Synthesis, X-ray, Hirshfeld, and AIM Studies on Zn(II) and Cd(II) Complexes with Pyridine Ligands
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Physical Measurements
2.2. Synthesis of Complexes 1–3
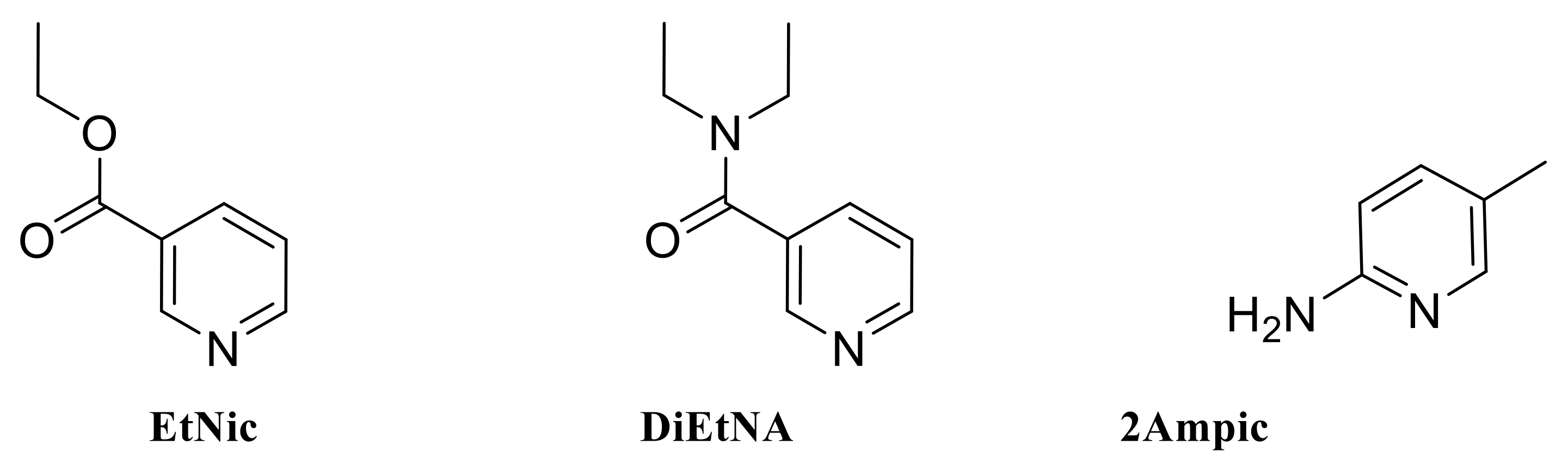
2.2.1. [Zn(EtNic)2Cl2] (1)
2.2.2. [Zn(DiEtNA)(H2O)4(SO4)]·H2O (2)
2.2.3. [Cd(OAc)2(2Ampic)2] (3)
2.3. Crystal Structure Determination
2.4. Hirshfeld Surface Analysis
2.5. DFT Calculations
3. Results and Discussion
3.1. X-ray Structure Description
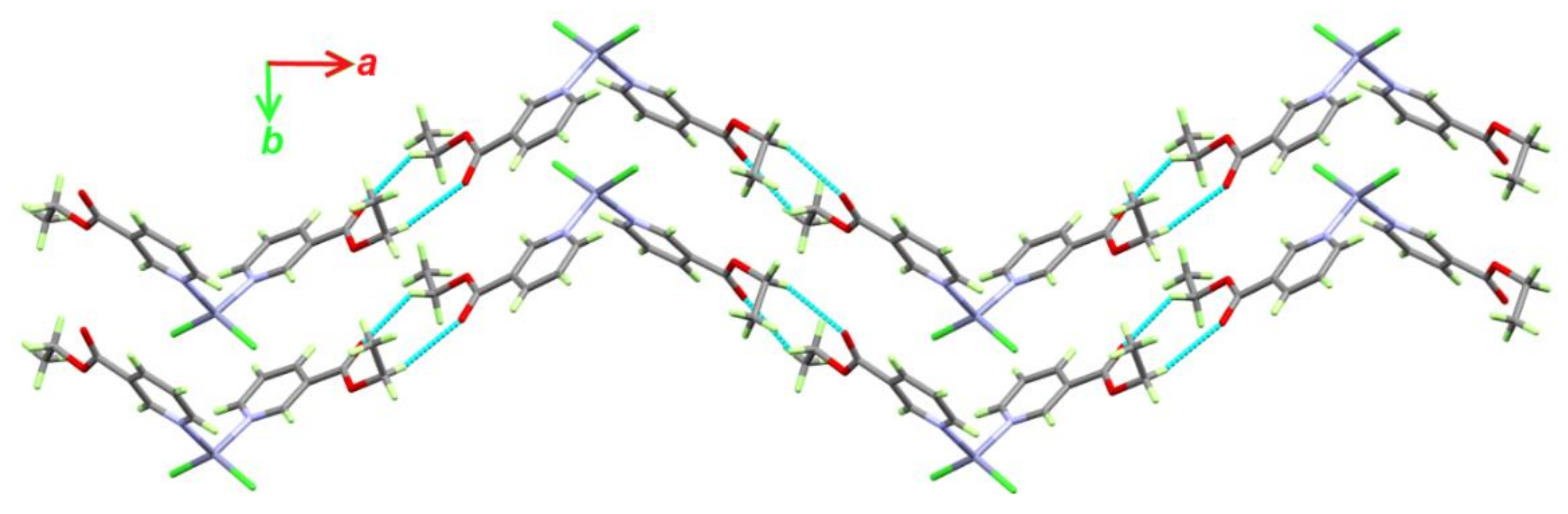
3.1.1. Structure of [Zn(EtNic)2Cl2]; 1
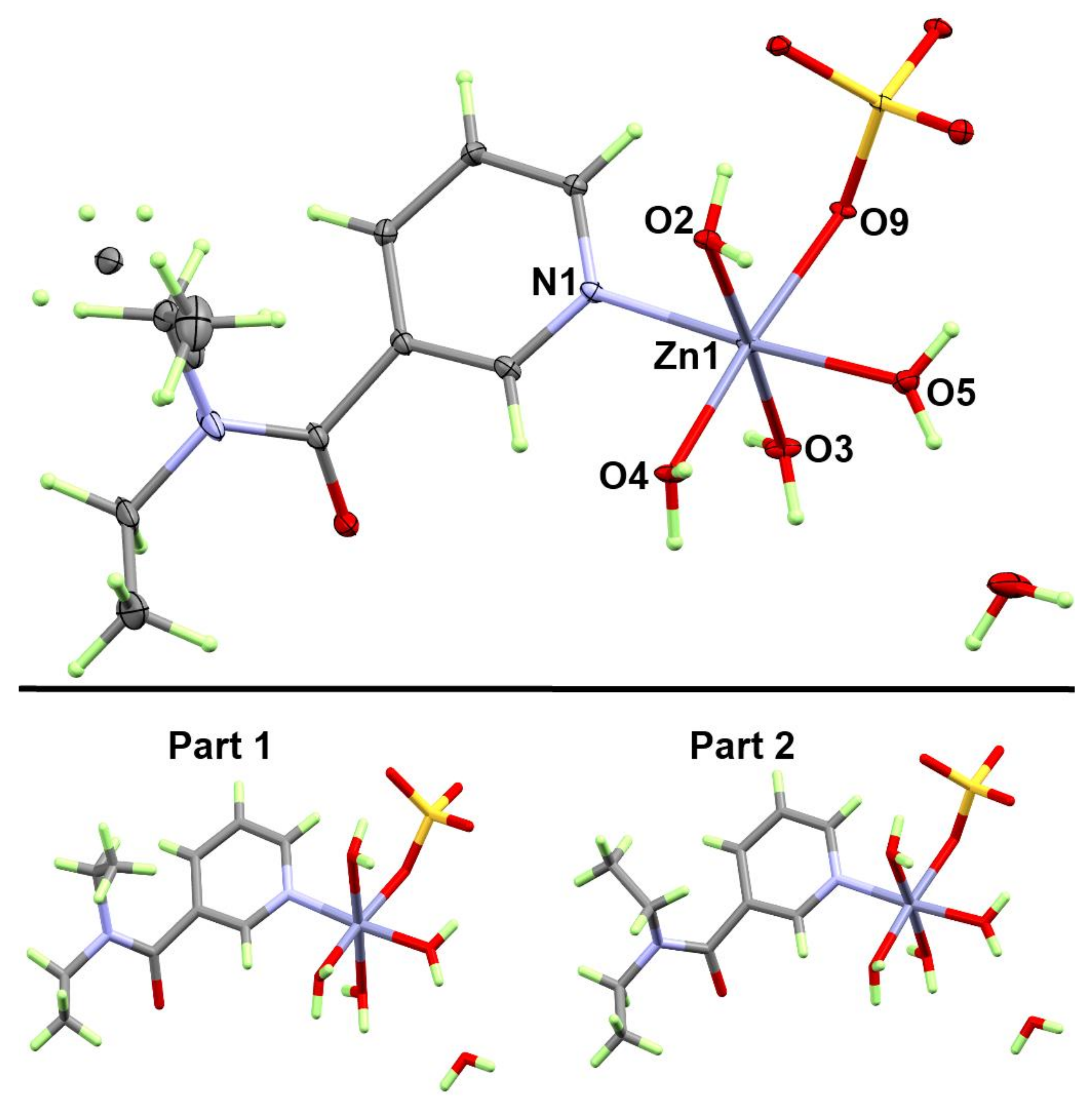
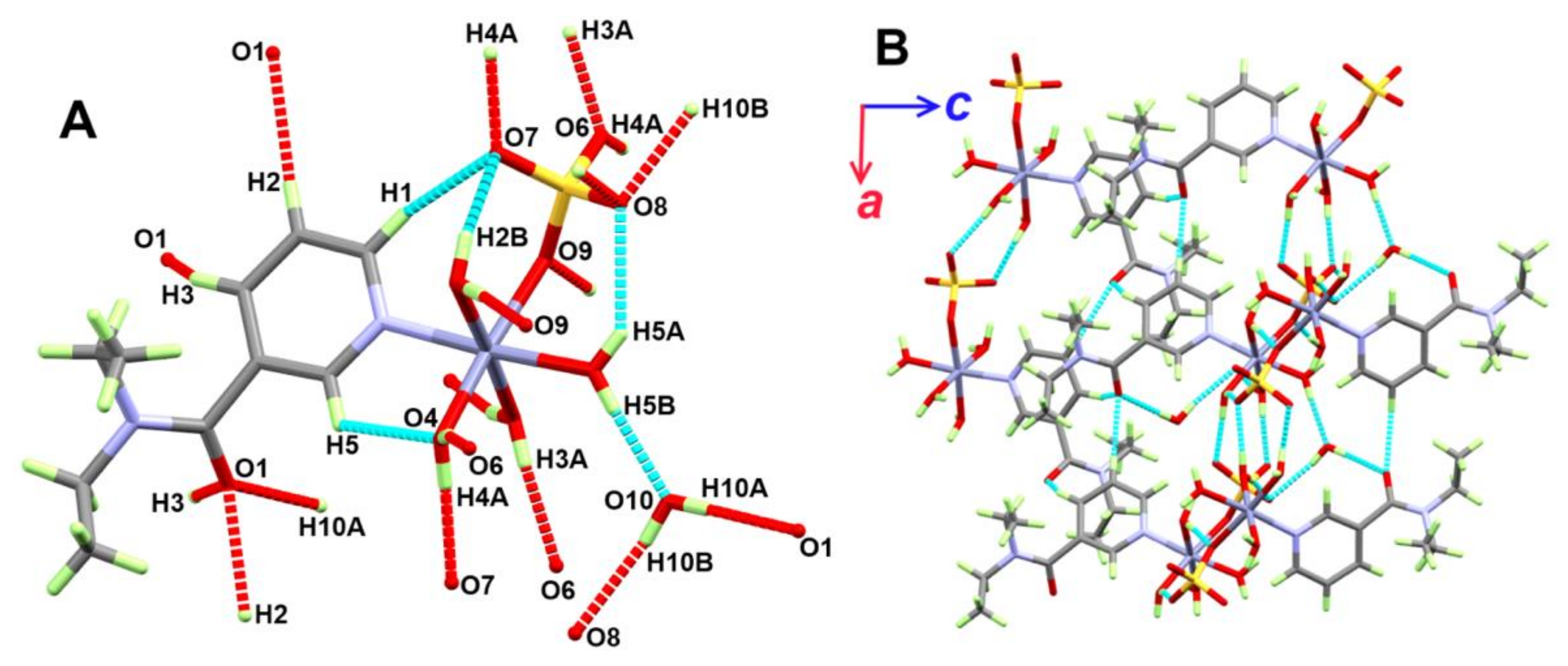
3.1.2. Structure of [Zn(DiEtNA)(H2O)4(SO4)]·H2O; 2
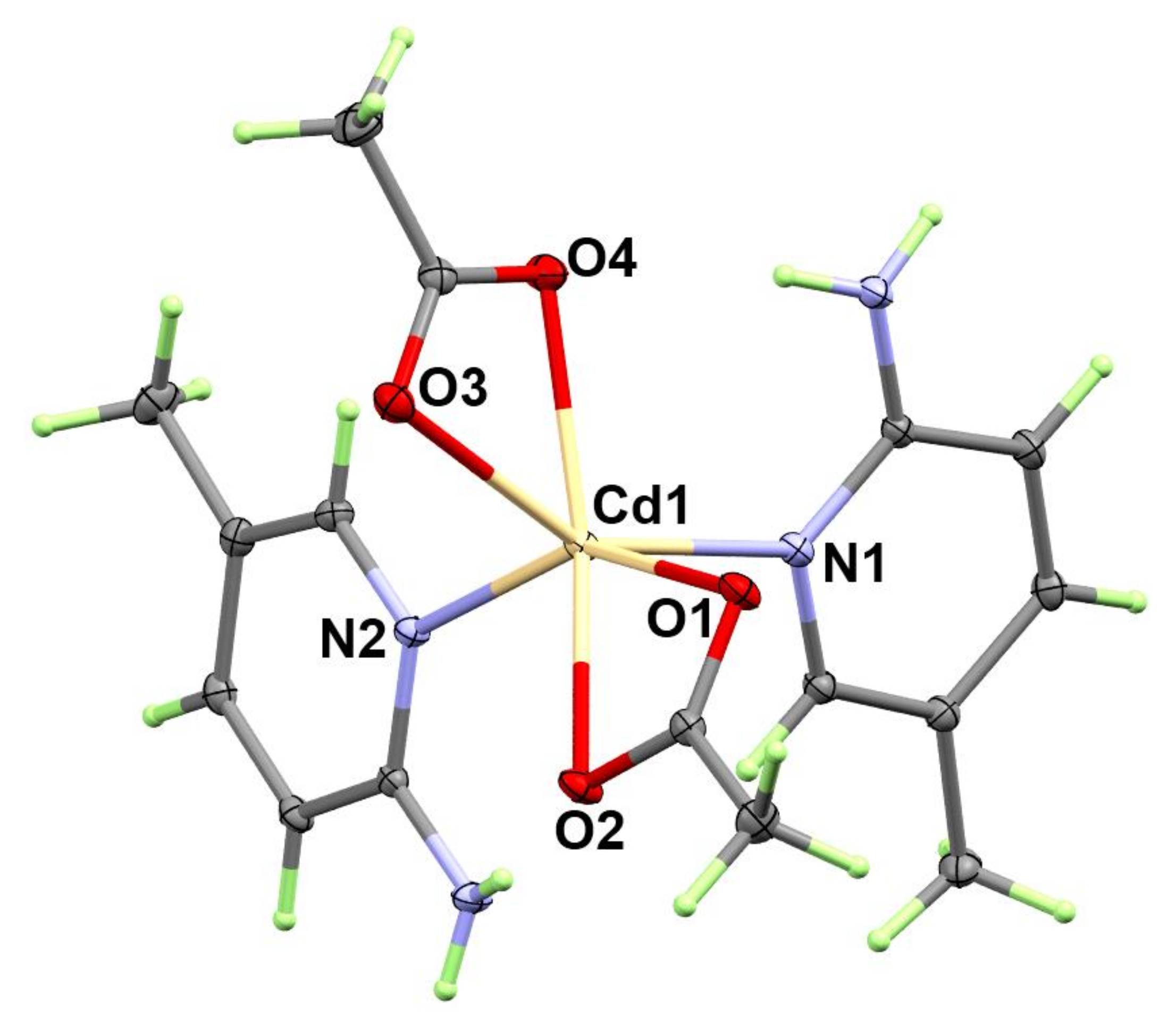
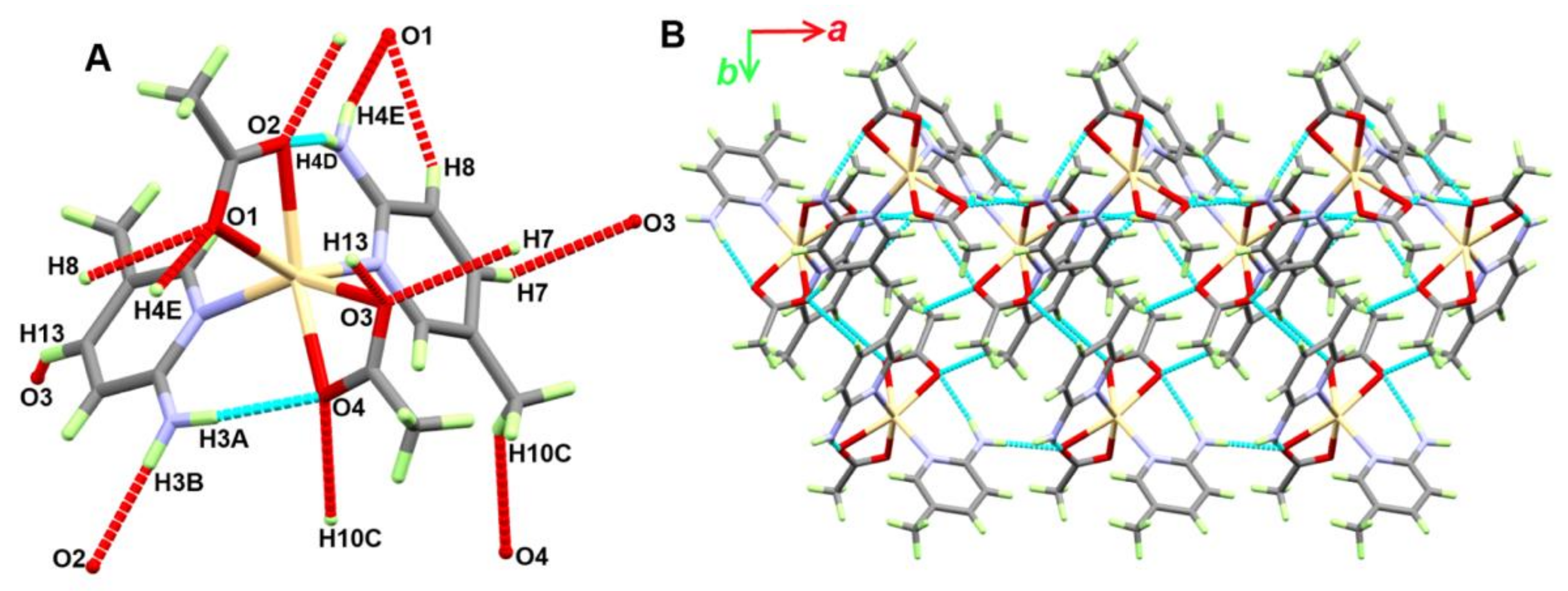
3.1.3. Structure of [Cd(OAc)2(2Ampic)2]; 3
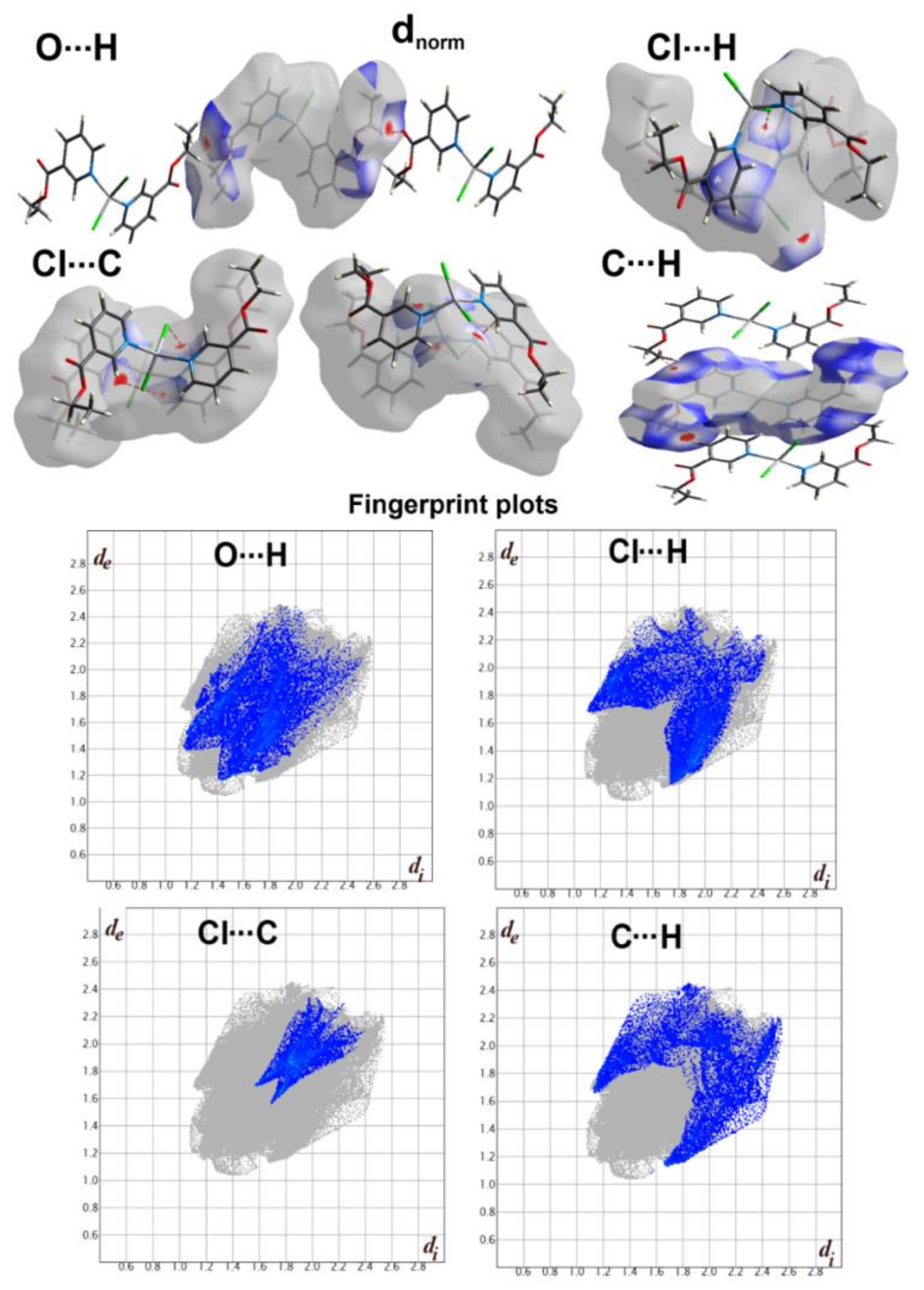
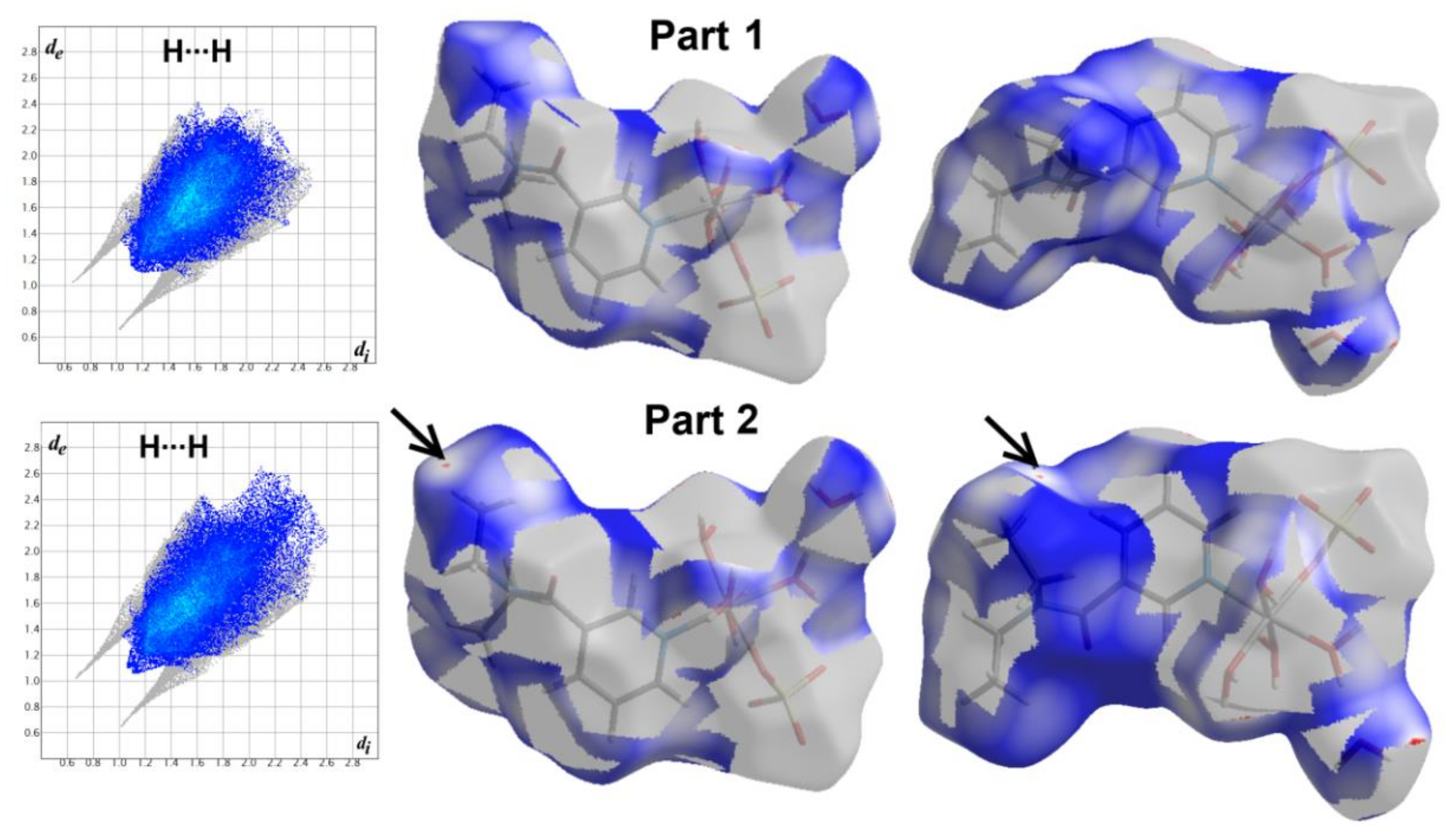
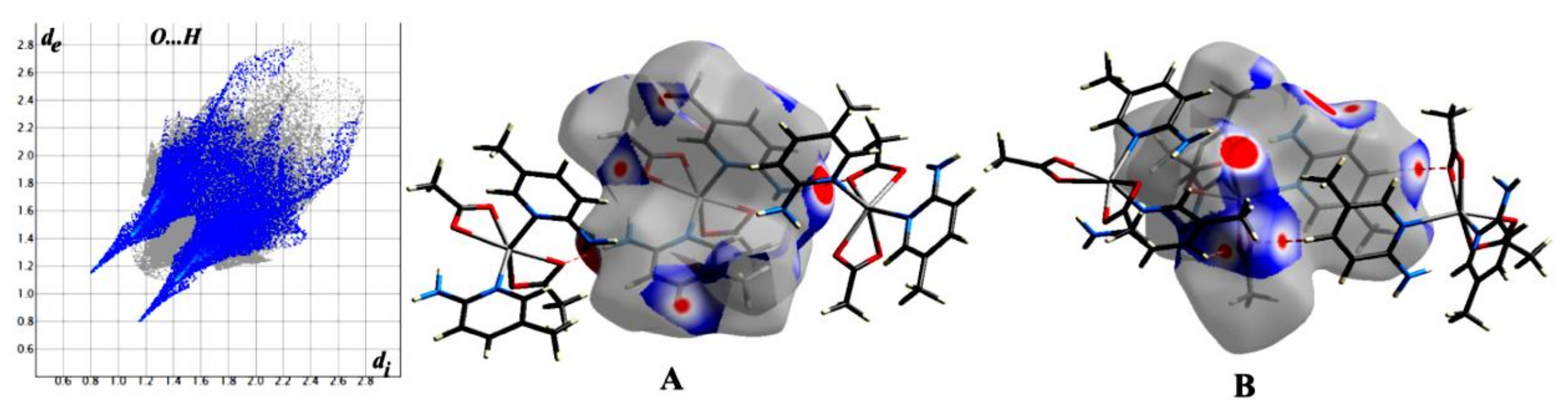
3.2. Hirshfeld Surface Analysis
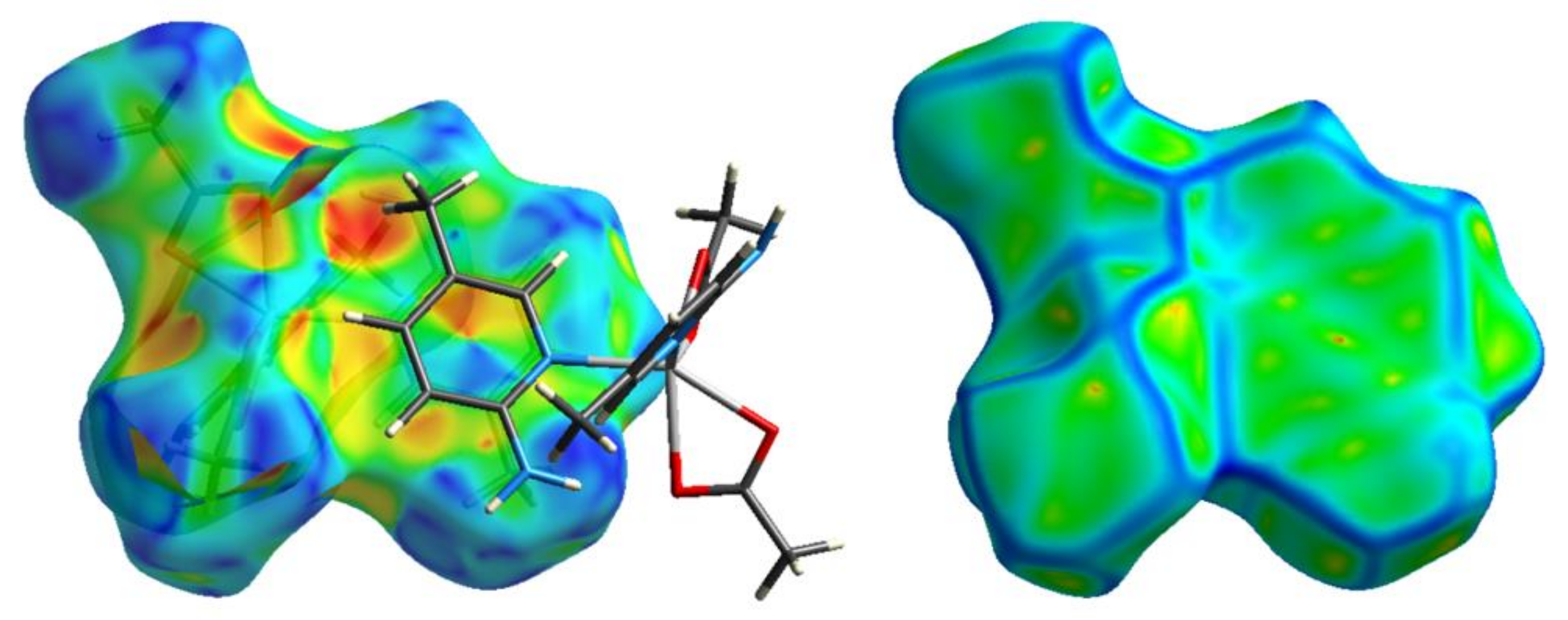
3.3. Natural Charge Distribution
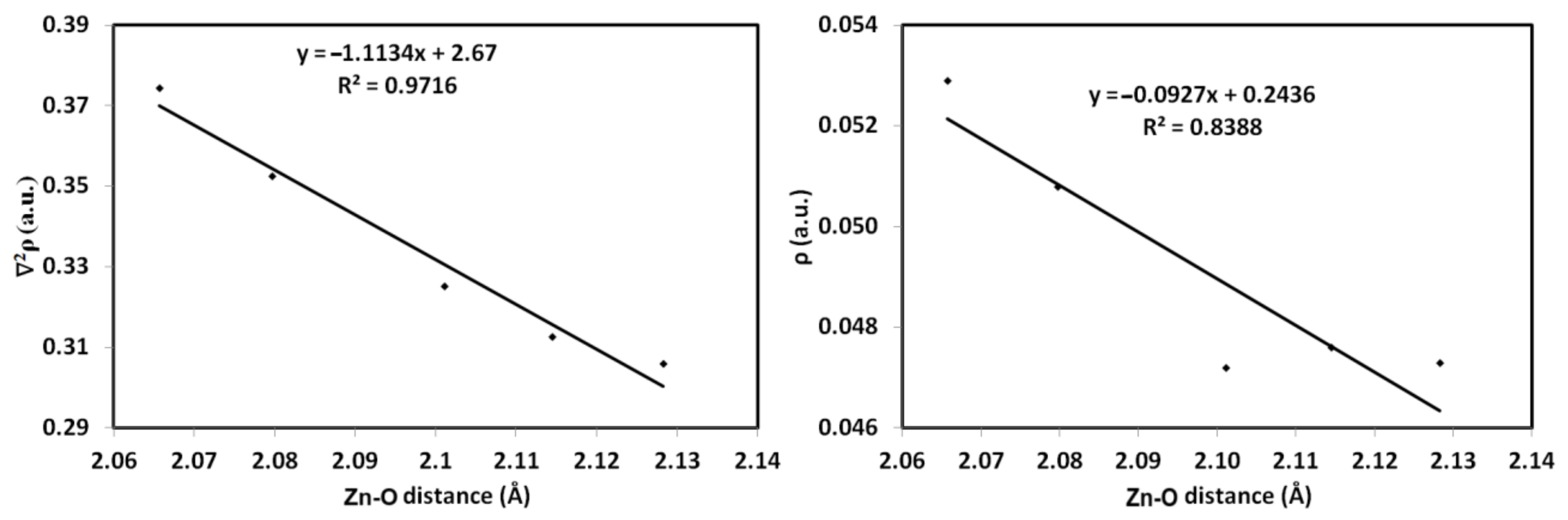
3.4. The Atoms in Molecules (AIM) Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.-L.; Xiang, S.-C.; Qian, G.-D. Metal−Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Batten, S.R.; Robson, R. Interpenetrating Nets: Ordered, Periodic Entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Zaworotko, M.J. Designer pores made easy. Nature 2008, 451, 410–411. [Google Scholar] [CrossRef] [PubMed]
- Neville, S.M.; Halder, G.J.; Chapman, K.W.; Duriska, M.B.; Moubaraki, B.; Murray, K.S.; Kepert, C.J. Guest tunable structure and spin crossover properties in a nanoporous coordination framework material. J. Am. Chem. Soc. 2009, 131, 12106–12108. [Google Scholar] [CrossRef] [PubMed]
- Weng, D.-F.; Wang, Z.-M.; Gao, S. Framework-structured weak ferromagnets. Chem. Soc. Rev. 2011, 40, 3157–3181. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Old materials with new tricks: Multifunctional open-framework materials. J. Chem. Soc. Rev. 2007, 36, 770–818. [Google Scholar] [CrossRef]
- Chen, X.-D.; Du, M.; Mak, T.C.W. Controlled generation of heterochiral or homochiral coordination polymer: Helical conformational polymorphs and argentophilicity-induced spontaneous resolution. Chem. Commun. 2005, 35, 4417–4419. [Google Scholar] [CrossRef]
- Zou, R.-Q.; Sakurai, H.; Xu, Q. Preparation, adsorption properties, and catalytic activity of 3D porous metal-organic frameworks composed of cubic building blocks and alkali-metal ions. Angew. Chem. Int. Ed. 2006, 45, 2542–2546. [Google Scholar] [CrossRef]
- Guo, F.-S.; Leng, J.-D.; Liu, J.-L.; Meng, Z.-S.; Tong, M.-L. Polynuclear and polymeric gadolinium acetate derivatives with large magnetocaloric effect. Inorg. Chem. 2012, 51, 405–413. [Google Scholar] [CrossRef]
- Zhao, H.-Y.; Jin, Z.; Su, H.-M.; Jing, X.-F.; Sun, F.-X.; Zhu, G.-S. Targeted synthesis of a 2D ordered porous organic framework for drug release. Chem. Commun. 2011, 47, 6389–6391. [Google Scholar] [CrossRef]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef]
- Hosseini, M.W. Molecular Tectonics: From Simple Tectons to Complex Molecular Networks. Acc. Chem. Res. 2005, 38, 313–323. [Google Scholar] [CrossRef]
- Du, M.; Zhao, X.-J.; Guo, J.-H.; Batten, S.R. Direction of topological isomers of silver(i) coordination polymers induced by solvent, and selective anion-exchange of a class of PtS-type host frameworks. Chem. Commun. 2005, 38, 4836–4838. [Google Scholar] [CrossRef]
- Zhou, Y.-F.; Hong, M.-C.; Wu, X.-T. Lanthanide–transition metal coordination polymers based on multiple N- and O-donor ligands. Chem. Commun. 2006, 2, 135–143. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry: Occurrence and Taxonomy of Nets and Grammar for the Design of Frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef]
- Zhang, W.; Xiong, R.-G.; Huang, S.-D. 3D Framework Containing Cu4Br4 Cubane as Connecting Node with Strong Ferroelectricity. J. Am. Chem. Soc. 2008, 130, 10468–10469. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, R.-F.; Xing, S.-K.; Liu, X.-M.; Hu, T.-L.; Bu, X.-H. A Highly Selective On/Off Fluorescence Sensor for Cadmium(II). Inorg. Chem. 2011, 50, 10041–10046. [Google Scholar] [CrossRef] [PubMed]
- Shan, G.-G.; Li, H.-B.; Cao, H.-T.; Zhu, D.-X.; Li, P.; Su, Z.-M.; Liao, Y. Reversible piezochromic behavior of two new cationic iridium(iii) complexes. Chem. Commun. 2012, 48, 2000–2002. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppier, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-X.; Xiang, S.-C.; Zhang, W.-W.; Zhang, Z.-X.; Wang, L.; Bai, J.-F.; Chen, B.-L. A new MOF-505 analog exhibiting high acetylene storage. Chem. Commun. 2009, 48, 7551–7553. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Z.; Li, M.-A.; Li, Z.; Hou, J.-Z.; Huang, X.-C.; Li, D. Concomitant and controllable chiral/racemic polymorphs: From achirality to isotactic, syndiotactic, and heterotactic chirality. Angew. Chem. Int. Ed. 2008, 47, 6371–6374. [Google Scholar] [CrossRef]
- Lang, J.; Vagnerova, K.; Czernek, J.; Lhotak, P. Flip–flop Motion of Circular Hydrogen Bond Array in Thiacalix [4]arene. Supramol. Chem. 2006, 18, 371–381. [Google Scholar] [CrossRef]
- Srivastava, K.; Khan, E.; Shimpi, M.R.; Tandon, P.; Sinha, K.; Velaga, S.P. Molecular structure and hydrogen bond interactions of a paracetamol–4,4′-bipyridine cocrystal studied using a vibrational spectroscopic and quantum chemical approach. CrystEngComm 2018, 20, 213–222. [Google Scholar] [CrossRef]
- Perpetuo, G.J.; Janczak, J. Supramolecular architectures in crystals of melamine and aromatic carboxylic acids. J. Mol. Struct. 2008, 891, 429–436. [Google Scholar] [CrossRef][Green Version]
- Roesky, H.W.; Andruh, M. The interplay of coordinative, hydrogen bonding and π–π stacking interactions in sustaining supramolecular solid-state architectures. A study case of bis(4-pyridyl)- and bis(4-pyridyl-N-oxide) tectons. Coord. Chem. Rev. 2003, 236, 91–119. [Google Scholar] [CrossRef]
- Han, L.L.; Li, Z.H.; Chen, J.S.; Wang, X.P.; Sun, D. Solution and Mechanochemical Syntheses of Two Novel Cocrystals: Ligand Length Modulated Interpenetration of Hydrogen-Bonded 2D 63-hcb Networks Based on a Robust Trimeric Heterosynthon. Cryst. Growth Des. 2014, 14, 1221–1226. [Google Scholar] [CrossRef]
- Sun, D.; Liu, F.J.; Huang, R.B.; Zheng, L.S. Three guest-dependent nitrate–water aggregations encapsulated in silver(i)–bipyridine supramolecular frameworks. CrystEngComm 2012, 14, 7872–7876. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.H.; Hao, H.J.; Liu, F.J.; Wen, Y.M.; Huang, R.B.; Zheng, L.S. Solvent-Controlled Rare Case of a Triple Helical Molecular Braid Assembled from Proton-Transferred Sebacic Acid. Cryst. Growth Des. 2011, 11, 3323–3327. [Google Scholar] [CrossRef]
- Benabid, W.; Ouari, K.; Bendia, S.; Bourzami, R.; Ali, M.A. Crystal structure, spectroscopic studies, DFT calculations, cyclic voltammetry and biological activity of a copper (II) Schiff base complex. J. Mol. Struct. 2020, 1203, 127313. [Google Scholar] [CrossRef]
- Salvadeo, E.; Dubois, L.; Latour, J.-M. Trinuclear copper complexes as biological mimics: Ligand designs and reactivities. Coord. Chem. Rev. 2018, 374, 345–375. [Google Scholar] [CrossRef]
- Zhao, Q.-Q.; Wu, X.-H.; Ren, N.; Zhang, J.-J.; Geng, L.-N. Construction of two novel lanthanide complexes supported by 2-bromine-5-methoxybenzoate and 2,2′-bipyridine: Syntheses, crystal structures and thermodynamic properties. J. Chem. Thermodyn. 2017, 113, 124–131. [Google Scholar] [CrossRef]
- Basu, O.; Das, S.K. Supramolecular inorganic chemistry leading to functional materials. J. Chem. Sci. 2020, 132, 46–77. [Google Scholar] [CrossRef]
- Li, S.M.; Zheng, X.J.; Yuan, D.Q.; Ablet, A.; Jin, L.P. In Situ Formed White-Light-Emitting Lanthanide–Zinc–Organic Frameworks. Inorg. Chem. 2012, 51, 1201–1203. [Google Scholar] [CrossRef]
- Tanabe, K.K.; Allen, C.A.; Cohen, S.M. Photochemical Activation of a Metal–Organic Framework to Reveal Functionality. Angew. Chem. Int. Ed. 2010, 49, 9730–9733. [Google Scholar] [CrossRef]
- Feng, R.; Jiang, F.L.; Chen, L.; Yan, C.F.; Wu, M.Y.; Hong, M.C. Exceptionally high H2 storage by a metal–organic polyhedral framework. Chem. Commun. 2009, 9, 5296–5298. [Google Scholar] [CrossRef]
- Wang, H.L.; Qi, D.D.; Xie, Z.; Cao, W.; Wang, K.; Shang, H.; Jiang, J.Z. Synthesis and decarboxylative Wittig reaction of difluoromethylene phosphobetaine. Chem. Commun. 2013, 49, 889–891. [Google Scholar] [CrossRef]
- Rachuri, Y.; Parmar, B.; Bisht, K.K.; Suresh, E. Solvothermal self-assembly of Cd2+ coordination polymers with supramolecular networks involving N-donor ligands and aromatic dicarboxylates: Synthesis, crystal structure and photoluminescence studies. Dalton Trans. 2017, 46, 3623–3630. [Google Scholar] [CrossRef]
- Amiri, M.G.; Morsali, A.; Bigdeli, F. A New Metal–Organic ZnII Supramolecular Assembled via Hydrogen Bonds and N···N Interactions as a New Precursor for Preparation Zinc(II) Oxide Nanoparticles, Thermal, Spectroscopic and Structural Studies. J. Inorg. Organomet. Polym. Mater. 2011, 21, 195–200. [Google Scholar] [CrossRef]
- Cooke, N.H.C.; Viavattene, R.L.; Eksteen, R.; Wong, W.S.; Davies, G.; Karger, B.L. Use of metal ions for selective separations in high-performance liquid chromatography. J. Chromatogr. 1978, 149, 391–415. [Google Scholar] [CrossRef]
- Le Page, J.N.; Lindner, W.; Davies, G.; Seitz, D.E.; Karger, B.L. Resolution of the optical isomers of dansyl amino acids by reversed phase liquid chromatography with optically active metal chelate additives. Anal. Chem. 1979, 51, 433–435. [Google Scholar] [CrossRef]
- Lindner, W. HPLC-Enantiomerentrennung an gebundenen chiralen Phasen. Nat. Schaften 1980, 67, 354–356. [Google Scholar] [CrossRef]
- Uddin, N.; Sirajuddin, M.; Uddin, N.; Ullah, H.; Ali, S.; Tariq, M.; Tirmizi, S.A.; Khan, A.R. Synthesis, spectroscopic characterization, biological screenings, DNA binding study and POM analyses of transition metal carboxylates. Spectrochim. Acta A 2015, 140, 563–574. [Google Scholar] [CrossRef]
- Agarwala, B.V.; Hingorani, M.S. Characteristic IR and Electronic Spectral Studies on Novel Mixed Ligand Complexes of Copper(II) with Thiosemicarbazones and Heterocyclic Bases. Synth. Synth. React. Inorg. Met.-Org. Chem. 1990, 20, 123–132. [Google Scholar]
- Hayashi, M.; Matsushima, S.; Noro, A.; Matsushita, Y. Mechanical Property Enhancement of ABA Block Copolymer-Based Elastomers by Incorporating Transient Cross-Links into Soft Middle Block. Macromolecules 2015, 48, 421–431. [Google Scholar] [CrossRef]
- Chen, Y.N.; Zhao, W.; Zhang, J.C. Preparation of 4-vinylpyridine (4VP) resin and its adsorption performance for heavy metal ions. RSC Adv. 2017, 7, 4226–4236. [Google Scholar] [CrossRef]
- McCurdie, M.P.; Belfiore, L.A. Spectroscopic analysis of transition-metal coordination complexes based on poly(4-vinylpyridine) and dichlorotricarbonylruthenium(II). Polymer 1999, 40, 2889–2902. [Google Scholar] [CrossRef]
- Oconnell, E.M.; Yang, C.Z.; Root, T.W.; Cooper, S.L. Spectroscopic studies of pyridine-containing polyurethanes blended with metal acetates. Macromolecules 1996, 29, 6002–6010. [Google Scholar] [CrossRef]
- Noro, A.; Matsushima, S.; He, X.D.; Hayashi, M.; Matsushita, Y. Thermoreversible Supramolecular Polymer Gels via Metal-Ligand Coordination in an Ionic Liquid. Macromolecules 2013, 46, 8304–8310. [Google Scholar] [CrossRef]
- Martinez-Bulit, P.; Garza-Ortiz, A.; Mijangos, E.; Barron-Sosa, L.; Sanchez-Bartez, F.; Gracia-Mora, I.; Flores-Parra, A.; Contreras, R.; Reedijk, J.; Barba-Behrens, N. 2,6-Bis(2,6-diethylphenyliminomethyl)pyridine coordination compounds with cobalt(II), nickel(II), copper(II), and zinc(II): Synthesis, spectroscopic characterization, X-ray study and in vitro cytotoxicity. J. Inorg. Biochem. 2015, 142, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pierrat, P.; Gros, P.C.; Fort, Y. Solid phase synthesis of pyridine-based derivatives from a 2-chloro-5-bromopyridine scaffold. J. Comb. Chem. 2005, 7, 879–886. [Google Scholar] [CrossRef]
- Schlosser, M.; Mongin, F. Pyridine elaboration through organometallic intermediates: Regiochemical control and completeness. Chem. Soc. Rev. 2007, 36, 1161–1172. [Google Scholar] [CrossRef]
- Asath, R.M.; Premkumar, R.; Mathavan, T.; Benial, A.M.F. Structural, spectroscopic and molecular docking studies on 2-amino-3-chloro-5-trifluoromethyl pyridine: A potential bioactive agent. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 175, 51–60. [Google Scholar] [CrossRef]
- Jose, S.P.; Mohan, S. Vibrational spectra and normal co-ordinate analysis of 2-aminopyridine and 2-amino picoline. Spectrochim Acta A Mol. Biomol. Spectrosc. 2006, 64, 240–245. [Google Scholar] [CrossRef]
- Machura, B.; Nawrot, I.; Michalik, K.B.; Machura, I.; Nawrot, K. Michalik, Novel thiocyanate complexes of cadmium(II)-Synthesis, spectroscopic characterization, X-ray studies and DFT calculations. Polyhedron 2011, 30, 2619–2626. [Google Scholar] [CrossRef]
- Ma, Q.; Zhu, M.L.; Yuan, C.X.; Feng, S.S.; Lu, L.P.; Wang, Q.M. A Molecular Helix: Self-Assembly of Coordination Polymers from d10 Metal Ions and 1,10-Phenanthroline-5,6-dione (pdon) with the Bridges of SCN− and Cl−Anions. Cryst. Growth Des. 2010, 10, 1706–1714. [Google Scholar] [CrossRef]
- Tao, J.; Tong, M.L.; Chen, X.M. Hydrothermal synthesis and crystal structures of three-dimensional coordination frameworks constructed with mixed terephthalate (tp) and 4,4′-bipyridine (4,4′-bipy) ligands: [M(tp)(4,4′-bipy)] (M = CoII, CdII or ZnII). J. Chem. Soc. Dalton Trans. 2000, 20, 3669–3674. [Google Scholar] [CrossRef]
- Lo, S.M.L.; Chui, S.S.Y.; Shek, L.Y.; Lin, Z.Y.; Zhang, X.X.; Wen, G.H.; Williams, I.D. Solvothermal Synthesis of a Stable Coordination Polymer with Copper-I−Copper-II Dimer Units: [Cu4{1,4-C6H4(COO)2}3(4,4‘-bipy)2]n. J. Am. Chem. Soc. 2000, 122, 6293–6294. [Google Scholar] [CrossRef]
- Shi, Z.; Feng, S.H.; Sun, Y.; Hua, J. Novel Coordination Polymers with Mixed Ligands and Orientated Enantiomers. Inorg. Chem. 2001, 40, 5312–5313. [Google Scholar] [CrossRef]
- Konar, S.; Mukherjee, P.S.; Ribas, J.; Drew, M.G.B.; Ribas, J.; Chaudhuri, N.R. Syntheses of Two New 1D and 3D Networks of Cu(II) and Co(II) Using Malonate and Urotropine as Bridging Ligands: Crystal Structures and Magnetic Studies. Inorg. Chem. 2003, 42, 2545–2552. [Google Scholar] [CrossRef]
- Bourosh, P.; Bulhac, I.; Covaci, O.; Zubareva, V.; Mitina, T. Iron(II) Bis-α-Benzyldioximate Complexes with 3- and 4-Pyridine Hemiacetals as Axial Ligands: Synthesis, Structure, and Physicochemical Properties. Russ. J. Coord. Chem. 2018, 44, 507–517. [Google Scholar] [CrossRef]
- Rechitskaya, E.D.; Kuratieva, N.V.; Lider, E.V.; Eremina, J.A.; Klyushova, L.S.; Eltsov, I.V.; Kostin, G.A. Tuning of cytotoxic activity by bio-mimetic ligands in ruthenium nitrosyl complexes. J. Mol. Struct. 2020, 1219, 128565. [Google Scholar] [CrossRef]
- Wang, N.; Sun, X.; Wan, D.; Chen, J.; Li, B. Chloridobis(dimethylglyoximatok2N,N’)(ethyl pyridine-3-carboxylatekN) cobalt(III). Acta Cryst. E 2012, 68, m20. [Google Scholar]
- Yildirim, T.; Köse, D.A.; Avci, G.A.; Þahin, O.; Akkurt, F. Novel coordination compounds: Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) cations with acesulfame/N,N-diethylnicotinamide ligands. J. Coord. Chem. 2019, 72, 3502–3517. [Google Scholar] [CrossRef]
- Bigoli, F.; Braibant, A.; Pellinghelli, M.A.; Tiripicchio, A. The Crystal and Molecular Structure of Diaquobis-(N,N-diethylnicotinamide)-diisothiocyanatozinc. Acta Cryst. B 1973, 29, 2344. [Google Scholar] [CrossRef]
- Cherkasova, T.G.; Pervukhina, N.V.; Kuratieva, N.V.; Cherkasova, E.V.; Isakova, I.V.; Mizinkina, Y.A.; Tatarinova, E.S. Mixed-Ligand Cobalt(II) and Nickel(II) Complexes with N,N,-Diethylpyridine-3-carboxamide: Synthesis and Crystal Structure. Russ. J. Inorg. Chem. 2020, 65, 1160–1165. [Google Scholar] [CrossRef]
- Maa, Z.; Sutradhar, M.; Gurbanov, A.V.; Maharramov, A.M.; Aliyeva, R.A.; Aliyeva, F.S.; Bahmanova, F.N.; Mardanova, V.I.; Chyragov, F.M.; Mahmudov, K.T. CoII, NiII and UO2II complexes with β-diketones and their arylhydrazone derivatives: Synthesis, structure and catalytic activity in Henry reaction. Polyhedron 2015, 101, 14–22. [Google Scholar] [CrossRef]
- Bykov, M.; Emelina, A.; Kiskin, M.; Sidorov, A.; Aleksandrov, G.; Bogomyakov, A.; Dobrokhotova, Z.; Novotortsev, V.; Eremenko, I. Coordination polymer [Li2Co2(Piv)6(l-L)2]n (L = 2-amino-5-methylpyridine) as a new molecular precursor for LiCoO2 cathode material. Polyhedron 2009, 28, 3628–3634. [Google Scholar] [CrossRef]
- LI, G.-S.; ZHANGJ, H.-L. Synthesis, Characterization And Crystal Structure of A Polymeric Silver(I) Complex With Cytotoxic Property. Chil. Chem. Soc. 2015, 60, 2677–2680. [Google Scholar] [CrossRef][Green Version]
- Pakhmutova, E.V.; Malkov, A.E.; Mikhailova, T.B.; Sidorov, A.A.; Fomina, I.G.; Aleksandrov, G.G.; Novotortsev, V.M.; Ikorskii, V.N.; Eremenkoa, I.L. Formation of bi and tetranuclear cobalt(II) trimethylacetate complexes with 2amino5methylpyridine and 2,6diaminopyridine. Russ. Chem. Bullet 2003, 52, 2117–2124. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; ScienceOpen: Burlington, VT, USA, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Hirshfeld, F.L. Bonded-Atom Fragments for Describing Molecular Charge Densities. Theor. Chim. Acta 1977, 44, 129–138. [Google Scholar] [CrossRef]
- Bernstein, J.; Davis, R.E.; Shimoni, L.; Chang, N.-L. Patterns in Hydrogen Bonding: Functionality and Graph Set Analysis in Crystals. Angew. Chemie Int. Ed. Engl. 1995, 34, 1555–1573. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. GAUSSIAN 09; Revision A02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F. NBO, version 3.1; CI; University of Wisconsin: Madison, WI, USA, 1998. [Google Scholar]
- Adamo, C.; Barone, V. Exchange functionals with improved long-range behavior and adiabatic connection methods without adjustable parameters: The mPW and mPW1PW models. J. Chem. Phys. 1998, 108, 664–675. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comp. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: Oxford, UK, 1990. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comp. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Cryst. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Matta, C.F.; Hernandez-Trujillo, J.; Tang, T.-H.; Bader, R.F.W. Hydrogen–Hydrogen Bonding: A Stabilizing Interaction in Molecules and Crystals. Chem. Eur. J. 2003, 9, 1940–1951. [Google Scholar] [CrossRef]
- Grabowski, S.J.; Pfitzner, A.; Zabel, M.; Dubis, A.T.; Palusiak, M. Intramolecular H···H Interactions for the Crystal Structures of [4-((E)-But-1-enyl)-2,6-dimethoxyphenyl]pyridine-3-carboxylate and [4-((E)-Pent-1-enyl)-2,6-dimethoxyphenyl]pyridine-3-carboxylate; DFT Calculations on Modeled Styrene Derivatives. J. Phys. Chem. B 2004, 108, 1831–1837. [Google Scholar] [CrossRef]
- Matta, C.F.; Castillo, N.; Boyd, R.J. Characterization of a Closed-Shell Fluorine−Fluorine Bonding Interaction in Aromatic Compounds on the Basis of the Electron Density. J. Phys. Chem. A 2005, 109, 3669–3681. [Google Scholar] [CrossRef]
- Pendás, A.M.; Francisco, E.; Blanco, M.A.; Gatti, C. Bond Paths as Privileged Exchange Channels. Chem. Eur. J. 2007, 13, 9362–9371. [Google Scholar] [CrossRef]
- Oliveira, B.G.; Pereira, F.S.; de Araujo, R.C.M.C.; Ramos, M.N. The hdyrogen bond strength: New proposals to evaluate the intermolecular interaction using DFT calculations and the AIM theory. Chem. Phys. Lett. 2006, 427, 181–184. [Google Scholar]
- Bader, R.F.W.; Essén, H. The characterization of atomic interactions. J. Chem. Phys. 1984, 80, 1943–1960. [Google Scholar] [CrossRef]
- Bone, R.G.A.; Bader, R.F.W. Identifying and Analyzing Intermolecular Bonding Interactions in van der Waals Molecules. J. Phys. Chem. 1996, 100, 10892–10911. [Google Scholar] [CrossRef]
- Bobrov, M.F.; Popova, G.V.; Tsirelson, V.G. A topological analysis of electron density and chemical bonding in cyclophosphazenes PnNnX2n (X = H, F, Cl; N = 2, 3, 4). Russ. J. Phys. Chem. 2006, 80, 584–590. [Google Scholar] [CrossRef]
- Gatti, C. Chemical bonding in crystals: New directions. Z. Für Krist.-Cryst. Mater. 2005, 220, 399–457. [Google Scholar] [CrossRef]
- Gibbs, G.V.; Downs, R.T.; Cox, D.F.; Ross, N.L.; Boisen, M.B.; Rosso, K.M. Shared and Closed-Shell O−O Interactions in Silicates. J. Phys. Chem. A 2008, 112, 3693–3699. [Google Scholar] [CrossRef]
- Love, I. Polar covalent bonds: An AIM analysis of S,O bonds. J. Phys. Chem. A 2009, 113, 2640–2646. [Google Scholar] [CrossRef] [PubMed]
- Cremer, D.; Kraka, E. Chemical Bonds without Bonding Electron Density—Does the Difference Electron-Density Analysis Suffice for a Description of the Chemical Bond? Angew. Chem. Int. Ed. Engl. 1984, 23, 627–628. [Google Scholar] [CrossRef]
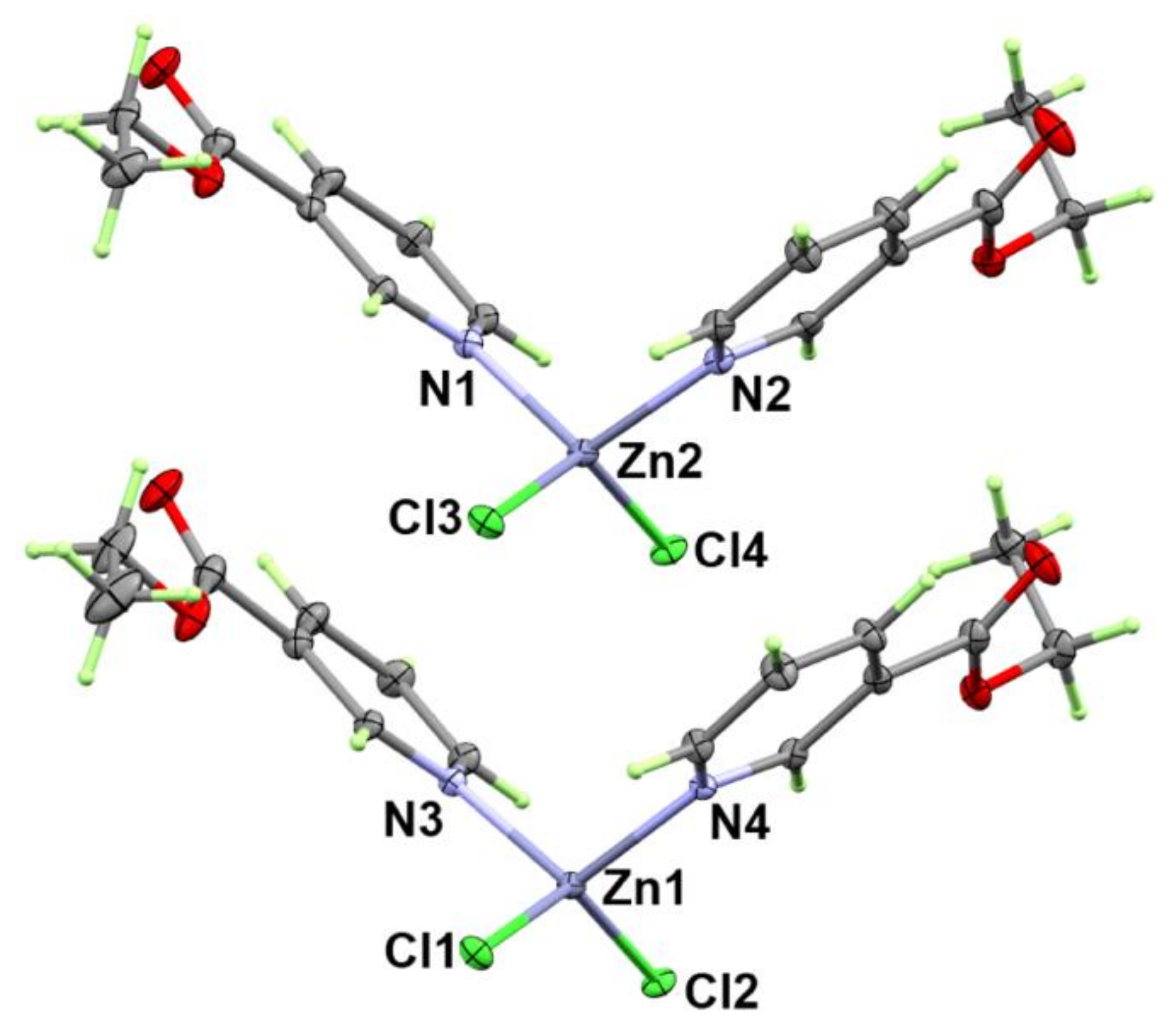

| Bond | Distance | Bond | Distance |
| Zn1-N4 | 2.057(3) | Zn2-N2 | 2.059(3) |
| Zn1-N3 | 2.057(3) | Zn2-N1 | 2.061(3) |
| Zn1-Cl1 | 2.222(1) | Zn2-Cl3 | 2.2221(1) |
| Zn1-Cl2 | 2.224(1) | Zn2-Cl4 | 2.226(1) |
| Bonds | Angle | Bonds | Angle |
| N4-Zn1-N3 | 110.84(13) | N2-Zn2-N1 | 110.68(13) |
| N4-Zn1-Cl1 | 103.87(8) | N2-Zn2-Cl3 | 104.85(8) |
| N3-Zn1-Cl1 | 104.79(9) | N1-Zn2-Cl3 | 105.46(9) |
| N4-Zn1-Cl2 | 103.58(8) | N2-Zn2-Cl4 | 102.29(9) |
| N3-Zn1-Cl2 | 103.84(8) | N1-Zn2-Cl4 | 103.91(8) |
| Cl1-Zn1-Cl2 | 129.46(4) | Cl3-Zn2-Cl4 | 129.13(4) |
| Bond | Distance | Bond | Distance |
| Zn1-O4 | 2.0657(11) | Zn1-O2 | 2.1146(11) |
| Zn1-O3 | 2.0796(11) | Zn1-O9 | 2.1284(10) |
| Zn1-O5 | 2.1012(11) | Zn1-N1 | 2.1388(12) |
| Bonds | Angle | Bonds | Angle |
| O4-Zn1-O3 | 92.35(4) | O5-Zn1-O9 | 83.99(4) |
| O4-Zn1-O5 | 90.56(5) | O2-Zn1-O9 | 92.84(4) |
| O3-Zn1-O5 | 92.27(5) | O4-Zn1-N1 | 92.91(4) |
| O4-Zn1-O2 | 85.66(4) | O3-Zn1-N1 | 89.26(5) |
| O3-Zn1-O2 | 177.53(5) | O5-Zn1-N1 | 176.15(5) |
| O5-Zn1-O2 | 89.20(5) | O2-Zn1-N1 | 89.39(5) |
| O4-Zn1-O9 | 174.37(4) | O9-Zn1-N1 | 92.50(4) |
| O3-Zn1-O9 | 89.28(4) |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) | Symm. Code |
|---|---|---|---|---|---|
| O2-H2A···O9 | 0.86(2) | 1.90(2) | 2.7561(15) | 173(2) | −x,−1/2 + y,1/2 − z |
| O2-H2B···O7 | 0.845(16) | 1.996(17) | 2.7789(14) | 153.7(18) | |
| O3-H3A···O6 | 0.82(2) | 1.93(2) | 2.7429(15) | 176(2) | 1 + x,y,z |
| O3-H3B···O8 | 0.83(2) | 2.00(2) | 2.8216(15) | 170(2) | −x,1/2 + y,1/2 − z |
| O4-H4A···O7 | 0.831(16) | 1.829(16) | 2.6591(15) | 177(2) | 1 + x,y,z |
| O4-H4B···O6 | 0.82(2) | 1.91(2) | 2.7266(14) | 170(3) | −x,−1/2 + y,1/2 − z |
| O5-H5A···O8 | 0.81(2) | 2.32(2) | 2.9958(16) | 141(2) | |
| O5-H5B···O10 | 0.77(3) | 1.92(3) | 2.680(2) | 174(3) | |
| O10-H10A···O1 | 0.77(2) | 1.97(3) | 2.734(3) | 178(3) | 1 − x,−1/2 + y,1/2 − z |
| O10-H10B···O8 | 0.81(3) | 2.09(3) | 2.8874(19) | 169(4) | 1 + x,y,z |
| C1-H1···O7 | 0.95 | 2.54 | 3.3586(18) | 144 | |
| C2-H2···O1 | 0.95 | 2.35 | 3.2622(19) | 160 | −1 + x,y,z |
| C3-H3···O1 | 0.95 | 2.58 | 3.260(2) | 129 | −1/2 + x,3/2 − y,−z |
| C5-H5···O4 | 0.95 | 2.53 | 3.0950(18) | 118 |
| Bond | Distance | Bond | Distance |
| Cd1-N2 | 2.2723(8) | Cd1-O4 | 2.3356(7) |
| Cd1-O1 | 2.2993(7) | Cd1-O3 | 2.3813(8) |
| Cd1-N1 | 2.3210(8) | Cd1-O2 | 2.4484(7) |
| Bonds | Angle | Bonds | Angle |
| N2-Cd1-O1 | 150.61(3) | N1-Cd1-O3 | 150.41(3) |
| N2-Cd1-N1 | 93.39(3) | O4-Cd1-O3 | 55.67(3) |
| O1-Cd1-N1 | 91.69(3) | N2-Cd1-O2 | 96.04(2) |
| N2-Cd1-O4 | 101.74(3) | O1-Cd1-O2 | 55.08(2) |
| O1-Cd1-O4 | 106.61(3) | N1-Cd1-O2 | 108.94(3) |
| N1-Cd1-O4 | 95.09(3) | O4-Cd1-O2 | 149.13(3) |
| N2-Cd1-O3 | 96.88(3) | O3-Cd1-O2 | 97.50(3) |
| O1-Cd1-O3 | 92.77(3) |
| D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) | Symm. Code |
|---|---|---|---|---|---|
| N3-H3A···O4 | 0.845(16) | 2.102(16) | 2.9333(12) | 167.6(16) | |
| N3-H3B···O2 | 0.862(15) | 2.151(15) | 3.0047(11) | 70.9(15) | −1 + x,y,z |
| N4-H4D···O2 | 0.827(17) | 2.188(17) | 3.0046(11) | 169.4(15) | |
| N4-H4E···O1 | 0.839(16) | 2.097(16) | 2.9181(12) | 165.9(15) | 1/2 + x,1/2 − y,1/2 + z |
| C7-H7···O3 | 0.95 | 2.55 | 3.2791(13) | 134 | 2 − x,1 − y,2 − z |
| C8-H8···O1 | 0.95 | 2.56 | 3.3046(13) | 135 | 1/2 + x,1/2 − y,1/2 + z |
| C10-H10C···O4 | 0.98 | 2.56 | 3.4072(14) | 145 | 1 − x,1 − y,2 − z |
| C13-H13···O3 | 0.95 | 2.57 | 3.3091(12) | 135 | −1/2 + x,1/2 − y,1/2 + z |
| Contact | Zn1 Unit | Zn2 Unit |
|---|---|---|
| Zn···N | 0.5 | 0.5 |
| Zn···C | 1.1 | 1.1 |
| Cl···N | 1.5 | 1.5 |
| Cl···O | 1.1 | 1.1 |
| Cl···C | 6.3 | 6.3 |
| Cl···H | 13.6 | 13.8 |
| N···C | 2.3 | 2.3 |
| N···H | 0.8 | 0.7 |
| O···O | 0.2 | 0.2 |
| O···C | 2.2 | 2.2 |
| O···H | 15.6 | 15.5 |
| C···C | 0.4 | 0.4 |
| C···H | 10.6 | 10.3 |
| H···H | 43.8 | 44 |
| Contact | Distance (Å) | Contact | Distance(Å) |
|---|---|---|---|
| O4···H31B | 2.520 | Cl3···C25 | 3.337 |
| O5···H7A | 2.533 | Cl4···C29 | 3.406 |
| Cl4···H29 | 2.799 | Cl4···C17 | 3.264 |
| Cl1···C9 | 3.349 | C16···H19 | 2.734 |
| Cl2···C1 | 3.270 | C24···H11 | 2.604 |
| Cl2···C13 | 3.396 |
| Contact | Distance (Å) | Contact | Distance(Å) |
|---|---|---|---|
| O1…H3 | 2.502 | O7…H4A | 1.677 |
| O1…H10A | 1.751 | O8…H3B | 1.851 |
| O1…H2 | 2.230 | O8…H10B | 1.918 |
| O6…H3A | 1.762 | O9…H2A | 1.779 |
| O6…H4B | 1.754 | O10…H31C | 2.558 |
| Moiety | 1 (Zn1 Unit) | 1 (Zn2 Unit) | Moiety | 2 | Moiety | 3 |
|---|---|---|---|---|---|---|
| Zn | 0.8775 | 0.8773 | Zn | 1.0559 | Cd | 1.2193 |
| 2Cl− | −1.1873 | −1.187 | SO42− | −1.7287 | 2OAc− | −1.4678 a |
| 2(EtNic) | 0.3099 a | 0.3097 | DiEtNA | 0.1307 | 2(2Ampic) | 0.2484 a |
| 4H2O | 0.5421 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altowyan, M.S.; Fathalla, E.M.; Hawas, D.; Albering, J.H.; Barakat, A.; Abu-Youssef, M.A.M.; Soliman, S.M.; Kassem, T.S.; Badr, A.M.A. Synthesis, X-ray, Hirshfeld, and AIM Studies on Zn(II) and Cd(II) Complexes with Pyridine Ligands. Crystals 2022, 12, 590. https://doi.org/10.3390/cryst12050590
Altowyan MS, Fathalla EM, Hawas D, Albering JH, Barakat A, Abu-Youssef MAM, Soliman SM, Kassem TS, Badr AMA. Synthesis, X-ray, Hirshfeld, and AIM Studies on Zn(II) and Cd(II) Complexes with Pyridine Ligands. Crystals. 2022; 12(5):590. https://doi.org/10.3390/cryst12050590
Chicago/Turabian StyleAltowyan, Mezna Saleh, Eman M. Fathalla, Dalia Hawas, Jörg H. Albering, Assem Barakat, Morsy A. M. Abu-Youssef, Saied M. Soliman, Taher S. Kassem, and Ahmed M. A. Badr. 2022. "Synthesis, X-ray, Hirshfeld, and AIM Studies on Zn(II) and Cd(II) Complexes with Pyridine Ligands" Crystals 12, no. 5: 590. https://doi.org/10.3390/cryst12050590
APA StyleAltowyan, M. S., Fathalla, E. M., Hawas, D., Albering, J. H., Barakat, A., Abu-Youssef, M. A. M., Soliman, S. M., Kassem, T. S., & Badr, A. M. A. (2022). Synthesis, X-ray, Hirshfeld, and AIM Studies on Zn(II) and Cd(II) Complexes with Pyridine Ligands. Crystals, 12(5), 590. https://doi.org/10.3390/cryst12050590









