Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces
Abstract
:1. Introduction
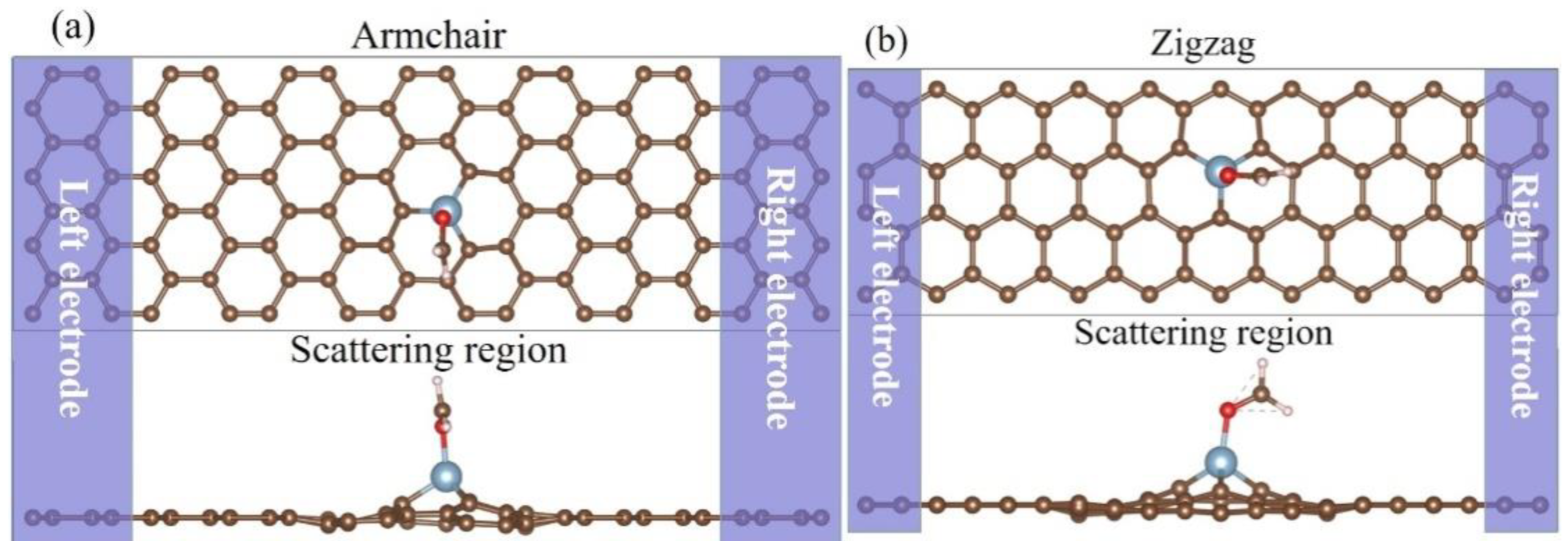
2. Computation and Method Details
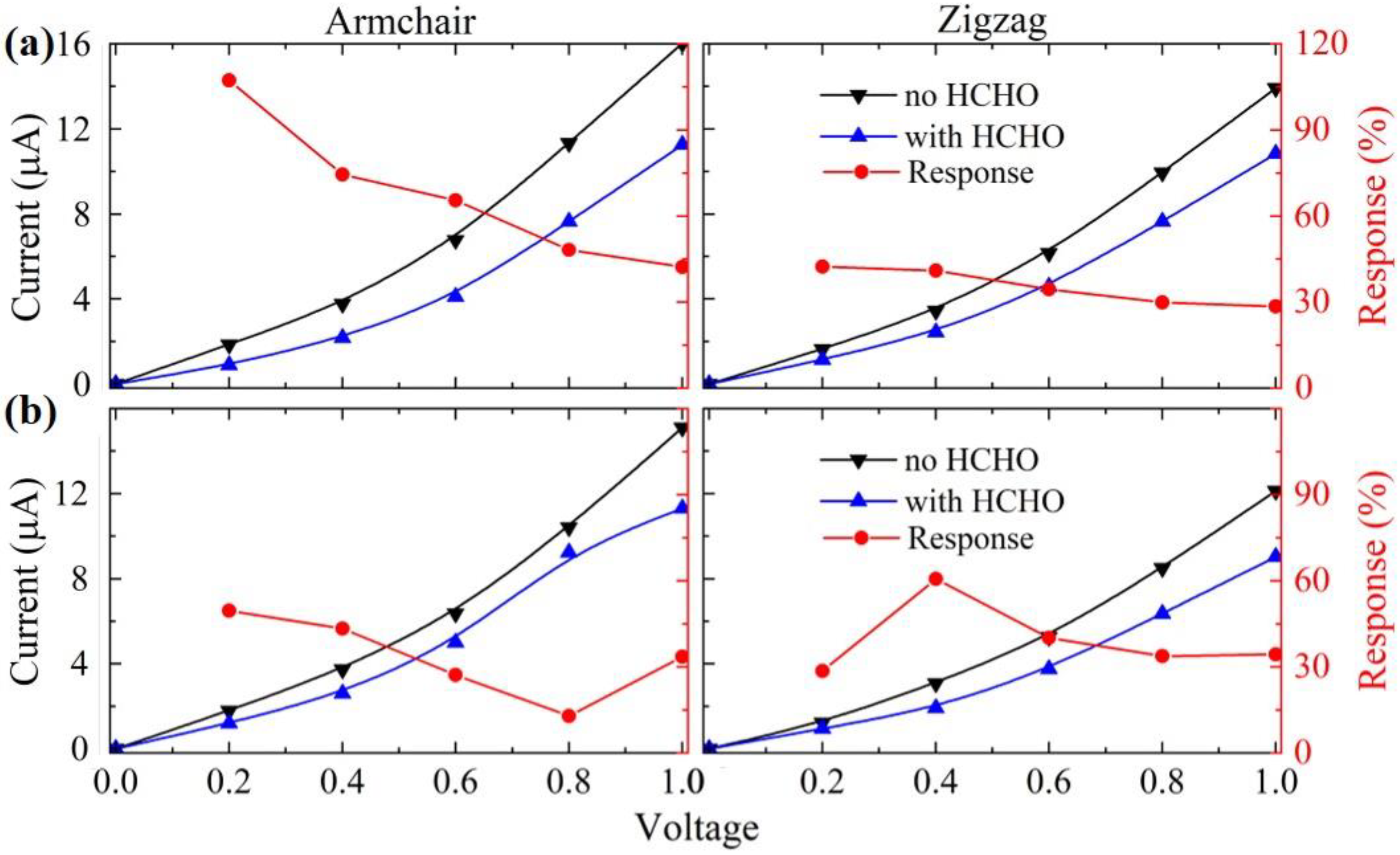
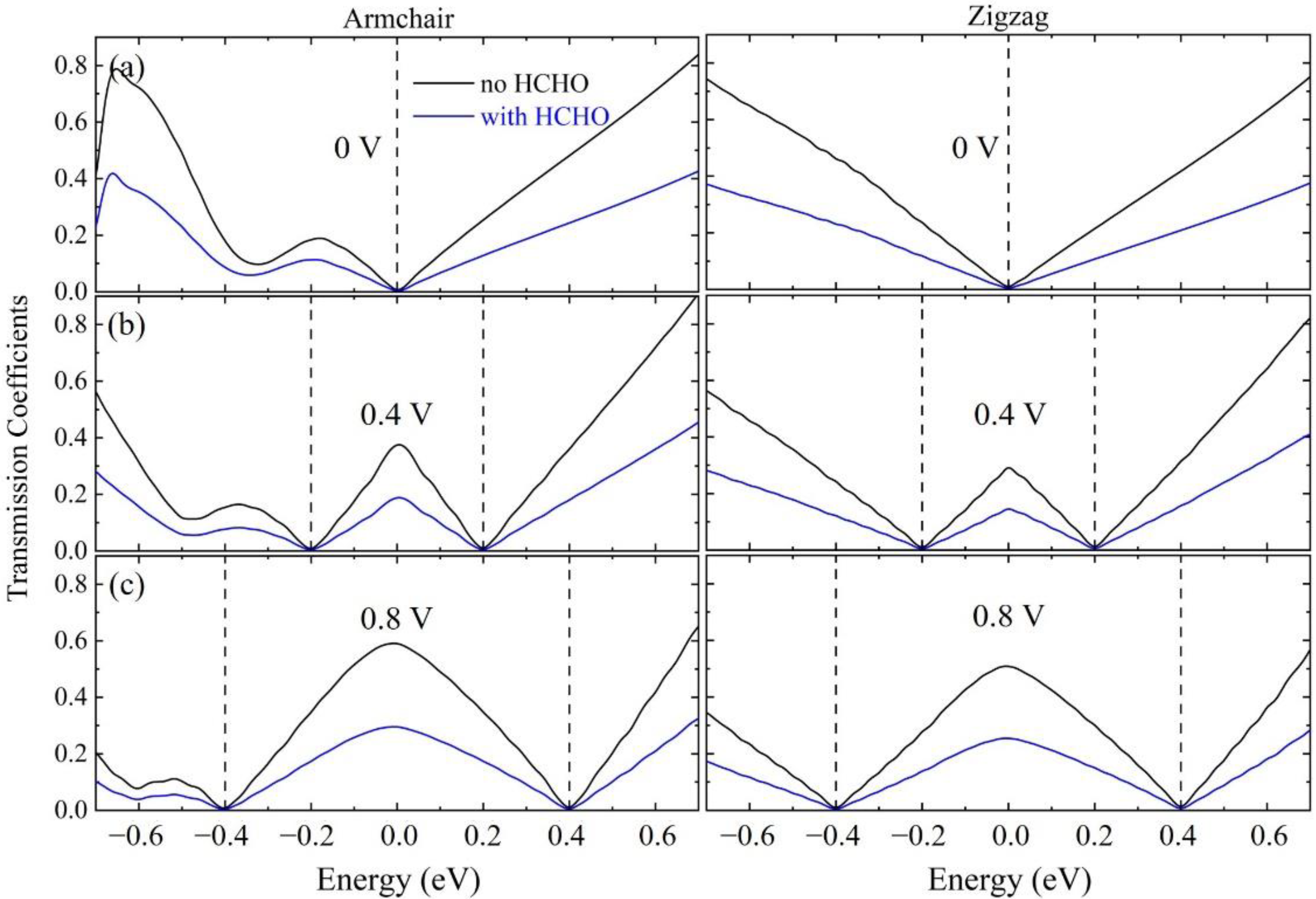
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockcroft, D.W.; Hoeppner, V.H.; Dolovtch, J. Occupational asthma caused by cedar urea formaldehyde particle board. Chest 1982, 82, 49–53. [Google Scholar] [CrossRef]
- Tang, X.; Bai, Y.; Duong, A.; Smith, M.T.; Li, L.; Zhang, L. Formaldehyde in China: Production, consumption, exposure levels, and health effects. Environ. Int. 2009, 35, 1210–1224. [Google Scholar] [CrossRef]
- Korpan, Y.I.; Gonchar, M.V.; Sibirny, A.A.; Martelet, C.; El’skaya, A.V.; Gibson, T.D.; Soldatkin, A.P. Development of highly selective and stable potentiometric sensors for formaldehyde determination. Biosens. Bioelectron. 2000, 15, 77–83. [Google Scholar] [CrossRef]
- Grammer, L.C.; Harris, K.E.; Cugell, D.W.; Patterson, R. Evaluation of a worker with possible formaldehyde-induced asthma. J. Allergy Clin. Immunol. 1993, 92, 29–33. [Google Scholar] [CrossRef]
- Dou, K.; Chen, G.; Yu, F.; Liu, Y.; Chen, L.; Cao, Z.; Chen, T.; Li, Y.; You, J. Bright and sensitive ratiometric fluorescent probe enabling endogenous FA imaging and mechanistic exploration of indirect oxidative damage due to FA in various living systems. Chem. Sci. 2017, 8, 7851–7861. [Google Scholar] [CrossRef] [Green Version]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the indoor environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef]
- Indoor Air Quality Standars. Available online: http://www.gb688.cn/bzgk/gb/newGbInfo?hcno=59392CE8FD4422912D711459014EA4A7 (accessed on 18 March 2022).
- Giovanni, M.; Poh, H.L.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Sanek, F.; Khezri, B.; Webster, R.D.; Pumera, M. Noble metal (Pd, Ru, Rh, Pt, Au, Ag) doped graphene hybrids for electrocatalysis. Nanoscale 2012, 4, 5002–5008. [Google Scholar] [CrossRef]
- Hu, X.; Meng, F. Structure and gap opening of graphene with Fe doped bridged trivacancy. Comput. Mater. Sci. 2016, 117, 65–70. [Google Scholar] [CrossRef]
- Krasheninnikov, A.V.; Lehtinen, P.O.; Foster, A.S.; Pyykko, P.; Nieminen, R.M. Embedding transition-metal atoms in graphene: Structure, bonding, and magnetism. Phys. Rev. Lett. 2009, 102, 126807. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Lu, Y.H.; Cai, Y.Q.; Zhang, C.; Feng, Y.P. Adsorption of gas molecules on transition metal embedded graphene: A search for high-performance graphene-based catalysts and gas sensors. Nanotechnology 2011, 22, 385502. [Google Scholar] [CrossRef]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S: A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Chen, Y.B.; Zhou, K.G.; Liu, C.H.; Zeng, J.; Zhang, H.L.; Peng, Y. Improving gas sensing properties of graphene by introducing dopants and defects: A first-principles study. Nanotechnology 2009, 20, 185504. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Zhang, J.M.; Xu, K.W.; Ji, V. Improving SO2 gas sensing properties of graphene by introducing dopant and defect: A first-principles study. Appl. Surf. Sci. 2014, 313, 405–410. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, J.M. Formaldehyde molecule adsorbed on doped graphene: A first-principles study. Appl. Surf. Sci. 2014, 293, 216–219. [Google Scholar] [CrossRef]
- Lv, R.; Dos Santos, M.C.; Antonelli, C.; Feng, S.; Fujisawa, K.; Berkdemir, A.; Cru-Silva, R.; Elias, A.L.; Perea-Lopez, N.; Lopez-Urias, F.; et al. Large-area Si-doped graphene: Controllable synthesis and enhanced molecular sensing. Adv. Mater. 2014, 26, 7593–7599. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Taylor, J.; Guo, H.; Wang, J. Ab initio modeling of quantum transport properties of molecular electronic devices. Phys. Rev. B 2001, 63, 245407. [Google Scholar] [CrossRef] [Green Version]
- Brandbyge, M.; Mozos, J.L.; Ordejón, P.; Taylor, J.; Stokbro, K. Density-functional method for nonequilibrium electron transport. Phys. Rev. B 2002, 65, 165401. [Google Scholar] [CrossRef] [Green Version]
- Büttiker, M.; Imry, Y.; Landauer, R.; Pinhas, S. Generalized many-channel conductance formula with application to small rings. Phys. Rev. B 1985, 31, 6207–6215. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, Q.; Sun, Q.; Chen, X.S.; Kawazoe, Y.; Jena, P. Ferromagnetism in semihydrogenated graphene sheet. Nano Lett. 2009, 9, 3867–3870. [Google Scholar] [CrossRef]
- Tian, Y.L.; Ren, J.F.; Yue, W.W.; Hu, G.C.; Yuan, X.B. Adsorption of chloroform on N-doped and Al-doped graphene: A first-principle study. Chem. Phys. Lett. 2017, 685, 344–348. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Dai, X.; Yang, Z.; Chen, W.; Ma, D.; Lu, Z. Theoretical study on the Si-doped graphene as an efficient metal-free catalyst for CO oxidation. Appl. Surf. Sci. 2014, 308, 402–407. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Formaldehyde gas sensing properties of transition metal-doped graphene: A first-principles study. J. Mater. Sci. 2021, 56, 12256–12269. [Google Scholar] [CrossRef]
- Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Tunable formaldehyde sensing properties of palladium cluster decorated graphene. RSC Adv. 2021, 11, 37120–37130. [Google Scholar] [CrossRef]
- Chen, X.; Xu, L.; Liu, L.L.; Zhao, L.S.; Chen, C.P.; Zhang, Y.; Wang, X.C. Adsorption of formaldehyde molecule on the pristine and transition metal doped graphene: First-principles study. Appl. Surf. Sci. 2017, 396, 1020–1025. [Google Scholar] [CrossRef]
- Chi, M.; Zhao, Y.P. Adsorption of formaldehyde molecule on the intrinsic and Al-doped graphene: A first principle study. Comput. Mater. Sci. 2009, 46, 1085–1090. [Google Scholar] [CrossRef]
- Topsakal, M.; Bagci, V.M.K.; Ciraci, S. Current-voltage (I−V) characteristics of armchair graphene nanoribbons under uniaxial strain. Phys. Rev. B 2010, 81, 205437. [Google Scholar] [CrossRef] [Green Version]
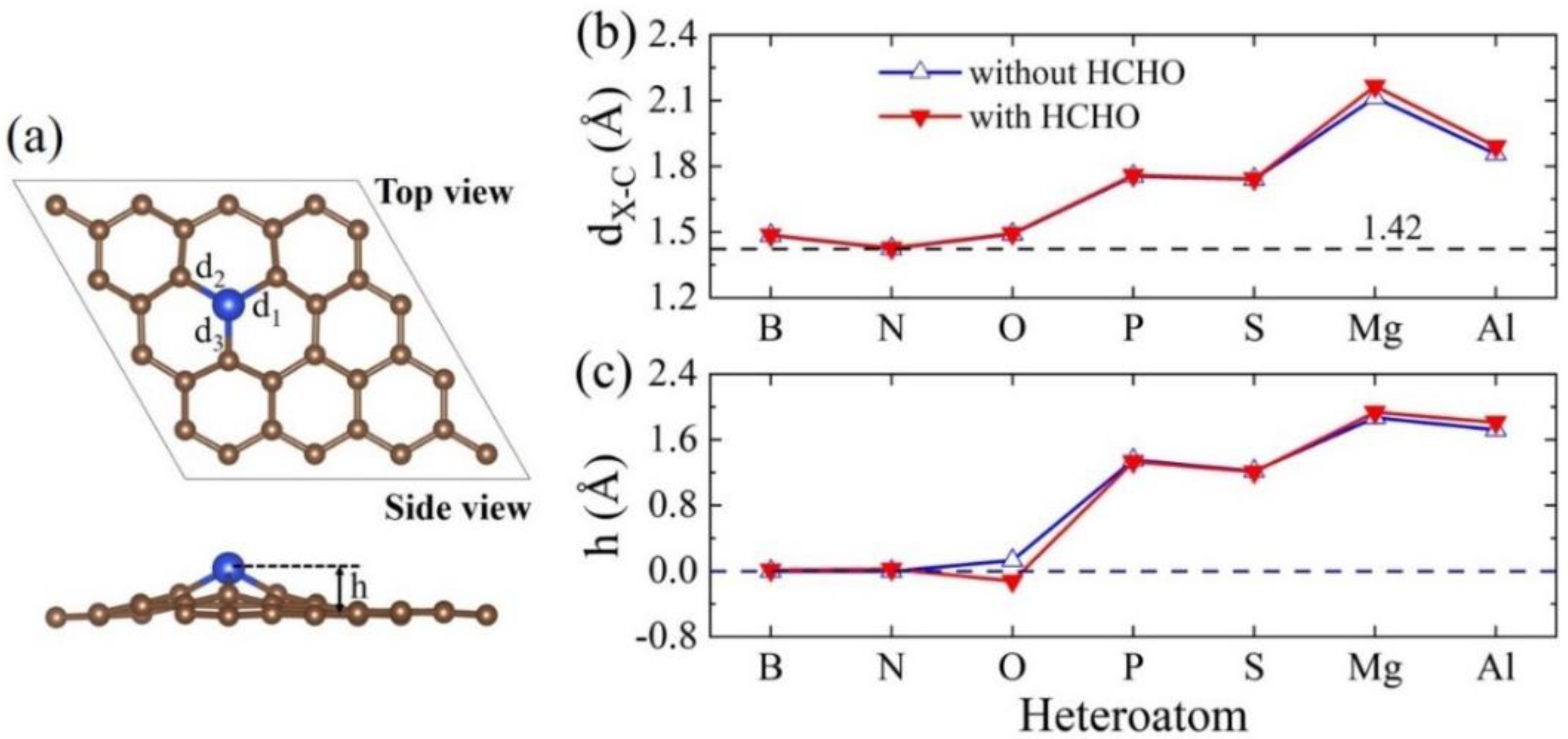
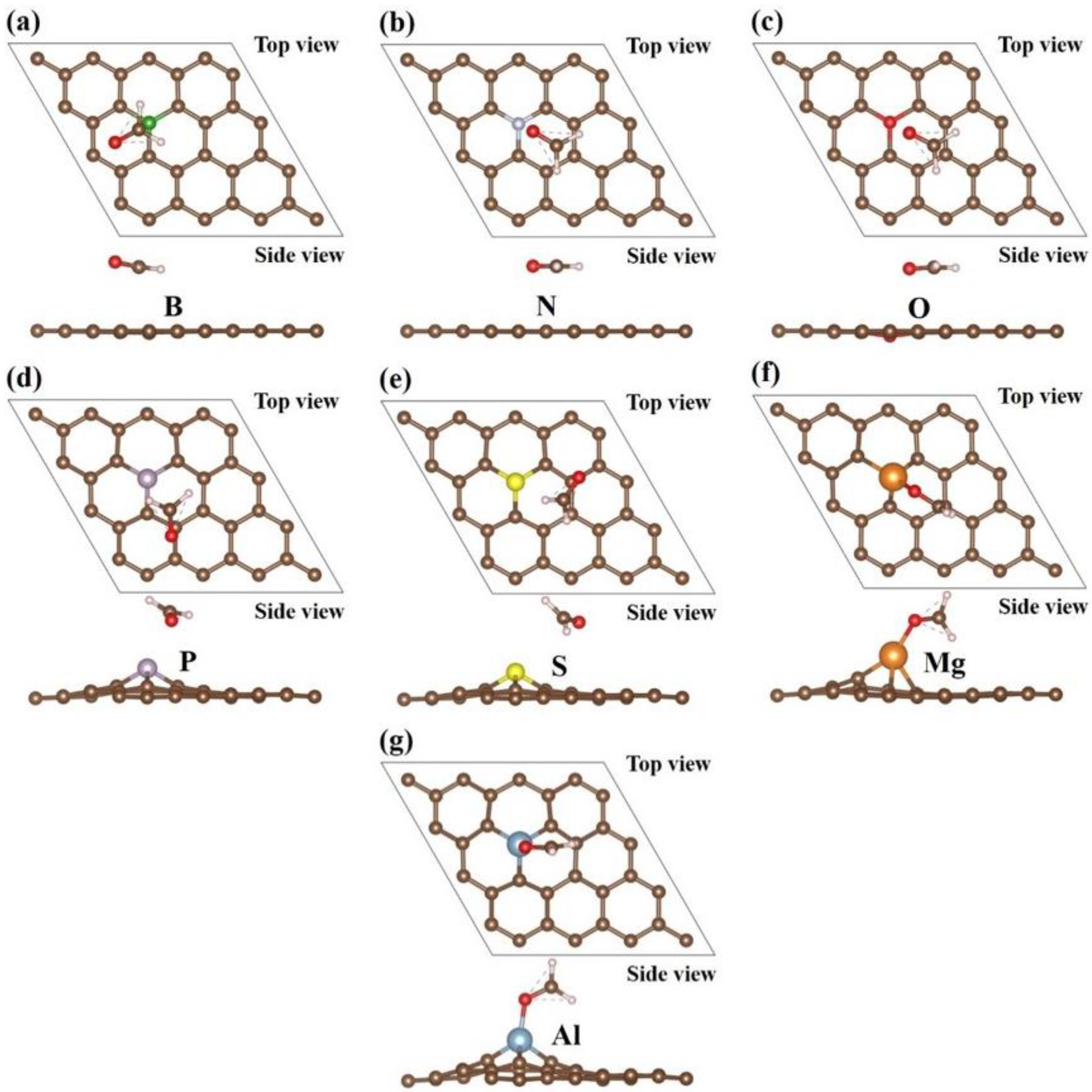
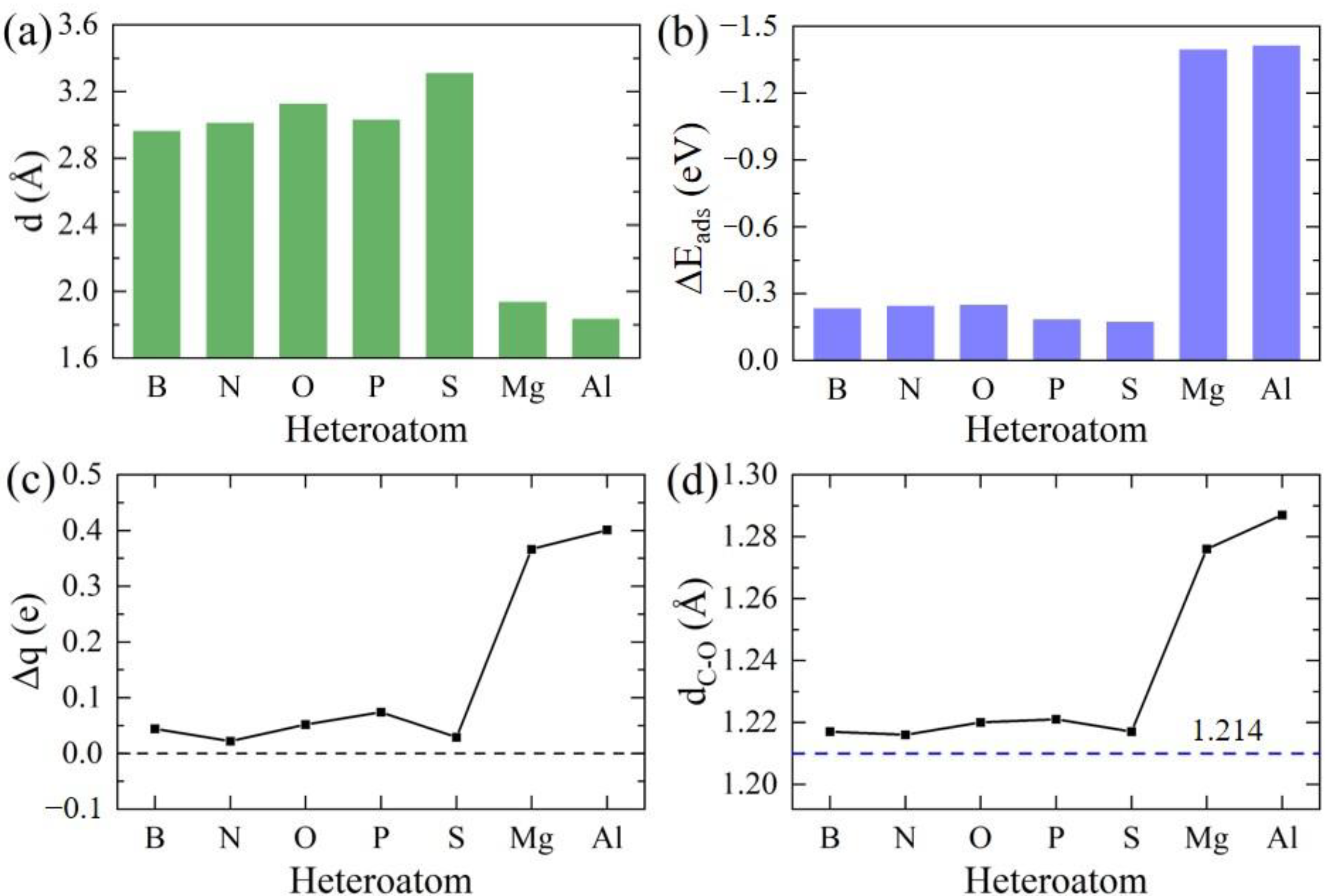
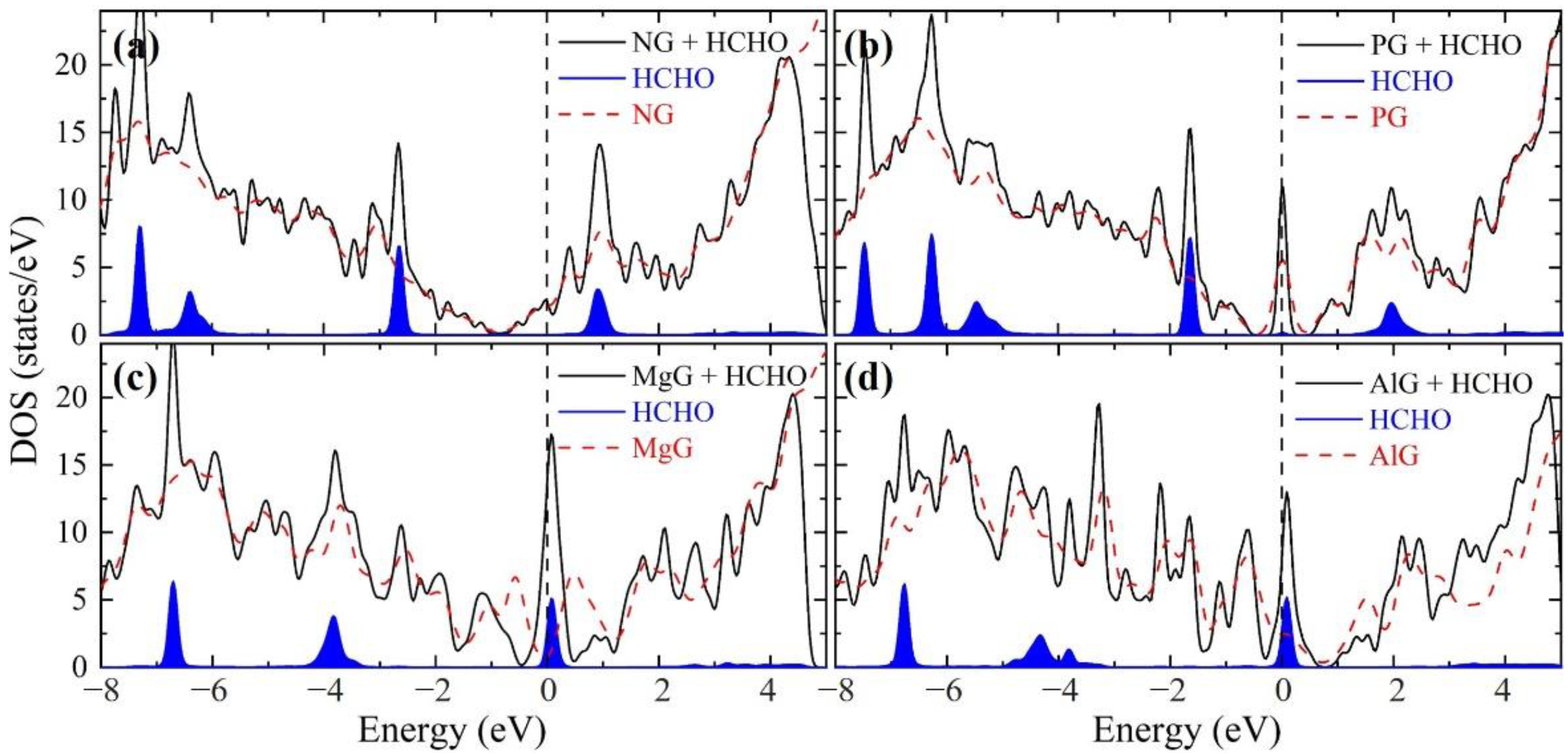

| Heteroatom | B | N | O | P | S | Mg | Al |
|---|---|---|---|---|---|---|---|
| d/Å | 2.946 | 3.011 | 3.125 | 3.029 | 3.311 | 1.944 | 1.838 |
| ∆q/e | 0.044 | 0.022 | 0.052 | 0.074 | 0.029 | 0.366 | 0.401 |
| ∆EZPE/eV | 0.025 | 0.032 | 0.031 | 0.030 | 0.020 | 0.035 | 0.042 |
| ∆Eads/eV | −0.233 | −0.245 | −0.252 | −0.184 | −0.173 | −1.396 | −1.413 |
| dC−O/Å | 1.217 | 1.216 | 1.220 | 1.221 | 1.217 | 1.276 | 1.287 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Xiao, W.; Wang, J.; Li, X.; Wang, L. Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces. Crystals 2022, 12, 553. https://doi.org/10.3390/cryst12040553
Yang L, Xiao W, Wang J, Li X, Wang L. Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces. Crystals. 2022; 12(4):553. https://doi.org/10.3390/cryst12040553
Chicago/Turabian StyleYang, Lunwei, Wei Xiao, Jianwei Wang, Xiaowu Li, and Ligen Wang. 2022. "Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces" Crystals 12, no. 4: 553. https://doi.org/10.3390/cryst12040553
APA StyleYang, L., Xiao, W., Wang, J., Li, X., & Wang, L. (2022). Adsorption and Sensing Properties of Formaldehyde on Chemically Modified Graphene Surfaces. Crystals, 12(4), 553. https://doi.org/10.3390/cryst12040553











