Scintillation Properties of Pr-Doped Lanthanum Pyrosilicate Single Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Sample Conditions
3.2. PL Properties
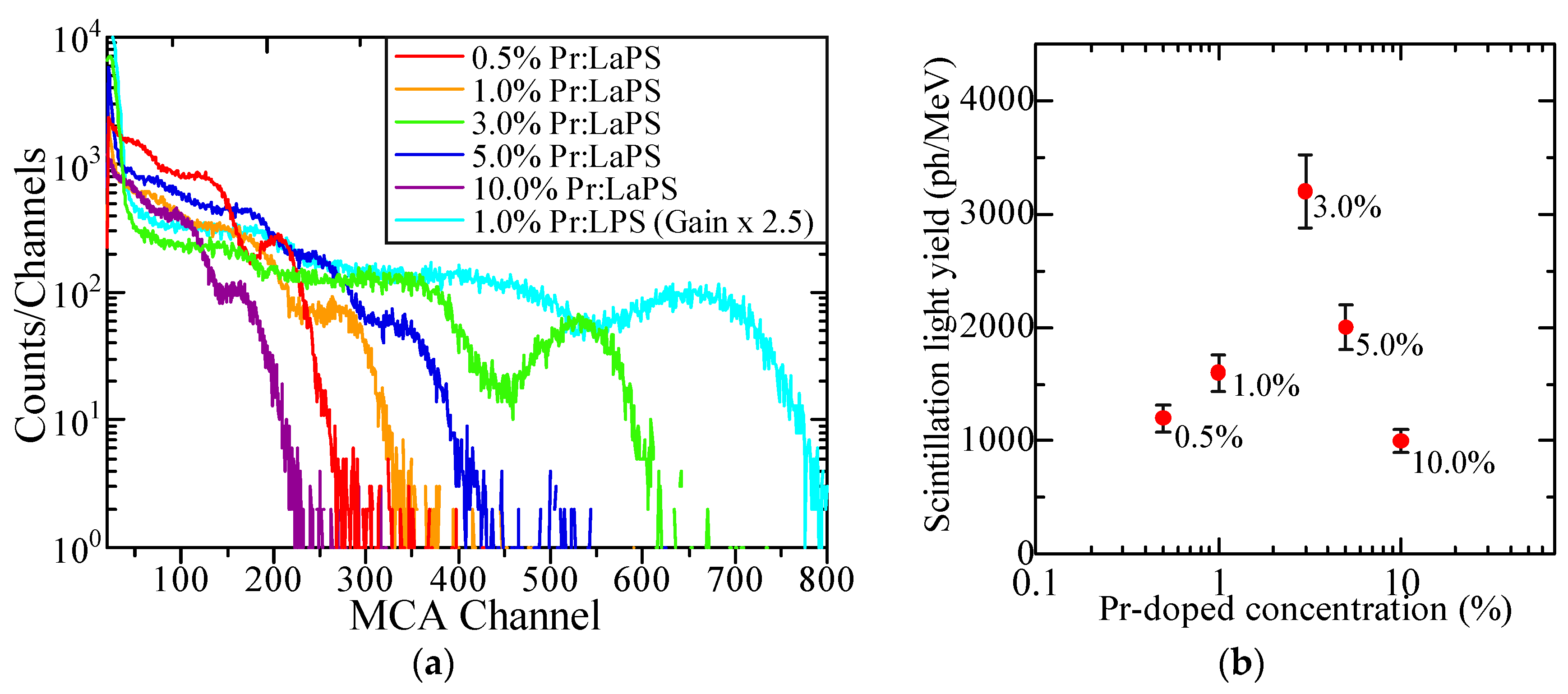
3.3. Scintillation Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yanagida, T. Study of rare-earth-doped scintillators. Opt. Mater. 2013, 35, 1987–1992. [Google Scholar] [CrossRef]
- Van Eijk, C.W.E. Inorganic-scintillator development. Nucl. Instrum. Methods Phys. Res. A 2001, 460, 1–14. [Google Scholar] [CrossRef]
- Koshimizu, M.; Yanagida, T.; Kamishima, R.; Fujimoto, Y.; Asai, K. Scintillation Properties and α-ray Detection Capabilities of Thin-film Plastic Scintillators. Sens. Mater. 2019, 31, 1233. [Google Scholar] [CrossRef]
- Takashima, D.; Ozaki, K.; Nishimura, M.; Okada, N.; Akai, D.; Ishida, M. Enhanced Detection Efficiency of Plastic Scintillators upon Incorporation of Zirconia Nanoparticles. Sens. Mater. 2015, 27, 1. [Google Scholar] [CrossRef]
- Masai, H.; Yanagida, T.; Okada, G.; Koreeda, A.; Ohkubo, T. X-ray-induced luminescence of SnO-SrO-B2O3 glasses prepared under different preparation conditions. Sens. Mater. 2017, 29, 1391–1398. [Google Scholar] [CrossRef][Green Version]
- Limkitjaroenporn, P.; Sangwaranatee, N.; Yonphan, S.; Borisut, P.; Kothan, S.; Wongdamnern, N.; Kim, H.J.; Kaewkhao, J. The radioluminescence investigation of lead sodium borate doped with Sm3+ glass scintillator. Radiat. Phys. Chem. 2022, 192, 109887. [Google Scholar] [CrossRef]
- Shiratori, D.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. X-ray-induced scintillation via energy transfer from Gd3+ to Ce3+ in silicate glasses composed of heavy elements. Sens. Mater. 2020, 32, 1365–1372. [Google Scholar] [CrossRef]
- Kunikata, T.; Kato, T.; Shiratori, D.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Scintillation Properties of Li-doped ZnO Translucent Ceramic. Sens. Mater. 2022, 34, 661. [Google Scholar] [CrossRef]
- Kimura, H.; Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Radiation-induced luminescence properties of SrBr2 transparent ceramics doped with different eu concentrations. Sens. Mater. 2020, 32, 1381–1387. [Google Scholar] [CrossRef]
- Okazaki, K.; Onoda, D.; Nakauchi, D.; Kawano, N.; Fukushima, H.; Kato, T.; Kawaguchi, N.; Yanagida, T. Scintillation Properties of an Organic–Inorganic Lead Iodide Perovskite Single Crystal Having Quantum Well Structures. Sens. Mater. 2022, 34, 575. [Google Scholar] [CrossRef]
- Onoda, D.; Akatsuka, M.; Kawano, N.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Photoluminescence and scintillation properties of (C6H5C2H4NH3)2Pb1−xZnxBr4 as a two-dimensional quantum-confined scintillator. J. Mater. Sci. Mater. Electron. 2020, 31, 20798–20804. [Google Scholar] [CrossRef]
- Fukushima, H.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Photoluminescence and Scintillation Properties of Ce-doped SrHfO3. Sens. Mater. 2019, 31, 1273. [Google Scholar] [CrossRef]
- Kawaguchi, N.; Kimura, H.; Akatsuka, M.; Okada, G.; Kawano, N.; Fukuda, K.; Yanagida, T. Scintillation characteristics of Pr:CaF2 crystals for charged-particle detection. Sens. Mater. 2018, 30, 1585–1590. [Google Scholar] [CrossRef]
- Akatsuka, M.; Daisuke, N.; Takumi, K.; Kawaguchi, N.; Yanagida, T. Scintillation Properties of Nd-doped LuVO4 Single Crystals. Sens. Mater. 2022, 34, 619. [Google Scholar] [CrossRef]
- McConnell, M.L.; Bloser, P.F.; Legere, J.; Ryan, J.M. Applications for New Scintillator Technologies in Gamma Ray Astronomy. J. Phys. Conf. Ser. 2016, 763, 012008. [Google Scholar] [CrossRef]
- Lesparre, N.; Marteau, J.; Déclais, Y.; Gibert, D.; Carlus, B.; Nicollin, F.; Kergosien, B. Design and operation of a field telescope for cosmic ray geophysical tomography. Geosci. Instrum. Methods Data Syst. 2012, 1, 33–42. [Google Scholar] [CrossRef]
- Glodo, J.; Wang, Y.; Shawgo, R.; Brecher, C.; Hawrami, R.H.; Tower, J.; Shah, K.S. New Developments in Scintillators for Security Applications. Phys. Procedia 2017, 90, 285–290. [Google Scholar] [CrossRef]
- Sanada, Y.; Torii, T. Aerial radiation monitoring around the Fukushima Dai-ichi nuclear power plant using an unmanned helicopter. J. Environ. Radioact. 2015, 139, 294–299. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Kurosawa, S.; Kamada, K.; Takahashi, H.; Fukazawa, Y.; Nikl, M.; Chani, V. Temperature dependence of scintillation properties of bright oxide scintillators for well-logging. Jpn. J. Appl. Phys. 2013, 52, 3–9. [Google Scholar] [CrossRef]
- Melcher, C.L. Scintillation Crystals for PET. J. Nucl. Med. 2000, 41, 1051–1055. [Google Scholar]
- Dujardin, C.; Auffray, E.; Bourret-Courchesne, E.; Dorenbos, P.; Lecoq, P.; Nikl, M.; Vasil’ev, A.N.; Yoshikawa, A.; Zhu, R.-Y. Needs, Trends, and Advances in Inorganic Scintillators. IEEE Trans. Nucl. Sci. 2018, 65, 1977–1997. [Google Scholar] [CrossRef]
- Yanagida, T.; Yoshikawa, A.; Yokota, Y.; Kamada, K.; Usuki, Y.; Yamamoto, S.; Miyake, M.; Baba, M.; Kumagai, K.; Sasaki, K.; et al. Development of Pr:LuAG Scintillator Array and Assembly for Positron Emission Mammography. IEEE Trans. Nucl. Sci. 2010, 57, 1492–1495. [Google Scholar] [CrossRef]
- Pidol, L.; Khan-Harari, A.; Viana, B.; Ferrand, B.; Dorenbos, P.; De Haas, J.T.M.; Van Eijk, C.W.E.; Virey, E. Scintillation properties of Lu2Si2O7:Ce3+, a fast and efficient scintillator crystal. J. Phys. Condens. Matter 2003, 15, 2091–2102. [Google Scholar] [CrossRef]
- Kantuptim, P.; Akatsuka, M.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Scintillation properties of Pr-doped Lu2Si2O7 single crystal. Radiat. Meas. 2020, 134, 106320. [Google Scholar] [CrossRef]
- Kantuptim, P.; Akatsuka, M.; Kawaguchi, N.; Yanagida, T. Optical and scintillation properties of Pr-doped Y2Si2O7 single crystal. Jpn. J. Appl. Phys. 2020, 59, SCCB17. [Google Scholar] [CrossRef]
- Kantuptim, P.; Akatsuka, M.; Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Scintillation Characteristics of Pr-doped Gd2Si2O7 Single Crystal. Sens. Mater. 2020, 32, 1357–1364. [Google Scholar] [CrossRef]
- Kantuptim, P.; Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Ce concentration dependence of optical and scintillation properties on Ce-doped La2Si2O7 crystal. Jpn. J. Appl. Phys. 2022, 61, SB1038. [Google Scholar] [CrossRef]
- Yanagida, T.; Kamada, K.; Fujimoto, Y.; Yagi, H.; Yanagitani, T. Comparative study of ceramic and single crystal Ce:GAGG scintillator. Opt. Mater. 2013, 35, 2480–2485. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Ito, T.; Uchiyama, K.; Mori, K. Development of X-ray-induced afterglow characterization system. Appl. Phys. Express 2014, 7, 18–21. [Google Scholar] [CrossRef]
- Yanagida, T.; Kawaguchi, N.; Fujimoto, Y.; Fukuda, K.; Watanabe, K.; Yamazaki, A.; Uritani, A. Scintillation properties of LiF-SrF2 and LiF-CaF2 eutectic. J. Lumin. 2013, 144, 212–216. [Google Scholar] [CrossRef]
- Yanagida, T.; Watanabe, K.; Okada, G.; Kawaguchi, N. Optical, scintillation and radiation tolerance properties of Pr-doped pyrosilicate crystals. Jpn. J. Appl. Phys. 2018, 57, 106401. [Google Scholar] [CrossRef]
- Kobayashi, K.; Sakka, Y. Rudimental research progress of rare-earth silicate oxyapatites: Their identification as a new compound until discovery of their oxygen ion conductivity. J. Ceram. Soc. Jpn. 2014, 122, 649–663. [Google Scholar] [CrossRef]
- Gorbenko, V.; Zorenko, Y.; Savchyn, V.; Zorenko, T.; Pedan, A.; Shkliarskyi, V. Growth and luminescence properties of Pr3+-doped single crystalline films of garnets and perovskites. Radiat. Meas. 2010, 45, 461–464. [Google Scholar] [CrossRef]
- Nakauchi, D.; Okada, G.; Koshimizu, M.; Yanagida, T. Scintillation and thermally-stimulated luminescence properties of Pr-doped SrAl2O4 single crystals. Radiat. Meas. 2017, 106, 170–174. [Google Scholar] [CrossRef]
- Yanagida, T.; Fujimoto, Y.; Kamada, K.; Totsuka, D.; Yagi, H.; Yanagitani, T.; Futami, Y.; Yanagida, S.; Kurosawa, S.; Yokota, Y.; et al. Scintillation properties of transparent ceramic pr:LuAG for different pr concentration. IEEE Trans. Nucl. Sci. 2012, 59, 2146–2151. [Google Scholar] [CrossRef]
- Kantuptim, P.; Fukushima, H.; Kimura, H.; Nakauchi, D.; Kato, T.; Koshimizu, M.; Kawaguchi, N.; Yanagida, T. VUV- and X-ray-induced Properties of Lu2Si2O7, Y2Si2O7, and Gd2Si2O7 Single Crystals. Sens. Mater. 2021, 33, 2195. [Google Scholar] [CrossRef]
- Fidelus, J.D.; Yatsunenko, S.; Godlewski, M.; Paszkowicz, W.; Werner-Malento, E.; Łojkowski, W. Relation between structural properties of Pr3+-doped yttria-stabilized zirconia nanopowders and their luminescence efficiency. Scr. Mater. 2009, 61, 415–418. [Google Scholar] [CrossRef]
- Melcher, C.L.; Schweitzer, J.S. A promising new scintillator: Cerium-doped lutetium oxyorthosilicate. Nucl. Instrum. Methods Phys. Res. A 1992, 314, 212–214. [Google Scholar] [CrossRef]
- Takagi, K.; Fukazawa, T. Cerium-activated Gd2 SiO5 single crystal scintillator. Appl. Phys. Lett. 1983, 42, 43–45. [Google Scholar] [CrossRef]
- Nagornaya, L.; Onyshchenko, G.; Pirogov, E.; Starzhinskiy, N.; Tupitsyna, I.; Ryzhikov, V.; Galich, Y.; Vostretsov, Y.; Galkin, S.; Voronkin, E. Production of the high-quality CdWO4 single crystals for application in CT and radiometric monitoring. Nucl. Instrum. Methods Phys. Res. A 2005, 537, 163–167. [Google Scholar] [CrossRef]
- Nakauchi, D.; Kato, T.; Kawaguchi, N.; Yanagida, T. Characterization of Eu-doped Ba2SiO4, a high light yield scintillator. Appl. Phys. Express 2020, 13, 2–5. [Google Scholar] [CrossRef]


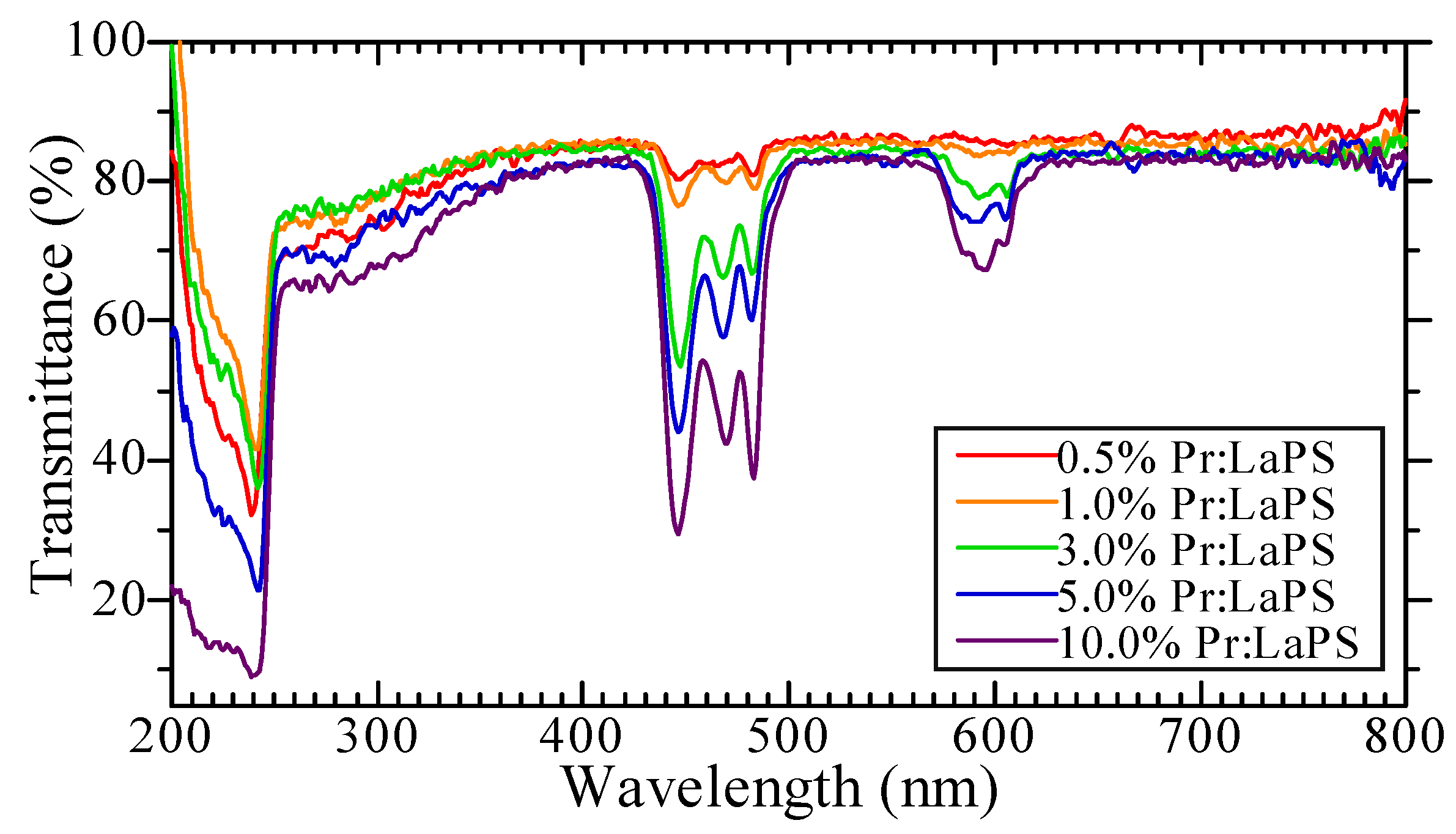
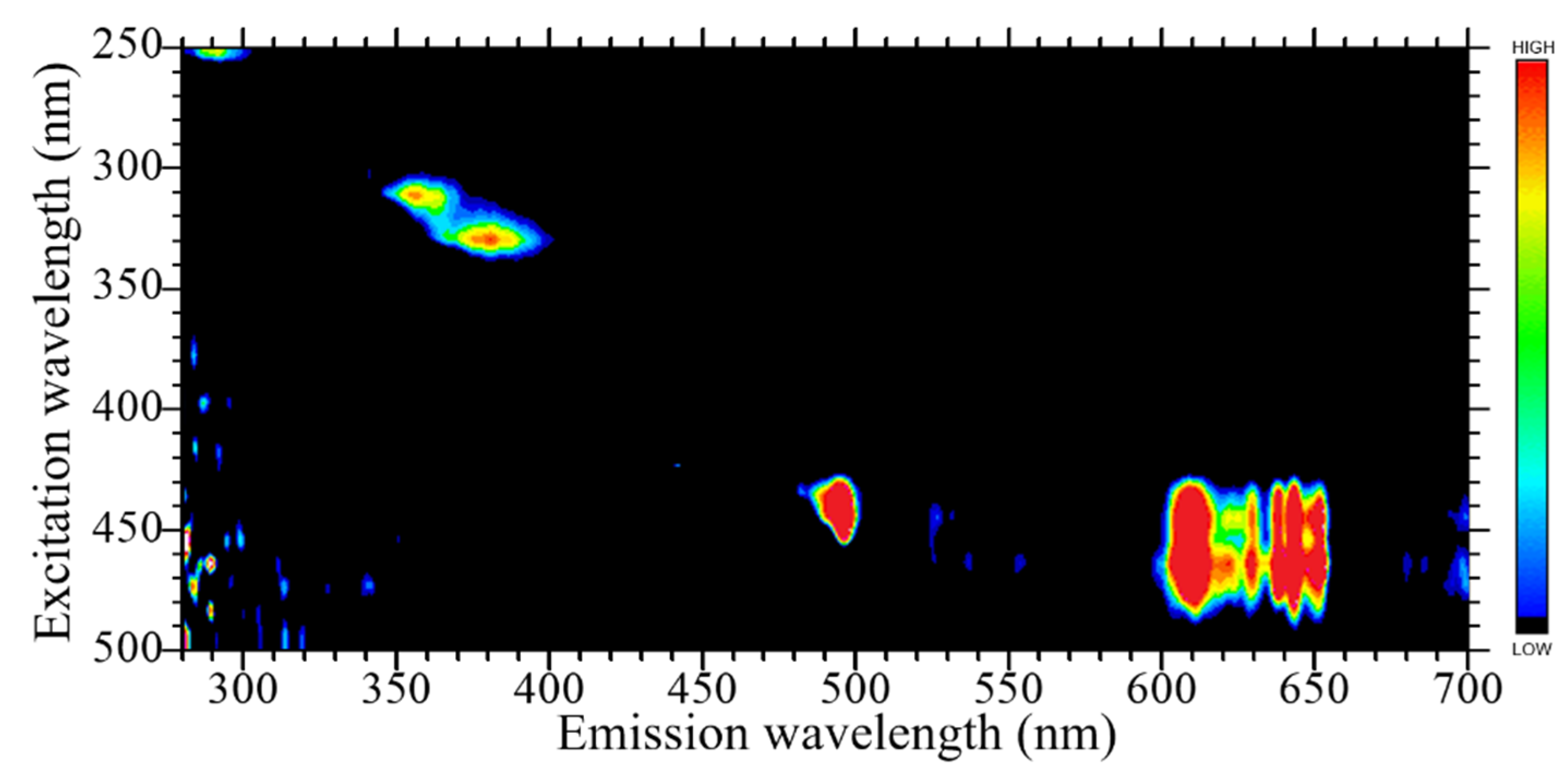

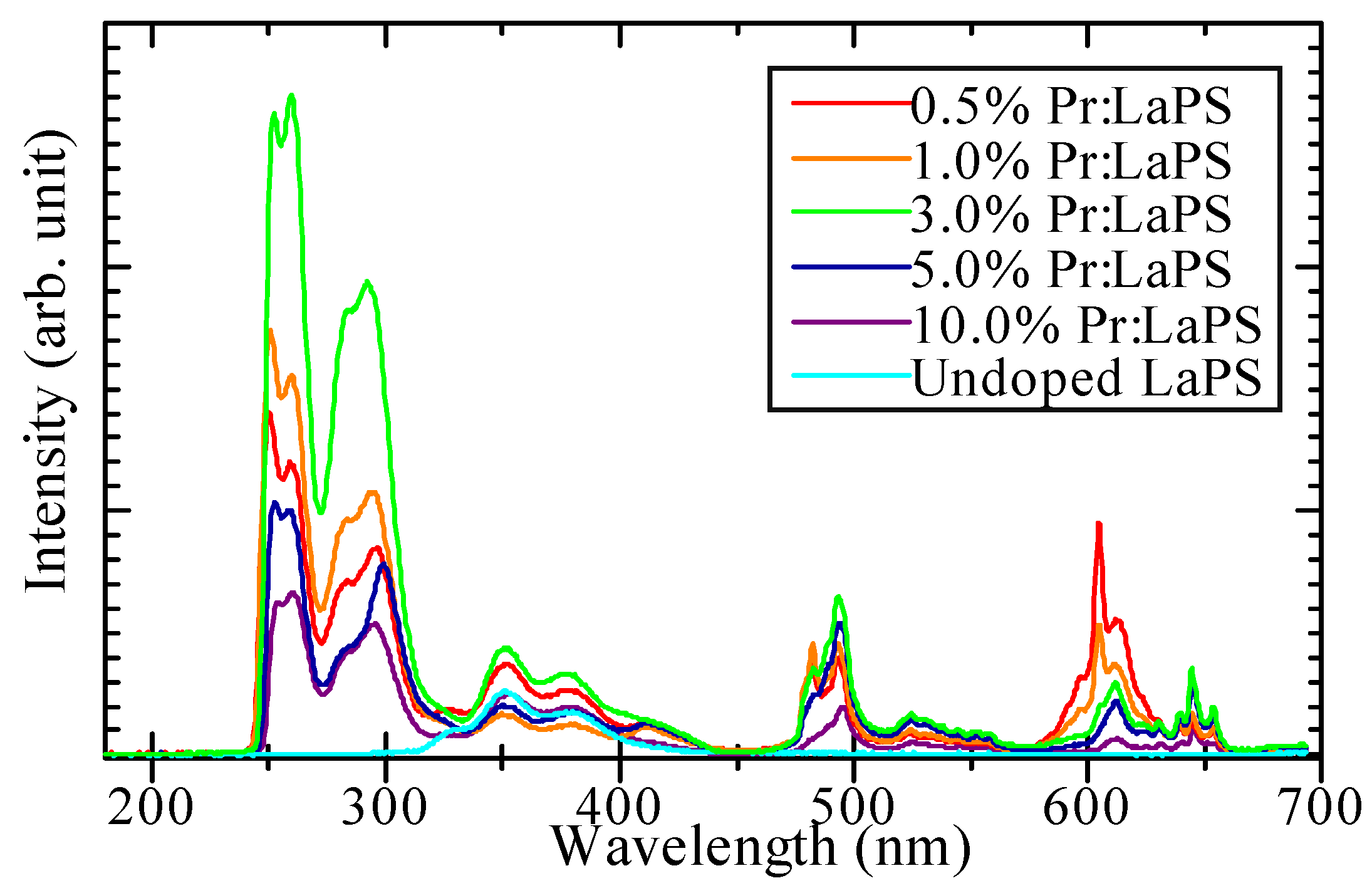

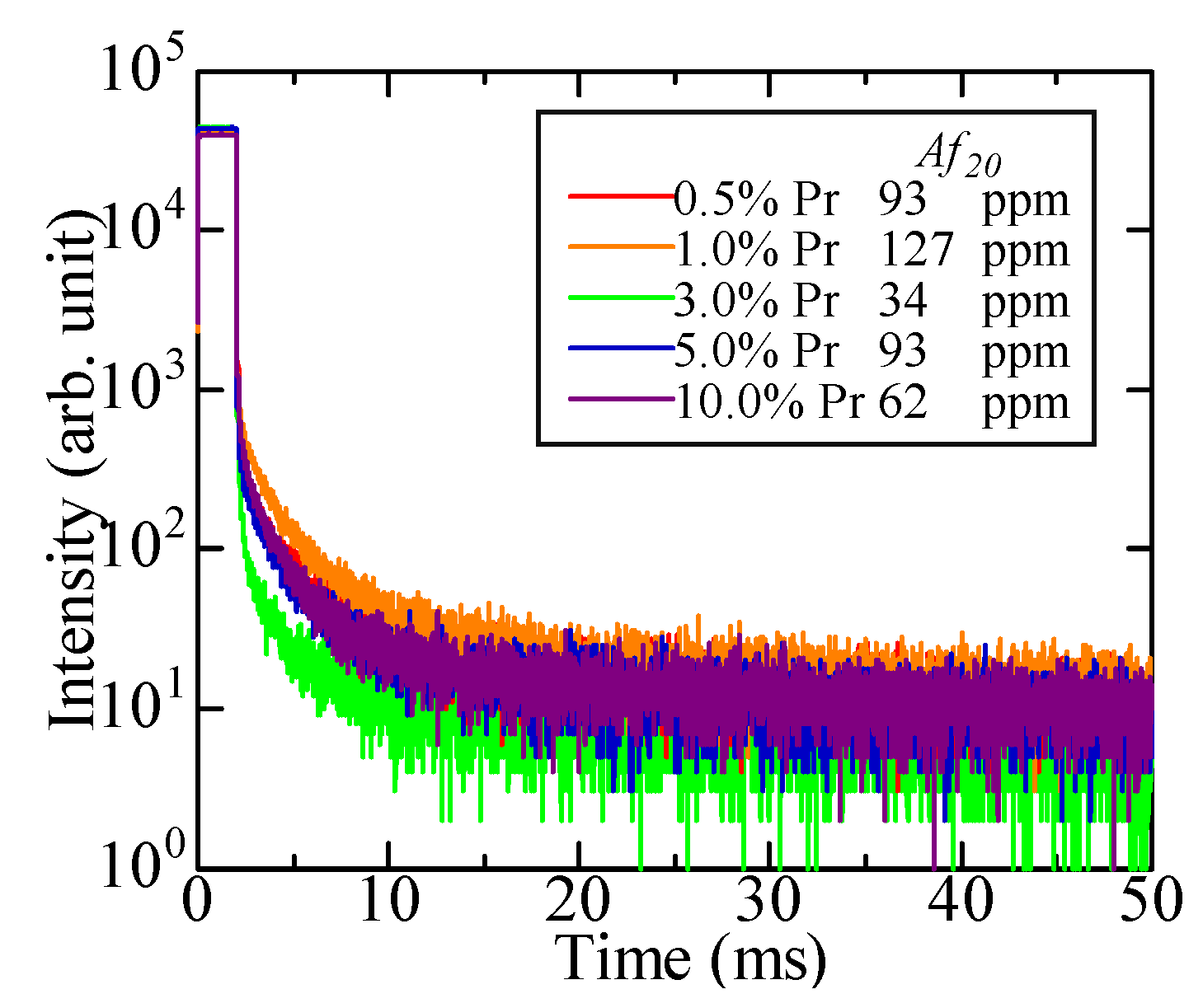
| Sample | Density (g/cm3) | Zeff | PL Emission Wavelength (nm) | PL Decay Time (ns) | Scintillation Wavelength (nm) | Scintillation Decay Time (ns) | Af20 (ppm) | 662 keV γ-ray Scintillation Light Yield (ph/MeV) |
|---|---|---|---|---|---|---|---|---|
| 0.5% Pr-doped LaPS | 4.64 | 50.6 | 280–310 (5d–4f), 350–400 (STE), 490–500 + 600–660 (4f–4f) | 18.1 | 250–310 (5d–4f), 350–400 (STE), 480–500 + 600–650 (4f–4f) | 26.7, 419.6 | 93 | 1200 |
| 1.0% Pr-doped LaPS | 4.56 | 17.9 | 25.7, 532.5 | 127 | 1600 | |||
| 3.0% Pr-doped LaPS | 4.43 | 19.2 | 27.4, 201.6 | 34 | 3200 | |||
| 5.0% Pr-doped LaPS | 4.68 | 19.0 | 25.4, 177.2 | 93 | 2000 | |||
| 10.0% Pr-doped LaPS | 4.74 | 19.8 | 25.5, 190.2 | 62 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kantuptim, P.; Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Scintillation Properties of Pr-Doped Lanthanum Pyrosilicate Single Crystals. Crystals 2022, 12, 459. https://doi.org/10.3390/cryst12040459
Kantuptim P, Kato T, Nakauchi D, Kawaguchi N, Yanagida T. Scintillation Properties of Pr-Doped Lanthanum Pyrosilicate Single Crystals. Crystals. 2022; 12(4):459. https://doi.org/10.3390/cryst12040459
Chicago/Turabian StyleKantuptim, Prom, Takumi Kato, Daisuke Nakauchi, Noriaki Kawaguchi, and Takayuki Yanagida. 2022. "Scintillation Properties of Pr-Doped Lanthanum Pyrosilicate Single Crystals" Crystals 12, no. 4: 459. https://doi.org/10.3390/cryst12040459
APA StyleKantuptim, P., Kato, T., Nakauchi, D., Kawaguchi, N., & Yanagida, T. (2022). Scintillation Properties of Pr-Doped Lanthanum Pyrosilicate Single Crystals. Crystals, 12(4), 459. https://doi.org/10.3390/cryst12040459







