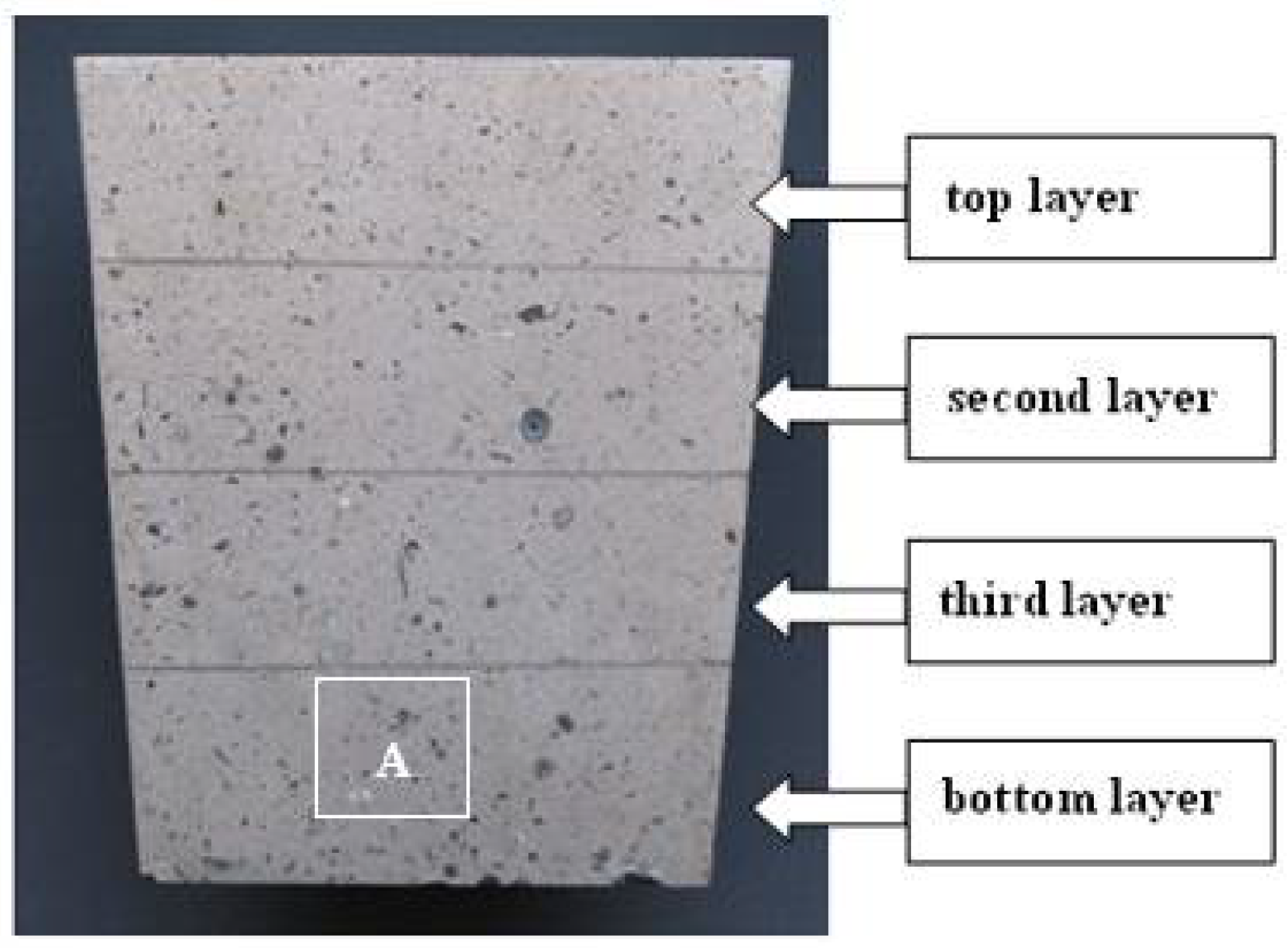
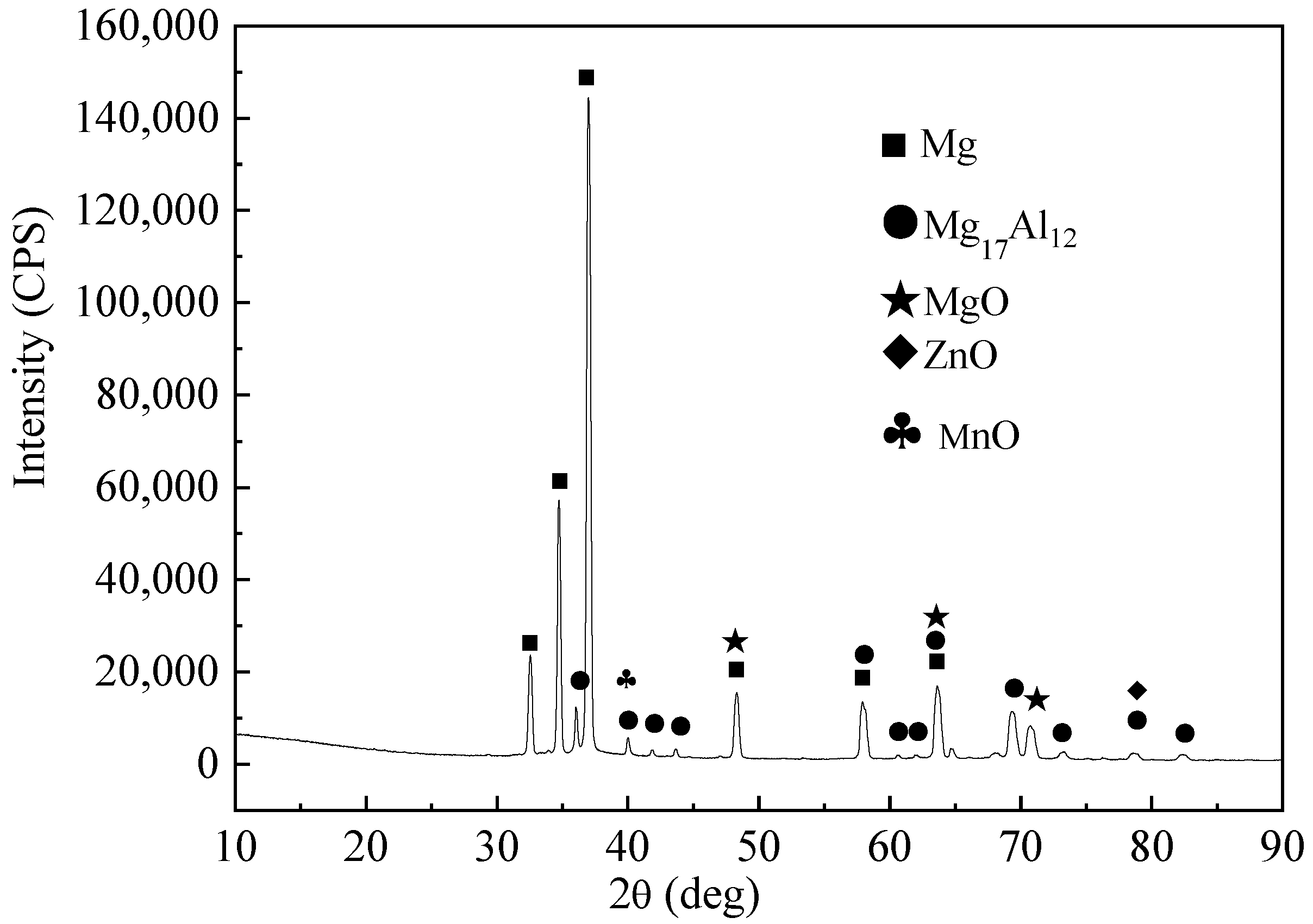
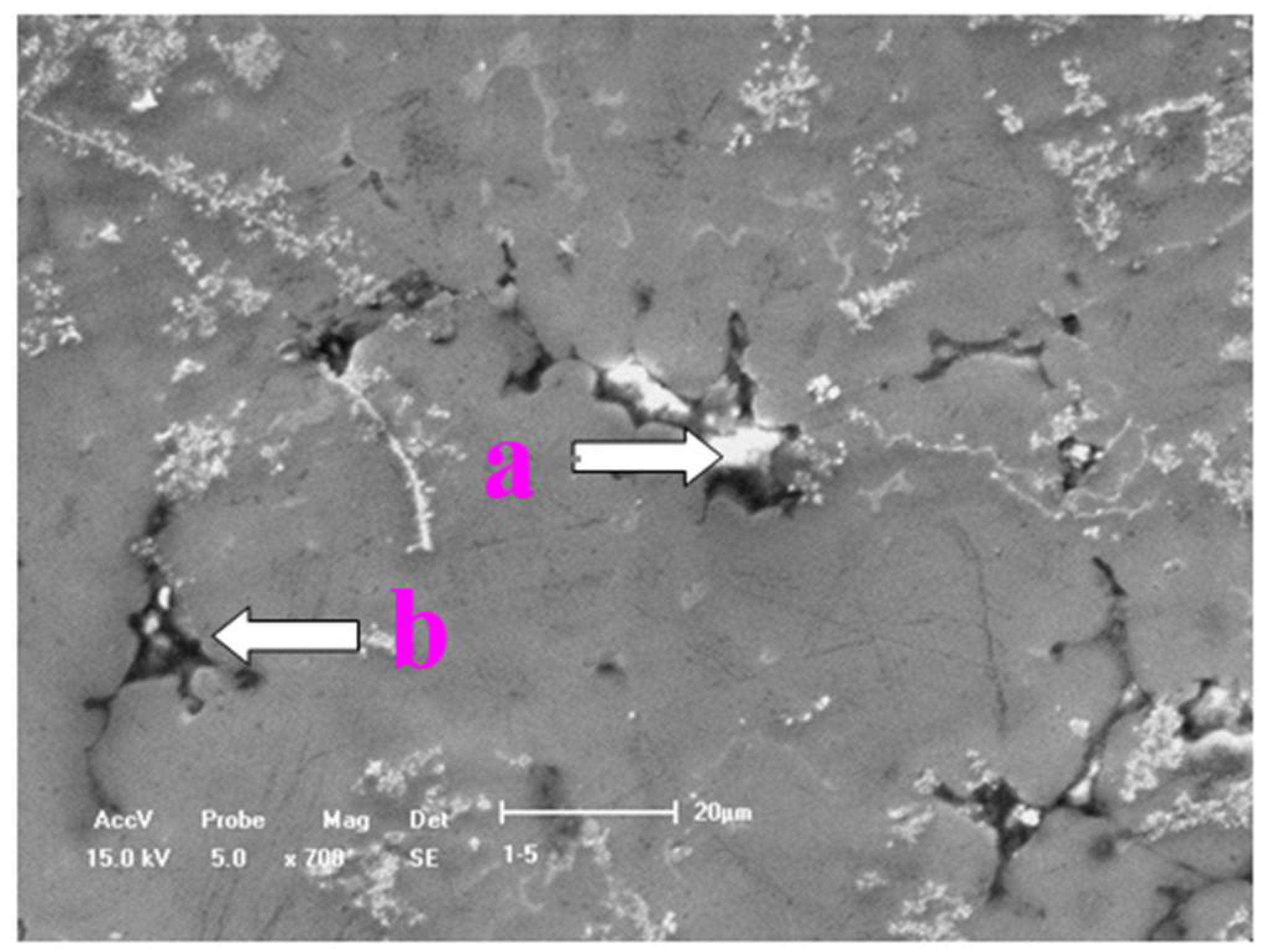

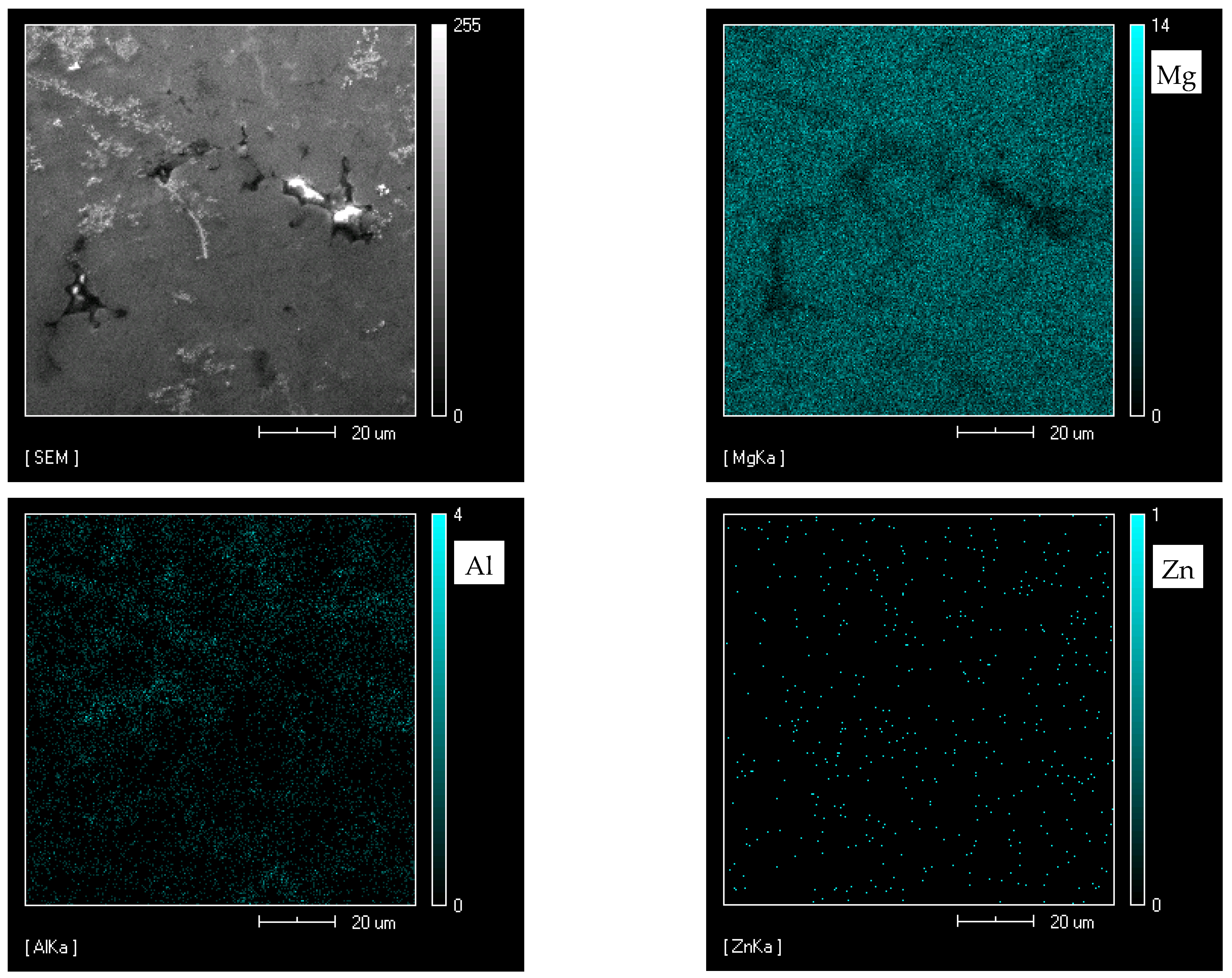
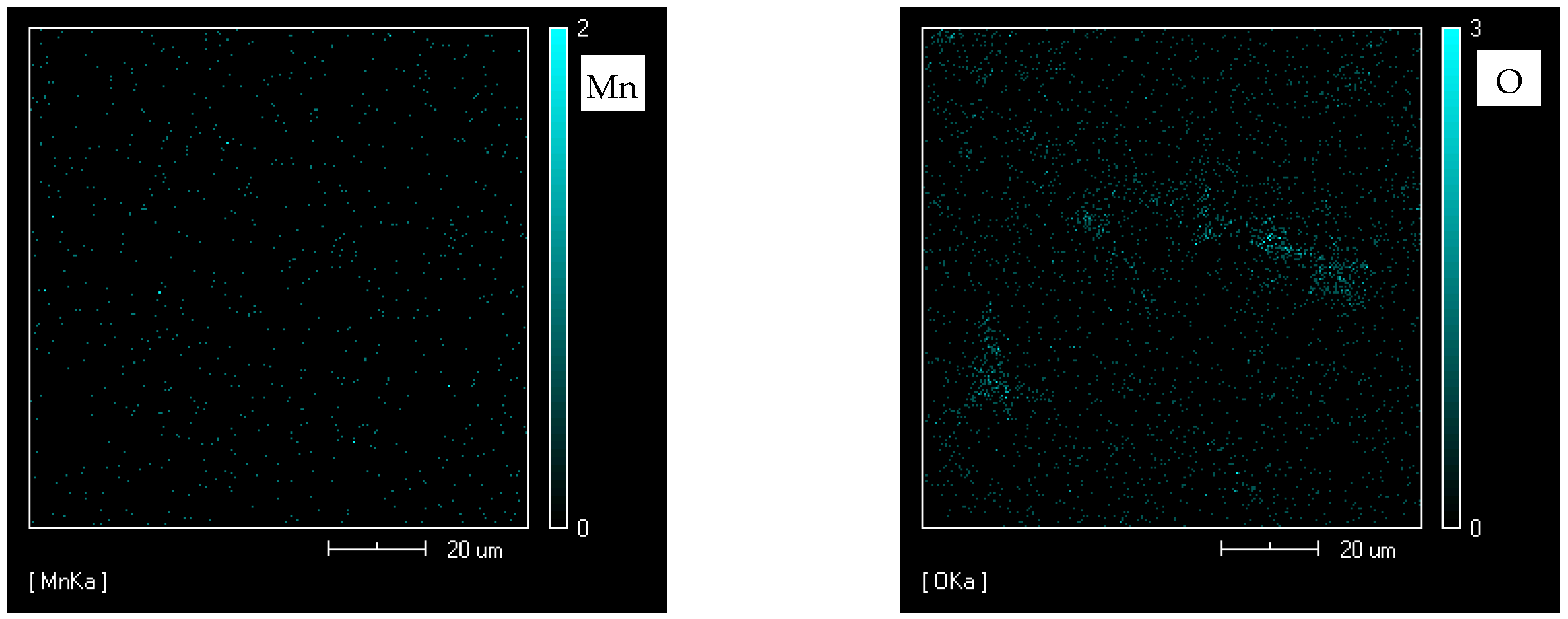


| Element | Al | Zn | Mn | Fe | Mg |
|---|---|---|---|---|---|
| AZ80 | 7.8 | 0.524 | 0.19 | 0.003 | Bal. |
| AZ91 | 9.1 | 0.82 | 0.20 | 0.020 | Bal. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Yu, B.; Yu, Y. Purification of AZ80 and Degassing of AZ91 Alloy by Ultrasonic Treatment. Crystals 2022, 12, 637. https://doi.org/10.3390/cryst12050637
Jia Z, Yu B, Yu Y. Purification of AZ80 and Degassing of AZ91 Alloy by Ultrasonic Treatment. Crystals. 2022; 12(5):637. https://doi.org/10.3390/cryst12050637
Chicago/Turabian StyleJia, Zheng, Bing Yu, and Yongzhi Yu. 2022. "Purification of AZ80 and Degassing of AZ91 Alloy by Ultrasonic Treatment" Crystals 12, no. 5: 637. https://doi.org/10.3390/cryst12050637
APA StyleJia, Z., Yu, B., & Yu, Y. (2022). Purification of AZ80 and Degassing of AZ91 Alloy by Ultrasonic Treatment. Crystals, 12(5), 637. https://doi.org/10.3390/cryst12050637





