Anion-Dependent Cu(II) Coordination Polymers: Geometric, Magnetic and Luminescent Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Syntheses
2.3. X-ray Crystallography
3. Results and Discussion
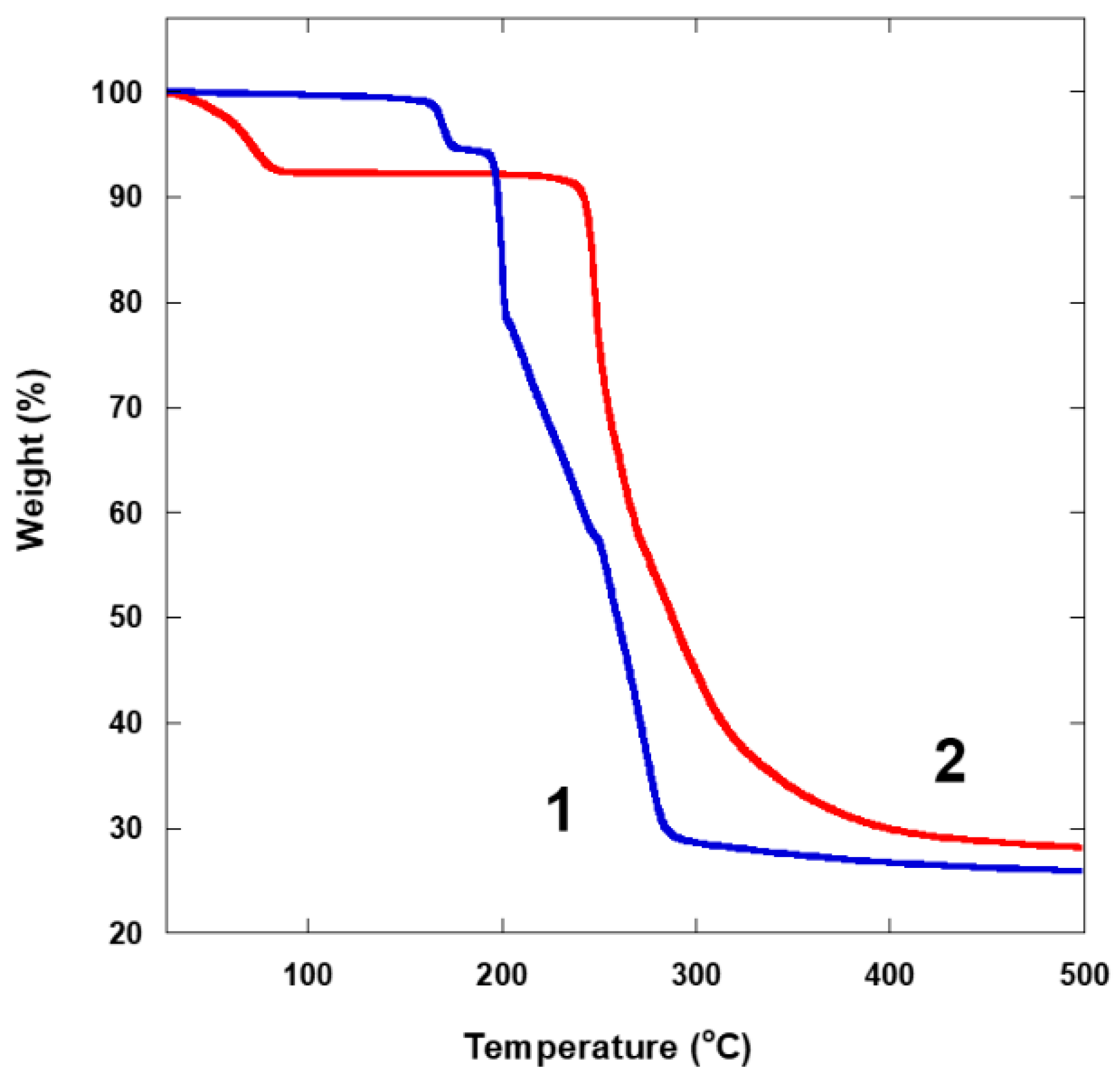
3.1. Synthesis and Characterization
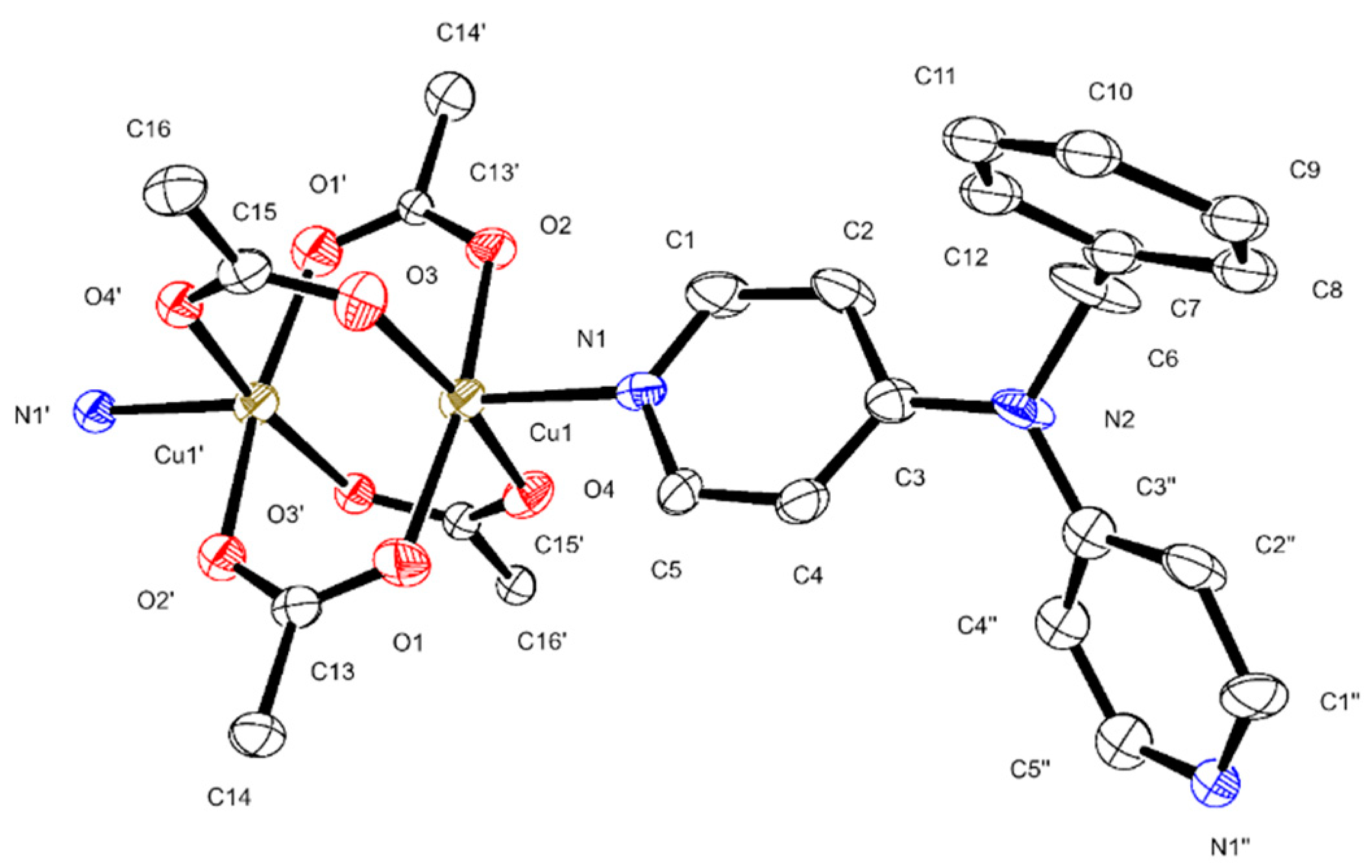
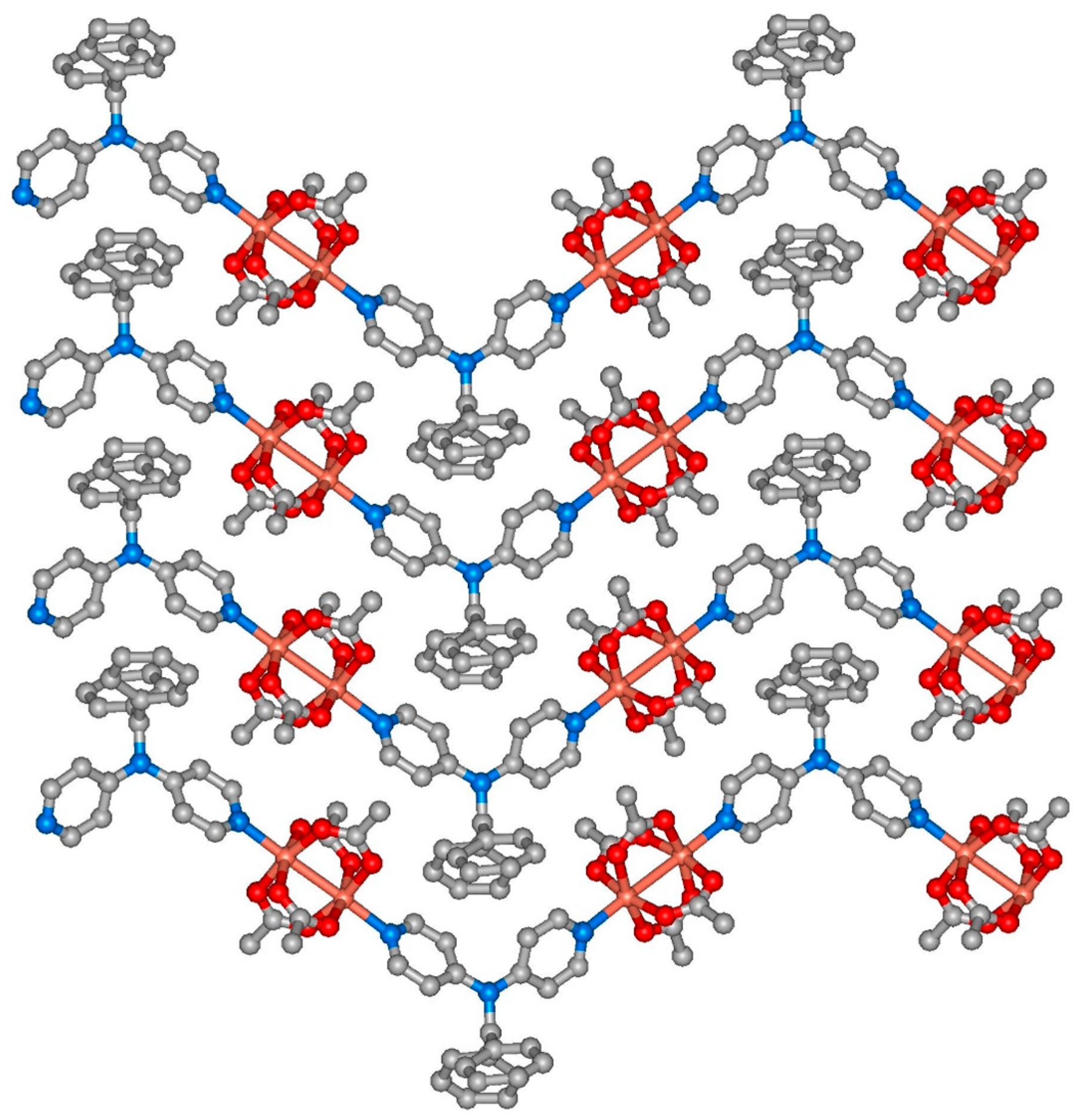
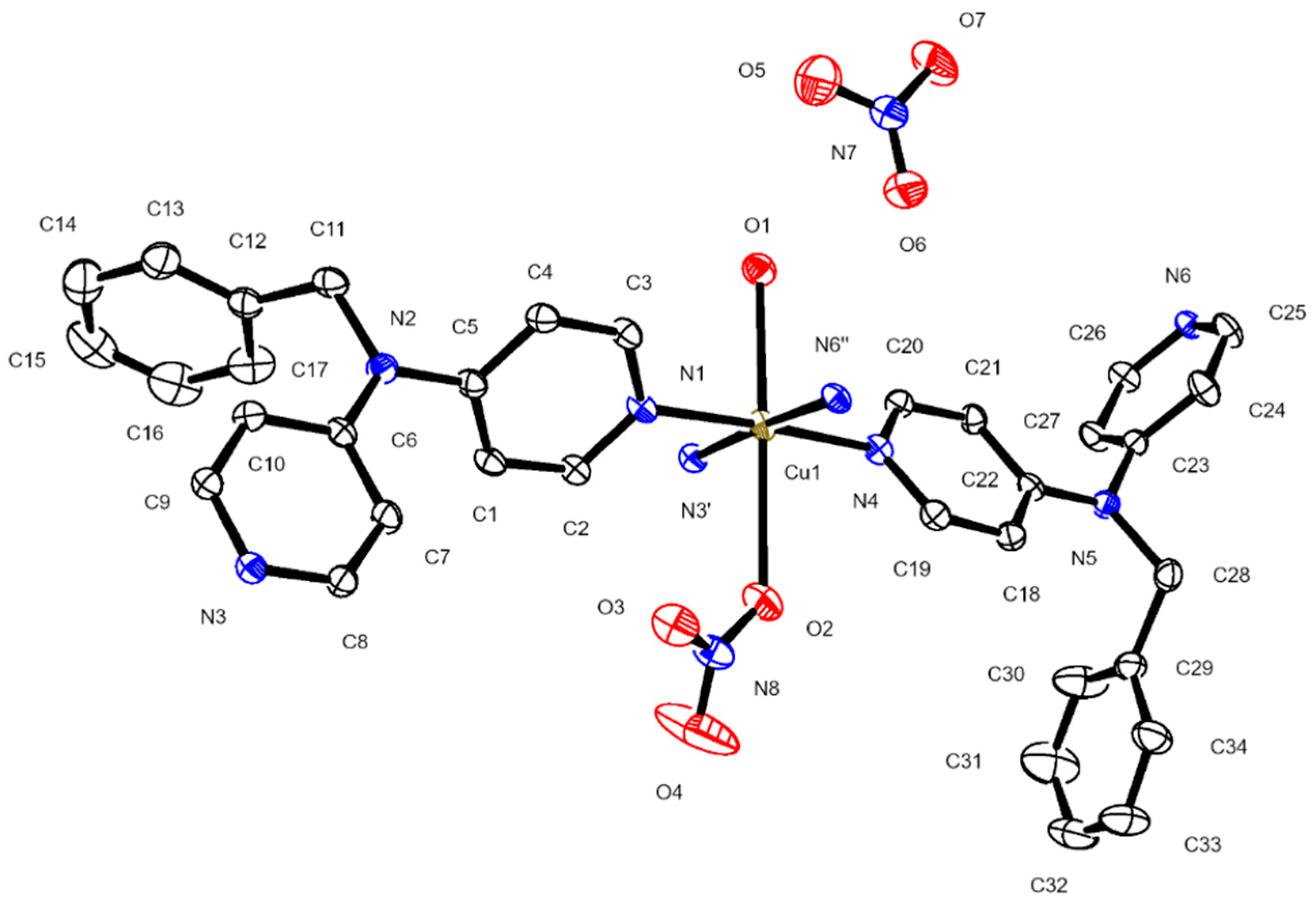
3.2. Description of the Crystal Structures
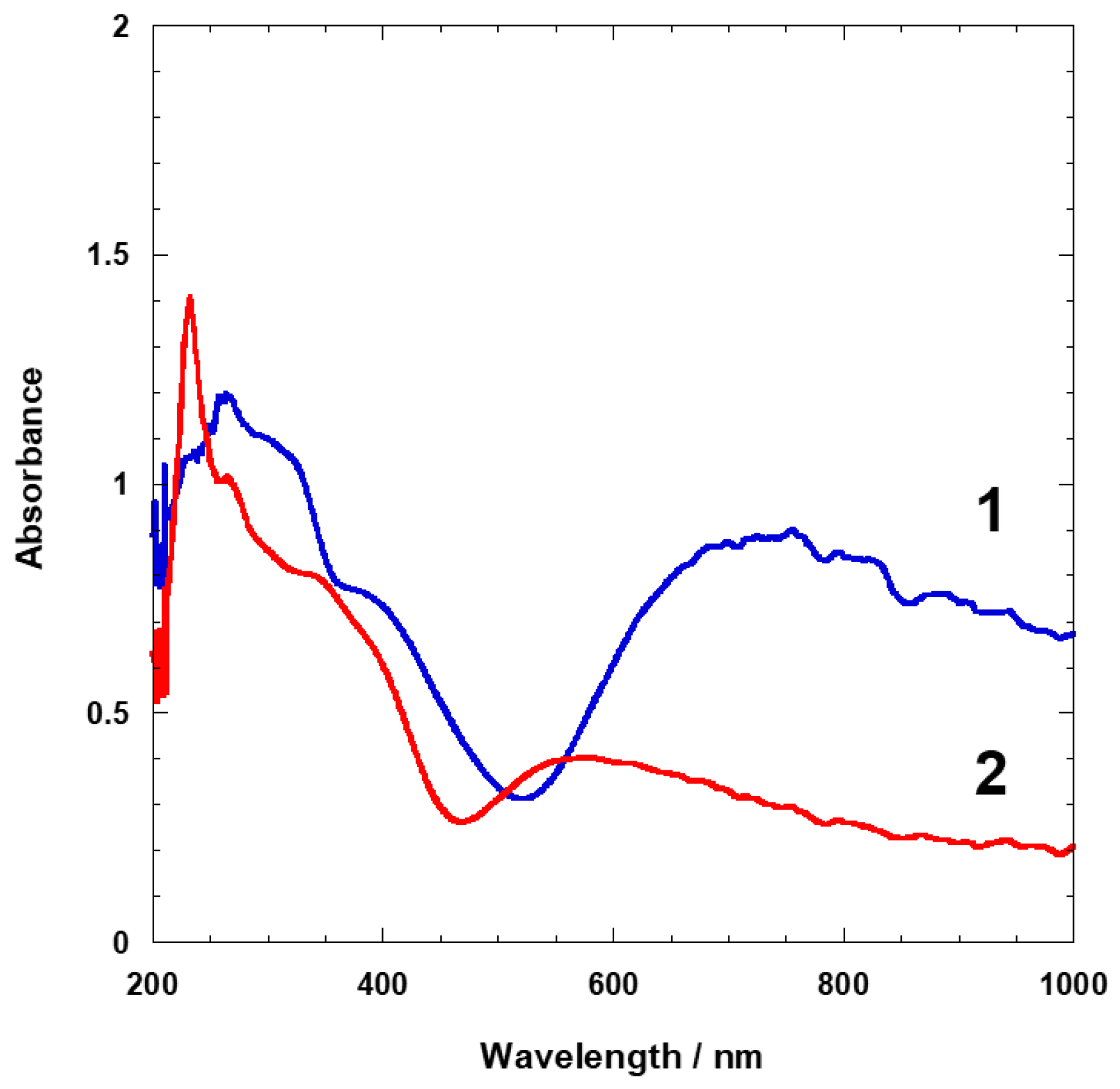
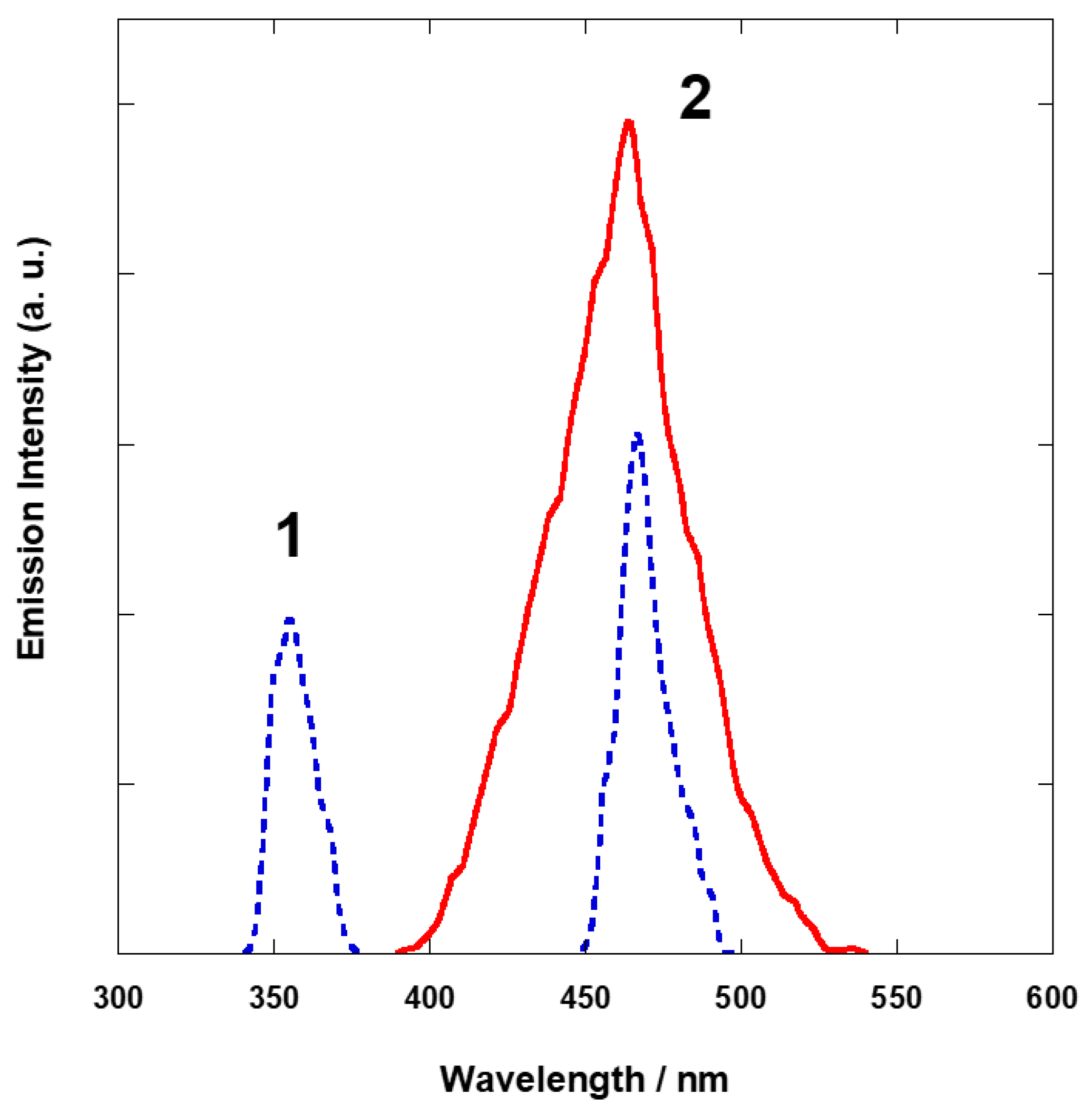
3.3. Photoluminescence Properties of 1 and 2
3.4. Magnetic Properties of 1 and 2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, G.; Han, Y. Two-in-One MOF Structure with Tunable Porosity for Enhanced Separation. ACS Cent. Sci. 2022, 8, 150–152. [Google Scholar] [CrossRef]
- Du, R.; Wu, Y.; Yang, Y.; Zhai, T.; Zhou, T.; Shang, Q.; Zhu, L.; Shang, C.; Guo, Z. Porosity Engineering of MOF-Based Materials for Electrochemical Energy Storage. Adv. Energy Mater. 2021, 11, 2100154. [Google Scholar] [CrossRef]
- Jeong, A.R.; Shin, J.W.; Jeong, J.H.; Jeoung, S.; Moon, H.R.; Kang, S.; Min, K.S. Porous and nonporous coordination polymers induced by pseudohalide ions for luminescence and gas sorption. Inorg. Chem. 2020, 59, 15987–15999. [Google Scholar] [CrossRef] [PubMed]
- Samanta, P.; Let, S.; Mandal, W.; Dutta, S.; Ghosh, S.K. Luminescent metal–organic frameworks (LMOFs) as potential probes for the recognition of cationic water pollutants. Inorg. Chem. Front. 2020, 7, 1801–1821. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Song, I.; Yoon, S.M. The Magnetism of Metal–Organic Frameworks for Spintronics. Bull. Korean Chem. Soc. 2021, 42, 1170–1183. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–Organic Framework Magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Shao, L.; Hu, X.; Sikligar, K.; Baker, G.A.; Atwood, J.L. Coordination Polymers Constructed from Pyrogallol[4]arene-Assembled Metal–Organic Nanocapsules. Acc. Chem. Res. 2021, 54, 3191–3203. [Google Scholar] [CrossRef]
- Persson, I.; Persson, P.; Sandström, M.; Ullström, A.-S. Structure of Jahn–Teller distorted solvated copper(II) ions in solution, and in solids with apparently regular octahedral coordination geometry. J. Chem. Soc. Dalton Trans. 2002, 1256–1265. [Google Scholar] [CrossRef]
- Trivedi, M.; Yadav, A.K.; Singh, G.; Kumar, A.; Kumar, G.; Husain, A.; Rath, N.P. Synthetic, spectral, structural and catalytic activity of infinite 3-D and 2-D copper(II) coordination polymers for substrate size-dependent catalysis for CO2 conversion. Dalton Trans. 2019, 48, 10078–10088. [Google Scholar]
- Hussain, N.; Bhardwaj, V.K. The influence of different coordination environments on one-dimensional Cu(II) coordination polymers for the photo-degradation of organic dyes. Dalton Trans. 2016, 45, 7697–7707. [Google Scholar] [CrossRef]
- Wang, X.-P.; Zhao, Y.-Q.; Jagličić, Z.; Wang, S.-N.; Lin, S.-J.; Li, X.-Y.; Sun, D. Controlled in situ reaction for the assembly of Cu(II) mixed-ligand coordination polymers: Synthesis, structure, mechanistic insights, magnetism and catalysis. Dalton Trans. 2015, 44, 11013–11020. [Google Scholar] [CrossRef] [PubMed]
- Köberl, M.; Cokoja, M.; Herrmann, W.A.; Kühn, F.E. From molecules to materials: Molecular paddle-wheel synthons of macromolecules, cage compounds and metal–organic frameworks. Dalton Trans. 2011, 40, 6834–6859. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, N.; Ikram, M.; Rehman, S.; Ali, S.; Akhtar, M.N.; AlDamen, M.A.; Schulzke, C. A paddle wheel dinuclear copper(II) carboxylate: Crystal structure, thermokinetic and magnetic properties. J. Mol. Struct. 2019, 1196, 754–759. [Google Scholar]
- Nesterova, O.V.; Kirillova, M.V.; Guedes da Silva, M.F.C.; Boča, R.; Pombeiro, A.J.L. How to force a classical chelating ligand to a metal non-chelating bridge: The observation of a rare coordination mode of diethanolamine in the 1D complex {[Cu2(Piv)4(H3tBuDea)](Piv)}n. CrystEngComm 2014, 16, 775–783. [Google Scholar] [CrossRef]
- Soldevila-Sanmartín, J.; Ayllón, J.A.; Calvet, T.; Font-Bardia, M.; Domingo, C.; Pons, J. Synthesis, crystal structure and magnetic properties of a Cu(II) paddle-wheel complex with mixed bridges. Inorg. Chem. Commun. 2016, 71, 90–93. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Masoudiasl, A.; Afkhami, F.A.; White, J.M.; Zangrando, E.; Gurbanov, A.V.; Frontera, A.; Safin, D.A. A new coordination polymer constructed from Pb(NO3)2 and a benzylideneisonicotinohydrazide derivative: Coordination-induced generation of a π-hole towards a tetrel-bonding stabilized structure. J. Mol. Struct. 2021, 1234, 130139. [Google Scholar] [CrossRef]
- Jeong, A.R.; Shin, H.J.; Jang, Y.J.; Min, K.S. Two-dimensional zinc(II) and copper(I) coordination polymers for photoluminescence. J. Mol. Struct. 2022, 1251, 132031. [Google Scholar] [CrossRef]
- Shin, J.W.; Bae, J.M.; Kim, C.; Min, K.S. Three-Dimensional Zinc(II) and Cadmium(II) Coordination Frameworks with N,N,N′,N′-Tetrakis(pyridin-4-yl)methanediamine: Structure, Photoluminescence, and Catalysis. Inorg. Chem. 2013, 52, 2265–2267. [Google Scholar] [CrossRef]
- Yang, W.; Yi, F.-Y.; Li, X.-D.; Wang, L.; Dang, S.; Sun, Z.-M. Construction of Cu(II) coordination polymers based on semi-rigid tetrahedral pyridine ligands. RSC Adv. 2013, 3, 25065–25070. [Google Scholar] [CrossRef]
- Winter, S.; Weber, E.; Eriksson, L.; Csöregh, I. New coordination polymer networks based on copper(II) hexafluoroacetylacetonate and pyridine containing building blocks: Synthesis and structural study. New J. Chem. 2006, 30, 1808–1819. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Wang, J.-L.; Bai, Y.; Dang, D.B.; Zhao, Y.-Q. Two copper coordination polymers with pyridine imine-based ligand: Synthesis, crystal structure and luminescent properties. Synth. Met. 2012, 162, 1126–1132. [Google Scholar] [CrossRef]
- Ding, B.; Wu, J.; Wu, X.X.; Huo, J.Z.; Zhu, Z.Z.; Liu, Y.Y.; Shi, F.X. Syntheses, structural diversities and characterization of a series of coordination polymers with two isomeric oxadiazol-pyridine ligands. RSC Adv. 2017, 7, 9704–9718. [Google Scholar] [CrossRef]
- Shin, J.W.; Cho, H.J.; Min, K.S. Synthesis, structure and photoluminescence properties of silver(I) coordination polymers with bis(4-pyridyl)benzylamine. Inorg. Chem. Commun. 2012, 16, 12–16. [Google Scholar] [CrossRef]
- Saint Plus, Version 6.02; Bruker Analytical X-ray: Madison, WI, USA, 1999.
- SADABS, Version 2.03; Bruker AXS Inc.: Madison, WI, USA, 2000.
- Sheldrick, G.M. Phase annealing in SHELX-90: Direct methods for larger structures. Acta Cryst. 1990, A46, 467–473. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Nakamoto, K. 2. Application in Organometallic Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; WILEY: Hoboken, NJ, USA, 2009; Volume 2, pp. 288–290. [Google Scholar]
- Zeybel, L.; Köse, D.A. Acesulfame complex compounds of some lanthanide group metal cations. Synthesis and characterization. J. Mol. Struct. 2021, 1226, 129399. [Google Scholar] [CrossRef]
- Pagliai, M.; Mancini, G.; Carnimeo, I.; De Mitri, N.; Barone, V. Electronic absorption spectra of pyridine and nicotine in aqueous solution with a combined molecular dynamics and polarizable QM/MM approach. J. Comput. Chem. 2017, 38, 319–335. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Applications; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Nakamoto, K. 1. Applications in Coordination Chemistry. In Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; Wiley: Hoboken, NJ, USA, 2009; Volume 1, pp. 92–94. [Google Scholar]
- Uysal, S.; Kurşunlu, A.N. The Synthesis and characterization of star shaped metal complexes of triazine cored schiff bases: Their thermal decompositions and magnetic moment values. J. Inorg. Organomet. Polym. 2011, 21, 291–296. [Google Scholar] [CrossRef]
- Kocyigit, O.; Kursunlu, A.N.; Guler, E. Complexation properties and synthesis of a novel Schiff base with triphenylene nucleus. J. Hazard. Mater. 2010, 183, 334–340. [Google Scholar] [CrossRef]
- Appavoo, D.; Spencer, L.C.; Guzei, I.A.; Gomez-Garcıa, C.J.; van Wyk, J.L.; Darkwa, J. Ring opening polymerization of D,L-lactide and ε-caprolactone catalysed by (pyrazol-1-yl)copper(II) carboxylate complexes. RSC Adv. 2021, 11, 13475–13485. [Google Scholar] [CrossRef]
- Mastropietro, T.F.; Armentano, D.; Grisolia, E.; Zanchini, C.; Lloret, F.; Julve, M.; De Munno, G. Guanine-containing copper(II) complexes: Synthesis, X-ray structures and magnetic properties. Dalton Trans. 2008, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Chun, M.K.; Jeong, A.R.; Min, K.S.; Jeong, J.H. Synthesis, crystal structure, and magnetic property of a stepped tetranuclear copper(II) complex of 3,5-diisopropylpyrazole-1-methoxide. Inorg. Chem. Comm. 2017, 78, 82–84. [Google Scholar] [CrossRef]
- Shabana, S.Y.; Ramadan, A.M.; Ibrahim, M.M.; Elshami, F.I.; van Eldik, R. Square planar versus square pyramidal copper(II) complexes containing N3O moiety: Synthesis, structural characterization, kinetic and catalytic mimicking activity. Inorg. Chim. Acta 2019, 486, 608–616. [Google Scholar] [CrossRef]
- Yu, F.; Ji, B.-Q.; Jagodič, M.; Su, Y.-M.; Zhang, S.S.; Feng, L.; Kurmoo, M.; Jagličić, Z.; Sun, D. Copper(II)-Assisted Ligand Fragmentation Leading to Three Families of Metallamacrocycle. Inorg. Chem. 2020, 59, 13524–13532. [Google Scholar] [CrossRef]
- Park, H.W.; Sung, S.M.; Min, K.S.; Bang, H.; Suh, M.P. 1-D Zigzag Coordination Polymers of Copper(II) and Nickel(II) with Mixed Ligands: Syntheses and Structures. Eur. J. Inorg. Chem. 2001, 2857–2863. [Google Scholar] [CrossRef]
- Min, K.S.; Suh, M.P. Self-Assembly and Selective Guest Binding of Three-Dimensional Open-Framework Solids from Macrocyclic Complex as a Trifunctional Metal Building. Chem. Eur. J. 2001, 7, 303–313. [Google Scholar] [CrossRef]
- Veselska, O.; Cai, L.; Podbevšek, D.; Ledoux, G.; Guillou, N.; Pilet, G.; Fateeva, A.; Demessence, A. Structural Diversity of Coordination Polymers Based on a Heterotopic Ligand: Cu(II)-Carboxylate vs Cu(I)-Thiolate. Inorg. Chem. 2018, 57, 2736–2743. [Google Scholar] [CrossRef]
- Hazra, M.; Dolai, T.; Pandey, A.; Dey, S.K.; Patra, A. Fluorescent copper(II) complexes: The electron transfer mechanism, interaction with bovine serum albumin (BSA) and antibacterial activity. J. Saudi Chem. Soc. 2017, 21, S240–S247. [Google Scholar] [CrossRef]
- Jeong, A.R.; Min, K.S. Zinc(II) and cadmium(II) coordination polymers with bis(4-pyridyl)benzylamine: Structure and photoluminescence. J. Incl. Phenom. Macrocycl. Chem. 2021, 101, 243–252. [Google Scholar] [CrossRef]
- Lorenza, V.; Liebing, P.; Suta, M.; Engelhardt, F.; Hilfert, L.; Busse, S.; Wang, S.; Wickleder, C.; Edelmann, F.T. Synthesis, structure, complexation, and luminescence properties of the first metal-organic curcumin compound bis(4-triphenylsiloxy)curcumin. J. Lumin. 2019, 211, 243–250. [Google Scholar] [CrossRef]
- Chumakov, Y.R.; Danilescu, O.; Kulikova, O.V.; Bourosh, P.; Bulhac, I.; Croitor, L. Metal ions impact on the isostructurality and properties of 2D coordination polymers. CrystEngComm 2022, 24, 4430–4439. [Google Scholar] [CrossRef]
- Shi, X.; Qu, X.; Chai, J.; Tong, C.; Fan, Y.; Wang, L. Stable coordination polymers with linear dependence color tuning and luminescent properties for detection of metal ions and explosives. Dye. Pigment. 2019, 170, 107583. [Google Scholar] [CrossRef]
- Nath, J.K.; Mondal, A.; Powell, A.K.; Baruah, J.B. Structures, Magnetic Properties, and Photoluminescence of Dicarboxylate Coordination Polymers of Mn, Co, Ni, Cu Having N-(4-Pyridylmethyl)-1,8-naphthalimide. Cryst. Growth Des. 2014, 14, 4735–4748. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Baslak, C. A Bodipy-bearing pillar[5]arene for mimicking photosynthesis: Multi-fluorophoric light harvesting system. Tetrahedron Lett. 2018, 59, 1958–1962. [Google Scholar] [CrossRef]
- Yoon, S.; Kim, H.-C.; Kim, Y.; Huh, S. Photophysical Properties and Electrochromism of Viologen Encapsulated Viologen@InBTB Metal–Organic Framework. Bull. Korean Chem. Soc. 2021, 42, 326–332. [Google Scholar] [CrossRef]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Shin, J.W.; Jeong, A.R.; Hayami, S.; Moon, D.; Min, K.S. Synthesis, structure, and magnetic properties of dicopper and tricobalt complexes based on N-(2-pyridylmethyl)iminodiethanol. Inorg. Chem. Front. 2015, 2, 763–770. [Google Scholar] [CrossRef]
- Güdel, H.U.; Stebler, A.; Furrer, A. Direct Observation of Singlet-Triplet Separation in Dimeric Copper(II) Acetate by Neutron Inelastic Scattering Spectroscopy. Inorg. Chem. 1979, 18, 1021–1023. [Google Scholar] [CrossRef]
- Min, K.S.; Suh, M.P. Self-Assembly, Structures, and Magnetic Properties of Ladder-Like Copper(II) Coordination Polymers. J. Solid State Chem. 2000, 152, 183–190. [Google Scholar] [CrossRef][Green Version]
- Soto, L.; Garcia, J.; Escriva, E.; Legros, J.-P.; Tuchagues, J.-P.; Dahan, F.; Fuertes, A. Synthesis, Characterization, and Magnetic Properties of µ-Oxalato- and µ-Oxamido-Bridged Copper(II) Dimers. Crystal and Molecular Structures of [Cu2(mepirizole)2(C2O4)(H2O)2](PF6)2∙mepirizole∙3H2O and [Cu2(mepirizole)2(C2O4)(NO3)2(H2O)]2[Cu2(mepirizole)2(C2O4)(NO3)2]. Inorg. Chem. 1989, 28, 3378–3386. [Google Scholar]
- Osiry, H.; Cano, A.; Lemus-Santana, A.A.; Rodríguez, A.; Carbonio, R.E.; Reguera, E. Intercalation of organic molecules in 2D copper(II) nitroprusside: Intermolecular interactions and magnetic properties. J. Soild State Chem. 2015, 230, 374–380. [Google Scholar] [CrossRef]
- Tang, J.; Costa, J.S.; Golobič, A.; Kozlevčar, B.; Robertazzi, A.; Vargiu, A.V.; Gamez, P.; Reedijk, J. Magnetic Coupling between Copper(II) Ions Mediated by Hydrogen-Bonded (Neutral) Water Molecules. Inorg. Chem. 2009, 48, 5473–5479. [Google Scholar] [CrossRef] [PubMed]


| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C25H27Cu2N3O8 | C35H40CuN8O10 |
| Formula weight | 624.58 | 796.29 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | C2/c | P21/c |
| a, Å | 14.737(1) | 17.917(1) |
| b, Å | 7.569(1) | 12.966(1) |
| c, Å | 25.6870(14) | 17.518(1) |
| α, deg | 90 | 90 |
| β, deg | 97.3060(10) | 113.399(2) |
| γ, deg | 90 | 90 |
| V, Å3 | 2842.0(3) | 3735.0(6) |
| Z | 4 | 4 |
| dcalc, g cm−3 | 1.460 | 1.416 |
| λ, Å | 0.71073 | 0.71073 |
| T, K | 200(2) | 200(2) |
| μ, mm−1 | 1.546 | 0.652 |
| F(000) | 1280 | 1660 |
| Reflections collected/2θmax | 9465/56.55 | 22,765/52.02 |
| Independent reflections | 3294 | 7321 |
| Reflections with I > 2σ(I) | 2048 | 4064 |
| Goodness-of-fit on F2 | 1.073 | 0.958 |
| Final R indices [I > 2σ(I)] a | R1 = 0.0767 wR2 = 0.1660 | R1 = 0.0628 wR2 = 0.1621 |
| CCDC | 2,183,299 | 2,183,300 |
| 1 | 2 | ||
|---|---|---|---|
| Cu1–N1 | 2.175(5) | Cu1–N1 | 2.045(4) |
| Cu1–O1 | 1.990(4) | Cu1–N4 | 2.009(4) |
| Cu1–O2 | 1.963(4) | Cu1–N3′ | 2.023(4) |
| Cu1–O3 | 1.975(4) | Cu1–N6′′ | 2.045(4) |
| Cu1–O4 | 1.993(4) | Cu1–O1 | 2.367(3) |
| Cu1–Cu1′ | 2.621(2) | Cu1–O2 | 2.644(4) |
| N1–Cu1–O1 | 95.83(19) | N1–Cu1–O1 | 89.00(13) |
| N1–Cu1–O2 | 95.68(19) | N4–Cu1–O1 | 94.67(13) |
| N1–Cu1–O3 | 94.92(18) | N3′–Cu1–O1 | 92.21(13) |
| N1–Cu1–O4 | 96.39(18) | N6′′–Cu1–O1 | 91.29(13) |
| O1–Cu1–O2 | 168.4(2) | N1–Cu1–N4 | 176.27(15) |
| O1–Cu1–O3 | 90.4(2) | N1–Cu1–N3′ | 90.22(15) |
| O1–Cu1–O4 | 88.2(2) | N1–Cu1–N6′′ | 89.81(15) |
| O2–Cu1–O3 | 89.69(18) | N4–Cu1–N3′ | 90.33(15) |
| O2–Cu1–O4 | 89.50(18) | N4–Cu1–N | 89.42(15) |
| O3–Cu1–O4 | 168.68(19) | N3′–Cu1–N6′′ | 176.50(15) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, I.; Shin, J.W.; Tokunaga, R.; Hayami, S.; Shin, H.J.; Min, K.S. Anion-Dependent Cu(II) Coordination Polymers: Geometric, Magnetic and Luminescent Properties. Crystals 2022, 12, 1096. https://doi.org/10.3390/cryst12081096
Ullah I, Shin JW, Tokunaga R, Hayami S, Shin HJ, Min KS. Anion-Dependent Cu(II) Coordination Polymers: Geometric, Magnetic and Luminescent Properties. Crystals. 2022; 12(8):1096. https://doi.org/10.3390/cryst12081096
Chicago/Turabian StyleUllah, Ihsan, Jong Won Shin, Ryuya Tokunaga, Shinya Hayami, Hye Jin Shin, and Kil Sik Min. 2022. "Anion-Dependent Cu(II) Coordination Polymers: Geometric, Magnetic and Luminescent Properties" Crystals 12, no. 8: 1096. https://doi.org/10.3390/cryst12081096
APA StyleUllah, I., Shin, J. W., Tokunaga, R., Hayami, S., Shin, H. J., & Min, K. S. (2022). Anion-Dependent Cu(II) Coordination Polymers: Geometric, Magnetic and Luminescent Properties. Crystals, 12(8), 1096. https://doi.org/10.3390/cryst12081096








