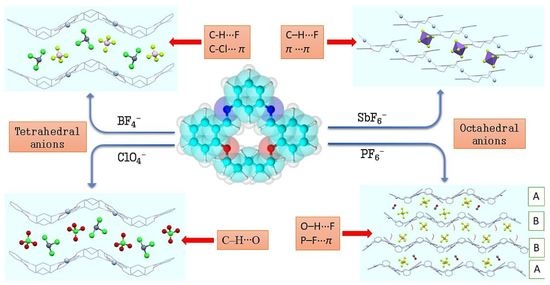
Anion Influence on Supramolecular Interactions in Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycle
Abstract
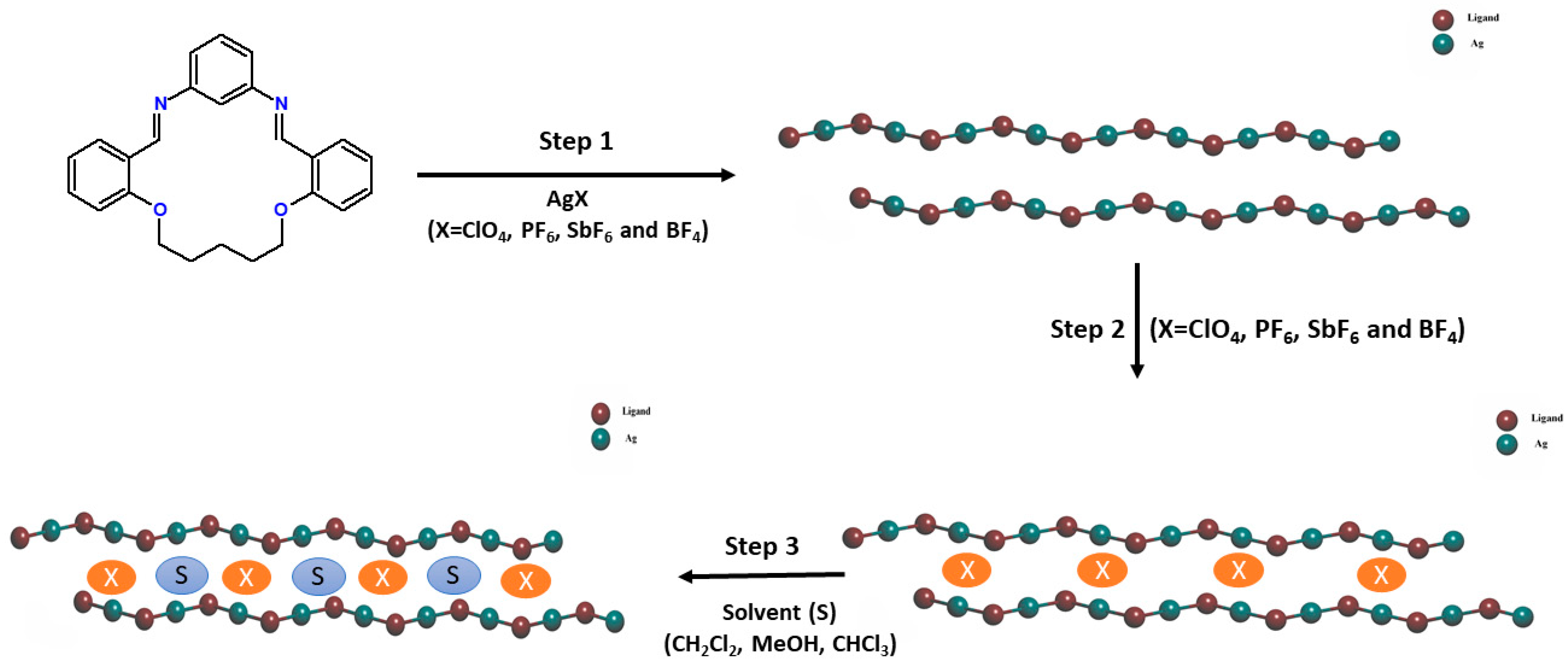
1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. X-Ray Crystallography
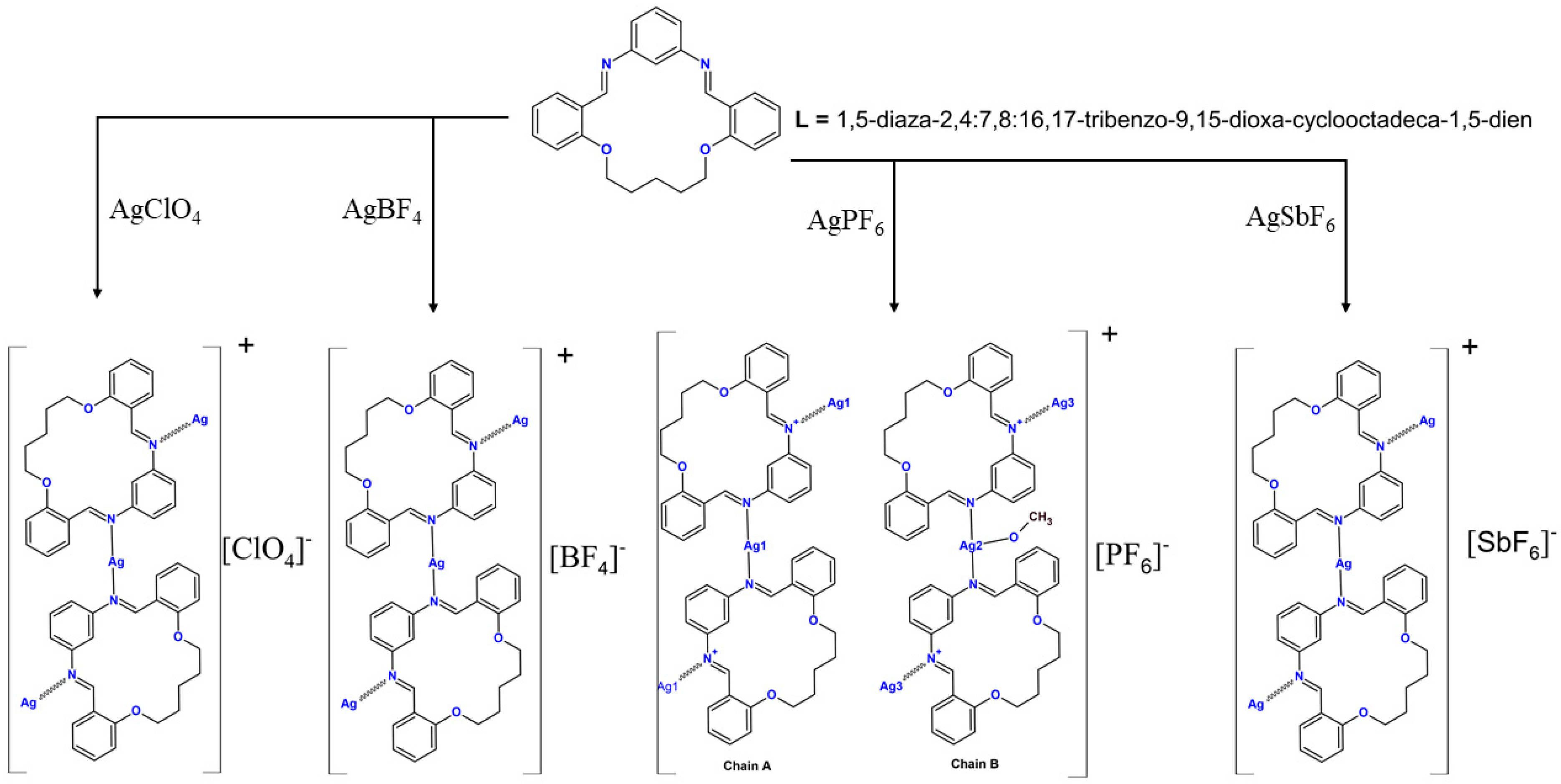
2.3. Synthesis
- Preparation of the silver(I) coordination polymers
- 2.
- Preparation of AgLPF6
- 3.
- Preparation of AgLSbF6
- 4.
- Preparation of AgLBF4
- 5.
- Preparation of AgLClO4
3. Results and Discussion
3.1. Synthesis, Structure of the Ligand (L), and IR Spectroscopy
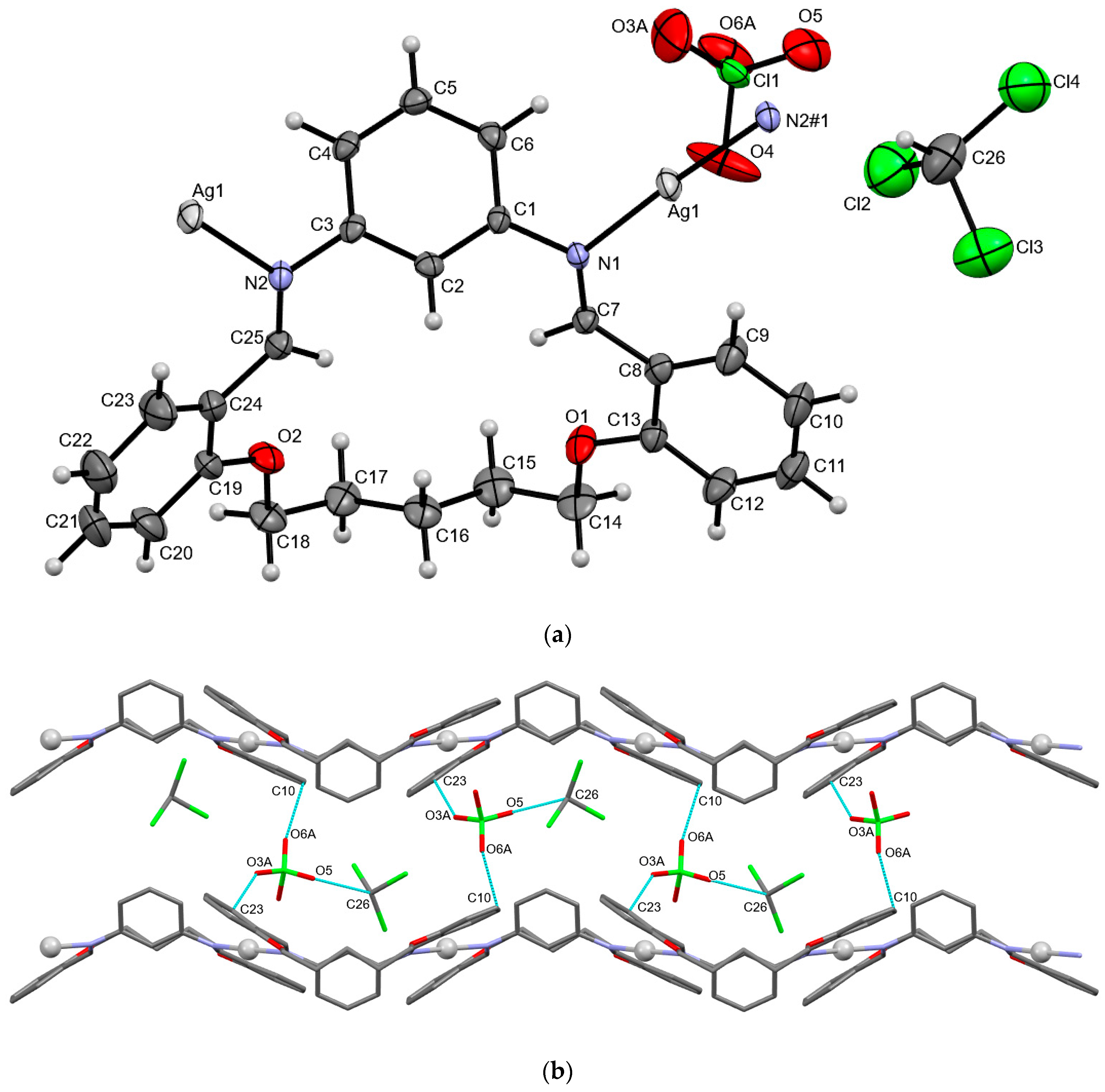
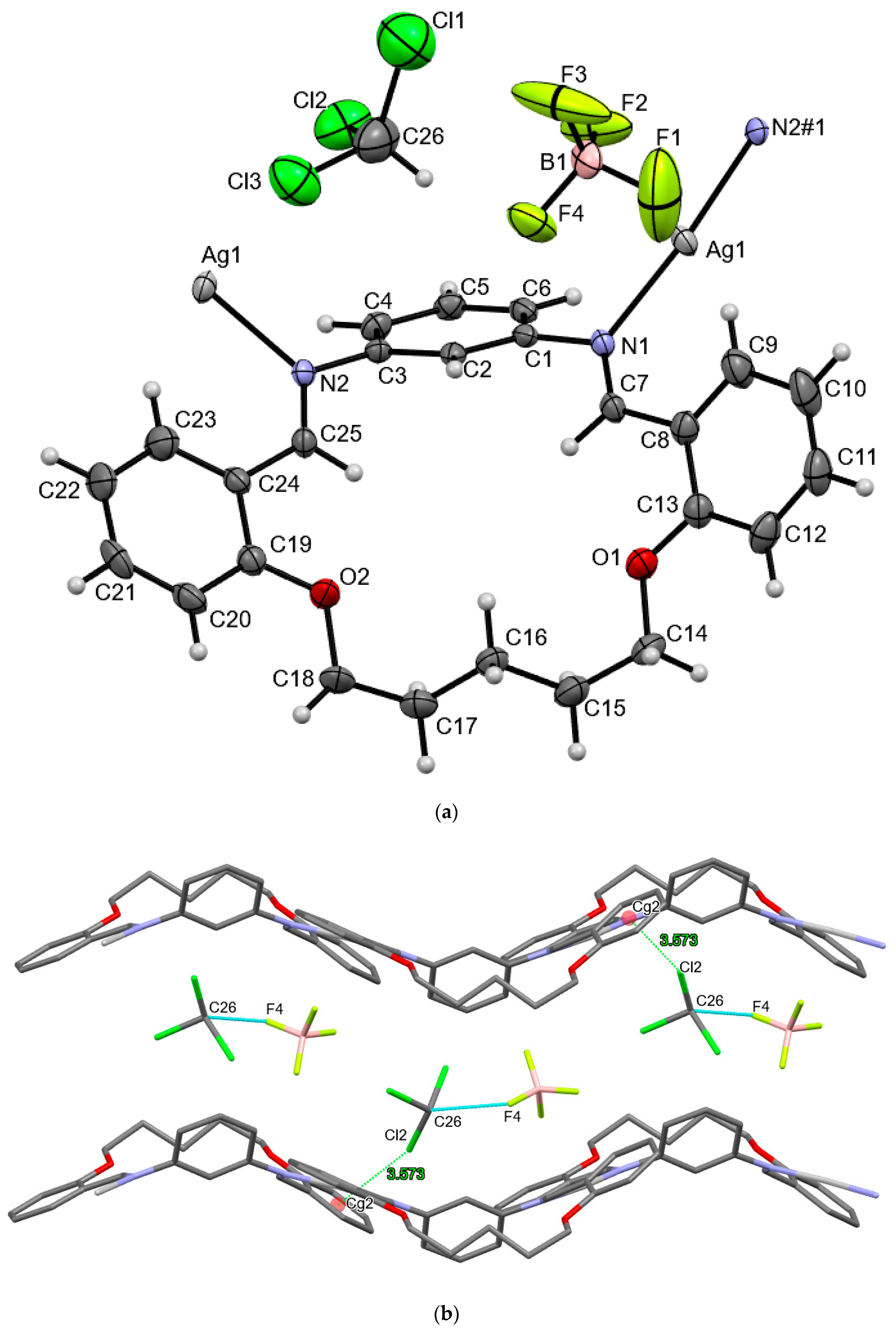
3.2. Crystal Structures of Ag(I) Coordination Polymers
3.3. Anion Influence on Supramolecular Assembly
3.4. Thermal Analysis (TG/DSC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andres, A.; Bazzicalupi, C.; Bencini, A.; Bianchi, A.; Fusi, V.; Garcia-Espana, E.; Paoletti, P.; Valtancoli, B. Oxa-Aza Macrocyclic Molecules as Receptors for Metal Cations. Inorg. Chem. 1994, 33, 617–620. [Google Scholar] [CrossRef]
- Evans, N.H.; Beer, P.D. Advances in Anion Supramolecular Chemistry: From Recognition to Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef]
- Korica, M.; Balić, I.; van Wyk, L.M.; van Heerden, D.P.; Nikolayenko, V.I.; Barbour, L.J.; Jednačak, T.; Đilović, I.; Balić, T. Inclusion of CO2, NH3, SO2, Cl2 and H2S in Porous N4O4-Donor Macrocyclic Schiff Base. Microporous Mesoporous Mater. 2022, 332, 111708. [Google Scholar] [CrossRef]
- Balić, T.; Marković, B.; Jaźwiński, J.; Matković-Čalogović, D. Synthesis and Structural Characterization of Microporous N4O4-Donor Schiff Base Macrocycle: Study of Host–Guest Interactions and Iodine Sorption. Microporous Mesoporous Mater. 2016, 226, 53–60. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Jones, L.O.; Liu, W.; Zhang, L.; Song, B.; Chen, X.; Stern, C.L.; Schatz, G.C.; Stoddart, J.F. Selective Separation of Hexachloroplatinate(IV) Dianions Based on Exo-Binding with Cucurbit[6]Uril. Angew. Chem. Int. Ed. 2021, 60, 17587–17594. [Google Scholar] [CrossRef]
- Balić, T.; Perdih, F.; Mršo, T.; Balić, I. Ligand Influence on the Formation of Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycles and the Role of Anion in Supramolecular Aggregation. Polyhedron 2020, 190, 114774. [Google Scholar] [CrossRef]
- Paurević, M.; Dandić, A.; Šrajer Gajdošik, M.; Vidović, B.; Perdih, F.; Balić, T. Efficient Synthesis of New 17-, 18-, 19- and 20-Membered N2O2-Donor Macrocycles by NaBH4 Reduction and Metal Picrate Extraction Studies. J. Inclusion Phenom. Macrocyclic Chem. 2020, 97, 87–98. [Google Scholar] [CrossRef]
- Vasilescu, I.M.; Bray, D.J.; Clegg, J.K.; Lindoy, L.F.; Meehan, G.V.; Wei, G. Rational Ligand Design for Metal Ion Recognition. Synthesis of a N-Benzylated N2S3-Donor Macrocycle for Enhanced Silver(i) Discrimination. Dalton Trans. 2006, 43, 5115–5117. [Google Scholar] [CrossRef][Green Version]
- Price, J.R.; Fainerman-Melnikova, M.; Fenton, R.R.; Gloe, K.; Lindoy, L.F.; Rambusch, T.; Skelton, B.W.; Turner, P.; White, A.H.; Wichmann, K. Macrocyclic Ligand Design. Structure–Function Relationships Involving the Interaction of Pyridinyl-Containing, Oxygen–Nitrogen Donor Macrocycles with Selected Transition and Post Transition Metal Ions on Progressive N-Benzylation of Their Secondary Amines. Dalton Trans. 2004, 21, 3715–3726. [Google Scholar] [CrossRef]
- Lee, E.; Lee, S.-G.; Park, I.-H.; Kim, S.; Ju, H.; Jung, J.H.; Ikeda, M.; Habata, Y.; Lee, S.S. Endo- and Exocyclic Coordination of a 20-Membered N2O2S2-Macrocycle and Cascade Complexation of a 40-Membered N4O4S4-Macrocycle. Inorg. Chem. 2018, 57, 6289–6299. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, H.-J.; Lee, S.-Y.; Lee, J.-Y.; Lee, S.-S. Endo- and Exo-Coordinated Mercury(II) Complexes of O3S2 Macrocycles: Effect of Dibenzo-Substituents on Coordination Mode. Bull. Korean Chem. Soc. 2007, 28, 2041–2044. [Google Scholar]
- Lee, E.; Lee, S.Y.; Lindoy, L.F.; Lee, S.S. Metallacycles Derived from Metal Complexes of Exo-Coordinated Macrocyclic Ligands. Coord. Chem. Rev. 2013, 257, 3125–3138. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, K.-Q.; Kou, H.-Z. Tunable Gas Adsorption Properties of Porous Coordination Polymers by Modification of Macrocyclic Metallic Tectons. CrystEngComm 2016, 18, 4084–4093. [Google Scholar] [CrossRef]
- Campbell, K.; Kuehl, C.J.; Ferguson, M.J.; Stang, P.J.; Tykwinski, R.R. Coordination-Driven Self-Assembly: Solids with Bidirectional Porosity. J. Am. Chem. Soc. 2002, 124, 7266–7267. [Google Scholar] [CrossRef]
- Kilpin, K.J.; Gower, M.L.; Telfer, S.G.; Jameson, G.B.; Crowley, J.D. Toward the Self-Assembly of Metal−Organic Nanotubes Using Metal−Metal and π-Stacking Interactions: Bis(Pyridylethynyl) Silver(I) Metallo-Macrocycles and Coordination Polymers. Inorg. Chem. 2011, 50, 1123–1134. [Google Scholar] [CrossRef]
- Custelcean, R. Anions in Crystal Engineering. Chem. Soc. Rev. 2010, 39, 3675. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.Y.; Park, K.-M.; Lee, S.S. Supramolecular Networking of Macrocycles Based on Exo-Coordination: From Discrete to Continuous Frameworks. Acc. Chem. Res. 2011, 45, 391–403. [Google Scholar] [CrossRef]
- Počkaj, M.; Cerc Korošec, R.; Popović, Z.; Balić, I.; Sućeska, M.; Dobrilović, M.; Balić, T. The Role of Anion in Supramolecular Aggregation and Energetic Properties in a Series of Cd Picolinamide Complexes. Polyhedron 2022, 228, 116152. [Google Scholar] [CrossRef]
- Sundaresan, S.; Kühne, I.; Kelly, C.; Barker, A.; Salley, D.; Müller-Bunz, H.; Powell, A.; Morgan, G. Anion Influence on Spin State in Two Novel Fe(III) Compounds: [Fe(5F-Sal2333)]X. Crystals 2018, 9, 19. [Google Scholar] [CrossRef]
- Liu, J.-J.; Xia, S.-B.; Duan, Y.-L.; Liu, T.; Cheng, F.-X.; Sun, C.-K. Anion-Controlled Architecture and Photochromism of Naphthalene Diimide-Based Coordination Polymers. Polymers 2018, 10, 165. [Google Scholar] [CrossRef]
- Amini, M.M.; Najafi, E.; Saeidian, H.; Mohammadi, E.; Shahabi, S.M.; Ng, S.W. Effect of Pseudohalogen Groups on the Optical Properties and the Structures of Diorganotin Coordination Compounds Based on the Flexible Ligand 1,2,3,4-Tetra-(4-Pyridyl)-Butane. Appl. Organomet. Chem. 2017, 31, e3884. [Google Scholar] [CrossRef]
- Vigato, P.A.; Tamburini, S. The Challenge of Cyclic and Acyclic Schiff Bases and Related Derivatives. Coord. Chem. Rev. 2004, 248, 1717–2128. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Ahmed, F.M.; Zordok, W.A.; El-Shwiniy, W.H.; Sadeek, S.A.; Elshafie, H.S. Novel Enrofloxacin Schiff Base Metal Complexes: Synthesis, Spectroscopic Characterization, Computational Simulation and Antimicrobial Investigation against Some Food and Phyto-Pathogens. Inorganics 2022, 10, 177. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Meehan, G.V.; Vasilescu, I.M.; Kim, H.J.; Lee, J.-E.; Lee, S.S. Transition and Post-Transition Metal Ion Chemistry of Dibenzo-Substituted, Mixed-Donor Macrocycles Incorporating Five Donor Atoms. Coord. Chem. Rev. 2010, 254, 1713–1725. [Google Scholar] [CrossRef]
- Khlobystov, A.N.; Blake, A.J.; Champness, N.R.; Lemenovskii, D.A.; Majouga, A.G.; Zyk, N.V.; Schröder, M. Supramolecular Design of One-Dimensional Coordination Polymers Based on Silver(I) Complexes of Aromatic Nitrogen-Donor Ligands. Coord. Chem. Rev. 2001, 222, 155–192. [Google Scholar] [CrossRef]
- Pickering, A.L.; Long, D.-L.; Cronin, L. Coordination Networks through the Dimensions: From Discrete Clusters to 1D, 2D, and 3D Silver(I) Coordination Polymers with Rigid Aliphatic Amino Ligands. Inorg. Chem. 2004, 43, 4953–4961. [Google Scholar] [CrossRef]
- Robin, A.Y.; Fromm, K.M. Coordination Polymer Networks with O- and N-Donors: What They Are, Why and How They Are Made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Fromm, K.M. Silver Coordination Compounds with Antimicrobial Properties. Appl. Organomet. Chem. 2013, 27, 683–687. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef]
- Balić, T.; Marković, B.; Jaźwiński, J.; Matković-Čalogović, D. Synthesis and structural characterization of new N2O2-donor Schiff base macrocycles and their silver (I) coordination polymers. Inorg. Chim. Acta 2015, 435, 283–291. [Google Scholar] [CrossRef]
- STARe, Software, Version 10.0; Mettler-Toledo GmbH: Gießen, Germany, 2009.
- CrysAlisPro, Version 1.171.39.64; Rigaku Oxford Diffraction: Yarnton, UK, 2019.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Found. Adv. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON: A Multipurpose Crystallographic Tool; Ultrecht University: Ultrecht, The Netherlands, 1998. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and Analysis of Crystal Structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Balić, T.; Marković, B. Crystal Structure of 2,2′-[Pentane-1,5-Diylbis(Oxy)]Dibenzaldehyde, C19H20O4. Z. Für Krist.—New Cryst. Struct. 2016, 231, 619–621. [Google Scholar] [CrossRef]
- Tăbăcaru, A.; Pettinari, C.; Bușilă, M.; Dinică, R.M. New Antibacterial Silver(I) Coordination Polymers Based on a Flexible Ditopic Pyrazolyl-Type Ligand. Polymers 2019, 11, 1686. [Google Scholar] [CrossRef]
- Cao, R.; McCarthy, B.D.; Lippard, S.J. Immobilization, Trapping, and Anion Exchange of Perrhenate Ion Using Copper-Based Tripodal Complexes. Inorg. Chem. 2011, 50, 9499–9507. [Google Scholar] [CrossRef]
- Świetlik, R.; Jankowski, D.; Fourmigué, M.; Yakushi, K. Infrared and Raman Studies of the Anion Ordering Transitions in Paramagnetic Organometallic Radical Cation Salts [Cp2Mo(Dmit)]X (X = PF6, SbF6). Vib. Spectrosc. 2011, 55, 195–200. [Google Scholar] [CrossRef]
- Grabowski, S.J. Hydrogen Bonds with BF4− Anion as a Proton Acceptor. Crystals 2020, 10, 460. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready-Reference Book of Chemical and Physical Data; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mingos, D.M.P.; Rohl, A.L. Size and Shape Characteristics of Inorganic Molecules and Ions and Their Relevance to Molecular Packing Problems. Dalton Trans. 1991, 12, 3419–3425. [Google Scholar] [CrossRef]
- ACD/ChemSketch (Freeware), 2021.1.3 ACD/Labs 2021.1.3 (File Version C25E41, Build 123835, 29 August 2021); Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2021.
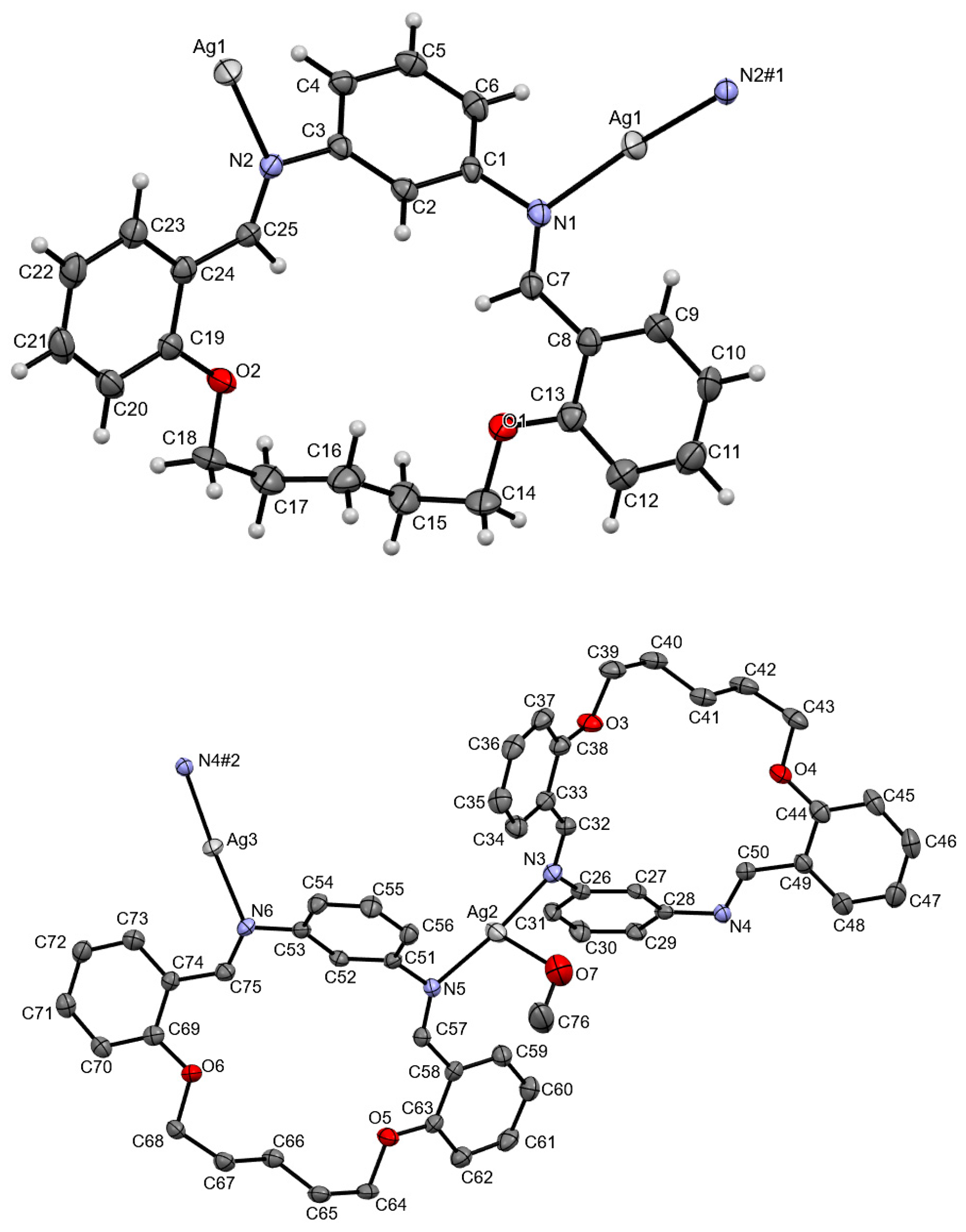
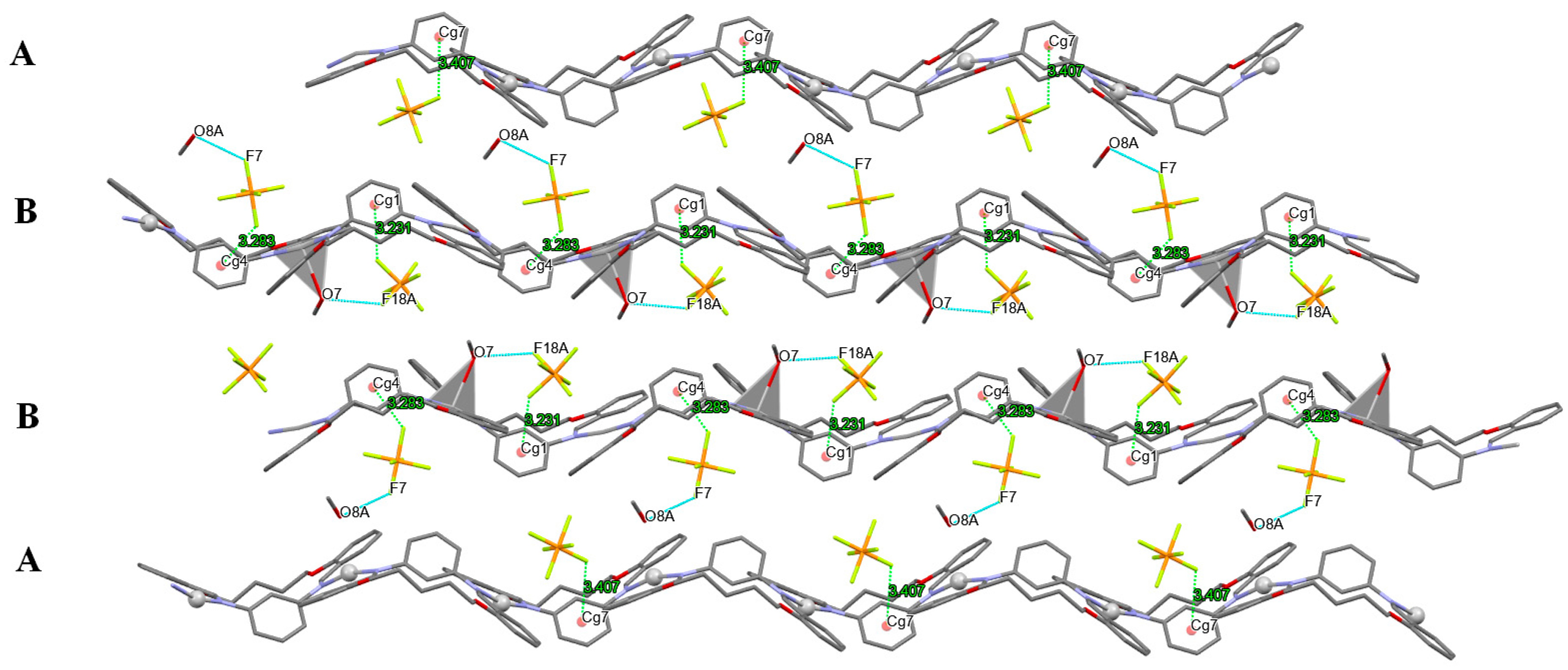

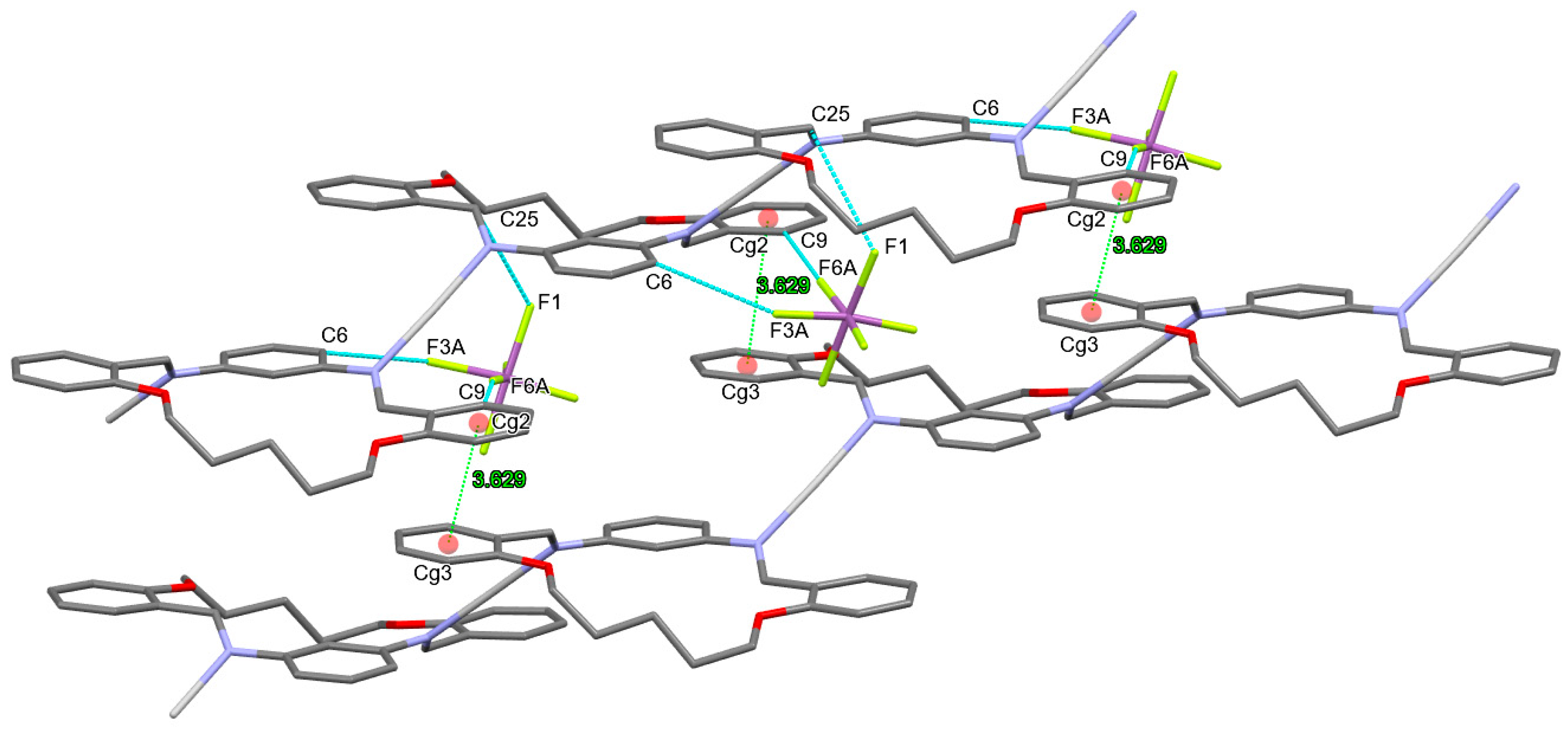
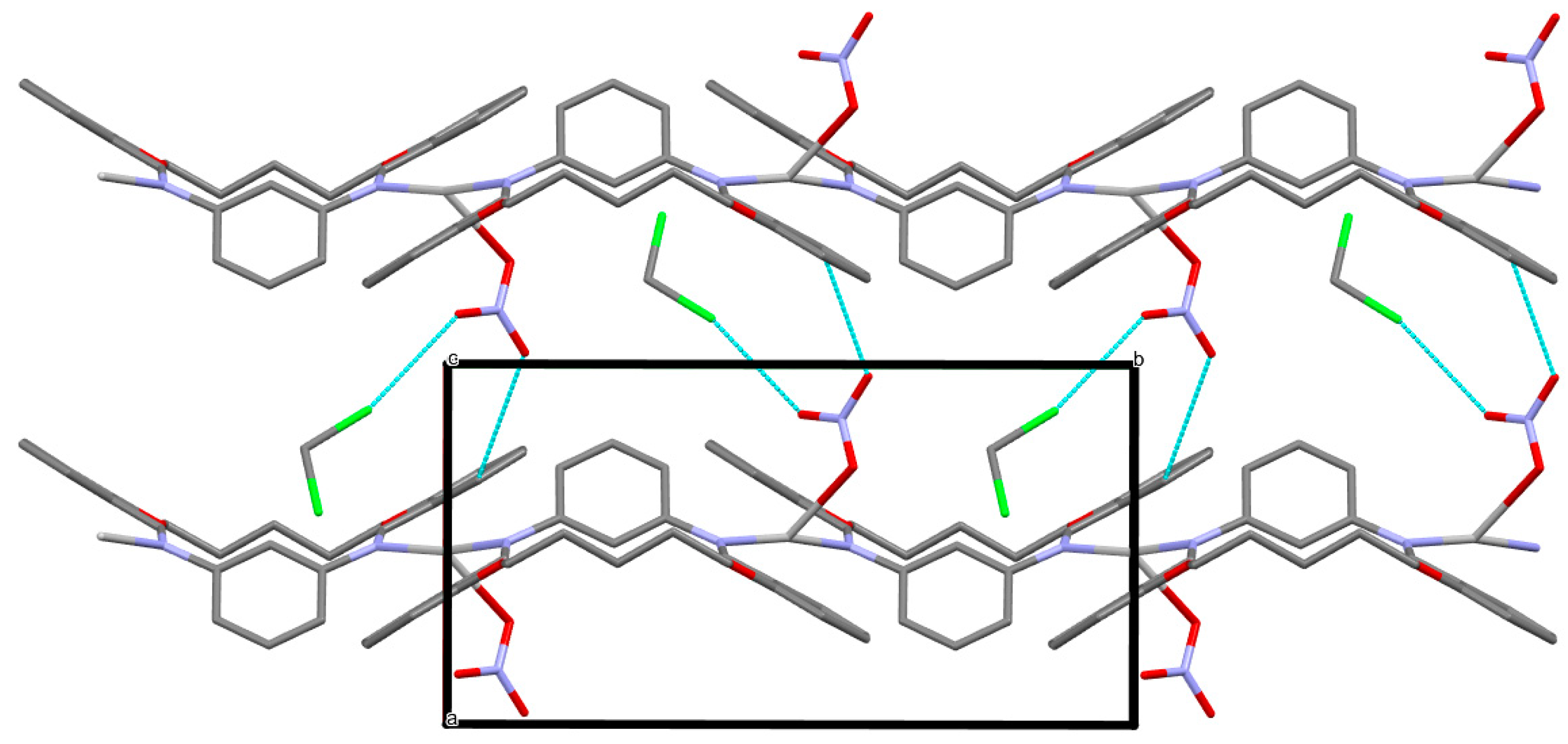
| Compound | AgLClO4 | AgLPF6 | AgLBF4 | AgLSbF6 |
|---|---|---|---|---|
| CCD number | 2223301 | 2223302 | 2223300 | 2223303 |
| Empirical formula | C26H25AgCl4N2O6 | C77H80Ag3F18N6O8P3 | C26H24AgBCl3F4N2O2 | C25H23AgF6N2O2Sb |
| Formula weight | 711.15 | 1975.99 | 697.50 | 727.092 |
| Temperature/K | 150.00(10) | 150.00(10) | 150.00(10) | 150.00(10) |
| Crystal system | orthorhombic | orthorhombic | orthorhombic | monoclinic |
| Space group | P212121 | P212121 | P212121 | P21/n |
| a/Å | 8.2849(4) | 14.6441(4) | 8.2608(7) | 10.4581(3) |
| b/Å | 15.7717(6) | 21.4869(5) | 15.7088(6) | 23.7924(9) |
| c/Å | 21.1305(8) | 24.9257(10) | 21.0174(10) | 11.9083(6) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 90 | 90 | 90 | 95.943(4) |
| γ/° | 90 | 90 | 90 | 90 |
| Volume/Å3 | 2761.1(2) | 7843.0(4) | 2727.4(3) | 2947.1(2) |
| Z | 4 | 4 | 4 | 4 |
| ρcalcg/cm3 | 1.711 | 1.673 | 1.699 | 1.639 |
| μ/mm−1 | 1.162 | 0.905 | 1.089 | 1.641 |
| F(000) | 1432.0 | 3984.0 | 1396.0 | 1415.1 |
| Crystal size/mm3 | 0.2 × 0.15 × 0.1 | 0.25 × 0.2 × 0.1 | 0.2 × 0.2 × 0.2 | 0.35 × 0.25 × 0.2 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) | Mo Kα (λ = 0.71073) |
| 2Θ range for data collection/° | 4.64 to 54.97 | 4.702 to 54.966 | 4.664 to 54.968 | 4.86 to 54.96 |
| Index ranges | −9 ≤ h ≤ 10, −20 ≤ k ≤ 19, −25 ≤ l ≤ 27 | −13 ≤ h ≤ 19, −27 ≤ k ≤ 27, −19 ≤ l ≤ 32 | −7 ≤ h ≤ 10, −20 ≤ k ≤ 20, −26 ≤ l ≤ 27 | −14 ≤ h ≤ 13, −32 ≤ k ≤ 33, −16 ≤ l ≤ 16 |
| Reflections collected | 13345 | 36345 | 9867 | 27761 |
| Independent reflections | 6328 [Rint = 0.0322, Rsigma = 0.0497] | 17962 [Rint = 0.0267, Rsigma = 0.0455] | 5858 [Rint = 0.0251, Rsigma = 0.0512] | 6762 [Rint = 0.0300, Rsigma = 0.0318] |
| Data/restraints/parameters | 6328/0/380 | 17962/12/1088 | 5858/0/352 | 6762/48/371 |
| Goodness-of-fit on F2 | 1.069 | 1.030 | 1.062 | 1.077 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0541, wR2 = 0.1421 | R1 = 0.0373, wR2 = 0.0739 | R1 = 0.0566, wR2 = 0.1527 | R1 = 0.0449, wR2 = 0.1067 |
| Final R indexes [all data] | R1 = 0.0667, wR2 = 0.1551 | R1 = 0.0515, wR2 = 0.0815 | R1 = 0.0697, wR2 = 0.1660 | R1 = 0.0569, wR2 = 0.1163 |
| Largest diff. peak/hole/e Å−3 | 1.26/−1.41 | 1.04/−0.68 | 1.40/−1.72 | 1.01/−1.05 |
| Flack parameter | 0.01(2) | 0.297(19) | −0.01(2) |
| AgLClO4 | |||||
|---|---|---|---|---|---|
| d(D–H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D–H⋅⋅⋅A) | symmetry operator | |
| C23–H23···O3A | 0.95 | 2.193 | 2.935(7) | 134(9) | −x + 1, +y + 1/2, −z + 1/2 |
| C26–H26···O5 | 1.00 | 2.236 | 3.107(1) | 144(8) | x, y, z |
| C10–H10···O6A | 0.95 | 2.881 | 3.072(1) | 92(1) | x, y, z |
| AgLBF4 | |||||
| d(D–H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D–H⋅⋅⋅A) | symmetry operator | |
| C26–H26···F4 | 1.00 | 2.208 | 3.087(7) | 145(8) | x, y, z |
| Y–X···Cg | X···Cg (Å) | Y···Cg | γ | Y–X···Cg | symmetry operator |
| C26–Cl2···Cg2(C8→C13) | 3.573(1) | 5.213(1) | 16.36 | 159(3) | x, y, z |
| AgLPF6 | |||||
| d(D–H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D–H⋅⋅⋅A) | symmetry operator | |
| O7–H7A···F18A | 0.84 | 2.08 | 2.906(4) | 169(1) | −1/2 + x, 1/2 − y, 1 − z |
| O8A–H8A···F7 | 0.84 | 2.04 | 2.849(7) | 163(1) | x, y, z |
| C6–H6···F7 | 0.95 | 2.42 | 3.144(7) | 133(1) | 1/2 + x, 1/2 − y, −z |
| C20–H20···F16A | 0.95 | 2.39 | 3.201(9) | 143(1) | 1/2 − x, −y, −1/2 + z |
| C29–H29···F6 | 0.95 | 2.30 | 3.054(7) | 136(1) | −1 + x, y, z |
| C37–H37···F3 | 0.95 | 2.41 | 3.341(3) | 167(1) | 1 − x, −1/2 + y, 1/2 − z |
| C70–H70···F10 | 0.95 | 2.48 | 3.403(9) | 165(1) | 1 − x, 1/2 + y, 1/2 − z |
| C42–H42B···F11 | 0.99 | 2.44 | 3.384(8) | 160(1) | −x, −1/2 + y, 1/2 − z |
| Y–X···Cg | X···Cg (Å) | Y···Cg | γ | Y–X···Cg | symmetry operator |
| P1–F5···Cg7(C1→C6) | 3.4064 | 4.3990 | 27.29 | 119 | x, y, z |
| P2–F12···Cg4(C51→C56) | 3.2838 | 4.3559 | 20.63 | 123 | x, y, z |
| P3–F15A···Cg1(C26→C31) | 3.2308 | 4.4960 | 20.84 | 133 | 1/2 + x, 1/2 − y, 1 − z |
| X–H···Cg | X···Cg (Å) | H···Cg | γ | X–H···Cg | symmetry operator |
| C68–H68A···Cg9(C19→C24) | 3.5634 | 2.85 | 12.84 | 130 | 1 − x, 1/2 + y, 1/2 − z |
| AgLSbF6 | |||||
| d(D–H) | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(D–H⋅⋅⋅A) | symmetry operator | |
| C6–H6···F3A | 0.95 | 2.57 | 3.463(1) | 156(3) | x + 1/2, −y + 1/2 + 1, +z − 1/2 |
| C9–H9···F6A | 0.95 | 2.45 | 3.397(1) | 171(8) | x + 1/2, −y + 1/2 + 1, +z − 1/2 |
| C14–H14B···F4A | 0.99 | 2.39 | 3.354(1) | 163(4) | −x + 2, −y + 1, −z + 2 |
| C20–H20···F4A | 0.95 | 2.54 | 3.486(1) | 170(8) | −x + 1, −y + 1, −z + 2 |
| Cg···Cg (Å) | α | β | Cg···plane | symmetry operator | |
| Cg2 (C8→C13)··· Cg3 (C19→C24) | 3.629(4) | 6.296 | 21.41 | 3.445 | −1 + x, y, z |
| Cg3 (C19→C24) ···Cg2 (C8→C13) | 3.629(4) | 6.296 | 18.67 | 3.385 | 1 + x, y, z |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perdih, F.; Korica, M.; Šebalj, L.; Balić, T. Anion Influence on Supramolecular Interactions in Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycle. Crystals 2023, 13, 50. https://doi.org/10.3390/cryst13010050
Perdih F, Korica M, Šebalj L, Balić T. Anion Influence on Supramolecular Interactions in Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycle. Crystals. 2023; 13(1):50. https://doi.org/10.3390/cryst13010050
Chicago/Turabian StylePerdih, Franc, Milenko Korica, Lorena Šebalj, and Tomislav Balić. 2023. "Anion Influence on Supramolecular Interactions in Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycle" Crystals 13, no. 1: 50. https://doi.org/10.3390/cryst13010050
APA StylePerdih, F., Korica, M., Šebalj, L., & Balić, T. (2023). Anion Influence on Supramolecular Interactions in Exo-Coordinated Silver(I) Complexes with N2O2 Schiff Base Macrocycle. Crystals, 13(1), 50. https://doi.org/10.3390/cryst13010050