Hot-Pressed Two-Dimensional Amorphous Metals and Their Electronic Properties
Abstract
:1. Introduction
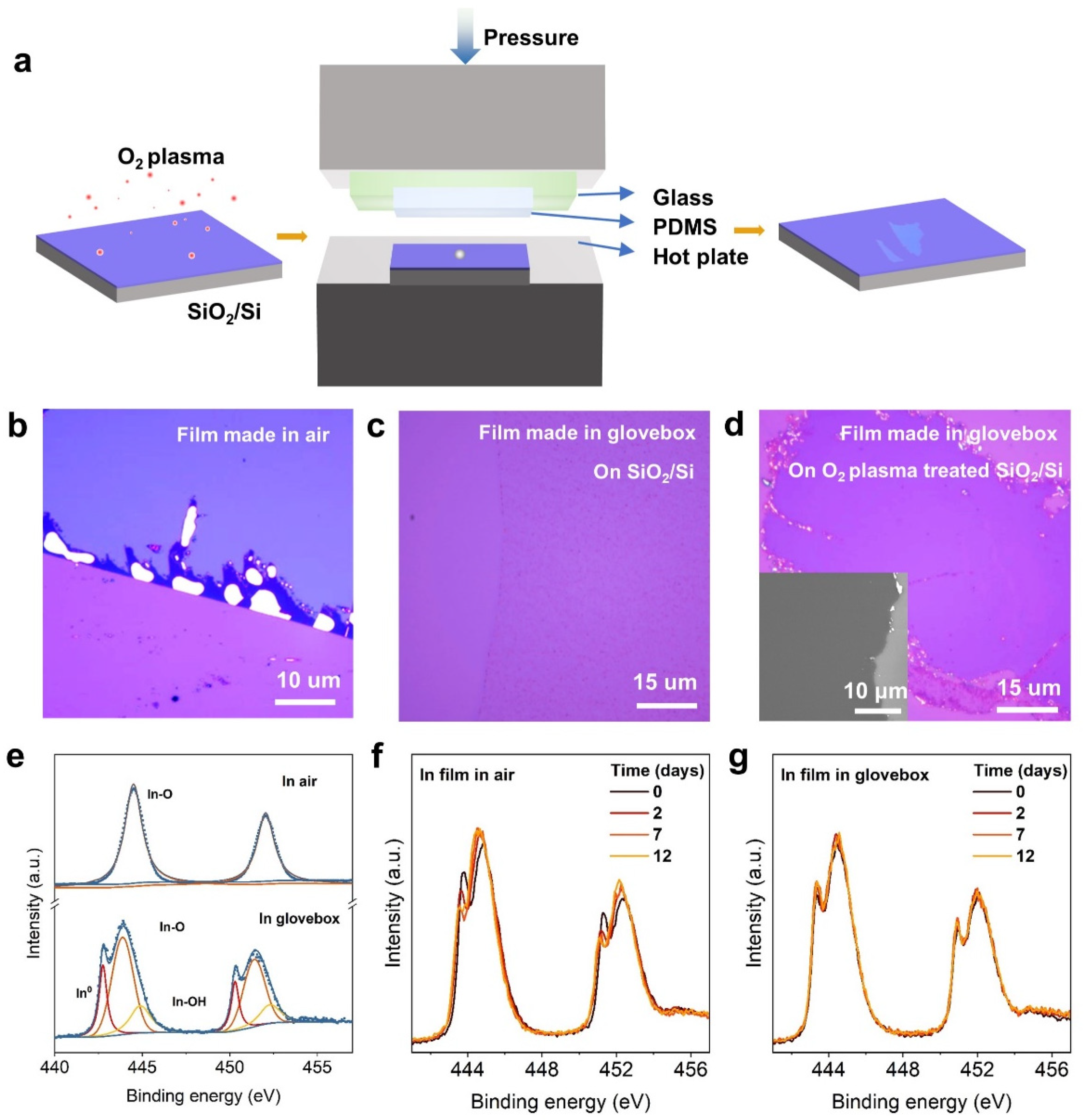
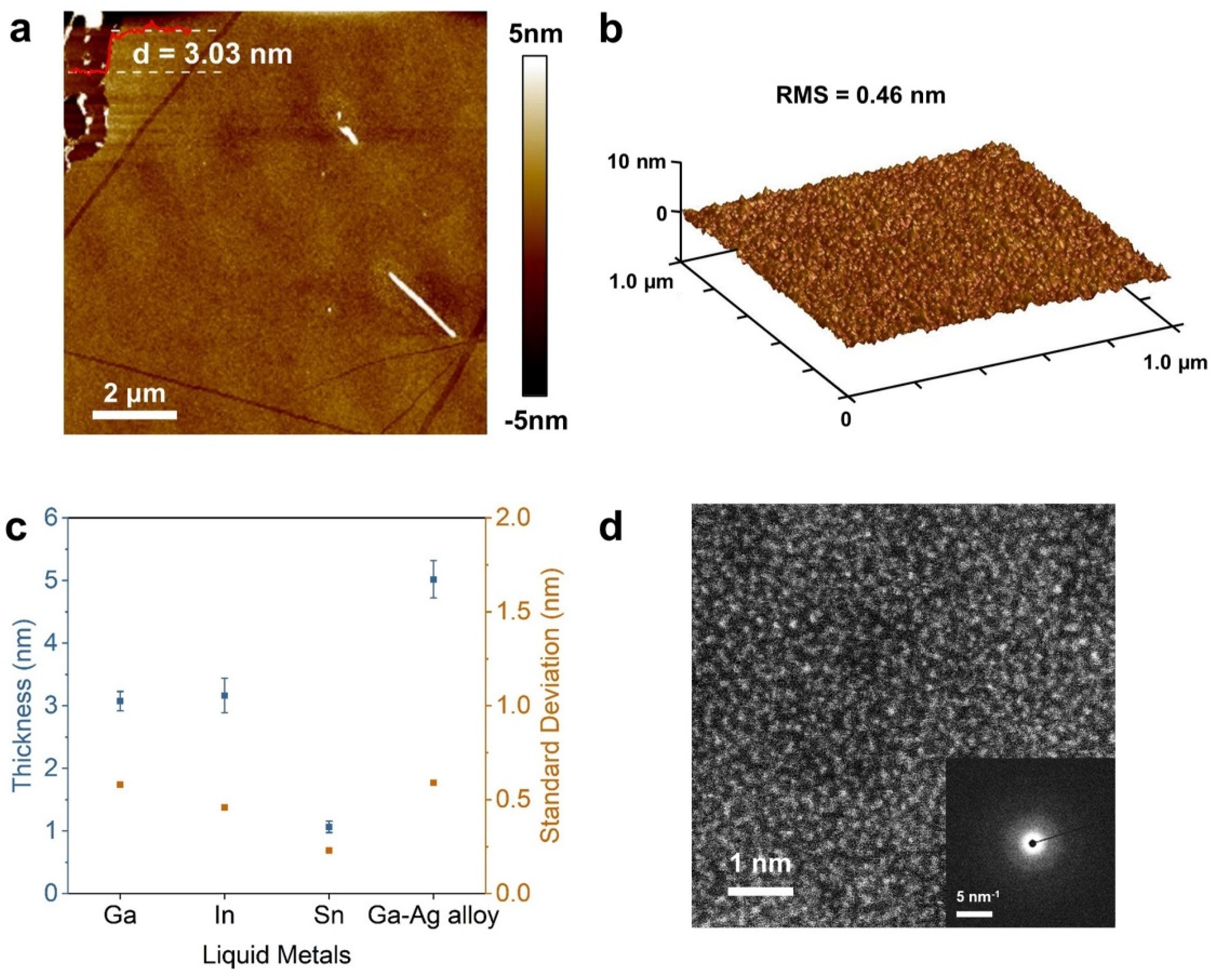
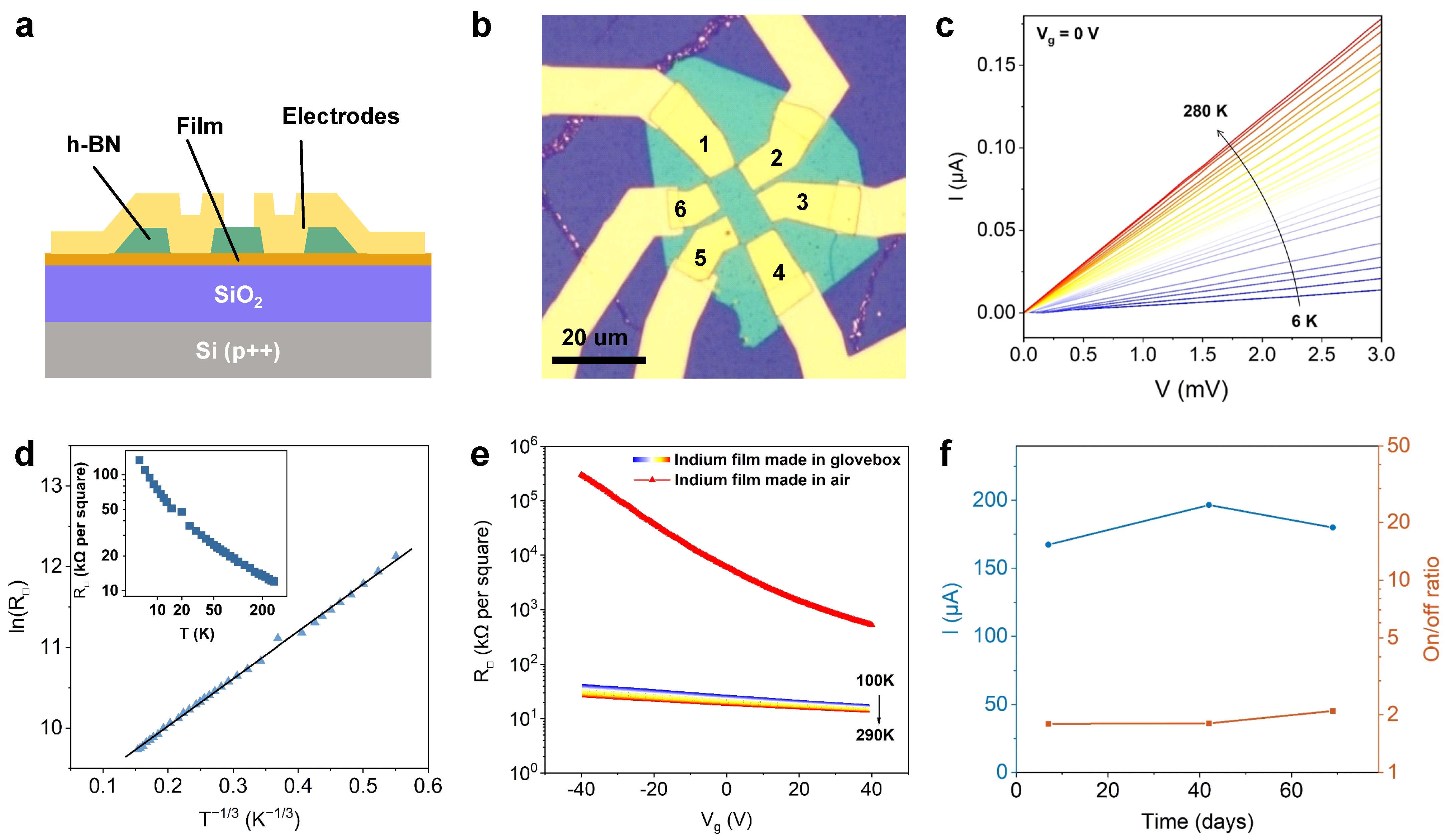
2. Results and Discussion
3. Methods
3.1. Fabrication of Metal Films in Glovebox
3.2. Characterizations
3.3. Device Fabrication and Electronic Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, C.; Cao, X.; Wu, X.-J.; He, Q.; Yang, J.; Zhang, X.; Chen, J.; Zhao, W.; Han, S.; Nam, G.-H.; et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017, 117, 6225–6331. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Huang, L.; Wang, J.; Han, L.; Li, S.; Zhang, S.; Zhang, H. Recent Progress in the Synthesis and Applications of 2D Metal Nanosheets. Nanotechnology 2019, 30, 222001. [Google Scholar] [CrossRef] [PubMed]
- Kumbhakar, P.; Chowde Gowda, C.; Mahapatra, P.L.; Mukherjee, M.; Malviya, K.D.; Chaker, M.; Chandra, A.; Lahiri, B.; Ajayan, P.M.; Jariwala, D.; et al. Emerging 2D Metal Oxides and Their Applications. Mater. Today 2021, 45, 142–168. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-zadeh, K. Liquid Metals: Fundamentals and Applications in Chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Fu, L. Liquid Metals: A Novel Possibility of Fabricating 2D Metal Oxides. Adv. Mater. 2021, 33, 2005544. [Google Scholar] [CrossRef]
- Huang, K.; Hou, J.; Zhang, Q.; Ou, G.; Ning, D.; Hussain, N.; Xu, Y.; Ge, B.; Liu, K.; Wu, H. Ultrathin Two-Dimensional Metals with Fully Exposed (111) Facets. Chem. Commun. 2018, 54, 160–163. [Google Scholar] [CrossRef]
- Lin, Z.; Du, C.; Yan, B.; Wang, C.; Yang, G. Two-Dimensional Amorphous NiO as a Plasmonic Photocatalyst for Solar H2 Evolution. Nat. Commun. 2018, 9, 4036. [Google Scholar] [CrossRef] [Green Version]
- Dral, A.P.; ten Elshof, J.E. 2D Metal Oxide Nanoflakes for Sensing Applications: Review and Perspective. Sens. Actuators B Chem. 2018, 272, 369–392. [Google Scholar] [CrossRef] [Green Version]
- Datta, R.S.; Syed, N.; Zavabeti, A.; Jannat, A.; Mohiuddin, M.; Rokunuzzaman, M.; Zhang, B.Y.; Rahman, M.A.; Atkin, P.; Messalea, K.A.; et al. Flexible Two-Dimensional Indium Tin Oxide Fabricated Using a Liquid Metal Printing Technique. Nat. Electron. 2020, 3, 51–58. [Google Scholar] [CrossRef]
- Yang, F.; Miao, L.; Wang, Z.F.; Yao, M.-Y.; Zhu, F.; Song, Y.R.; Wang, M.-X.; Xu, J.-P.; Fedorov, A.V.; Sun, Z.; et al. Spatial and Energy Distribution of Topological Edge States in Single Bi(111) Bilayer. Phys. Rev. Lett. 2012, 109, 016801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briggs, N.; Bersch, B.; Wang, Y.; Jiang, J.; Koch, R.J.; Nayir, N.; Wang, K.; Kolmer, M.; Ko, W.; De La Fuente Duran, A.; et al. Atomically Thin Half-van Der Waals Metals Enabled by Confinement Heteroepitaxy. Nat. Mater. 2020, 19, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Sun, Y.; Li, W.; Peng, J.P.; Song, C.L.; Xing, Y.; Zhang, Q.; Guan, J.; Li, Z.; Zhao, Y.; et al. Detection of a Superconducting Phase in a Two-Atom Layer of Hexagonal Ga Film Grown on Semiconducting GaN(0001). Phys. Rev. Lett. 2015, 114, 107003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fickert, M.; Assebban, M.; Canet-Ferrer, J.; Abellán, G. Phonon Properties and Photo-Thermal Oxidation of Micromechanically Exfoliated Antimonene Nanosheets. 2D Mater. 2021, 8, 015018. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.-F.; Bao, X.-Y.; Han, T.-Z.; Tang, Z.; Zhang, L.-X.; Zhu, W.-G.; Wang, E.G.; Niu, Q.; Qiu, Z.Q.; et al. Superconductivity Modulated by Quantum Size Effects. Science 2004, 306, 1915–1917. [Google Scholar] [CrossRef]
- Reis, F.; Li, G.; Dudy, L.; Bauernfeind, M.; Glass, S.; Hanke, W.; Thomale, R.; Schäfer, J.; Claessen, R. Bismuthene on a SiC Substrate: A Candidate for a High-Temperature Quantum Spin Hall Material. Science 2017, 357, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.; Xie, T.; Zhao, Y.; Li, Y. Single-Crystal Metal Nanoplatelets: Cobalt, Nickel, Copper, and Silver. Cryst. Growth Des. 2007, 7, 1904–1911. [Google Scholar] [CrossRef]
- Lim, B.S.; Rahtu, A.; Gordon, R.G. Atomic Layer Deposition of Transition Metals. Nat. Mater. 2003, 2, 749–754. [Google Scholar] [CrossRef]
- Hussain, N.; Liang, T.; Zhang, Q.; Anwar, T.; Huang, Y.; Lang, J.; Huang, K.; Wu, H. Ultrathin Bi Nanosheets with Superior Photoluminescence. Small 2017, 13, 1701349. [Google Scholar] [CrossRef]
- Messalea, K.A.; Carey, B.J.; Jannat, A.; Syed, N.; Mohiuddin, M.; Zhang, B.Y.; Zavabeti, A.; Ahmed, T.; Mahmood, N.; Della Gaspera, E.; et al. Bi2O3 Monolayers from Elemental Liquid Bismuth. Nanoscale 2018, 10, 15615–15623. [Google Scholar] [CrossRef]
- Daeneke, T.; Atkin, P.; Orrell-Trigg, R.; Zavabeti, A.; Ahmed, T.; Walia, S.; Liu, M.; Tachibana, Y.; Javaid, M.; Greentree, A.D.; et al. Wafer-Scale Synthesis of Semiconducting SnO Monolayers from Interfacial Oxide Layers of Metallic Liquid Tin. ACS Nano 2017, 11, 10974–10983. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Yang, B.; Zhang, C.; Xu, T.; Chen, L.; Yang, L.; Jin, X.; Liu, B. Template Approach to Large-Area Non-Layered Ga-Group Two-Dimensional Crystals from Printed Skin of Liquid Gallium. Chem. Mater. 2021, 33, 4568–4577. [Google Scholar] [CrossRef]
- Kochat, V.; Samanta, A.; Zhang, Y.; Bhowmick, S.; Manimunda, P.; Asif, S.A.S.; Stender, A.S.; Vajtai, R.; Singh, A.K.; Tiwary, C.S.; et al. Atomically Thin Gallium Layers from Solid-Melt Exfoliation. Sci. Adv. 2018, 4, e1701373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavabeti, A.; Ou, J.Z.; Carey, B.J.; Syed, N.; Orrell-Trigg, R.; Mayes, E.L.H.; Xu, C.; Kavehei, O.; O’Mullane, A.P.; Kaner, R.B.; et al. A Liquid Metal Reaction Environment for the Room-Temperature Synthesis of Atomically Thin Metal Oxides. Science 2017, 358, 332–335. [Google Scholar] [CrossRef] [Green Version]
- Bazaka, K.; Baranov, O.; Cvelbar, U.; Podgornik, B.; Wang, Y.; Huang, S.; Xu, L.; Lim, J.W.M.; Levchenko, I.; Xu, S. Oxygen Plasmas: A Sharp Chisel and Handy Trowel for Nanofabrication. Nanoscale 2018, 10, 17494–17511. [Google Scholar] [CrossRef]
- Dang, M.T.; Wantz, G.; Hirsch, L.; Wuest, J.D. Recycling Indium Tin Oxide (ITO) Anodes for Use in Organic Light-Emitting Diodes (OLEDs). Thin Solid Films 2017, 638, 236–243. [Google Scholar] [CrossRef]
- Shinde, D.V.; Ahn, D.Y.; Jadhav, V.V.; Lee, D.Y.; Shrestha, N.K.; Lee, J.K.; Lee, H.Y.; Mane, R.S.; Han, S.H. A Coordination Chemistry Approach for Shape Controlled Synthesis of Indium Oxide Nanostructures and Their Photoelectrochemical Properties. J. Mater. Chem. A 2014, 2, 5490–5498. [Google Scholar] [CrossRef]
- Zatsepin, D.A.; Boukhvalov, D.W.; Zatsepin, A.F.; Vines, L.; Gogova, D.; Shur, V.Y.; Esin, A.A. Bulk In2O3 Crystals Grown by Chemical Vapour Transport: A Combination of XPS and DFT Studies. J. Mater. Sci. Mater. Electron. 2019, 30, 18753–18758. [Google Scholar] [CrossRef]
- Jung, Y.; Choi, M.S.; Nipane, A.; Borah, A.; Kim, B.; Zangiabadi, A.; Taniguchi, T.; Watanabe, K.; Yoo, W.J.; Hone, J.; et al. Transferred via Contacts as a Platform for Ideal Two-Dimensional Transistors. Nat. Electron. 2019, 2, 187–194. [Google Scholar] [CrossRef]
- Mott, N.F. Conduction in Non-Crystalline Materials: III. Localized States in a Pseudogap and near Extremities of Conduction and Valence Bands. Philos. Mag. 1969, 19, 835–852. [Google Scholar] [CrossRef]
- Bishop, D.J.; Tsui, D.C.; Dynes, R.C. Nonmetallic Conduction in Electron Inversion Layers at Low Temperatures. Phys. Rev. Lett. 1980, 44, 1153–1156. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, T.; Wang, Z.; Ren, W.; Nan, H.; Ni, Z.; Chen, Q.; Yuan, S.; Miao, F.; Song, F.; et al. Hopping Transport through Defect-Induced Localized States in Molybdenum Disulphide. Nat. Commun. 2013, 4, 2642. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Jian, D.; Lin, Y.F.; Ku, M.M.; Jian, W.B. Electron Transport in the Two-Dimensional Channel Material—Zinc Oxide Nanoflake. Phys. B Condens. Matter 2018, 532, 135–138. [Google Scholar] [CrossRef]
- Long, G.; Xu, S.; Cai, X.; Wu, Z.; Han, T.; Lin, J.; Cheng, C.; Cai, Y.; Wang, X.; Wang, N. Gate-Tunable Strong-Weak Localization Transition in Few-Layer Black Phosphorus. Nanotechnology 2018, 29, 035204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Huang, S.; Wang, J.Y.; Xu, H.Q. Mott Variable-Range Hopping Transport in a MoS2 Nanoflake. RSC Adv. 2019, 9, 17885–17890. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wu, Z.; Lyu, Y.; Hao, J. Centimeter-Scale Growth of Two-Dimensional Layered High-Mobility Bismuth Films by Pulsed Laser Deposition. InfoMat 2019, 1, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Chen, W.; Xu, Y.; Gao, C.; Guan, D.; Liu, C.; Qian, D.; Zhang, S.-C.; Jia, J. Epitaxial Growth of Two-Dimensional Stanene. Nat. Mater. 2015, 14, 1020–1025. [Google Scholar] [CrossRef]
- Ismail, R.A.; Abdulrazaq, O.A.; Yahya, K.Z. Preparation and Characterization of In2O3 Thin Films for Optoelectronic Applications. Surf. Rev. Lett. 2005, 12, 515–518. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, X.; Zhai, J.; Yu, X.; Ding, L.; Zhang, W. Bottom-Gate Amorphous In2O3 Thin Film Transistors Fabricated by Magnetron Sputtering. Electron. Mater. Lett. 2013, 9, 279–282. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Fang, M.; Si, W.; Zhang, Q.; Huang, Z.; Gu, L.; Pan, W.; Yao, J.; Nan, C.; et al. 2D Metals by Repeated Size Reduction. Adv. Mater. 2016, 28, 8170–8176. [Google Scholar] [CrossRef]
| Ref. | Material | Processing Temperature [°C] | Method | Thickness [nm] | Oxidation Degree | Crystallinity | Electrical Properties |
|---|---|---|---|---|---|---|---|
| [40] | Ag, Au, Fe | RT a | Mechanical rolling method | 1~5 | Metal | Polycrystal (Ag) | N/A |
| [17] | Co, Ni, Cu, Ag | 100~110 | Hydro/solvothermal | ~8 | Metal | Crystal | N/A |
| [14] | Sb | RT a | Mechanical exfoliation | >14 | Metal Sb | Crystal | N/A |
| [15] | Pb | N/A | MBE | 12~28 layers | Metal Pb on Si(111) | Crystal | SC b |
| [36] | Bi | 100 | PLD | 1~15 | Metal Bi(111) on SiO2(300nm)/Si | Crystal | P-type |
| [19] | Bi | 150 | Hot-pressing | 2~10 | Metal Bi | Crystal | N/A |
| [20] | Bi | 300 | Squeeze transfer | 0.75 | α-Bi2O3 | Crystal | N-type semiconductor |
| [11] | Bi | N/A | MBE | Bilayer | Metal Bi on Bi2Te3 | Crystal | N/A |
| [16] | Bi | N/A | MBE | Monolayer | Metal Bi(111) on SiC | Crystal | N/A |
| [21] | Sn | 300 | Squeeze transfer | ~0.6 | SnO and SnO2 | Polycrystal | P-type semiconductor |
| [37] | Sn | N/A | MBE | 1~3 layers | Metal Sn on Bi2Te3(111) | α-Sn(111) | N/A |
| [22] | Ga | 50, 800 | PDMS-printing strategy and heat treatment | ~6 | β-Ga2O3 | Polycrystal | Insulator |
| [23] | Ga | 50 | Solid-melt exfoliation | ~4 | Metal Ga | Crystal | Conductor |
| [12] | Ga | 700~800 | Confinement heteroepitaxy | Trilayer | Metal graphene/Ga/SiC | Single crystal | SC b |
| In | Bilayer | Metal graphene/In/SiC | Single crystal | N/A | |||
| Sn | monolayer | Metal graphene/Sn/SiC | Single crystal | N/A | |||
| [13] | Ga | 650 | MBE | 0.552 | Metal Ga/GaN(0001) | Single crystal | SC b |
| [24] | Galinstan, EGaIn | RT a | Gas-injection method | 5.2 | Ga2O3, AI2O3, Hf2O3, Gd2O3 | Amorphous | N/A |
| This work | Ga | 50 | PDMS-hot-pressing | ~3.1 | Coexistence of metal Ga and Ga2O3 | Amorphous | Conductor, (Mott’s 2D VRH model) |
| In | 180 | ~3.2 | Coexistence of metal In and In2O3 | N/A | Conductor, (Mott’s 2D VRH model) | ||
| Sn | 260 | ~1.1 | Coexistence of SnO and SnO2 | N/A | N/A | ||
| Ga0.87Ag0.13 | 300 | ~5 | Coexistence of metal Ga, Ag and Ga2O3 | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tang, J.; Zhao, J.; Zhao, Y.; Shen, C.; Liao, M.; Wang, S.; Tian, J.; Chu, Y.; Li, J.; et al. Hot-Pressed Two-Dimensional Amorphous Metals and Their Electronic Properties. Crystals 2022, 12, 616. https://doi.org/10.3390/cryst12050616
Liu J, Tang J, Zhao J, Zhao Y, Shen C, Liao M, Wang S, Tian J, Chu Y, Li J, et al. Hot-Pressed Two-Dimensional Amorphous Metals and Their Electronic Properties. Crystals. 2022; 12(5):616. https://doi.org/10.3390/cryst12050616
Chicago/Turabian StyleLiu, Jieying, Jian Tang, Jiaojiao Zhao, Yanchong Zhao, Cheng Shen, Mengzhou Liao, Shuopei Wang, Jinpeng Tian, Yanbang Chu, Jiawei Li, and et al. 2022. "Hot-Pressed Two-Dimensional Amorphous Metals and Their Electronic Properties" Crystals 12, no. 5: 616. https://doi.org/10.3390/cryst12050616
APA StyleLiu, J., Tang, J., Zhao, J., Zhao, Y., Shen, C., Liao, M., Wang, S., Tian, J., Chu, Y., Li, J., Wei, Z., Long, G., Yang, W., Yang, R., Li, N., Shi, D., & Zhang, G. (2022). Hot-Pressed Two-Dimensional Amorphous Metals and Their Electronic Properties. Crystals, 12(5), 616. https://doi.org/10.3390/cryst12050616








