Synthesis, Crystal Structure, and Optical Properties of a Trinuclear Zinc(II) Complex with Rhodamine B
Abstract
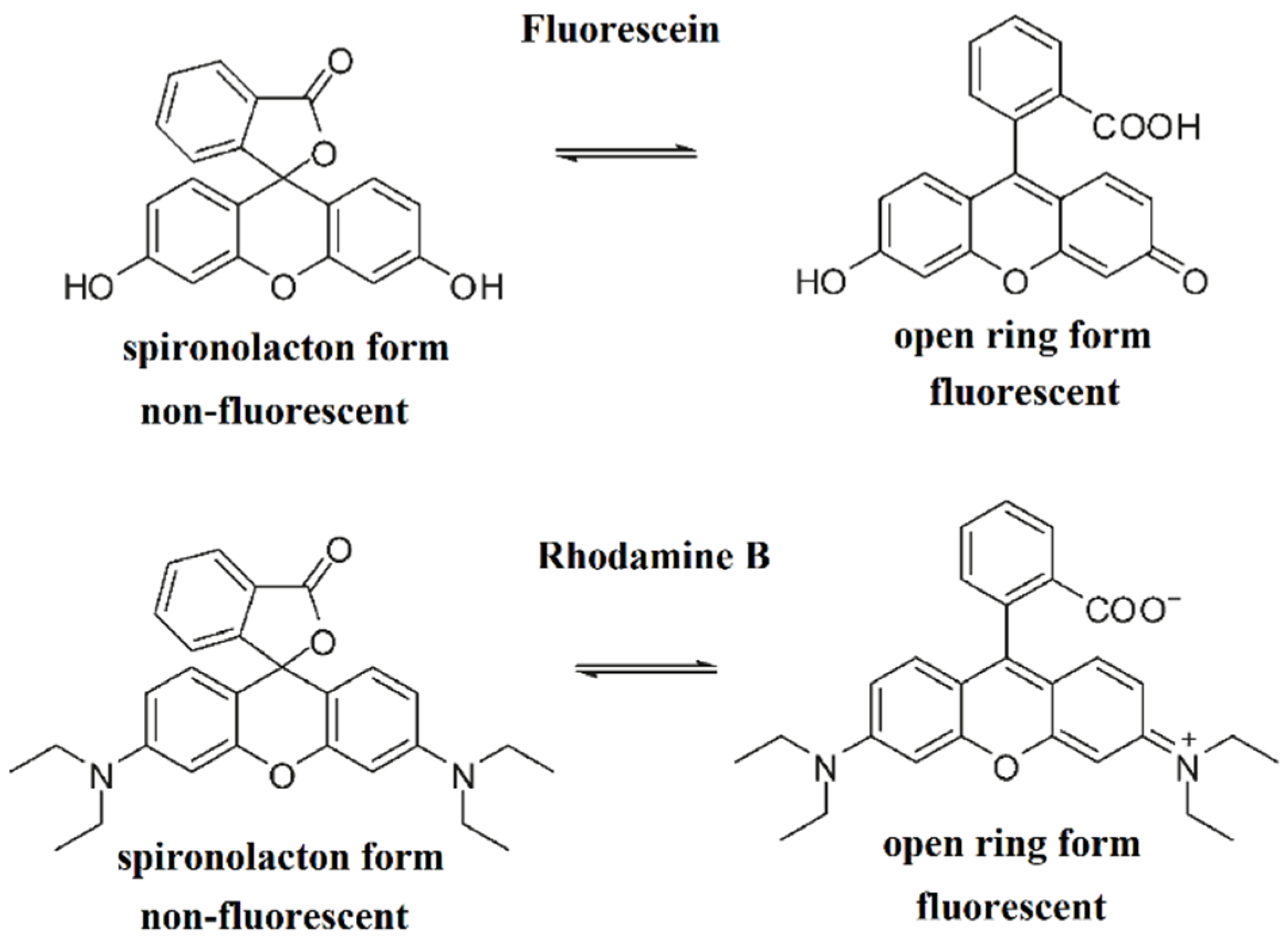
:1. Introduction
2. Materials and Methods
2.1. Synthesis of [Zn3(valhydr)2(RhB)2(EtOH)2](ClO4)2 (1)
2.2. Physical Measurements
2.3. X-ray Structure Determination
2.4. Hirshfeld Surface Analysis
3. Results
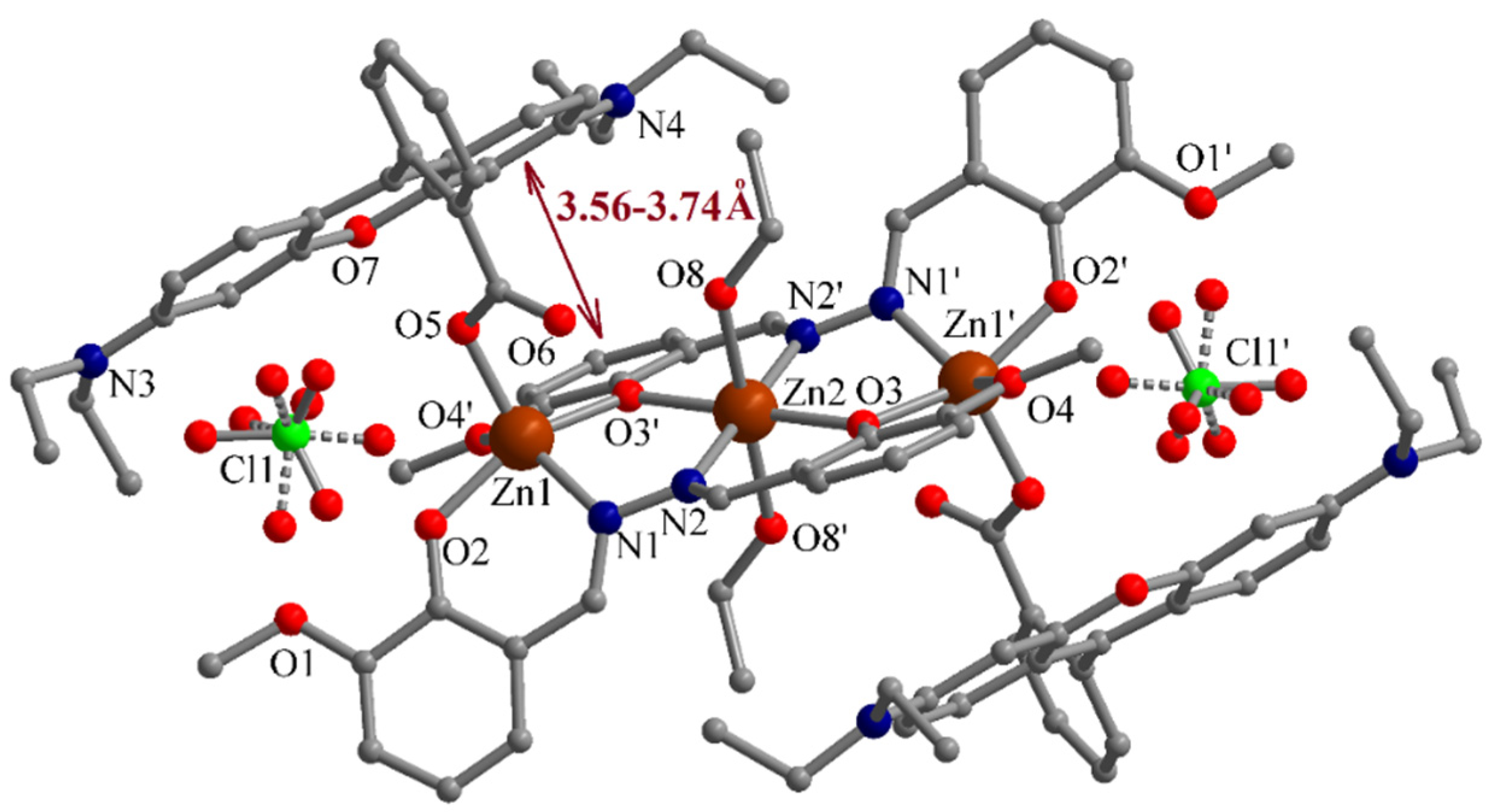
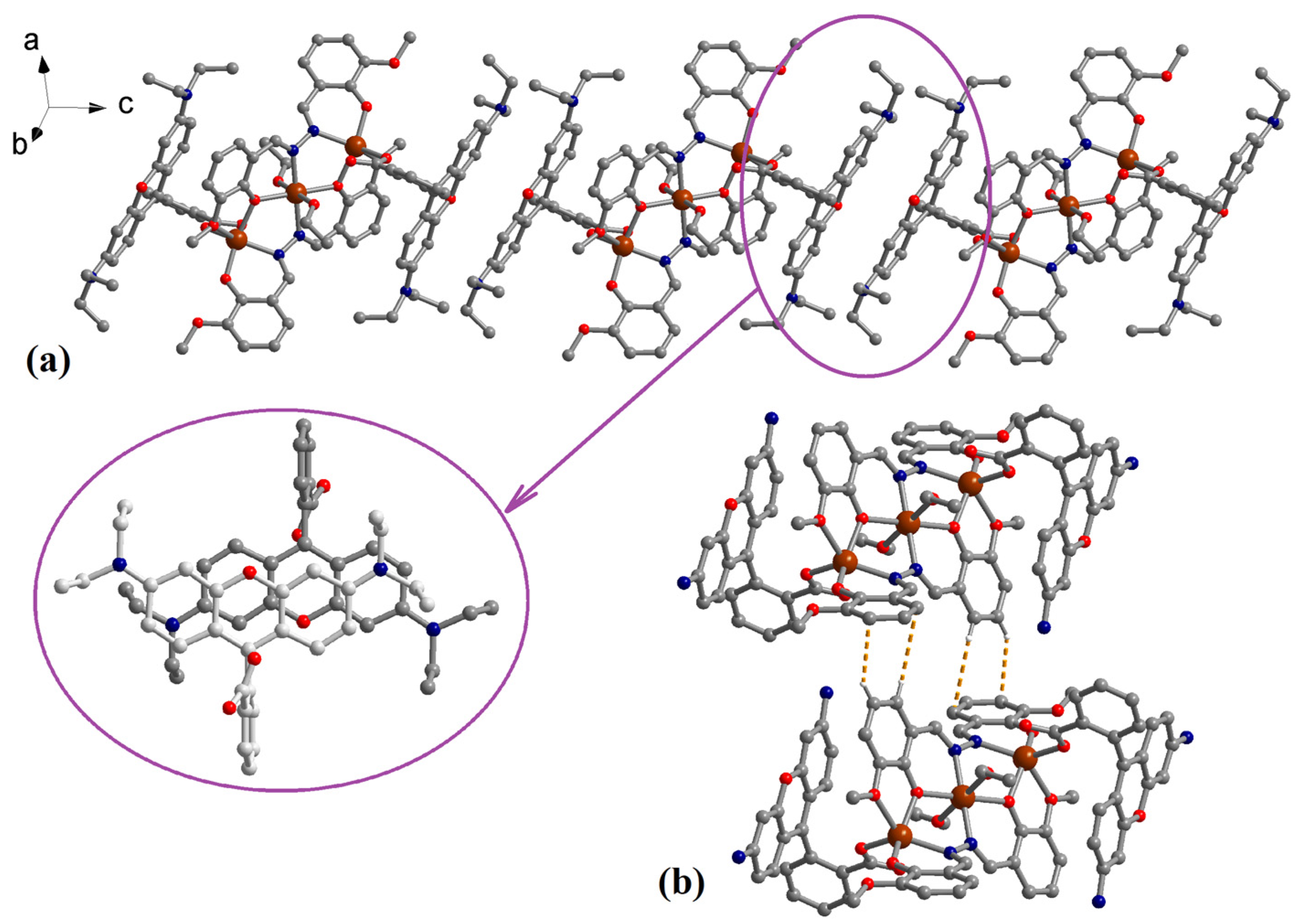
3.1. Description of the Structure
3.2. Spectral Properties
3.3. Thermal Behaviour
3.4. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, X.; Pradhan, T.; Wang, F.; Kim, J.S.; Yoon, J. Fluorescent Chemosensors Based on Spiroring-Opening of Xanthenes and Related Derivatives. Chem. Rev. 2012, 112, 1910–1956. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.S.T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar] [CrossRef] [PubMed]
- Răducă, M.; Ene, C.D.; Ionescu, S.; Florea, M.; Mădălan, A.M. Coordination polymers and a dinuclear complex constructed from zinc(II) ions and fluorescein: Iodine adsorption and optical properties. J. Coord. Chem. 2019, 72, 1222–1237. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Li, Y.; Xia, L.; Jiang, M.; Wu, P. Highly Efficient Visible-Light-Driven H2 Production via an Eosin Y-Based Metal–Organic Framework. Inorg. Chem. 2018, 57, 7495–7498. [Google Scholar] [CrossRef] [PubMed]
- García-Granda, S.; Díaz, M.R.; Gómez-Beltrán, F.; Blanco-Gomís, D. Structure of (4,7,13,16,21,24-hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane)lead(II) eosin dihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1993, 49, 884–886. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, C.; Li, B.; Wang, J.; Wang, M.; Zhang, N.; Feng, Y.; Wu, P. A visible-light responsive metal–organic framework as an eco-friendly photocatalyst under ambient air at room temperature. Inorg. Chem. Front. 2020, 7, 3541–3547. [Google Scholar] [CrossRef]
- Xu, X.W.; Zhou, Z.Y.; Tian, C.B.; Du, S.W. Two new coordination complexes based on eosin y dye ligand: Syntheses, crystal structures and magnetic properties. Jiegou Huaxue 2017, 36, 1593–1600. [Google Scholar]
- Thomas, J.; Ambili, K.S. Synthesis, crystal structure and luminescent properties of a new samarium-fluorescein metal-organic framework. J. Mol. Struct. 2015, 1098, 167–174. [Google Scholar] [CrossRef]
- Zhang, X.; Hayes, D.; Smith, S.J.; Friedle, S.; Lippard, S.J. New Strategy for Quantifying Biological Zinc by a Modified Zinpyr Fluorescence Sensor. J. Am. Chem. Soc. 2008, 130, 15788–15789. [Google Scholar] [CrossRef] [Green Version]
- Burdette, S.C.; Walkup, G.K.; Spingler, B.; Tsien, R.Y.; Lippard, S.J. Fluorescent Sensors for Zn 2+ Based on a Fluorescein Platform: Synthesis, Properties and Intracellular Distribution. J. Am. Chem. Soc. 2001, 123, 7831–7841. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Yan, S.; Wang, X.; Ren, X.; Gao, H.; Wang, N.; Li, Z.; Yu, T.; Zou, Z. A Possible Mechanism for Solar Hydrogen Production Over Rhodamine B. Sci. Adv. Mater. 2014, 6, 189–197. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, Q.; Wang, L.F.; Mu, L.J.; Huang, X.Y. The crystal structure of Zn(II) complex of rhodamine B {Zn(RB)(OAc)2·H2O, RB = 9-[2-(ethoxycarbonyl)phenyl]-3,6-bis(ethylamino)-2,7-dimethylxanthylium}. The first solid free radical species of rhodamine B metal complexes. Pol. J. Chem. 2000, 74, 639–647. [Google Scholar]
- Qu, J.-Q.; Wang, L.-F.; Li, Y.-Z.; Sun, G.-C.; Zhu, Q.-J.; Xia, C.-G. Synthesis and X-ray crystal structure of a Cd(II) complex of rhodamine b. Synth. React. Synth. React. Inorg. Met.-Org. Chem. 2001, 31, 1577–1585. [Google Scholar] [CrossRef]
- Gupta, M.; Kottilil, D.; Tomar, K.; Lu, S.; Vijayan, C.; Ji, W.; Bharadwaj, P.K. Two-Photon Absorption and Fluorescence in Micrometer-Sized Single Crystals of a Rhodamine B Coordinated Metal–Organic Framework. ACS Appl. Nano Mater. 2018, 1, 5408–5413. [Google Scholar] [CrossRef]
- Liu, L.; Dai, J.-C. [Zn(tp)(RdmB)(H2O)] and [Cd(tp)(RdmB)]: Two Unusual One-Dimensional Rhodamine B Coordination Polymeric Ribbons as Luminescent Sensors for Small Molecules and Metal Cations. Cryst. Growth Des. 2018, 18, 4460–4469. [Google Scholar] [CrossRef]
- Ju, L.; Qin, T.; Zhang, T.; Wang, P.; Sheng, L.; Xiao-An Zhang, S. Water-soluble and adjustable fluorescence copolymers con-taining a hydrochromic dye: Synthesis, characterization and properties. RSC Adv. 2018, 8, 13664–13670. [Google Scholar] [CrossRef] [Green Version]
- Weingarten, D.H.; Lacount, M.D.; Van De Lagemaat, J.; Rumbles, G.; Lusk, M.T.; Shaheen, S.E. Experimental demonstration of photon upconversion via cooperative energy pooling. Nat. Commun. 2017, 8, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Pepe, G.; Cole, J.M.; Waddell, P.G.; Perry, J.I. Rationalizing the suitability of rhodamines as chromophores in dye-sensitized solar cells: A systematic molecular design study. Mol. Syst. Des. Eng. 2016, 1, 416–435. [Google Scholar] [CrossRef]
- Porrès, L.; Holland, A.; Pålsson, L.-O.; Monkman, A.P.; Kemp, C.; Beeby, A. Absolute Measurements of Photoluminescence Quantum Yields of Solutions Using an Integrating Sphere. J. Fluoresc. 2006, 16, 267–273. [Google Scholar] [CrossRef]
- Olsen, A.L.; Washburn, E.R. An Interpolation Table for Refractive Index-Normality Relationship for Solutions of Hydrochloric Acid and Sodium Hydroxide. Trans. Kansas Acad. Sci. 1937, 40, 117. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Perth, Australia, 2017; Available online: http://hirshfeldsurface.net (accessed on 10 September 2022).
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Huang, X.R.; Yang, L.; Zhou, Y.J.; Zhang, S.H.; Zhang, H.Y.; Hai, H. Two Linear Trinuclear Clusters Based on Schiff Base: Syntheses, Structures and Magnetic Properties. J. Clust. Sci. 2015, 26, 2033–2042. [Google Scholar] [CrossRef]
- Sikdar, Y.; Goswami, R.; Modak, R.; Basak, M.; Heras Ojea, M.J.; Murrie, M.; Goswami, S. Diazine based ligand supported CoII3 and CoII4 coordination complexes: Role of anions. New J. Chem. 2018, 42, 17587–17596. [Google Scholar] [CrossRef] [Green Version]
- Adhikary, A.; Ghosh, K. Fine tuning of coordination environments by anions on a series of Cu(II) dihydrazide complexes: Syntheses, structures, magnetic properties and solution phase anion exchange. Polyhedron 2019, 168, 37–47. [Google Scholar] [CrossRef]
- Tan, Y.; Huang, J.; Lu, H.; Sun, J. Crystal structure of diaqua-dichlorido-bis(μ3-6,6′-(hydrazine-1,2-diylidenebis(methanylylidene))bis(2-methoxyphenolato)κ6O,N:N′,O′,O′:O′′)trizinc(II)—Ethanol (1/2), C36H44Cl2N4O12Zn3. Z. Für Krist.-New Cryst. Struct. 2019, 234, 431–432. [Google Scholar]
- Liu, E.; Zhang, Y.Z.; Tan, J.; Yang, C.; Li, L.; Golen, J.A.; Rheingold, A.L.; Zhang, G. Zn(II) and Co(III) metallosupramolecular assemblies derived from a rigid bis-Schiff base ligand. Polyhedron 2015, 102, 41–47. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kristoffersen, A.S.; Erga, S.R.; Hamre, B.; Frette, Ø. Testing Fluorescence Lifetime Standards using Two-Photon Excitation and Time-Domain Instrumentation: Rhodamine B, Coumarin 6 and Lucifer Yellow. J. Fluoresc. 2014, 24, 1015–1024. [Google Scholar] [CrossRef]



| Compound | 1 |
|---|---|
| Chemical formula | C92H100Cl2N8O24Zn3 |
| M (g mol−1) | 1968.80 |
| Temperature, (K) | 293 (2) |
| Wavelength, (Å) | 0.71073 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a (Å) | 11.4535 (9) |
| b (Å) | 12.3264 (9) |
| c (Å) | 17.7024 (13) |
| α (°) | 105.604 (6) |
| β (°) | 97.473 (6) |
| γ (°) | 101.851 (6) |
| V (Å3) | 2309.8 (3) |
| Z | 1 |
| Dc (g cm−3) | 1.415 |
| μ (mm−1) | 0.909 |
| F(000) | 1024 |
| Goodness-of-fit on F2 | 0.874 |
| Final R1, wR2 [I > 2σ(I)] | 0.0538, 0.1261 |
| R1, wR2 (all data) | 0.1193, 0.1530 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răducă, M.; Ionescu, S.; Mădălan, A.M. Synthesis, Crystal Structure, and Optical Properties of a Trinuclear Zinc(II) Complex with Rhodamine B. Crystals 2022, 12, 1813. https://doi.org/10.3390/cryst12121813
Răducă M, Ionescu S, Mădălan AM. Synthesis, Crystal Structure, and Optical Properties of a Trinuclear Zinc(II) Complex with Rhodamine B. Crystals. 2022; 12(12):1813. https://doi.org/10.3390/cryst12121813
Chicago/Turabian StyleRăducă, Mihai, Sorana Ionescu, and Augustin M. Mădălan. 2022. "Synthesis, Crystal Structure, and Optical Properties of a Trinuclear Zinc(II) Complex with Rhodamine B" Crystals 12, no. 12: 1813. https://doi.org/10.3390/cryst12121813
APA StyleRăducă, M., Ionescu, S., & Mădălan, A. M. (2022). Synthesis, Crystal Structure, and Optical Properties of a Trinuclear Zinc(II) Complex with Rhodamine B. Crystals, 12(12), 1813. https://doi.org/10.3390/cryst12121813







