Abstract
Crystallization remains a bottleneck for determining the three-dimensional X-ray structure of proteins. Many parameters influence the complexity of protein crystallization. Therefore, it is not easy to systematically examine all of these parameters individually during crystallization because of a limited quantity of purified protein. We studied several factors that influence crystallization including protein concentration, pH, temperature, age, volume of crystallization, inhibitors, metal ions, seeding, and precipitating agents on RuBisCO samples from Alcaligenes eutrophus which are not only freshly purified, but are also dissolved both individually and in combination from microcrystals and precipitated droplets of recycled RuBisCO. Single-, twin-, and/or microcrystals are dependent upon the concentration of RuBisCO by both RuBisCO samples. The morphology, either orthorhombic- or monoclinic-space group, depends upon pH. Furthermore, ammonium sulfate((NH4)2SO4) concentration at 20 °C (22% saturated) and/or at 4 °C (28% saturated) affected the crystallization of RuBisCO differently from one another. Finally, the age of RuBisCO also affected more uniformity and forming sharp edge during crystallization. Unexpected surprising monoclinic RuBisCO crystals were grown from dissolved microcrystals and precipitated droplets recycled RuBisCO samples. This quaternary RuBisCO single crystal, which contained Mg2+ and HCO3 for an activated ternary complex and is inhibited with a transition substrate analogue, CABP (2-carboxyarabinitol-1,5-bisphosphate)−, diffracts better than 2.2 Å. It is different from Hansen S. et al. reported RuBisCO crystals which were grown ab initio in absence of Mg2+, HCO3− and CABP, a structure which was determined at 2.7 Å resolution.
1. Introduction
Proteins are relatively long, fragile chain molecules composed of 20 different amino acids. Their biological activities are optimal within a narrow range of temperature and pH [1]. They function in the cell as structural elements, catalysts, transport, regulators of various processes, messengers, receptors for messengers, cell markers, and defense against cells that carry foreign antigens. Some proteins bind to DNA (deoxyribonucleic acid) or RNA to regulate recombination, whereas others participate in the replication, transcription, or translation of genetic information [2,3].
Probably the most important proteins are the enzymes that act as catalysts during cell metabolism. They recognize a specific molecule, the substrate, and bind to it in a dynamic equilibrium. The characteristics of an enzyme reside in the fact that it can chemically change a substrate by lowering activation energy under physiological conditions. Usually, this change accompanies the formation or cleavage of a covalent chemical bond. Thus, the substrate can be broken down into two or more parts: a chemical group can be attached, or binding patterns present in substrate molecules may be rearranged. The reaction mechanism of an enzyme is divided into three steps: the enzyme binds to its component, a chemical reaction takes place, and the altered substrate (product) disappears again. All three of these steps are reversible.
One of the main goals in protein research is to clarify the structure and reaction mechanisms of these molecules. The only method used to completely determine the structure of a protein in detail is X-ray crystallographic analysis [4,5]. Approximately 85% of the protein structures registered in the PDB have been determined by this method [6]. The aim of this study was to identify parameters that influence the crystallization of the enzyme, RuBisCO, derived from A. eutrophus. RuBisCO is the most abundant protein in nature and catalyzes the first reaction step of the Calvin-Benson-Bassham (CBB) cycle [7,8], which is involved in both of photosynthesis and photorespiration. Agricultural researchers are interested in increasing the production of crops by increasing photosynthesis, while decreasing photorespiration through genetic manipulation of the active sites of RuBisCO [9]. RuBisCO, in most bacteria as well as in higher plants, is composed of eight large (M.W. = 55,000) and eight small (M.W. = 14,000) subunits yielding a total molecular weight of 550,000 (L8S8). Both catalytic and active sites reside on the large subunit [10]. RuBisCO from Rhodospirillum rubrum consists of only two large subunits (L2) [11]. However, it functions completely on its own in the cell without a small subunit. The role of the small subunit of RuBisCO, therefore, remains still unclear, although a mini review published about the role of the small subunit. It may regulate the structure or function of RuBisCO [12].
2. Material and Methods
Unless otherwise specified, materials from Sigma Aldrich (St. Louis, MO, USA) were used. Special chemicals or biochemicals have been purchased from the following companies, including ammonium peroxidosulfate (Merck, Taufkirchen, Germany), Amicon (MilliporeSigma, Burlington, MA, USA), Centricon 10 (MilliporeSigma, Burlington, MA, USA), RuBP(Ribulose 1,5-bisphosphate) (Sigma, Muenchen, Germany), NADH (Nicotinamide adenine dinucleotide), GDH (Glycerol-3-Phosphate Dehydrogenase)/TIM (triosephosphate isomerase), GAPDH (Glyceraldehyde 3-phosphate dehydrogenase)/PGK (Phosphoglycerate kinase), ATP (Adenosine triphosphate), EDTA (Ethylene-diamine-tetraacetic acid), PMSF (Phenylmethylsulfonyl fluoride), DTE (1,4-Dithioerythritol) (Roche, Basel, Switzerland).
Some of the experiments were undertaken at the Institute of Crystallography, Free University, Berlin, Germany and repeated at the laboratory of protein structure at the Department of Oriental Medicine Resources, College of Environmental and Bioresource Sciences, Jeonbuk National University, Republic of Korea.
2.1. Cell Cultivation and Purification of RuBisCO
A. eutrophus (ATCC 17699) was cultivated according to a culture manual and the harvested wet cells were collected by centrifugation (Hanil supra 30K, A50S-6, 4000 rpm, 4 °C, 15 min) and stored at −60 °C until use. For the isolation of RuBisCO, 10 g of wet cells were thawed and suspended in 100 mL isolation buffer (20 mM Tris/HCl, 50 mM NaHCO3, 10 mM MgCl2, 1 mM EDTA, 1 mM DTE, pH 8.0) with addition of 1/10 volume of 10 mM PMSF in ethanol and 10 mg DNase I. The cells were ruptured by sonication six times at 25 watts for 30 s with a 1-min break using an ultrasonicator R-4710-10 (Cole-Parmer, Vernon Hills, IL, USA) in an ice water bath. The samples were then centrifuged (Beckman coulter Optima XE-90, SW-28, 25,000 rpm, 4 °C, 30 min) and the pellets were discarded. Next, 500 μL were taken out from the supernatant and the absorption was measured at 260 and 280 nm using a UV-vis spectrometer (NEOGEN NEO-S490, Lansing, MI, USA). The O.D. (optical density) ratio (280/260) nm was approximately 0.84. According to the method of Warburg [13], the total amount of protein was approximately 2 g. Next, 80 mg protamine sulfate were added to eliminate nucleic acids and the samples were centrifuged (Hanil supra 30K, A50S-6, 20,000 rpm, 4 °C, 20 min). The resulting supernatant was mixed with 25% (NH4)2SO4 (percentages for (NH4)2SO4 in the description usually always refer to saturated = 100% solution). The samples were then re-centrifuged. The supernatant was adjusted to 40% saturation with (NH4)2SO4 and the suspensions were kept at 4 °C for overnight. The next day, the samples were centrifuged (Hanil supra 30K, A50S-6, 20,000 rpm, 4 °C, 20 min). The supernatant was discarded and the pellets were solubilized with 10–20 mL of isolation buffer. Dialysis tubes (cut-off 6000–8000 Daltons) were used to desalt the samples. Before dialysis, the tubes were boiled for one hour in a solution containing 1 mM EDTA and 100 mM NaHCO3. The samples were then dialyzed four times against the isolation buffer and the resulting solution served as a crude extract for RuBisCO enrichment.
The fresh purchased column materials were directly used for RuBisCO isolation, however, for recycling the column materials, the resin was washed on a glass filter with 1 M sodium acetate (pH 3, adjusted with acetic acid) until the pH of the eluate was 3. Thereafter, it was washed with 0.5 M NaOH to a pH of 14. It was re-washed with 1 M sodium acetate and then with isolation buffer until the pH value of the eluate was 8. The column material was finally degassed for 30 min in a suction flask. Before packing, the column material was stored at 4 °C.
The column material (DEAE-sepharose CL-6B) was poured into a column (d = 2 cm, h = 45 cm), which was placed vertically in a cold room (4 °C). A long glass rod was used so that no air bubbles could rise. Subsequently, the column was equilibrated with isolation buffer. The flow rate of the column was 1 mL/min. Then, 10–20 mL samples, which were dialyzed four times against the isolation buffers, were placed on the column and washed overnight with isolation buffer until no protein was detectable in the eluate. The proteins bound in the column material were then eluted by with a KCl gradient (0–0.4 M KCl) (Figure S1a). The eluate was divided into fractions of 7.5 mL each in the fractions in which enzyme activity was present. Purity was assessed by sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Figure S1b).
2.2. Determination of RuBisCO Concentration
The protein concentration of the crude extract was determined by measuring the absorption at 260 and 280 nm using a UV-vis spectrometer (NEOGEN NEO-S490). The protein concentration of the purified RuBisCO was measured according to Bowien B. et al. [14] by absorbance at 280 nm (The absorption coefficient at 280 nm of RuBisCO solution was 1 mg/mL = 1.22).
2.3. Determination of RuBisCO Activity
To determine enzyme activity, the modified method of Racker E. [15] was used. For this assay the reagents shown in Table 1 were mixed. The solution was filled to 1000 μL with H2O and the enzyme solution to be analyzed. The change in absorbance at 340 nm was recorded over time for 3 min.

Table 1.
RuBisCO activity reagents and volume.
2.4. Crystallization of RuBisCO
Hanging drop, sitting drop vapor diffusion, and microdialysis methods [16] were used for the crystallization of RuBisCO from A. eutrophus. For the salts in RuBisCO crystallization of this study, 100% saturated solutions in isolation buffer were used as stock solution. For polyethylenglycol (PEG) 6000, 40% solutions in buffer (w/v) were used as stock solutions. 2-methyl-2,4 pentandiol (MPD) solutions were prepared directly by using buffers. Among the precipitating agents tested, (NH4)2SO4 was shown to be the most suitable; therefore, it was used as a precipitating agent for testing the effects of parameters influenced by the crystallization for this study.
3. Results and Discussion
Protein crystallization is the process by which a metastable solid form, where highly purified homogeneous protein molecules are three dimensionally perfectly arranged into a protein crystal. In another word, it is a process of protein crystal formation via mechanisms of protein crystal growth. The protein crystallization point lies in general just below the precipitating point of a protein. There are many parameters that influence by the protein crystallization. We represent the results from the effects of parameters that influenced by RuBisCO crystallization from A. eutrophus. In the presence of Mg2+, HCO3− activated ternary complex, and substrate analogue, the CABP inhibited the quaternary RuBisCO single crystal was formed. This resulted from CABP inhibited microcrystal droplets and precipitate droplets that had been dissolved in an isolation buffer over a period of days at 4 °C without mechanical intervention. The dissolved RuBisCO samples were then purifieded with a gel filtration to separate the denatured RuBisCO before crystallization. These were then compared with those from the freshly purified RuBisCO crystals.
3.1. Effect of Temperature and pH Values
The dependence of protein solubility on temperature resulted from the change in the acid/base reaction constant of the protein side chains as a function of temperature [17,18]. In addition, the pKa values of the ionizable groups were strictly related to the median ionic strength.
To correlate this phenomenon with salt and the change in precipitation characteristics, changes in conductivity with respect to concentration were determined and the effect of some parameters (temperature, pH, precipitant) on crystallization of RuBisCO from A. eutrophus was confirmed by electrical conductivity as shown in Figure 1. This method is relatively simple and different from the method described in a review by Nanev C.N. [19].
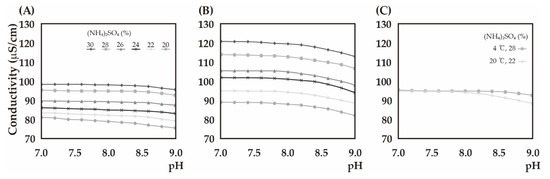
Figure 1.
The electrical conductivity depends on pH, temperature and concentration of the precipitate. (A) The conductivity values (pH 7–9) depending on various (NH4)2SO4 concentrations at 4 °C; (B) Those values at 20 °C (C) The overlapping conductivity values between 28% saturated (NH4)2SO4 at 4 °C and those of 22% (NH4)2SO4 at 20 °C. The conductivity values depended upon (NH4)2SO4 concentrations were represented differently marked lines in the broken line graphs.
Fresh purified and/or recycled from microcrystals and precipitates droplets of RuBisCOs from A. eutophus were crystallized separately with 22% saturated (NH4)2SO4 as a precipitating agent at 20 °C, while they were crystallized with 28% saturated (NH4)2SO4 at 4 °C. The conductivity values for 22% saturated (NH4)2SO4 at 20 °C and those of 28% (NH4)2SO4 at 4 °C were very similar to one another between pH 7.0 and 8.0. This suggests that the conductivity values can provide information as to how one can obtain crystals with different salts as precipitating agents. Crystallization conditions of other salts such as Na2SO4 or MgSO4 as precipitating agents shown in Table 2 were resulted from the conductivity measurement experiment.

Table 2.
Crystallization conditions and methods from both RuBisCO samples.
The monoclinic morphology of RuBisCO crystals from A. eutrophus as decribed Section 3.2 in detail, is an unexpected result of the temperature effect on protein crystallization. The linbro plate which contained precipitated RuBisCO droplet on the concave of the bridge had been transferred from a temperature at 20 °C into a cooling room at 4 °C. This process might be induced the solubility change of RuBisCO sample in the droplet. The temperature affected the normal or retrograde solubility of RuBisCO samples. This might be a scientific plausible explanation how this monoclinic morphology of RuBisCO crystals could grow. The other plausible explanation in detail has been described in Section 3.2.
pH influences the nature of protein-protein interactions, which modify the potential for salt bridge and hydrogen bond formation. This is important for the formation of specific crystal contacts [20]. RuBisCO from A. eutophus showed a maximal enzyme activity in the range of pH 7.8–8.2 [21]. The morphology of orthorhombic and/or monoclinic RuBisCO crystals was dependent upon pH of crystallization conditions.
The first RuBisCO with Mg2+ and HCO3− activated ternary complex was crystallized at room temperature by Bowien B. et al. [22]. Between pH 7.0 and pH 8.4 the quaternary RuBisCO with Mg2+, HCO3− and CABP was crystallized as orthorhombic which has been reported by Pal G.P. et al. [23]. The same quaternary RuBisCO samples either from fresh prepared or dissolved both from microcrystals droplets and precipitated droplets through a gel filtration recycled, were crystallized as monoclinic beyond pH 8.4 as represented in Figure 2, Figure 3D and Figure S2. This crystal morphology is new and indicates that pH change can induce to other morphologies of protein crystals. Whether this new crystal morphology has a merit for 3D structure determination or not is another matter. This result suggests that pH changes mainly influence to growing other morphologies of protein crystals.
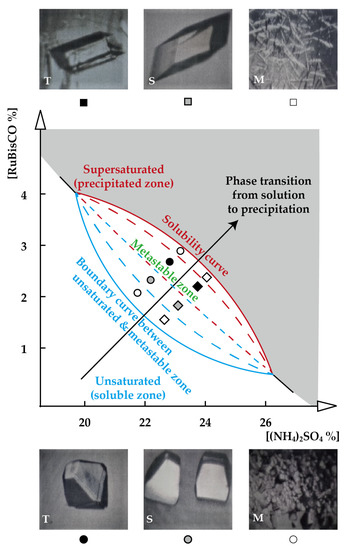
Figure 2.
A canoe-shaped RuBisCO crystallization phase diagram The numbers of crystallization droplets which were observed orthorhombic twin crystals (T) are represented as  , those of single crystals (S) are denoted as
, those of single crystals (S) are denoted as  , and those of microcrystals (M) represented as
, and those of microcrystals (M) represented as  , while the numbers of crystallization droplets observed monoclinic twin crystals (T) are marked as
, while the numbers of crystallization droplets observed monoclinic twin crystals (T) are marked as  , those of monoclinic single crystals (S) are represented as
, those of monoclinic single crystals (S) are represented as  , and those of microcrystals (M) are marked as
, and those of microcrystals (M) are marked as  . The individual symbols in the canoe-shaped diagram denote the areas within the boundaries where characteristic morphologies of RuBisCO crystals were grown. Two upper –and down parts’ areas, where microcrystals were grown in the diagram, are significantly different from each other. The extended lines from the two tips of the canoe-shaped diagram where do not exist the metastable zone, were observed either aggregates (precipitates) of RuBisCOs or occasionally salts crystals in the crystallization droplets.
. The individual symbols in the canoe-shaped diagram denote the areas within the boundaries where characteristic morphologies of RuBisCO crystals were grown. Two upper –and down parts’ areas, where microcrystals were grown in the diagram, are significantly different from each other. The extended lines from the two tips of the canoe-shaped diagram where do not exist the metastable zone, were observed either aggregates (precipitates) of RuBisCOs or occasionally salts crystals in the crystallization droplets.
 , those of single crystals (S) are denoted as
, those of single crystals (S) are denoted as  , and those of microcrystals (M) represented as
, and those of microcrystals (M) represented as  , while the numbers of crystallization droplets observed monoclinic twin crystals (T) are marked as
, while the numbers of crystallization droplets observed monoclinic twin crystals (T) are marked as  , those of monoclinic single crystals (S) are represented as
, those of monoclinic single crystals (S) are represented as  , and those of microcrystals (M) are marked as
, and those of microcrystals (M) are marked as  . The individual symbols in the canoe-shaped diagram denote the areas within the boundaries where characteristic morphologies of RuBisCO crystals were grown. Two upper –and down parts’ areas, where microcrystals were grown in the diagram, are significantly different from each other. The extended lines from the two tips of the canoe-shaped diagram where do not exist the metastable zone, were observed either aggregates (precipitates) of RuBisCOs or occasionally salts crystals in the crystallization droplets.
. The individual symbols in the canoe-shaped diagram denote the areas within the boundaries where characteristic morphologies of RuBisCO crystals were grown. Two upper –and down parts’ areas, where microcrystals were grown in the diagram, are significantly different from each other. The extended lines from the two tips of the canoe-shaped diagram where do not exist the metastable zone, were observed either aggregates (precipitates) of RuBisCOs or occasionally salts crystals in the crystallization droplets.
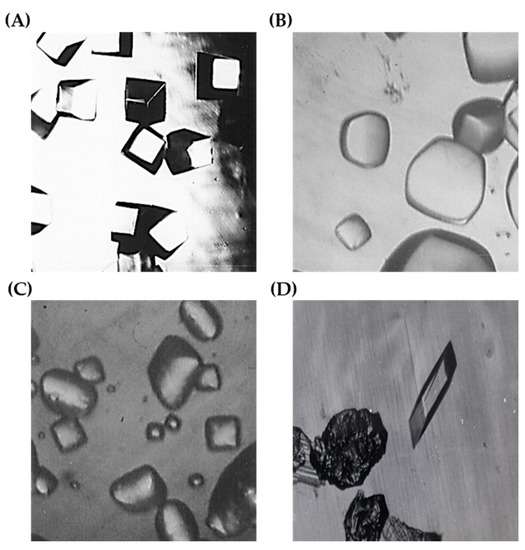
Figure 3.
Differences in RuBisCO crystals depend upon the ages of enzymes. (A) Crystals from fresh purified RuBisCO, (B) Crystals from 3-months-old purified RuBisCO kept by 50% (NH4)2SO4 suspension at 4 °C, (C) Crystals from 6 months old purified RuBisCO kept by 50% (NH4)2SO4 suspension at 4 °C. (D) Monoclinic morphology of RuBisCO single crystal and poly-crystals which were recycled from microcrystals droplets and precipitated droplets.
After the crystallization setup, we observed the droplets on the concave of the bridge which were sealed with cover glasses, manually every two days for three weeks under a microscope. Results were recorded in tables in the laboratory notebook. Photos were occasionally taken and recorded. To avoid confusion when controls were carried out, a serial number and setup date were indicated on the cover and the bottom of every linbro plate.
After one week, the morphology differences could be observed after a control check setup. Two different morphologies of crystals were studied with X-ray to determine the space group. Regardless of the crystal morphologies, crystal growth was dependent on the number of nucleation seeds. Crystals moved within a droplet. The movement was either a zig-zag motion or they would sometimes roll. These movements are necessary in searching neighbor RuBisCO molecules for their growth. Such movement could be caused by a twin formation of RuBisCO crystals.
3.2. Effect of Protein Concentration and Dependence on the Volume of the Crystallization Droplet
The effect of protein concentration was determined for a range of 1% to 4% at various pH values. After 3-weeks incubation, the formation of single-, twin- and/or micro crystals and even poly crystals depended heavily on protein concentration. It is interesting that the formations of single-, twin- and/or micro crystals and even poly crystals’ morphology of orthorhombic and monoclinic of RuBisCO crystals were not significantly different at 20 °C from those at 4 °C. However, 22% saturated (NH4)2SO4 concentrations at 20 °C and 28% saturated (NH4)2SO4 concentration at 4 °C for the crystallization of RuBisCO from A. eutrophus, are fairly different from one another. As shown in Figure S2, the boundary between orthorhombic and monoclinic morphologies of RuBisCO crystals was pH 8.4. This boundary is marked as a vertical red dotted line. Figure S2 represents the diagram of the effects of pH, protein concentration complexity by the crystallization of RuBisCO from A. eutrophus. We represent a canoe-shaped RuBisCO crystallization phase diagram in Figure 2. This crystallization phase diagram was deduced from the raw data of Figure S2. This crystallization phase diagram is unique for RuBisCO from A. eutrophus and is not consistent with either the diagram of human carbonic anhydrase IX by Koruza K. et al. [24] or nucleation and growth of protein crystals: general principles and assays in Methods in Enzymology [25]. We can understand easily from this canoe-shaped crystallization phase transition diagram both the salt-in effect and the salting out effect simultaneously which are required to crystallize a protein with precipitating agent as salts. A new insight into the well-established protein crystallization phase diagram might be arisen from the canoe-shaped RuBisCO crystallization phase diagram.
A surprising and unexpected result came by routine observation later in the month. From a 4% recycled RuBisCO sitting drop crystallization droplet was a perfectly grown single crystal and polycrystals as shown in Figure 3D. This unexpected RuBisCO crystal diffracts better than 2.2 Å. This crystal appeared from a droplet that was completely precipitated for a period of 3 weeks with regular checks. The linbro plate was left at 4 °C and was left unchecked for a month. It is hard to explain this phenomenon according to the theory that the crystallization point lies just below the precipitating point. A plausible explanation might be that we could not observe the nucleation of too tiny crystal nucleation seeds caused by too rapidly formed overclouded surrounded precipitates of RuBisCO molecules. The velocity of the transition from solution to precipitation was too rapid to observe and thereby grasp the crystallization point of RuBisCO as most of RuBisCO molecules themselves. However, after several days and even up to a month later, these nucleation seed crystals, in certain circumstances, came out from the surrounded precipitates and started to grow through sucking the precipitated, perfectly folded RuBisCO molecules. We could not observe this crystal growth under the microscope by through regular checks. During crystal growth, the surroundings of precipitates become clear because of decrease of the precipitates which are perfectly folded RuBisCO molecules. Because of the surrounding environment, growing RuBisCO crystal could not move freely. Therefore, rolling and zig-zag motion of RuBisCO crystals were not possible because of still much existing surrounded precipitates. Longer than a month, by chance could be seen wonderfully perfectly grown large single crystal as an outlier and too tiny crystal nucleation seeds which were located at almost the same position and suddenly covered with precipitates and by chance came out and started to grow finally as polycrystals of RuBisCO as represented in Figure 3D. Expectedly all precipitates surrounded crystal was totally disappeared. X-ray data analysis of this recycled unexpected RuBisCO crystal diffracted better than 2.2 Å is in progress.
Preliminary X-ray studies on the orthorhombic single and twin crystals from A. eutrophus have been reported [23]. However, monoclinic space group of RuBisCO crystals from A. eutrophus for the first resulted from this study. Preliminary X-ray data of differently crystallized RuBisCO crystals from A. eutrophus were represented in Table S1.
The optimal concentration varies with the protein used for the crystallization. Generally, the higher concentration of protein is more favorable as it appears to provide more opportunity for nucleation to occur. However, too high of a protein concentration can lead to an excess of nuclei and fewer large crystals and/or even polycrystals. Clearly, the more that is known about the solubility properties of the protein to be crystallized, the easier it will be to make the necessary adjustments for growing suitable single crystals [26] for X-ray analysis.
After determining the effect of protein concentration on crystallization, the amount of enzyme was varied (2 μL, 4 μL, 6 μL and 8 μL). The purpose of this experiment was to grow large single crystals. In crystallization sets where the volume of protein was 2–4 μL, a large number of small crystals grew. In contrast, with 8 μL, only 5–6 crystals grew in each crystallization set, which were usually 0.3 × 0.4 × 0.6 mm in size. The size of the crystals at a concentration of 2% was proportional to the amount of enzyme in the experiment.
3.3. Effect of Precipitating Agents
RuBisCO from A. eutrophus was crystallized using various precipitating agents and crystallization methods (Table 2).
Chemical compounds that reduce protein solubility are referred to as crystallizing (or precipitating) agents. They reinforce the attractions among bio-macromolecules and act either by altering the activity coefficient of water (salts) [27,28], changing the dielectric constant of the solvating medium (organic solvents), or increasing molecular crowding (high molecular weight polymers like PEG) [29]. Precipitants that act by different mechanisms show little exchangeability. Crystals obtained with one type of precipitant do not commonly form if the precipitant is changed with a functionally different one. However, it has been thoroughly demonstrated that combinations of mechanistically distinct precipitating agents can be synergistic and increase the probability of crystal growth.
3.4. Effect of Inhibitor and Dependence on RuBisCO Age
In general, it is more interesting to crystallize a protein together with a ligand as an apoenzyme. From the structure of such complexes, it may be possible to elucidate the biochemical reaction mechanism. For this reason, RuBisCO was co-crystallized with a transition state analog of its substrate. This analog CABP, binds very strongly to RuBisCO (Kd < 10 pM) [30]. Since CABP at a pH below 6 is present as a lactone, the ligand was added to an equal volume of 1 M Tris-HCl, pH 9, and incubated for 24 h at 20 °C. CABP was used in the crystallization experiments at a molar ratio of 1:16 (100% over excess), as each of the eight catalytic centers binds to CABP. When the buffered CABP solution was added to the protein-containing isolation buffer, the pH and ionic strength (conductivity) were changed. The 100% over excess of CABP was removed through a gel filtration, therefore in the outlier crystal in Figure 3D is the molar ratio between RuBisCO and CABP is 1:8. The Kd value of CABP to RuBisCO is extremely low, therefore dissociation of CABP from RuBisCO through a gel filtration is excluded. Holzenburg A. et al. presented 5 Å 3D structure model for the A. eutrophus RuBisCO in Nature (Table S2) [31]. They reported that the local 4-fold axes of the two L4S4 halves do not coincide but are shifted by 36 Å. This shift is caused by CABP bound to an activated ternary RuBisCO complex. However, there were many suspects about this structure model. Choe H.-W. et al. could not observe such a shift of 36 Å in solution between ternary activated RuBisCO and quaternary CABP bound inhibited RuBisCO through combining photon correlation and sedimentation analysis [32]. The structure of inactivated RuBisCO from A. eutrophus has been determined to 2.7 Å resolution by Hansen S. et al. and published [33]. They reported that the crystal structure of RuBisCO from A. eutrophus reveals a novel central eight-stranded beta-barrel formed by beta-strands from four subunits.
However, RuBisCO crystal by Hansen S. et al. and RuBisCO crystal in the current study are different from each other. RuBisCO crystals by Hansen S. et al. were crystallized in absence of Mg2+, HCO3−, therefore totally ab initio inactivated RuBisCO (Table S2) [33]. The RuBisCO in current crystal has been crystallized in presence of Mg2+, HCO3−, and inactivated through binding of CABP. This is a quaternary structure of RuBisCO. The current representing crystal structure analysis is required for clarification of a 36 Å shift between the two L4S4 halves through CABP bound to activated RuBisCO complex which could not observed by Choe H.-W. et al. in solution between ternary activated RuBisCO and quaternary CABP bound inhibited RuBisCO through combining photon correlation and sedimentation analysis. Presently, there are still not better than 5 Å resolution X-ray structures either with Mg2+, HCO3− activated ternary complex or a Mg2+, HCO3− and CABP inactivated quaternary complex of X-ray 3D structures from A. eutrophus [32].
RuBisCO from A. eutrophus lost less than 10% of its activity in a 50% (NH4)2SO4 suspension in isolation buffer within 6 months at 4 °C. Crystallization experiments were conducted with protein stored for different lengths of time. It is clear that the quality of the crystals clearly depends on storage time (Figure 3A–C). Using fresh protein, crystals were obtained that showed more uniformity, better morphology, and were more suitable for X-ray study compared with crystals formed from aged proteins. The recycled CABP bound RuBisCO samples from dissolving microcrystals and precipitated droplets within a month could be grown and were not significantly different from the fresh purified RuBisCO samples. CABP bound RuBisCO samples which were dissolved from microcrystals and precipitated droplets, have been undertaken a gel filtration to separate the denatured RuBisCO before. As an outlier recycled CABP bound RuBisCO crystal diffracts better than 2.2 Å. This crystal picture is represented in Figure 3D. This crystal picture represents how the uniformity of crystals can be improved by dissolving the aged microcrystals and precipitated samples through a simple gel filtration to separate the denatured proteins. This simple gel filtration might be a clue to grow a much better diffracted RuBisCO crystal. This sample was a homogeneous stoichiometric exact quaternary complex of RuBisCO. This sample was contained neither the denatured RuBisCOs nor the excess of CABP through a simple gel filtration.
3.5. Effect of Metal Ions
The active form of RuBisCO is a ternary complex with an allosteric effector, CO2 [34], and a divalent metal ion [35,36,37]. As a Me2+ ion, Mg2+ exhibited the highest carboxylation activation. For the oxygenase reaction, however, Mn2+ and Co2+ as cofactors were more effective [38,39], which indicates different binding sites for the cations in the two reactions. For this reason, experiments were done using Mn2+ or Co2+ ions instead of Mg2+ for crystallization. Both ternary and quaternary complexes were crystallized with 10 mM Co(NO3)2 and MnC12, respectively, instead of MgC12 in the isolation buffer. With Mn2+ and Co2+, RuBisCO crystals of the same morphology were formed as with Mg2+ (Figure 3A). The crystals of the complex with Mn2+ exhibited round edges. For the flame tests, Mn2+ or Co2+ contained RuBisCO crystals were washed thoroughly with isolation buffer without metal ions, respectively. The flame test was performed with dissolved crystal solutions. We could see the characteristic flame colors of Mn2+ or Co2+. We, therefore, are sure RuBisCO crystal bound Mn2+ or Co2+ ions in crystals. However, showing the positions of each metal ion in the RuBisCO molecule is beyond the scope of this study, although it is very interesting question. The refined crystal 3D structures of both BuBisCO crystals either with Mn2+ or Co2+ will give the exact positions in the RuBisCO structures.
A procedure for the use of additives has recently been proposed [40,41]. In this technique, known as the cross-influence procedure, each crystallization trial utilizes four droplets containing equal volumes of the precipitating agent. The protein is added to one of the droplets, whereas additives (metallic salts) are placed in the others. Then, all drops are left to equilibrate against the same reservoir. In some cases, the ions are essential for biological activity and contribute to the maintenance of certain structural features of the protein. In other cases, metal ions stabilize intermolecular contacts in the crystal. Studies have shown that the application of biocompatible water-soluble ionic liquids, organic salts, and salts with melting points at or below 20 °C as crystallization additives provides very interesting results [42,43].
3.6. Seeding with Crystal Nucleus
It is often desirable to reproduce previously grown crystals of a protein in which either the formation of nuclei is limited or spontaneous nucleation occurs at such a profound level of supersaturation that poor growth patterns result. In such cases, it is desirable to induce growth in a directed fashion at low levels of supersaturation. This can sometimes be accomplished by seeding a metastable, supersaturated protein solution with crystals from earlier trials. These seeding techniques [44,45] fall into two categories: those employing microcrystals as seeds and those using larger macro seeds. For both methods, the fresh solution to be seeded should only be slightly supersaturated so that controlled, slow growth occurs. The two approaches have been described elsewhere in some detail [46,47].
The purpose of seeding is to limit the number of crystals in the crystallization set from the beginning. This increases the likelihood of obtaining large crystals. The collected microcrystals were washed with mother liquor and diluted (1:1000–1:5000) [48,49]. At 1 μL of the seeding, RuBisCO crystals grew, the quality of which was however not different from crystals obtained by other methods. It is recommended to seed at accurate time after set up the crystallization, 3–4 days after set up was the best time to seed by RuBisCO.
It depended on the protein concentration trials in the area of microcrystals without seeding, was more successful than those of high concentration for the RuBisCO crystallization. Caution is required when using crosslinked crystals or long kept crystals as they were not suitable for a seeding experiment. From freshly purified RuBisCO samples, grown microcrystals that are mechanically broken and diluted properly with crystallization buffer produced successfully grown single crystals. There can always be an exception in experimental research. Such an outlier can be a clue for a surprising result.
It is therefore important to continuing the persistent attempts to examine the effects of various parameters on the crystallization of a protein. Crystallization is a physical phenomenon. Observations of perfectly grown crystals under a polarizing microscope leave the impression that crystallization might be regarded not only as a science, but as a work of art.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12020196/s1, Figure S1: (a) Chromatogram of RuBisCO using by DEAE-Sepharose Cl -6B column. The diagonal line indi-cates the KCl concentration gradient. The peaks from I to V were loaded on the SDS-PAGE. Peak V indicates the RuBisCO fractions (Fraction number 62-71). (b) SDS-PAGE. Samples were taken out from the main fractions after the purification by DEAE- sepharose Cl-6B column. The lanes indicate as follows. (1) Marker, (2) Crude extract, (3) Ammonium sulfate fractionation (40%), (4) Peak I, (5) Peak II, (6) Peak III, (7) Peak IV, (8) Peak V, and (9) Side cuts of Peak V from the profile of the chromatogram shown as Figure S1a respectively; Figure S2: Influence of protein concentration on the crystallization of RuBisCO; Table S1: Preliminary X-ray data of differently crystallized RuBisCOs from A. eutrophus; Table S2: X-ray 3D structures of RuBisCOs from A. eutrophus.
Author Contributions
Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing—original draft, Writing—review & editing, H.-W.C. and Y.J.K.; funding acquisition, Y.J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Bio & Medical Technology Development Program and ICT (NRF-2017M3A9F6029733) and Basic Science Research Program of the National Research Foundation (NRF) funded by the Ministry of Science (NRF-2021R1I1A3060013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
H.-W. Choe thanks JBNU and DGIST for his chair professorship in the period of 2014–2021 at JBNU and in the period of 2017–2019 at DGIST. He is currently working as an emeritus professor at the Department of Chemistry, College of Natural Science, JBNU, Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schulz, G.E.; Schirmer, R.H. Principles of Protein Structure; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Doolittle, R.F. Proteins. Sci. Am. 1985, 253, 88. [Google Scholar] [CrossRef] [PubMed]
- Keene, J.D. Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc. Natl. Acad. Sci. USA 2001, 98, 7018–7024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fersht, A. Enzyme Structure and Mechanism; W. H. Freeman and Company: New York, NY, USA, 1977. [Google Scholar]
- Ilari, A.; Savino, C. Protein structure determination by X-ray crystallography. Bioinformatics 2008, 452, 63–87. [Google Scholar]
- Worldwide Protein Data Bank Consortium. Protein Data Bank: The single global archive for 3D macromolecular structure data. Nucleic Acids Res. 2019, 47, D520–D528. [Google Scholar] [CrossRef] [Green Version]
- Sainis, J.; Jawali, N. Channeling of the intermediates and catalytic facilitation to Rubisco in a multienzyme complex of Calvin cycle enzymes. Indian J. Biochem. Biophys. 1994, 31, 215–220. [Google Scholar] [PubMed]
- Ducat, D.C.; Silver, P.A. Improving carbon fixation pathways. Curr. Opin. Chem. Biol. 2012, 16, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ślesak, I.; Ślesak, H. The activity of RubisCO and energy demands for its biosynthesis. Comparative studies with CO2-reductases. J. Plant Physiol. 2021, 257, 153337. [Google Scholar] [CrossRef] [PubMed]
- Andersson, I.; Backlund, A. Structure and function of Rubisco. Plant Physiol. Biochem. 2008, 46, 275–291. [Google Scholar] [CrossRef]
- Choe, H.-W.; Jakob, R.; Hahn, U.; Pal, G.P. Crystallization of the activated ternary complex of ribulose-1, 5-bisphosphate carboxylase-oxygenase isolated from Rhodospirillum rubrum and from an Escherichia coli clone. J. Mol. Biol. 1985, 185, 781–783. [Google Scholar] [CrossRef]
- Spreitzer, R.J. Role of the small subunit in ribulose-1, 5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 2003, 414, 141–149. [Google Scholar] [CrossRef]
- Warburg, O. Isolation and crystallization of enolase. Biochem. Z. 1942, 310, 384–421. [Google Scholar]
- Bowien, B.; Mayer, F.; Codd, G.; Schlegel, H. Purification, some properties and quaternary structure of the D-ribulose 1, 5-diphosphate carboxylase of Alcaligenes eutrophus. Arch. Microbiol. 1976, 110, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Racker, E. [29a] Ribulose diphosphate carboxylase from spinach leaves: Ribulose diphosphate + CO2 + H2O → 2 3-P-Glycerate. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1962; Volume 5, pp. 266–270. [Google Scholar]
- McPherson, A.; Gavira, J.A. Introduction to protein crystallization. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2014, 70, 2–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosenberger, F.; Howard, S.; Sowers, J.; Nyce, T. Temperature dependence of protein solubility—Determination and application to crystallization in X-ray capillaries. J. Cryst. Growth 1993, 129, 1–12. [Google Scholar] [CrossRef]
- Chernov, A.; Komatsu, H. Principles of crystal growth in protein crystallization. In Science and Technology of Crystal Growth; Springer: Berlin/Heidelberg, Germany, 1995; pp. 329–353. [Google Scholar]
- Nanev, C.N. Recent insights into the crystallization process; protein crystal nucleation and growth peculiarities; Processes in the Presence of Electric Fields. Crystals 2017, 7, 310. [Google Scholar] [CrossRef] [Green Version]
- Dumetz, A.C.; Chockla, A.M.; Kaler, E.W.; Lenhoff, A.M. Effects of pH on protein–protein interactions and implications for protein phase behavior. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2008, 1784, 600–610. [Google Scholar] [CrossRef]
- Anderson, L.E.; Fuller, R. Photosynthesis in Rhodospirillum rubrum: IV. ISOLATION AND CHARACTERIZATION OF RIBULOSE 1, 5-DIPHOSPHATE CARBOXYLASE. J. Biol. Chem. 1969, 244, 3105–3109. [Google Scholar] [CrossRef]
- Bowien, B.; Mayer, F.; Spiess, E.; Pähler, A.; Englisch, U.; Saenger, W. On the structure of crystalline ribulosebisphosphate carboxylase from Alcaligenes eutrophus. Eur. J. Biochem. 1980, 106, 405–410. [Google Scholar] [CrossRef]
- Pal, G.P.; Jakob, R.; Hahn, U.; Bowien, B.; Saenger, W. Single and twinned crystals of ribulose-1, 5-bisphosphate carboxylase-oxygenase from Alcaligenes eutrophus. J. Biol. Chem. 1985, 260, 10768–10770. [Google Scholar] [CrossRef]
- Koruza, K.; Lafumat, B.; Nyblom, M.; Knecht, W.; Fisher, Z. From initial hit to crystal optimization with microseeding of human carbonic anhydrase IX—A case study for neutron protein crystallography. Crystals 2018, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Feher, G.; Kam, Z. [4] Nucleation and growth of protein crystals: General principles and assays. Methods Enzymol. 1985, 114, 77–112. [Google Scholar] [PubMed]
- Littlechild, J. Protein crystallization: Magical or logical: Can we establish some general rules? J. Phys. D Appl. Phys. 1991, 24, 111. [Google Scholar] [CrossRef]
- Hofmeister, F. Zur lehre von der wirkung der salze. Arch. Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Kunz, W.; Henle, J.; Ninham, B.W. ‘Zur Lehre von der Wirkung der Salze’ (about the science of the effect of salts): Franz Hofmeister’s historical papers. Curr. Opin. Colloid Interface Sci. 2004, 9, 19–37. [Google Scholar] [CrossRef]
- McPherson, A., Jr. Crystallization of proteins from polyethylene glycol. J. Biol. Chem. 1976, 251, 6300–6303. [Google Scholar] [CrossRef]
- Pierce, J.; Tolbert, N.; Barker, R. Interaction of ribulosebisphosphate carboxylase/oxygenase with transition-state analogs. Biochemistry 1980, 19, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Holzenburg, A.; Mayer, F.; Harauz, G.; Van Heel, M.; Tokuoka, R.; Ishida, T.; Harata, K.; Pal, G.; Saenger, W. Structure of D-ribulose-l, 5-bisphosphate carboxylase/oxygenase from Alcaligenes eutrophyus H16. Nature 1987, 325, 730–732. [Google Scholar] [CrossRef]
- Choe, H.-W.; Georgalis, Y.; Saenger, W. Comparative studies of ribulose-1, 5-biphosphate carboxylase/oxygenase from Alcaligenes eutrophus H16 cells, in the active and CABP-inhibited forms. J. Mol. Biol. 1989, 207, 621–623. [Google Scholar] [CrossRef]
- Hansen, S.; Vollan, V.B.; Hough, E.; Andersen, K. The crystal structure of Rubisco from Alcaligenes eutrophus reveals a novel central eight-stranded β-barrel formed by β-strands from four subunits. J. Mol. Biol. 1999, 288, 609–621. [Google Scholar] [CrossRef]
- Badger, M.; Andrews, T. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem. Biophys. Res. Commun. 1974, 60, 204–210. [Google Scholar] [CrossRef]
- Lorimer, G.H.; Badger, M.R.; Andrews, T.J. The activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions. Equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry 1976, 15, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Paech, C.; Tolbert, N. Active site studies of ribulose-1,5-bisphosphate carboxylase/oxygenase with pyridoxal 5′-phosphate. J. Biol. Chem. 1978, 253, 7864–7873. [Google Scholar] [CrossRef]
- Laing, W.A.; Christeller, J.T. A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem. J. 1976, 159, 563–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robison, P.D.; Martin, M.N.; Tabita, F.R. Differential effects of metal ions on Rhodospirillum rubrum ribulosebisphosphate carboxylase/oxygenase and stoichiometric incorporation of bicarbonate (1-) ion into a cobalt (III)-enzyme complex. Biochemistry 1979, 18, 4453–4458. [Google Scholar] [CrossRef]
- Wildner, G.F.; Henkel, J. Differential reactivation of ribulose 1,5-bisphosphate oxygenase with low carboxylase activity by Mn2+. FEBS Lett. 1978, 91, 99–103. [Google Scholar] [CrossRef] [Green Version]
- Tomčová, I.; Branca, R.M.M.; Bodó, G.; Bagyinka, C.; Kutá Smatanová, I. Cross-crystallization method used for the crystallization and preliminary diffraction analysis of a novel di-haem cytochrome c4. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2006, 62, 820–824. [Google Scholar] [CrossRef] [Green Version]
- Tomčová, I.; Smatanová, I.K. Copper co-crystallization and divalent metal salts cross-influence effect: A new optimization tool improving crystal morphology and diffraction quality. J. Cryst. Growth 2007, 306, 383–389. [Google Scholar] [CrossRef]
- Hekmat, D.; Hebel, D.; Joswig, S.; Schmidt, M.; Weuster-Botz, D. Advanced protein crystallization using water-soluble ionic liquids as crystallization additives. Biotechnol. Lett. 2007, 29, 1703–1711. [Google Scholar] [CrossRef]
- Judge, R.A.; Takahashi, S.; Longenecker, K.L.; Fry, E.H.; Abad-Zapatero, C.; Chiu, M.L. The effect of ionic liquids on protein crystallization and X-ray diffraction resolution. Cryst. Growth Des. 2009, 9, 3463–3469. [Google Scholar] [CrossRef]
- Bergfors, T. Seeds to crystals. J. Struct. Biol. 2003, 142, 66–76. [Google Scholar] [CrossRef]
- Stura, E.A.; Wilson, I.A. Applications of the streak seeding technique in protein crystallization. J. Cryst. Growth 1991, 110, 270–282. [Google Scholar] [CrossRef]
- Fitzgerald, P.M.; Madsen, N.B. Improvement of limit of diffraction and useful X-ray lifetime of crystals of glycogen debranching enzyme. J. Cryst. Growth 1986, 76, 600–606. [Google Scholar] [CrossRef]
- Thaller, C.; Eichele, G.; Weaver, L.; Wilson, E.; Karlsson, R.; Jansonius, J. [9] Seed enlargement and repeated seeding. Methods Enzymol. 1985, 114, 132–135. [Google Scholar] [PubMed]
- Thaller, C.; Weaver, L.; Eichele, G.; Wilson, E.; Karlsson, R.; Jansonius, J. Repeated seeding technique for growing large single crystals of proteins. J. Mol. Biol. 1981, 147, 465–469. [Google Scholar] [CrossRef]
- Smit, J.D.G.; Winterhalter, K.H. Crystallographic data for haemoglobin from the lanceolate fluke Dicrocoelium dendriticum. J. Mol. Biol. 1981, 146, 641–647. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).