RETRACTED: Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Sampling
2.2. Plant Material and Chemical Analysis
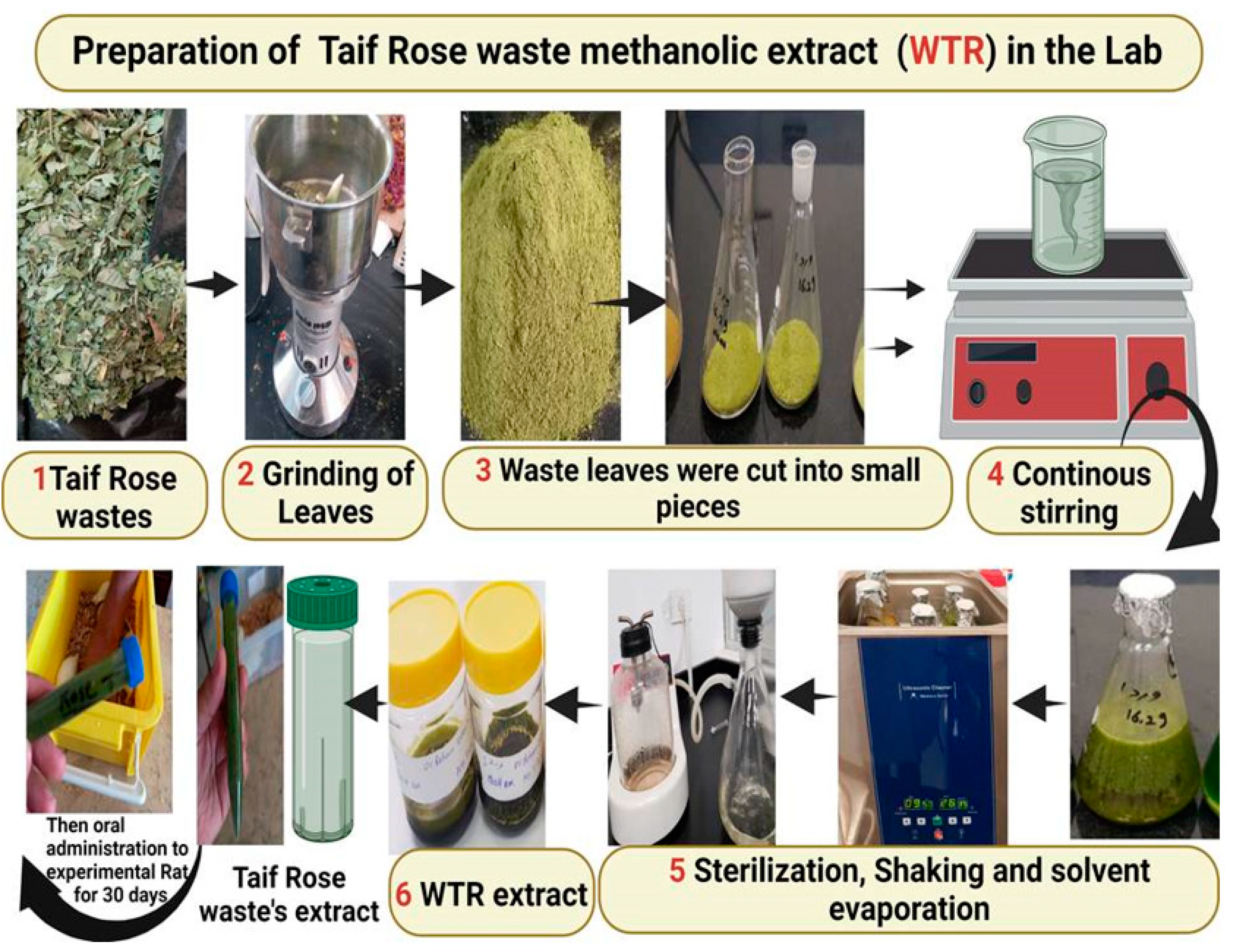
2.3. Extraction of WTR and Determination of Its Active Components
2.4. GC–MS Analysis of Concrete and Absolute Rose Oil
2.5. Cytotoxic Activity (IC50 Determination)
2.6. Cell Counting
2.7. Cell Culture
2.7.1. Cytotoxicity Assay
2.7.2. Analysis of Cell Cycle Distribution
2.7.3. Apoptosis Assay
2.7.4. Autophagy Assay
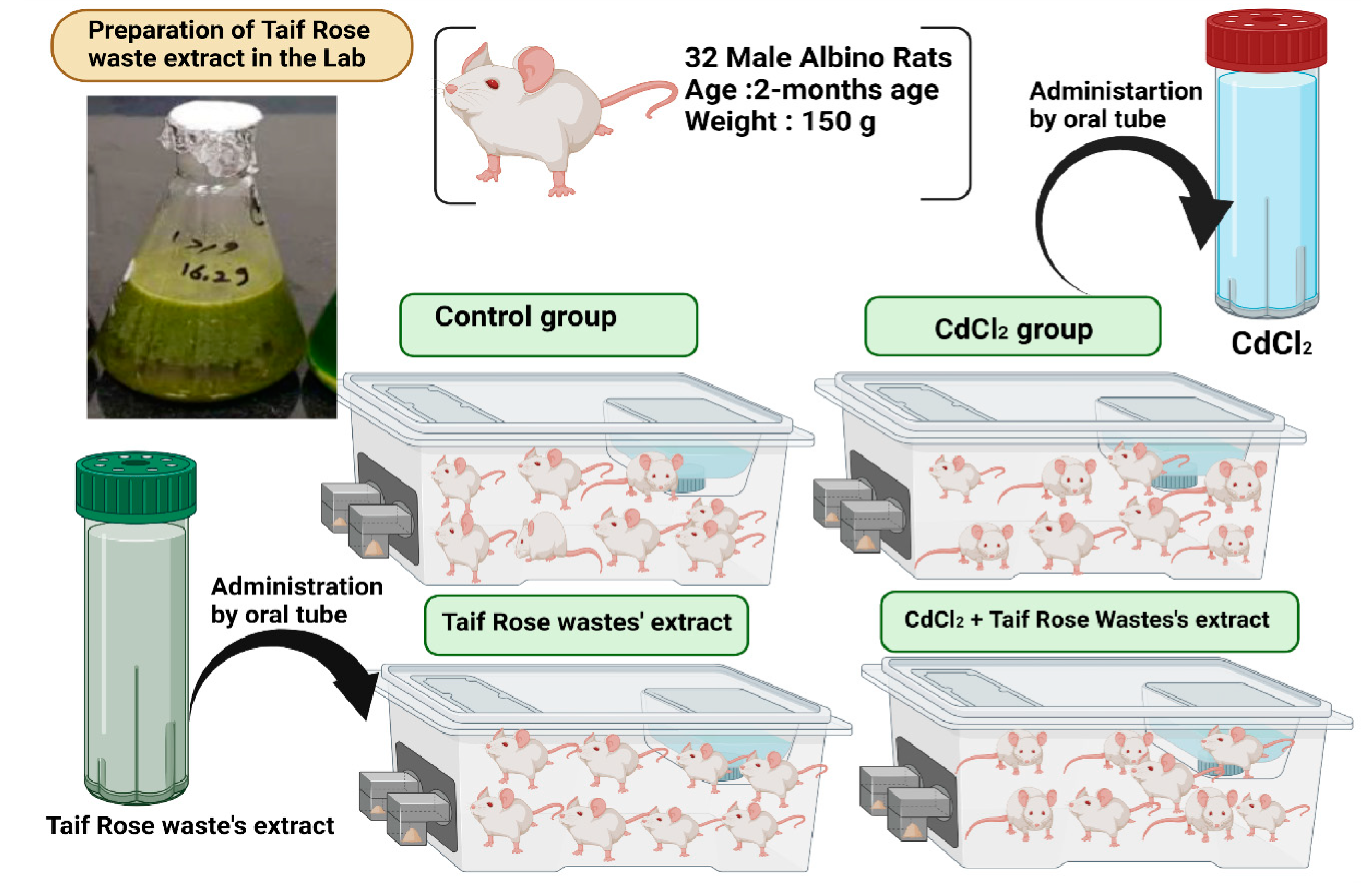
2.8. Animal Model
2.8.1. Experimental Design
2.8.2. Blood Samples
2.8.3. Hepatic Function Activities and Biomarkers
2.8.4. Preparation of Tissue Homogenates for the Determination of the Redox State
2.8.5. Determination of Oxidative Stress Biomarker Activities in Hepatic Tissues
2.8.6. Histological Changes and TEM Estimation
2.9. Statistical Analysis
3. Results
3.1. GC–MS Analysis of WTR Extract
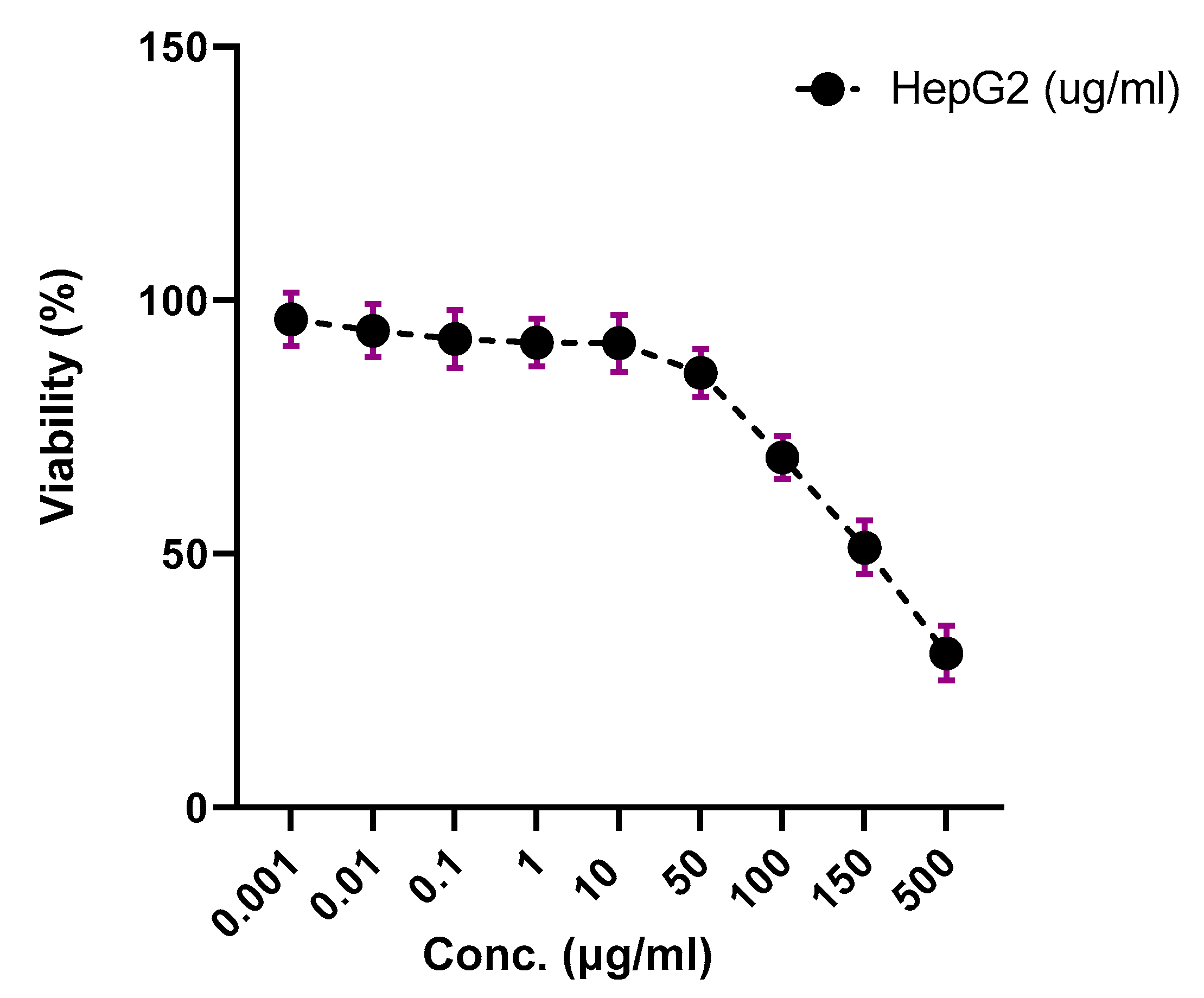
3.2. Screeningof Cytotoxic Activity of WTR Extract
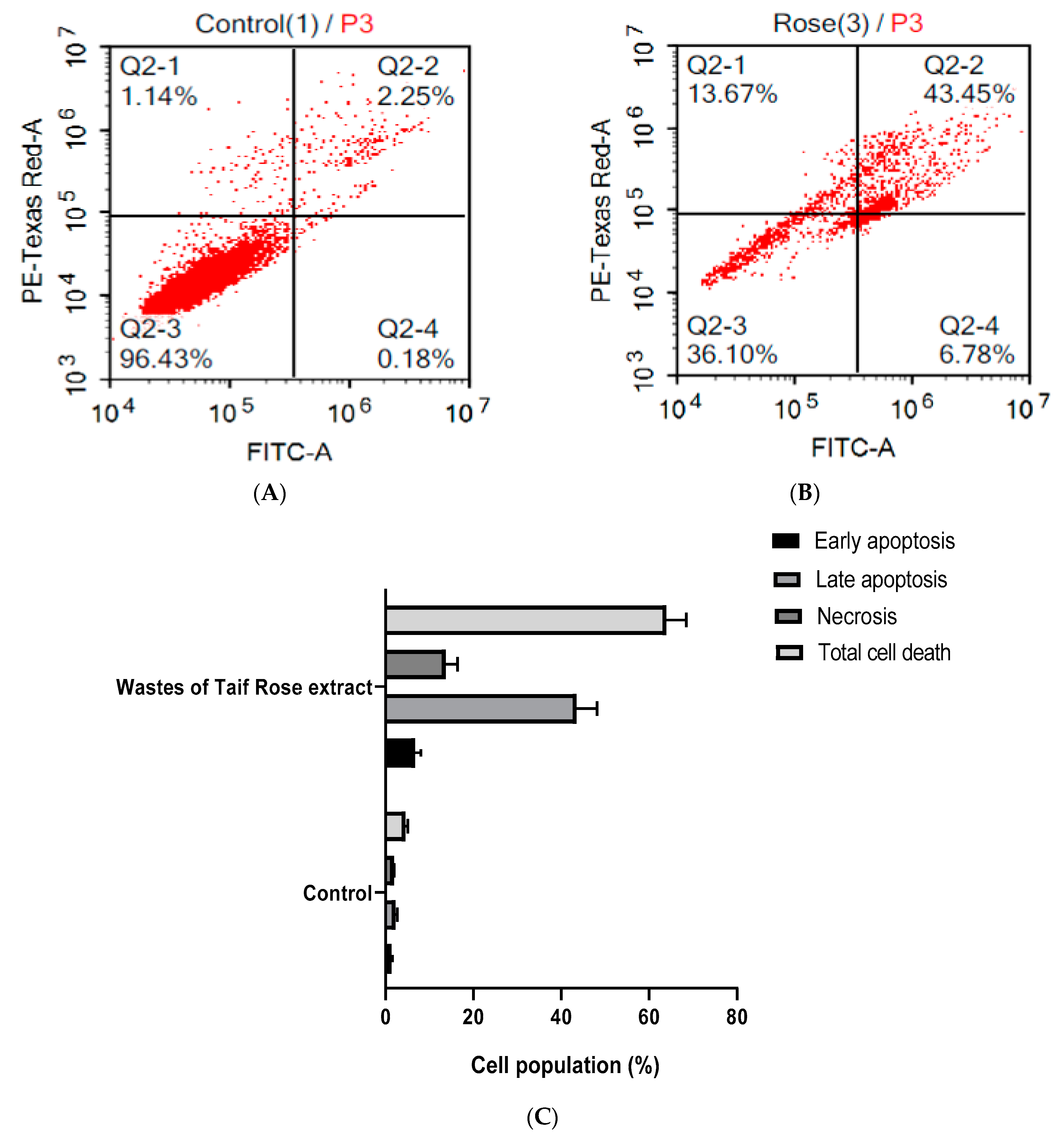
3.3. WTR Induced Apoptosis and Necrosis of HepG2 Cells
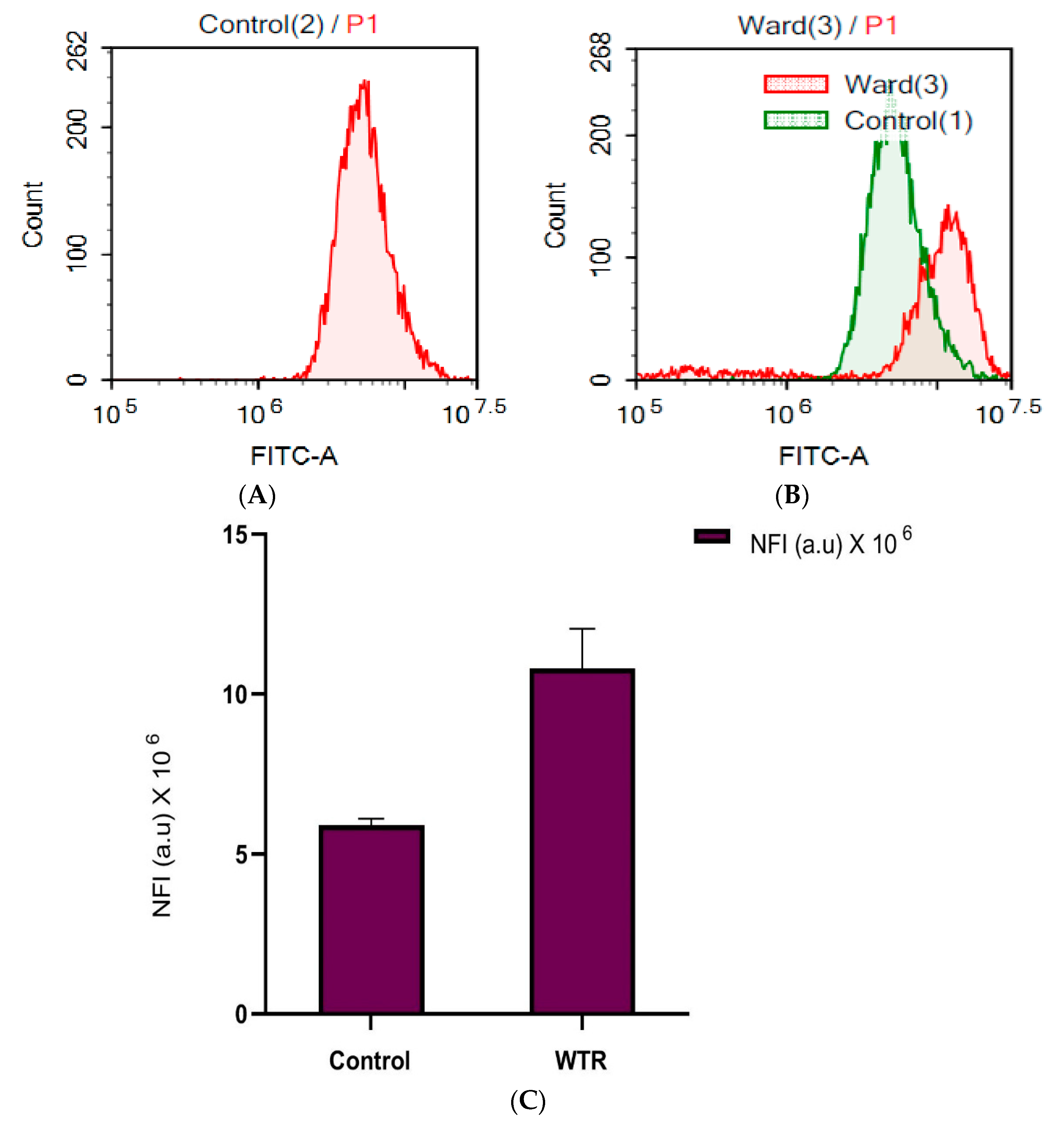
3.4. WTR Induced Autophagy in HepG2 Cells
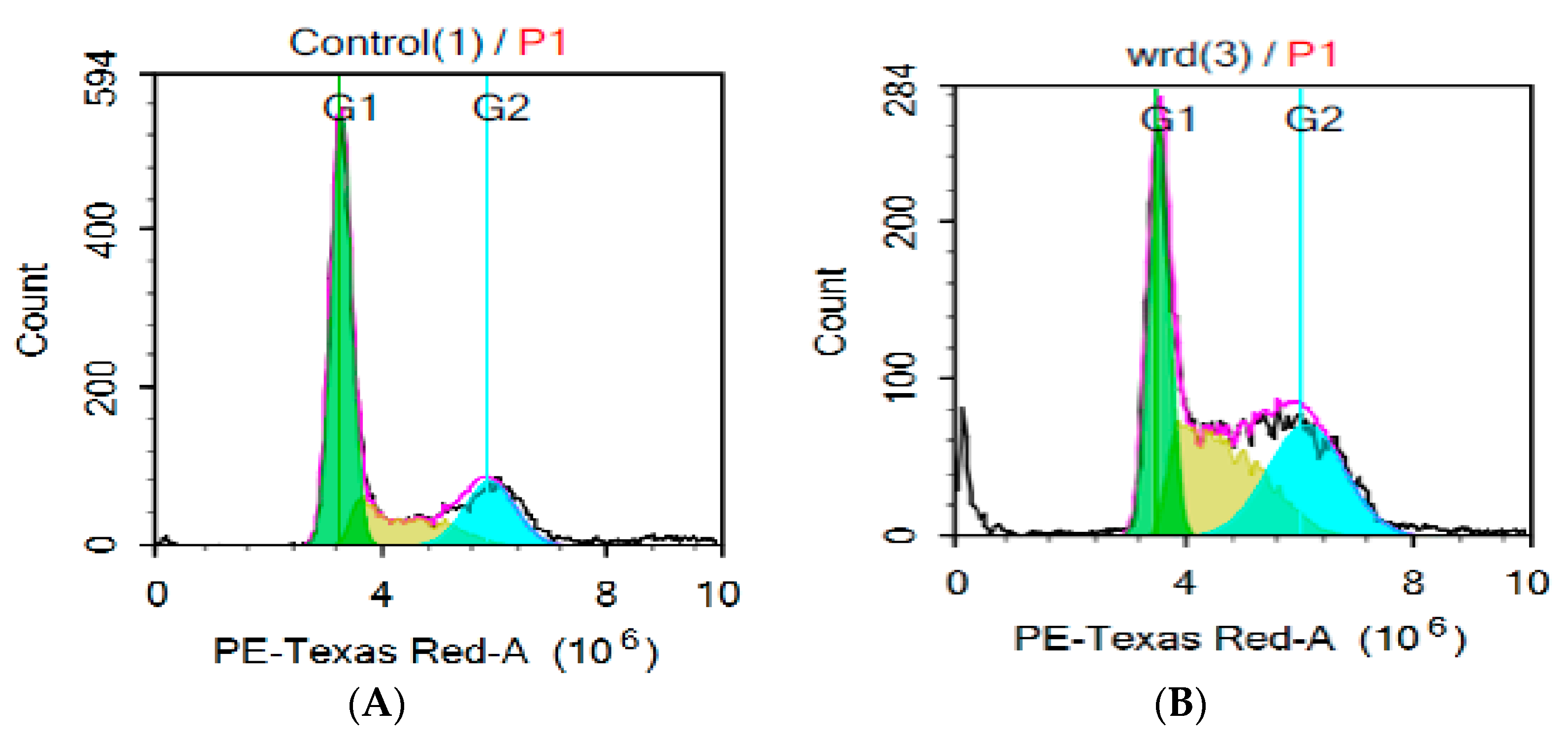
3.5. Cell Cycle Distribution Analysis of HepG2 Cells
3.6. Effect on Liver Functions
3.7. Effect of WTR on CdCl2 and Oxidative Stress Markers in Liver Tissues
3.8. Effect on Liver Histopathology, Ultrastructure, and DNA Damage
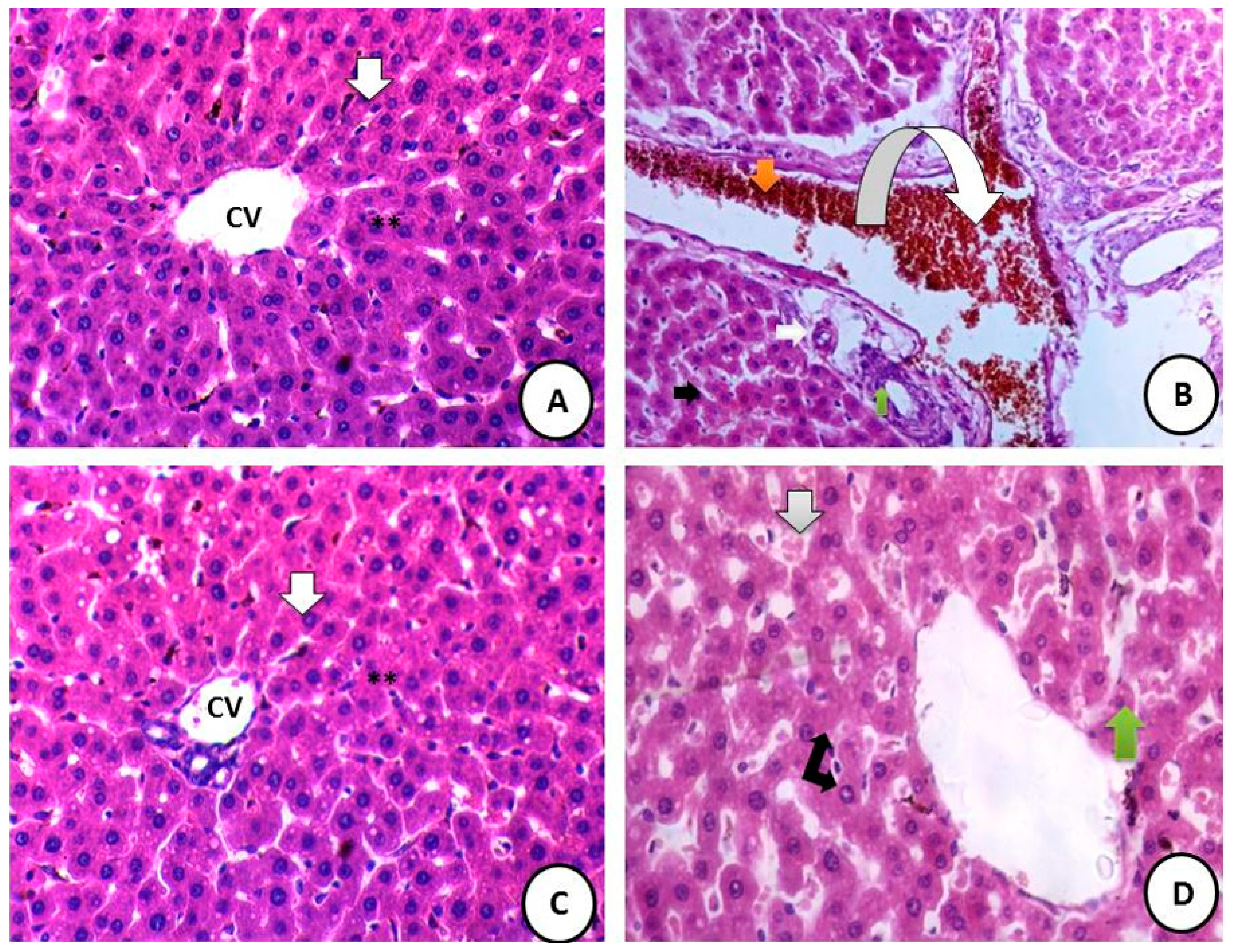
3.8.1. Liver Histopathology
3.8.2. Liver Ultrastructure (TEM Sections)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vamanu, E.; Nita, S. Antioxidant capacity and the correlation with major phenolic compounds, anthocyanin, and tocopherol content in various extracts from the wild edible Boletus edulis mushroom. BioMed Res. Int. 2013, 2013, 313905. [Google Scholar] [CrossRef] [PubMed]
- Kazaz, S.; Erbas, S.; Baydar, H.; Dilmacunal, T.; Koyuncu, M.A. Cold storage of oil rose (Rosa damascena Mill.) flowers. Sci. Hortic. 2010, 126, 284–290. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Xing, J.; Sun, M.; Zhan, Z.Q.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef]
- Baydar, N.G.; Baydar, H. Phenolic compounds, antiradical activity and antioxidant capacity of oil-bearing rose (Rosa damascena Mill.) extracts. Ind. Crops Prod. 2013, 41, 375–380. [Google Scholar] [CrossRef]
- Ali, E.F.; Al-Yasi, H.M.; Issa, A.A.; Hessini, K.; Hassan, F.A.S. Ginger extract and fulvic acid foliar applications as novel practical approaches to improve the growth and productivity of damask rose. Plants 2022, 11, 412. [Google Scholar] [CrossRef]
- Hessini, K.; Wasli, H.; Al-Yasi, H.M.; Ali, E.F.; Issa, A.A.; Hassan, F.A.S.; Siddique, K.H.M. Graded moisture deficit effect on secondary metabolites, antioxidant, and inhibitory enzyme activities in leaf extracts of Rosa damascena Mill. var. trigentipetala. Horticulturae 2022, 8, 177. [Google Scholar] [CrossRef]
- Ali, E.F.; Issa, A.A.; Al-Yasi, H.M.; Hessini, K.; Hassan, F.A.S. The efficacies of 1-methylcyclopropene and chitosan nanoparticles in preserving the postharvest quality of damask rose and their underlying biochemical and physiological mechanisms. Biology 2022, 11, 242. [Google Scholar] [CrossRef]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Ali, E.; Elshazly, S. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Biochem. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Iqbal, S.; Khalid, S.; Shahid, S. Pharmacological properties of Rosa damascena. Asian J. Pharm. Technol. 2020, 10, 183–186. [Google Scholar] [CrossRef]
- Galal, T.M.; Al-Yasi, H.M.; Fawzy, M.A.; Abdelkader, T.G.; Hamza, R.Z.; Eid, E.M.; Ali, E.F. Evaluation of the Phytochemical and Pharmacological Potential of Taif’s Rose (Rosa damascena Mill var. trigintipetala) for Possible Recycling of PruningWastes. Life 2022, 12, 273. [Google Scholar] [CrossRef]
- Villena-Tejada, M.; Vera-Ferchau, I.; Cardona-Rivero, A.; Cornejo, R.; Quispe-Florez, M.; Frisancho-Triveño, Z.; AbarcaMelendez, R.C.; Alvarez-Sucari, S.G.; Mejia, C.R.; Yañez, J.A. Use of medicinal plants for COVID-19 prevention and respiratory symptom treatment during the pandemic in Cusco, Peru: A cross-sectional survey. PLoS ONE 2021, 16, e0257165. [Google Scholar] [CrossRef] [PubMed]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; Ajabnoor, G.; Choudhry, H.; Al-Abd, A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018, 8, 11674. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, F.R.; Howlader, S.; Raihan, T.; Hasan, M. Plants Metabolites: Possibility of Natural Therapeutics Against the COVID-19 Pandemic. Front. Med. 2020, 7, 444. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Shohayeb, M.M.; El-Sayed, M.M.; El-Wakil, E.A. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. Eur. J. Med. Plants 2012, 2, 93–112. [Google Scholar] [CrossRef]
- Abu-El-Zahab, H.S.; Hamza, R.Z.; Montaser, M.M.; El-Mahdi, M.M.; Al-Harthi, W.A. Antioxidant, antiapoptotic, antigenotoxic, and hepatic ameliorative effects of L-carnitine and selenium on cadmium-induced hepatotoxicity and alterations in liver cell structure in male mice. Ecotoxicol. Environ. Saf. 2019, 173, 419–428. [Google Scholar] [CrossRef]
- Rani, A.; Kumar, A.; Lal, A.; Pant, M. Cellular mechanisms of cadmium-induced toxicity: A review. Int. J. Environ. Health Res. 2014, 24, 378–399. [Google Scholar] [CrossRef]
- Godt, J.; Franziska, S.; Christian, G.S.; Vera, E.; Paul, B.; Andrea, R.; David, A.G. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef]
- Staessen, J.A.; Roels, H.A.; Emelianov, D.; Kuznetsova, T.; Thijs, L.; Vangronsveld, J.; Fagard, R. Environmental exposure to cadmium, forearm bone density, and risk of fractures: Prospective population study. Public Health and Environmental Exposure to Cadmium (PheeCad) Study Group. Lancet 1999, 353, 1140–1144. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Behari, J.R.; Ashquin, M.; Tandon, S.K. Time-dependent protective effect of selenium against cadmium-induced nephrotoxicity and hepatotoxicity. Chem.-Biol. Interact 1982, 42, 345–351. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Fallah, H.; Sharififar, F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on α-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine 2009, 16, 935–941. [Google Scholar] [CrossRef]
- Abdel-Hameed, E.S.S.; Bazaid, S.A.; Hagag, H.A. Chemical characterization of Rosa damascena Miller var. trigintipetala Dieck essential oil and it’s in vitro genotoxic and cytotoxic properties. J. Essent. Oil Res. 2016, 28, 121–129. [Google Scholar] [CrossRef]
- Al-Abbasi, F.A.; Alghamdi, E.A.; Baghdadi, M.A.; Alamoudi, A.J.; El-Halawany, A.M.; El-Bassossy, H.M.; Aseeri, A.H.; Al-Abd, A.M. Gingerol synergizes the cytotoxic effects of doxorubicin against liver cancer cells and protects from its vascular toxicity. Molecules 2016, 21, 886. [Google Scholar] [CrossRef] [PubMed]
- Algehani, R.A.; Abou Khouzam, R.; Hegazy, G.A.; Alamoudi, A.A.; El-Halawany, A.M.; El Dine, R.S.; Ajabnoor, G.A.; Al-Abbasi, F.A.; Baghdadi, M.A.; Elsayed, I.; et al. Colossolactone-G synergizes the anticancer properties of 5-fluorouracil and gemcitabine against colorectal cancer cells. Biomed. Pharmacother. 2021, 140, 111730. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Cadmium-induced hepatotoxicity in rats and the protective effect of naringenin. Exp. Toxicol. Pathol. 2010, 62, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. Methods Enzym. Anal. 1983, 8, 273–286. [Google Scholar]
- Couri, D.; Abdel-Rahman, M.S. Effect of chlorine dioxide and metabolites on glutathione dependent system in rat, mouse and chicken blood. J. Environ. Pathol. Toxicol. 1979, 3, 451–460. [Google Scholar]
- Hafeman, D.G.; Sunde, R.A.; Hoekstra, W.G. Effect of dietary selenium on erythrocyte and liver glutathione peroxidase in the rat. J. Nutr. 1974, 104, 580–587. [Google Scholar] [CrossRef]
- Hayat, M. Basic Techniques for Transmission Electron Microscopy; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Hagag, H.A.; Bazaid, S.A.; Abdel-Hameed, E.S.S.; Salman, M. Cytogenetic, cytotoxic and GC–MS studies on concrete and absolute oils from Taif rose, Saudi Arabia. Cytotechnology 2014, 66, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Siswadi, S.; Saragih, G.S. Phytochemical analysis of bioactive compounds in ethanolic extract of Sterculia quadrifida R.Br. Int. Conf. Life Sci. Technol. 2020, 2353, 030098. [Google Scholar]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef] [PubMed]
- Baydar, H.A.S.A.N.; Özkan, G.; Erbaş, S.; Altındal, D. Yield, chemical composition and antioxidant properties of extracts and essential oils of sage and rosemary depending on seasonal variations. In Proceedings of the I International Medicinal and Aromatic Plants Conference on Culinary Herbs, Antalya, Turkey, 29 May–4 April 2007; Volume 826, pp. 383–390. [Google Scholar]
- Ju, C.; Song, S.; Hwang, S.; Kim, C.; Kim, M.; Gu, J.; Yu-Kyoung, O.; Lee, K.; Kwon, J.; Lee, K.; et al. Discovery of novel (1S)-(−)-verbenone derivatives with anti-oxidant and anti-ischemic effects. Bioorganic Med. Chem. Lett. 2013, 23, 5421–5425. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lim, J.H.; Hwang, S.; Lee, J.C.; Cho, G.S.; Kim, W.K. Anti-ischemic and anti-inflammatory activity of (S)-cis-verbenol. Free Radic. Res. 2010, 44, 541–551. [Google Scholar] [CrossRef]
- Hamza, R.Z.; El-Shenawy, N.S. Anti-inflammatory and antioxidant role of resveratrol on nicotine-induced lung changes in male rats. Toxicol. Rep. 2017, 4, 399–407. [Google Scholar] [CrossRef]
- De Castro, D.S.B.; Da Silva, D.B.; Tibúrcio, J.D.; Sobral, M.E.G.; Ferraz, V.; Taranto, A.G.; Serrão, J.E.; Siqueira, F.M.; Alves, S.N. Larvicidal activity of essential oil of Peumus boldus Molina and its ascaridole-enriched fraction against Culex quinquefasciatus. Exp. Parasitol. 2016, 171, 84–90. [Google Scholar] [CrossRef]
- Peramo, A.; Mura, S.; Yesylevskyy, S.O.; Cardey, B.; Sobot, D.; Denis, S.; Ramseyer, C.; Desmaële, D.; Couvreur, P. Squalene versus cholesterol: Which is the best nanocarrier for the delivery to cells of the anticancer drug gemcitabine? Comptes Rendus Chimie 2018, 21, 974–986. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Shill, M.C.; Karmakard, U.K.; Yarlag, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Rolf, J. Milk and Dairy Products. Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2002; Volume 16, pp. 3–6. [Google Scholar]
- Smedman, A.E.; Gustafsson, I.B.; Berglund, L.G.; Vessby, B.O. Pentadecanoic acid in serum as a marker for intake of milk fat: Relations between intake of milk fat and metabolic risk factors. Am. J. Clin. Nutr. 1999, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- El-Shenawy, N.S.; AL-Harbi, M.S.; Hamza, R.Z. Effect of vitamin E and selenium separately and in combination on biochemical, immunological and histological changes induced by sodium azide in male mice. Exp. Toxicol. Pathol. 2015, 67, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Harbi, M.S.; El-Shenawy, N.S. Ameliorative effect of vitamin E and selenium against oxidative stress induced by sodium azide in liver, kidney, testis and heart of male mice. Biomed. Pharmacother. 2017, 91, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Hamza, R.Z.; Al-Talhi, T.; Gobouri, A.A.; Alsanie, W.F.; El-Megharbel, S.M. Are favipiravir and acyclovir with IgG injections supplemented with vitamin D “suggested therapeutic option” can fight against COVID-19. Adv. Anim. Vet. Sci. 2021, 9, 549–554. [Google Scholar] [CrossRef]
- Alharthi, W.A.; Hamza, R.Z.; Elmahdi, M.M.; Abuelzahab, H.S.; Saleh, H. Selenium and L-carnitine ameliorate reproductive toxicity induced by cadmium in male mice. Biol. Trace Elem. Res. 2020, 197, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Refat, M.S.; Hamza, R.Z.; Adam, A.M.; Saad, H.A.; Gobouri, A.A.; Azab, E.; Al-Salmi, F.A.; Altalhi, T.A.; Khojah, E.; Gaber, A.; et al. Antioxidant, Antigenotoxic, and Hepatic Ameliorative Effects of Quercetin/Zinc Complex on Cadmium-Induced Hepatotoxicity and Alterations in Hepatic Tissue Structure. Coatings 2021, 11, 501. [Google Scholar] [CrossRef]
- Al-Baqami, N.M.; Hamza, R.Z. Protective Effect of Resveratrol against Hepatotoxicity of Cadmium in Male Rats: Antioxidant and Histopathological Approaches. Coatings 2021, 11, 594. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Al-Salmi, F.A.; Al-Harthi, S.; Alsolami, K.; Hamza, R.Z. Chitosan/Selenium Nanoparticles Attenuate Diclofenac Sodium-Induced Testicular Toxicity in Male Rats. Crystals 2021, 11, 1477. [Google Scholar] [CrossRef]
- El-Megharbel, S.M.; Al-Thubaiti, E.H.; Qahl, S.H.; Al-Eisa, R.A.; Hamza, R.Z. Synthesis and Spectroscopic Characterization of Dapagliflozin/Zn (II), Cr (III) and Se (IV) Novel Complexes That Ameliorate Hepatic Damage, Hyperglycemia and Oxidative Injury Induced by Streptozotocin-Induced Diabetic Male Rats and Their Antibacterial Activity. Crystals 2022, 12, 304. [Google Scholar]
- Newairy, A.A.; El-Sharaky, A.S.; Badreldeen, M.M.; Eweda, S.M.; Sheweita, S.A. The hepatoprotective effects of selenium against cadmium toxicity in rats. Toxicology 2007, 242, 23–30. [Google Scholar] [CrossRef]
- Al-Yasi, H.; El-Shazly, S.A.; Ahmed, E.F.; Alamer, K.H.; Hessini, K.Y.; Attia, H.A.; Alkafafy, M.E.; Mohamed, A.A.; Hassan, F.A. Protective Effects of Taif Rosewater Against Testicular Impairment Induced by Lead Intoxication in Rats. Andrologia 2021, 53, e14045. [Google Scholar]
- Hassan, F.; Al-Yasi, H.; Ali, E.; Alamer, K.; Hessini, K.; Attia, H.; El-Shazly, S. Mitigation of salt-stress effects by moringa leaf extract or salicylic acid through motivating antioxidant machinery in damask rose. Can. J. Plant Sci. 2020, 101, 157–165. [Google Scholar] [CrossRef]
- Kang, Y.; Wu, K.; Sun, J.; Liu, C.; Su, C.; Yi, F. Preparation of Kushui Rose (Rosa setate × Rosa rugosa) essential oil fractions by double molecular distillation: Aroma and biological activities. Ind. Crops Prod. 2022, 175, 114230. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Xing, S.; Zhang, C. Flavonoids of Rosa rugosa Thunb. inhibit tumor proliferation and metastasis in human hepatocellular carcinoma HepG2 cells. Food Sci. Hum. Wellness 2022, 11, 374–382. [Google Scholar] [CrossRef]
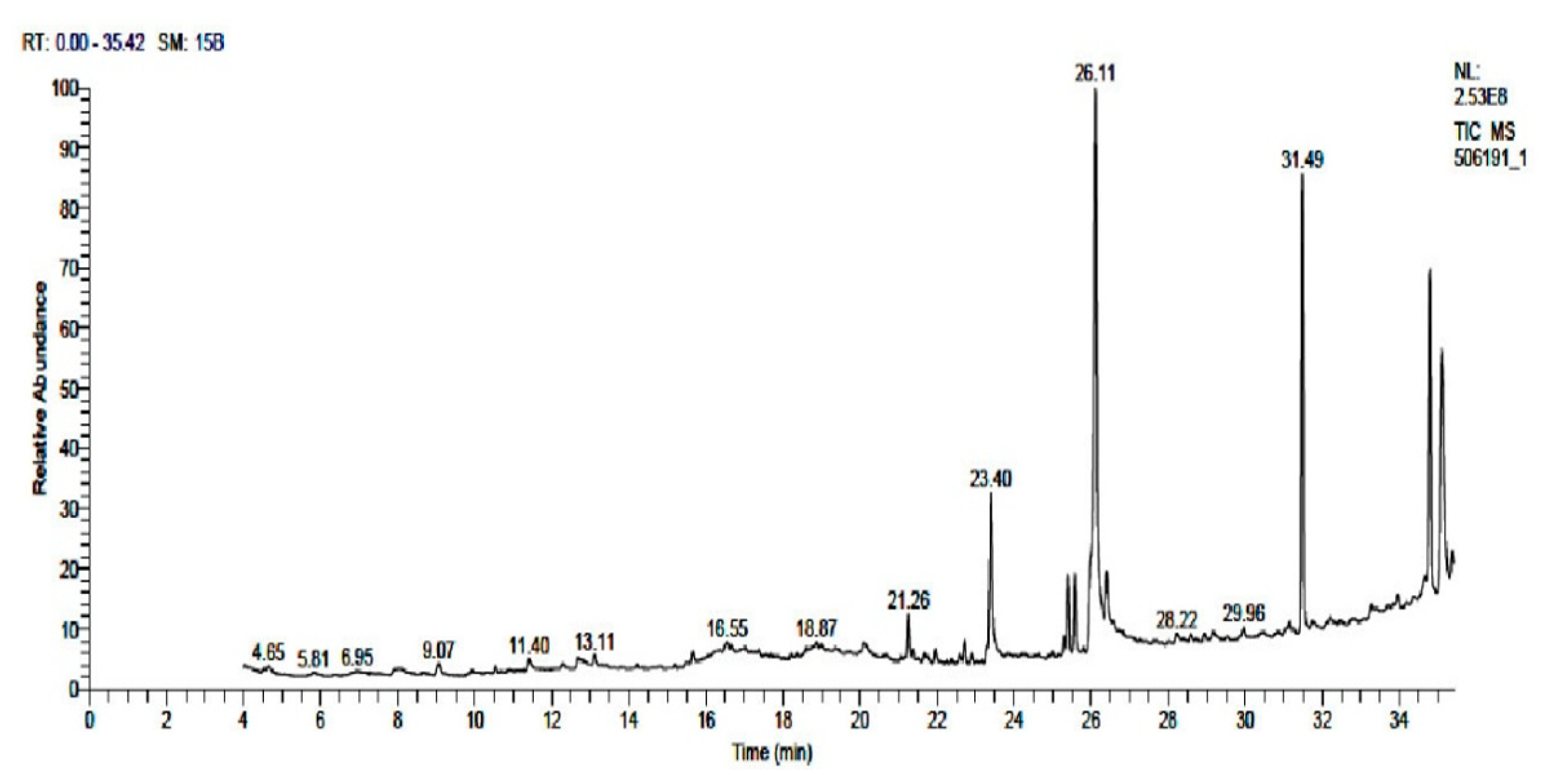

| S.No | RT (min) | Area % | Molecular Formula | Molecular Weight | Cas # | Compound Name | Library |
|---|---|---|---|---|---|---|---|
| 1 | 4.65 | 0.74 | C10H17NO6S | 279 | 5115-81-1 | Desulphosinigrin | mainlib |
| 2 | 9.07 | 0.62 | C10H14O | 150 | 18309-32-5 | D-Verbenone | mainlib |
| 3 | 11.40 | 0.54 | C10H16O3 | 184 | 135760-25-7 | Ascaridole epoxide | mainlib |
| 4 | 12.68 | 0.55 | C14H14O | 198 | 121189-99-9 | Naphthalene, 1 Methoxy-8-(1-Methylethenyl) | Wiley Registry8ed |
| 5 | 12.78 | 0.44 | C6H14N4O2 | 174 | 74-79-3 | 2-Amino-5-Guanidino-Pentanoic acid | Wiley Registry8ed |
| 6 | 15.66 | 0.43 | C18H34O2 | 282 | 112-80-1 | 9-Octadecenoic acid | Wiley Registry8ed |
| 7 | 16.55 | 0.50 | C30H52O3Si | 488 | 55759-94-9 | 1,25Dihydroxyvitamin D3, TMS derivative | mainlib |
| 8 | 20.15 | 0.54 | C15H30O2 | 242 | 1002-84-2 | Pentadecanoic acid | Wiley Registry8ed |
| 9 | 21.26 | 1.63 | C20H40O2 | 312 | 5353-25-3 | Ethanol | Wiley Registry8ed |
| 10 | 21.67 | 0.29 | C19H22O6 | 346 | NA | Isochiapin B | Wiley Registry8ed |
| 11 | 21.96 | 0.57 | C15H20O5 | 280 | NA | Tetraneurin-A-Diol | Wiley Registry8ed |
| 12 | 22.60 | 0.32 | C30H52O3Si | 488 | 55759-9 4-9 | 1,25-Dihydroxyvitamin D3, TMS derivative | mainlib |
| 13 | 22.90 | 0.31 | C37H68O3Si3 | 644 | NA | Tris –Trimethyl Silyl Ether Derivative of 1,25 Di-hydroxy vitamin D2 | Wiley Registry8ed |
| 14 | 23.29 | 0.23 | C23H36O4 | 376 | NA | Phthalic acid, butyl undecyl ester | mainlib |
| 15 | 23.40 | 6.44 | C16H32O2 | 256 | 57-10-3 | Hexadecanoic acid | Wiley Registry8ed |
| 16 | 25.30 | 0.58 | C20H36O2 | 308 | 544-35-4 | Linoleic acid ethyl ester | replib |
| 17 | 25.40 | 2.73 | C19H32O2 | 292 | 7361-8 0-0 | 9,12,15-Octadecatrienoic Acid, Methyl Ester | Wiley Registry8ed |
| 18 | 25.58 | 2.85 | C20H40O | 296 | 150-86-7 | Phytol | replib |
| 19 | 26.11 | 24.90 | C18H30O2 | 278 | 463-40-1 | 9,12,15-Octadecatrienoic acid | mainlib |
| 20 | 26.41 | 1.97 | C21H42O4 | 358 | 123-94-4 | Octadecanoic Acid | Wiley Registry8ed |
| 21 | 29.16 | 0.45 | C19H26O6 | 350 | NA | Isochiapin B %2< | Wiley Registry8ed |
| 22 | 29.96 | 0.44 | C22H28O3 | 340 | 51-98-9 | Norethindrone Acetate | replib |
| 23 | 31.13 | 0.57 | C27H30O15 | 594 | NA | Flavone | Wiley Registry8ed |
| 24 | 31.49 | 16.34 | C24H38O4 | 390 | NA | Phthalic acid | mainlib |
| 25 | 31.74 | 0.43 | C69H134O6 | 1058 | 18641-57-1 | Docosanoic Acid | Wiley Registry8ed |
| 26 | 33.27 | 0.62 | --- | 0 | NA | Hahnfett | Wiley Registry8ed |
| 27 | 33.95 | 0.51 | C27H44O | 384 | 601-54-7 | Cholest-5-EN-3-ONE | Wiley Registry8ed |
| 28 | 34.37 | 0.30 | C27H30O15 | 594 | NA | Flavone | Wiley Registry8ed |
| 29 | 34.79 | 12.07 | C30H50 | 410 | 111-02-4 | Squalene | replib |
| 30 | 35.11 | 12.52 | C29H50O2 | 430 | 59-02-9 | Vitamin E | replib |
| 31 | 35.37 | 0.67 | C27H30O15 | 594 | NA | Flavone 4′-OH,5-OH,7-DI-O- Glucoside | Wiley Registry8ed |
| Parameters | Normal Control | CdCl2 | WTR | CdCl2 + WTR |
|---|---|---|---|---|
| ALT (U/L) | 12.51 ± 0.42 c | 123.31 ± 6.69 a | 13.40 ± 0.82 b,c | 23.87 ± 3.14 b |
| AST (U/L) | 12.43 ± 0.37 c | 164.26 ± 5.27 a | 11.27 ± 0.52 c | 25.53 ± 3.20 b |
| LDH (U/L) | 84.05 ± 8.47 c | 567.42 ± 47.00 a | 133.56 ± 11.91 c | 220.02 ± 12.05 b |
| Parameters | Normal Control | CdCl2 | WTR | CdCl2 + WTR |
|---|---|---|---|---|
| CAT (U/g) | 5.68 ± 0.26 b | 1.99 ± 0.16 c | 7.19 ± 1.31 a | 5.18 ± 0.35 b |
| SOD (U/g) | 10.59 ± 0.26 b | 3.17 ± 0.25 d | 12.83 ± 0.34 a | 8.86 ± 0.39 c |
| GRx (U/g) | 7.26 ± 0.18 a | 2.30 ± 0.28 c | 7.52 ± 0.33 a | 5.39 ± 0.45 b |
| MDA (µg/mg) | 3.75 ± 0.15 c | 35.41 ± 1.30 a | 2.71 ± 0.68 b | 7.64 ± 0.68 b |
| GPx (U/g) | 13.22 ± 0.45 a | 3.75 ± 0.40 c | 14.22 ± 0.51 a | 9.56 ± 0.65 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamza, R.Z.; Al-Yasi, H.M.; Ali, E.F.; Fawzy, M.A.; Abdelkader, T.G.; Galal, T.M. RETRACTED: Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals 2022, 12, 460. https://doi.org/10.3390/cryst12040460
Hamza RZ, Al-Yasi HM, Ali EF, Fawzy MA, Abdelkader TG, Galal TM. RETRACTED: Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals. 2022; 12(4):460. https://doi.org/10.3390/cryst12040460
Chicago/Turabian StyleHamza, Reham Z., Hatim M. Al-Yasi, Esmat F. Ali, Mustafa A. Fawzy, Tharwat G. Abdelkader, and Tarek M. Galal. 2022. "RETRACTED: Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2)" Crystals 12, no. 4: 460. https://doi.org/10.3390/cryst12040460
APA StyleHamza, R. Z., Al-Yasi, H. M., Ali, E. F., Fawzy, M. A., Abdelkader, T. G., & Galal, T. M. (2022). RETRACTED: Chemical Characterization of Taif Rose (Rosa damascena Mill var. trigentipetala) Waste Methanolic Extract and Its Hepatoprotective and Antioxidant Effects against Cadmium Chloride (CdCl2)-Induced Hepatotoxicity and Potential Anticancer Activities against Liver Cancer Cells (HepG2). Crystals, 12(4), 460. https://doi.org/10.3390/cryst12040460







