Humidity Sensitivity of Hydration of Expansive Agent and Its Expansive Efficiency in Ultra-High Performance Concrete
Abstract
:1. Introduction
2. Materials and Methods
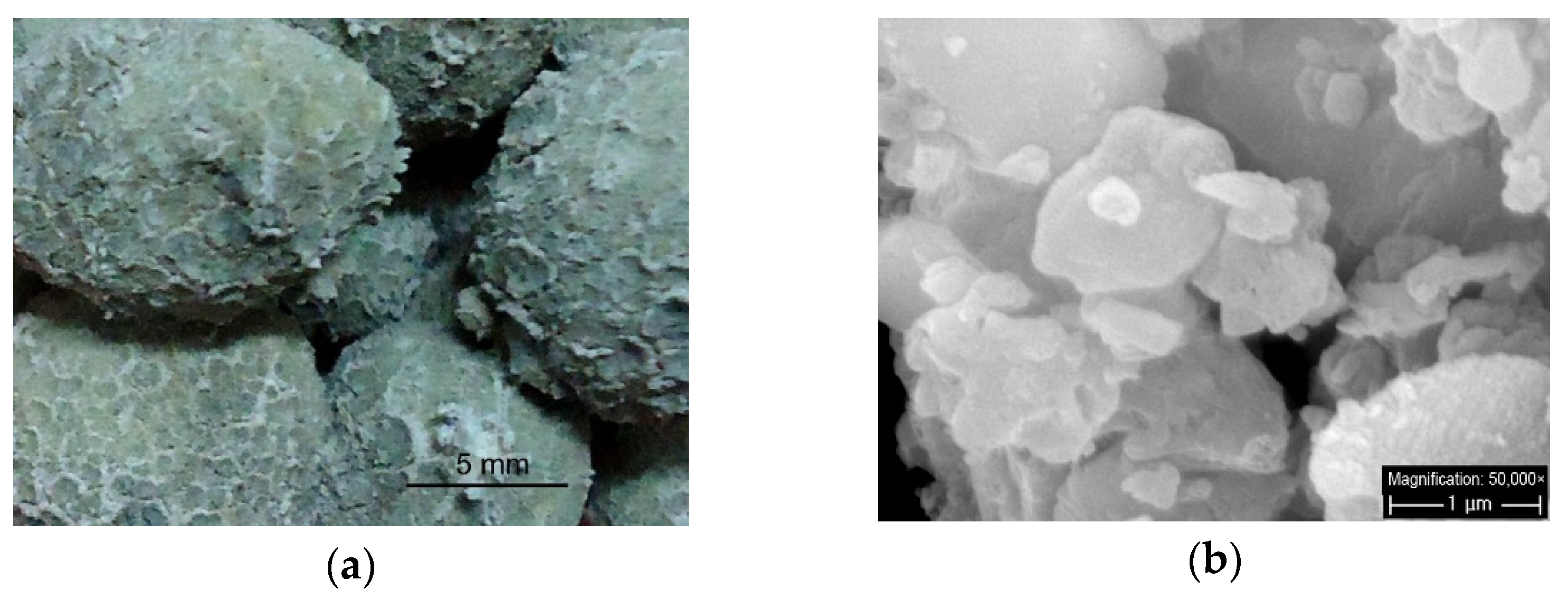
2.1. Materials
2.2. Autogenous Shrinkage
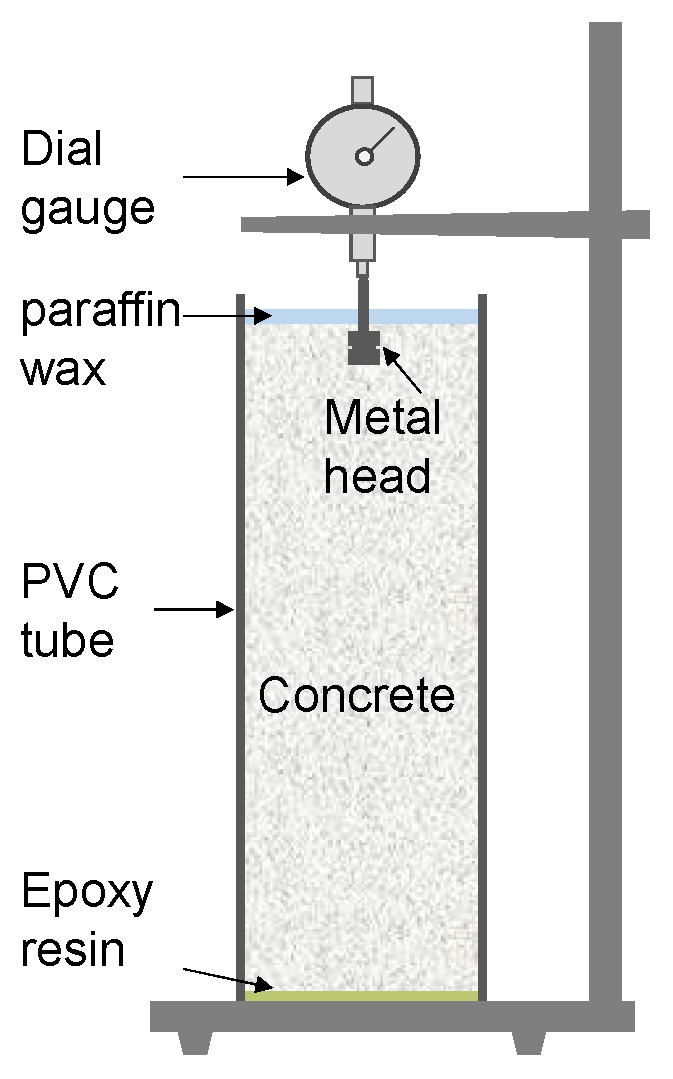
2.3. Internal Relative Humidity
2.4. Hydration of Expansive Agent under Different RH
2.5. Isothermal Calorimetry Measurements
2.6. Micrographs
3. Results and Discussion
3.1. Effect of Expansive Agent on Autogenous Shrinkage of UHPC
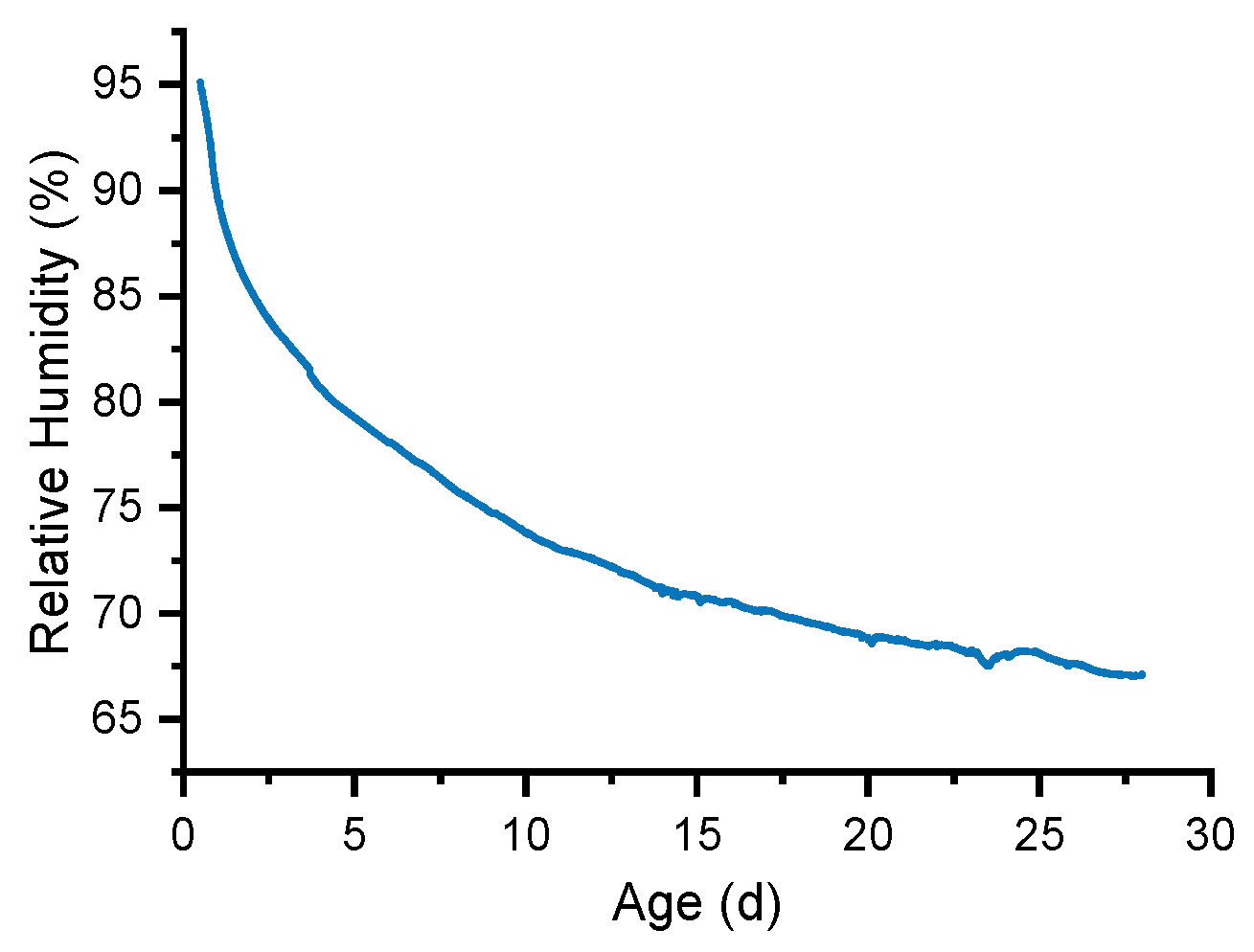
3.2. Internal RH of UHPC
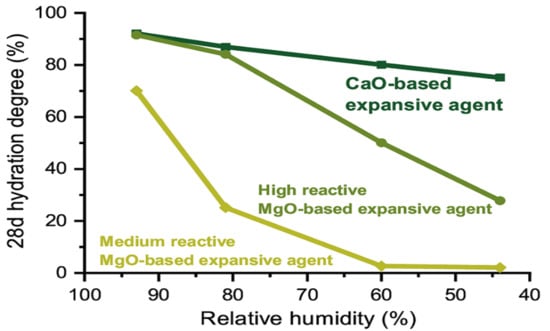
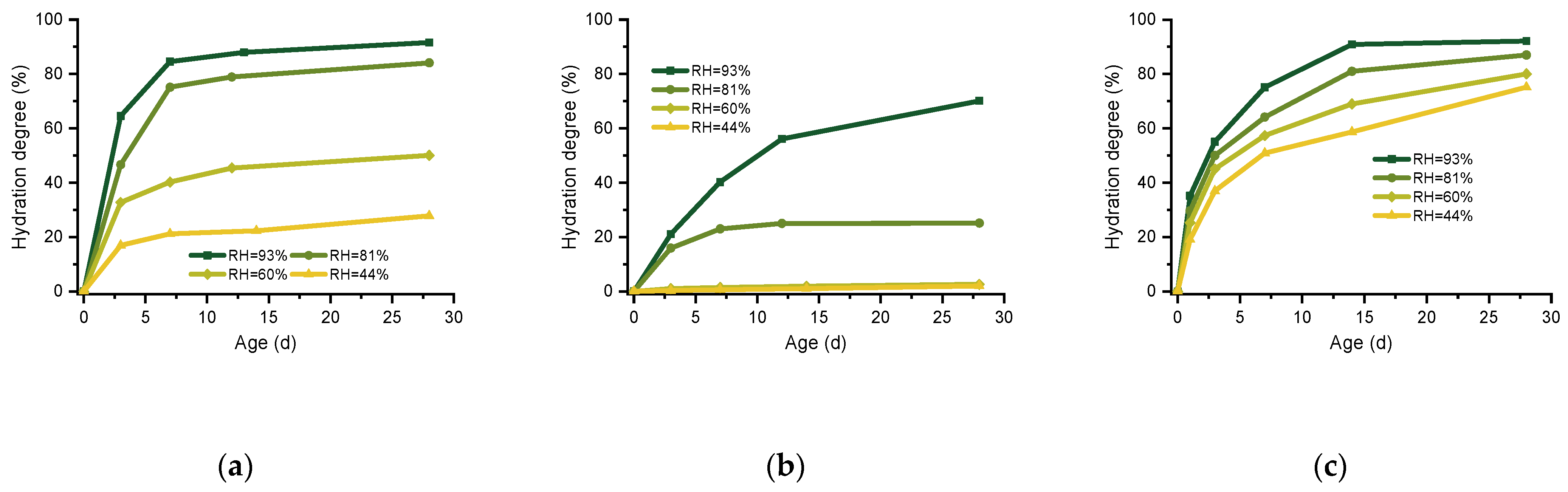
3.3. Hydration Degree of Expansive Agent at Different RH
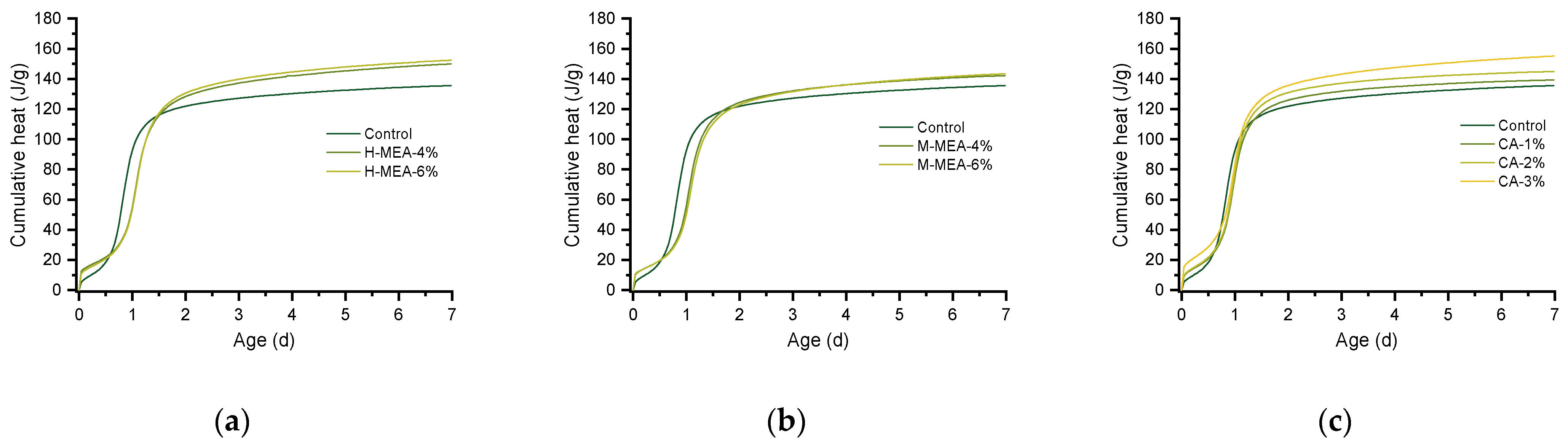
3.4. Hydration Heat of the Paste
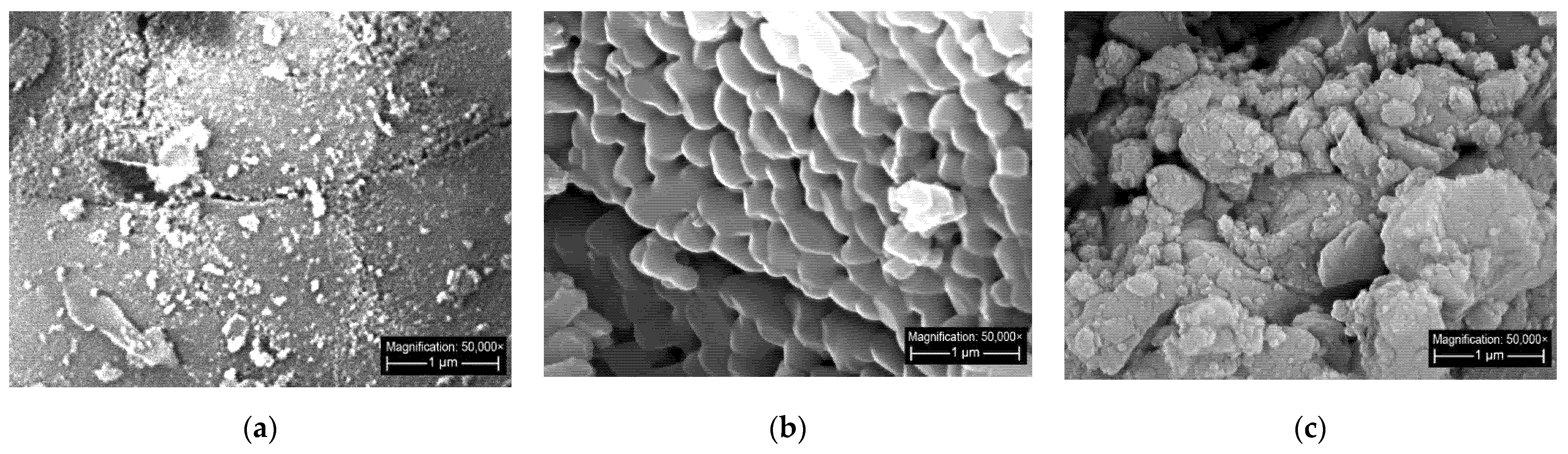
3.5. Micrographs Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Society for Testing and Materials (ASTM). ASTM C1856 Standard practice for fabricating and testing specimens of ultra-high performance concrete. In Book of ASTM Standards; American Society for Testing and Materials: Conshohocken, PA, USA, 2017. [Google Scholar]
- Shi, C.; Wu, Z.; Xiao, J.; Wang, D.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part I. Raw materials and mixture design. Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Wang, D.; Shi, C.; Wu, Z.; Xiao, J.; Huang, Z.; Fang, Z. A review on ultra high performance concrete: Part II. Hydration, microstructure and properties. Constr. Build. Mater. 2015, 96, 368–377. [Google Scholar] [CrossRef]
- Liu, K.; Yu, R.; Shui, Z.; Li, X.; Guo, C.; Yu, B.; Wu, S. Optimization of autogenous shrinkage and microstructure for Ultra-High Performance Concrete (UHPC) based on appropriate application of porous pumice. Constr. Build. Mater. 2019, 214, 369–381. [Google Scholar] [CrossRef]
- Koh, K.; Ryu, G.; Kang, S.; Park, J.; Kim, S. Shrinkage properties of ultra-high Performance concrete (UHPC). Adv. Sci. Lett. 2011, 4, 948–952. [Google Scholar] [CrossRef]
- Kheir, J.; Klausen, A.; Hammer, T.A.; de Meyst, L.; Hilloulin, B.; van Tittelboom, K.; Loukili, A.; de Belie, N. Early age autogenous shrinkage cracking risk of an ultra-high performance concrete (uhpc) wall: Modelling and Experimental Results. Eng. Fract. Mech. 2021, 257, 108024. [Google Scholar] [CrossRef]
- Yang, L.; Shi, C.; Wu, Z. Mitigation techniques for autogenous shrinkage of ultra-high-performance concrete—A review. Compos. Part B Eng. 2019, 178, 107456. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.A.; Costa, H.; Júlio, E.; Portugal, A.; Durães, L. Effect of supplementary cementitious materials on autogenous shrinkage of ultra-high performance concrete. Constr. Build. Mater. 2016, 127, 43–48. [Google Scholar] [CrossRef]
- Valipour, M.; Khayat, K.H. Coupled effect of shrinkage-mitigating admixtures and saturated lightweight sand on shrinkage of UHPC for overlay applications. Constr. Build. Mater. 2018, 184, 320–329. [Google Scholar] [CrossRef]
- Shen, P.; Lu, L.; He, Y.; Wang, F.; Lu, J.; Zheng, H.; Hu, S. Investigation on expansion effect of the expansive agents in ultra-high performance concrete. Cem. Concr. Compos. 2020, 105, 103425. [Google Scholar] [CrossRef]
- Li, S.; Mo, L.; Deng, M.; Cheng, S. Mitigation on the autogenous shrinkage of ultra-high performance concrete via using mgo expansive agent. Constr. Build. Mater. 2021, 312, 125422. [Google Scholar] [CrossRef]
- Soliman, A.M.; Nehdi, M.L. Effects of shrinkage reducing admixture and wollastonite microfiber on early-age behavior of ultra-high performance concrete. Cem. Concr. Compos. 2014, 46, 81–89. [Google Scholar] [CrossRef]
- Justs, J.; Wyrzykowski, M.; Bajare, D.; Lura, P. Internal curing by superabsorbent polymers in ultra-high performance concrete. Cem. Concr. Res. 2015, 76, 82–90. [Google Scholar] [CrossRef]
- Liu, J.; Farzadnia, N.; Khayat, K.H.; Shi, C. Effects of SAP characteristics on internal curing of UHPC matrix. Constr. Build. Mater. 2021, 280, 122530. [Google Scholar] [CrossRef]
- Liu, J.; Han, F.; Cui, G.; Zhang, Q.; Lv, J.; Zhang, L.; Yang, Z. Combined effect of coarse aggregate and fiber on tensile behavior of ultra-high performance concrete. Constr. Build. Mater. 2016, 121, 310–318. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Banthia, N. Mechanical properties of ultra-high-performance fiber-reinforced concrete: A review. Cem. Concr. Compos. 2016, 73, 267–280. [Google Scholar] [CrossRef]
- Li, P.P.; Yu, Q.L.; Brouwers, H.J.H. Effect of coarse basalt aggregates on the properties of Ultra-high Performance Concrete (UHPC). Constr. Build. Mater. 2018, 170, 649–659. [Google Scholar] [CrossRef]
- Szostak, B.; Golewski, G.L. Rheology of cement pastes with siliceous fly ash and the csh nano-admixture. Materials 2021, 14, 3640. [Google Scholar] [CrossRef]
- Hisseine, O.A.; Soliman, N.A.; Tolnai, B.; Tagnit-Hamou, A. Nano-engineered ultra-high performance concrete for controlled autogenous shrinkage using nanocellulose. Cem. Concr. Res. 2020, 137, 106217. [Google Scholar] [CrossRef]
- Liu, C.; He, X.; Deng, X.; Wu, Y.; Zheng, Z.; Liu, J.; Hui, D. Application of Nanomaterials in Ultra-High Performance Concrete: A Review. Nanotechnol. Rev. 2020, 9, 1427–1444. [Google Scholar] [CrossRef]
- Mehta, P.K. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Tian, Q.; Zhao, H.; Lu, A.; Liu, J. Temperature sensitivity of MgO expansive agent and its application in temperature crack mitigation in shiplock mass concrete. Constr. Build. Mater. 2018, 170, 613–618. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]
- Loukili, A.; Khelidj, A.; Richard, P. Hydration kinetics, change of relative humidity, and autogenous shrinkage of ultra-high-strength concrete. Cem. Concr. Res. 1999, 29, 577–584. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G. Examining the “time-zero” of autogenous shrinkage in high/ultra-high performance cement pastes. Cem. Concr. Res. 2017, 97, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Deng, M.; Tang, M.; Al-Tabbaa, A. MgO expansive cement and concrete in China: Past, present and future. Cem. Concr. Res. 2014, 57, 430–436. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Wang, Y.; Liu, J.; Tian, Q. Effect of CaO and MgO based expansive agent on deformation and mechanical properties of concrete-filled steel tubes. Constr. Build. Mater. 2020, 250, 118723. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). ASTM C403 Standard test method for time of setting of concrete mixtures by penetration resistance. In Book of ASTM Standards; American Society for Testing and Materials: Conshohocken, PA, USA, 2016. [Google Scholar]


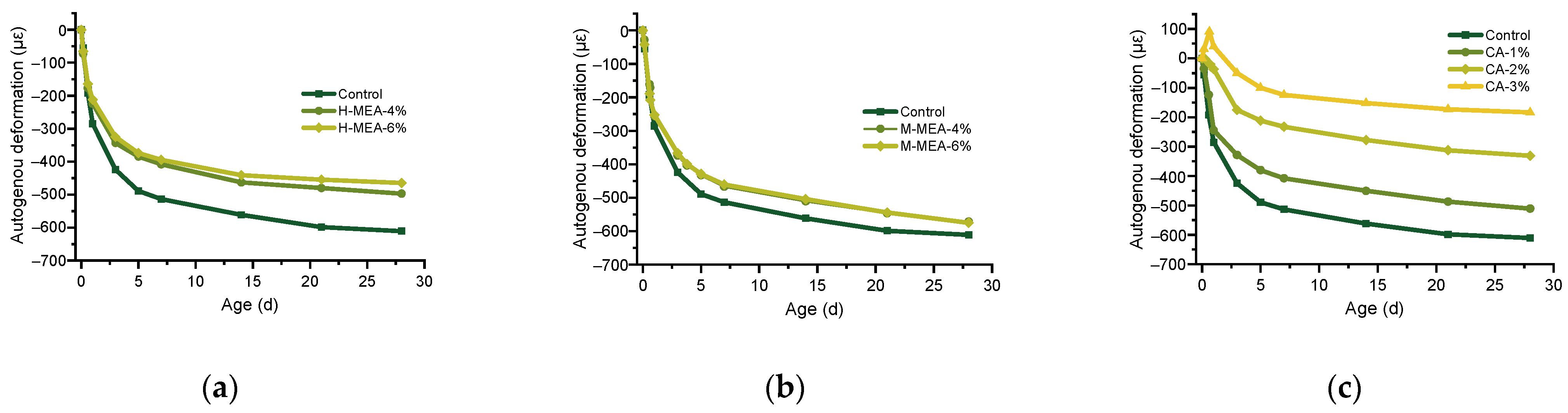
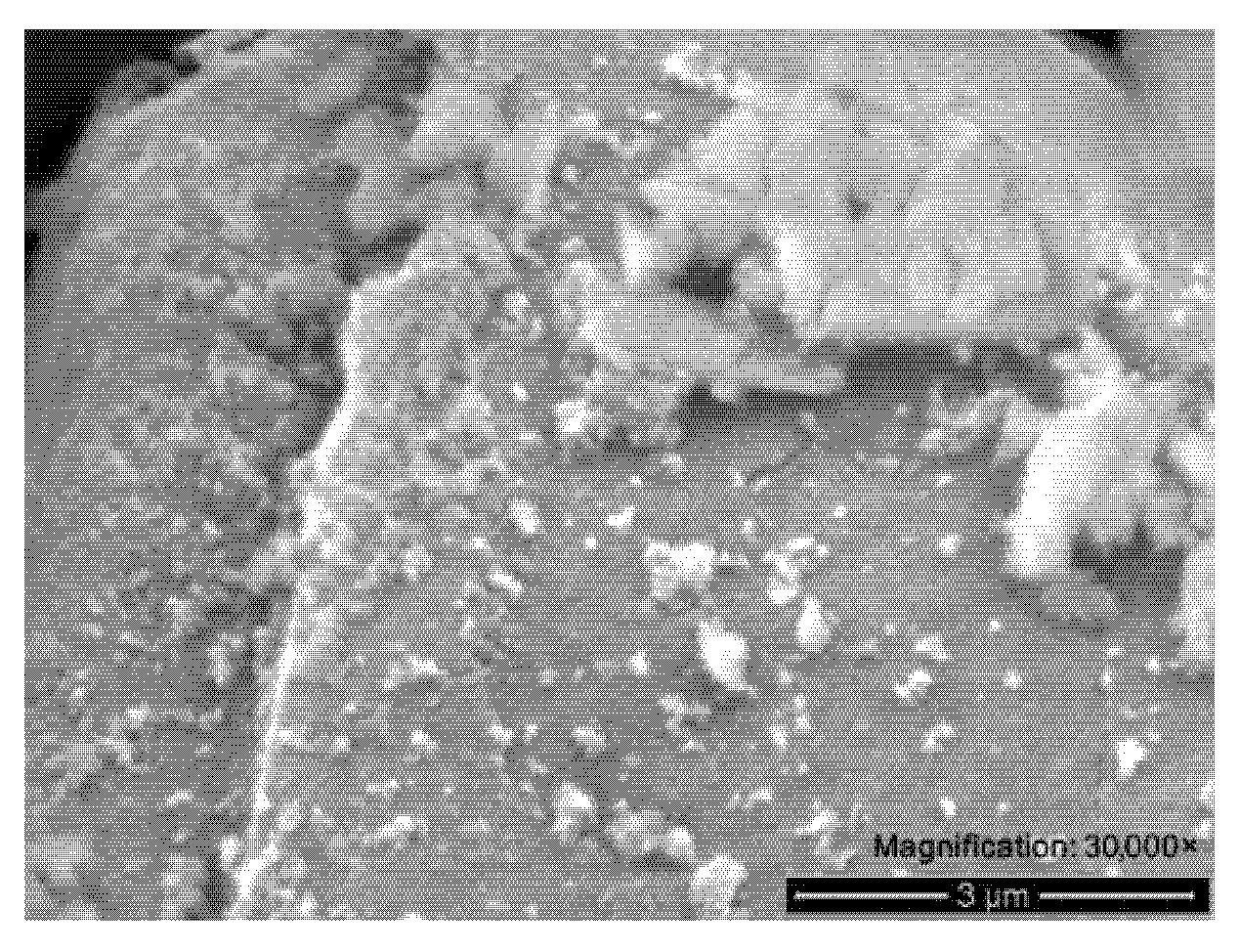
| CaO | MgO | Fe2O3 | Al2O3 | SiO2 | SO3 | Loss | |
|---|---|---|---|---|---|---|---|
| Cement | 64.21 | 1.55 | 3.94 | 4.29 | 19.89 | 3.25 | 2.31 |
| Silica fume | 0.56 | 2.10 | 0.75 | 0.06 | 93.55 | / | 2.98 |
| H-MEA | 1.78 | 94.8 | 0.93 | 0.18 | 0.52 | / | 1.76 |
| M-MEA | 1.86 | 95.8 | 1.02 | 0.17 | 0.54 | / | 0.57 |
| CA | 81.9 | 1.5 | 0.23 | 3.33 | 1.18 | 10.40 | 1.35 |
| Water | Cement | Silica Fume | Fine Aggregate | Coarse Aggregate | H-MEA | M-MEA | CA | Superplasticizer | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 201 | 1125 | 59 | 592 | 480 | / | / | / | 13 |
| H-MEA-4% | 201 | 1080 | 57 | 592 | 480 | 47 | / | / | 13 |
| H-MEA-6% | 201 | 1057 | 56 | 592 | 480 | 71 | / | / | 13 |
| M-MEA-4% | 201 | 1080 | 57 | 592 | 480 | / | 47 | / | 13 |
| M-MEA-6% | 201 | 1057 | 56 | 592 | 480 | / | 71 | / | 13 |
| CA-1% | 201 | 1114 | 59 | 592 | 480 | / | / | 12 | 13 |
| CA-2% | 201 | 1102 | 58 | 592 | 480 | / | / | 24 | 13 |
| CA-3% | 201 | 1091 | 57 | 592 | 480 | / | / | 36 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tian, Q.; Li, H.; Wang, Y. Humidity Sensitivity of Hydration of Expansive Agent and Its Expansive Efficiency in Ultra-High Performance Concrete. Crystals 2022, 12, 195. https://doi.org/10.3390/cryst12020195
Wang Y, Tian Q, Li H, Wang Y. Humidity Sensitivity of Hydration of Expansive Agent and Its Expansive Efficiency in Ultra-High Performance Concrete. Crystals. 2022; 12(2):195. https://doi.org/10.3390/cryst12020195
Chicago/Turabian StyleWang, Yujiang, Qian Tian, Hua Li, and Yang Wang. 2022. "Humidity Sensitivity of Hydration of Expansive Agent and Its Expansive Efficiency in Ultra-High Performance Concrete" Crystals 12, no. 2: 195. https://doi.org/10.3390/cryst12020195
APA StyleWang, Y., Tian, Q., Li, H., & Wang, Y. (2022). Humidity Sensitivity of Hydration of Expansive Agent and Its Expansive Efficiency in Ultra-High Performance Concrete. Crystals, 12(2), 195. https://doi.org/10.3390/cryst12020195