Investigation of Magnetocaloric Properties in the TbCo2-H System
Abstract
:1. Introduction
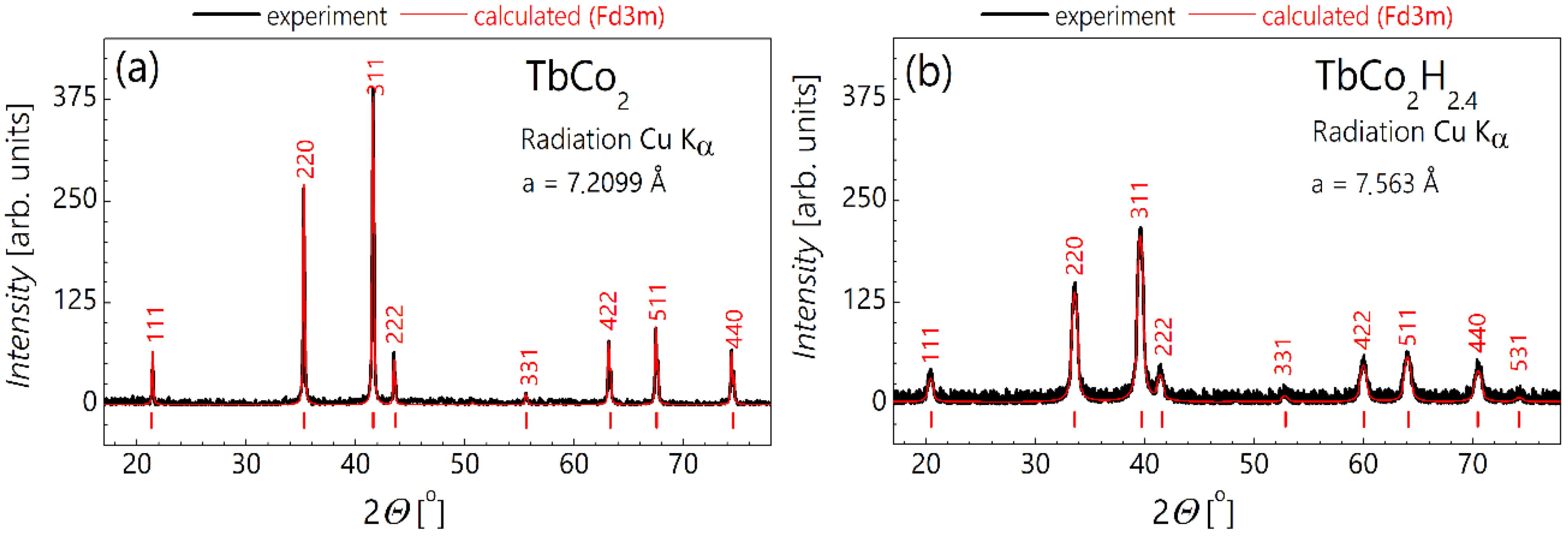
2. Materials and Methods
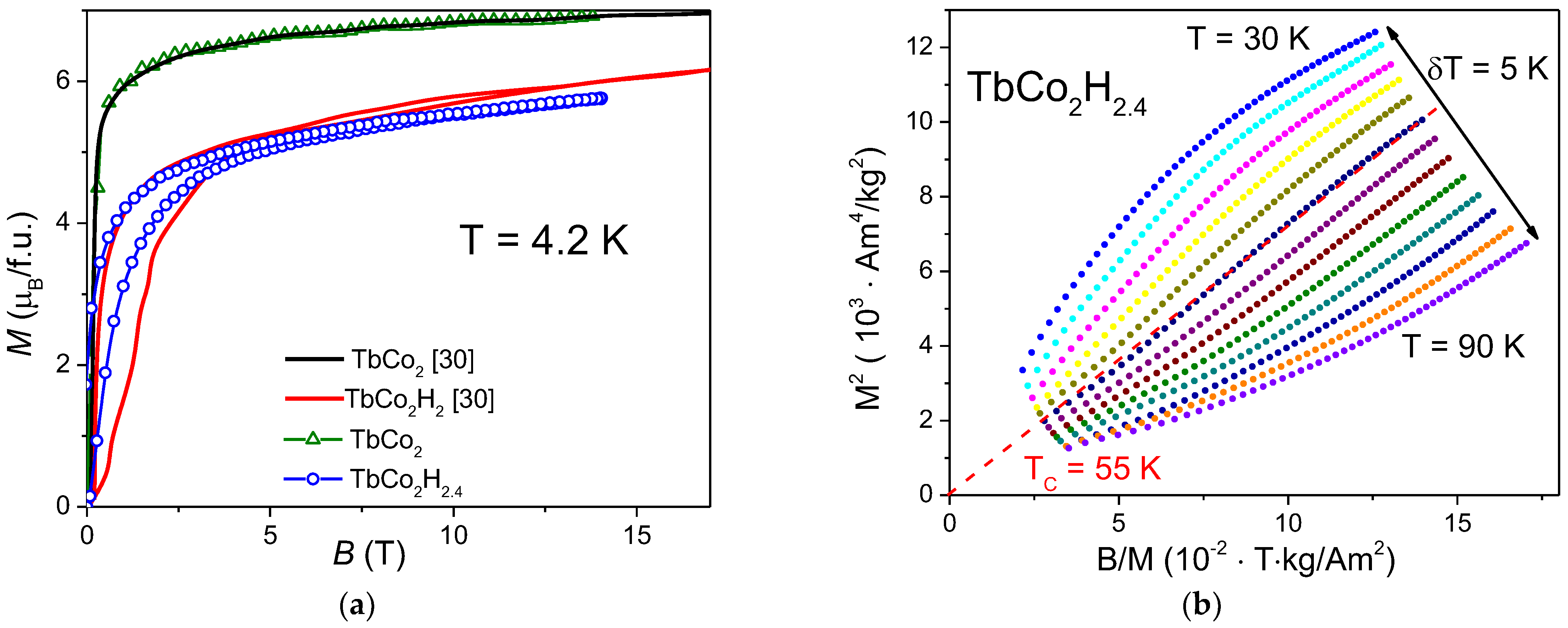
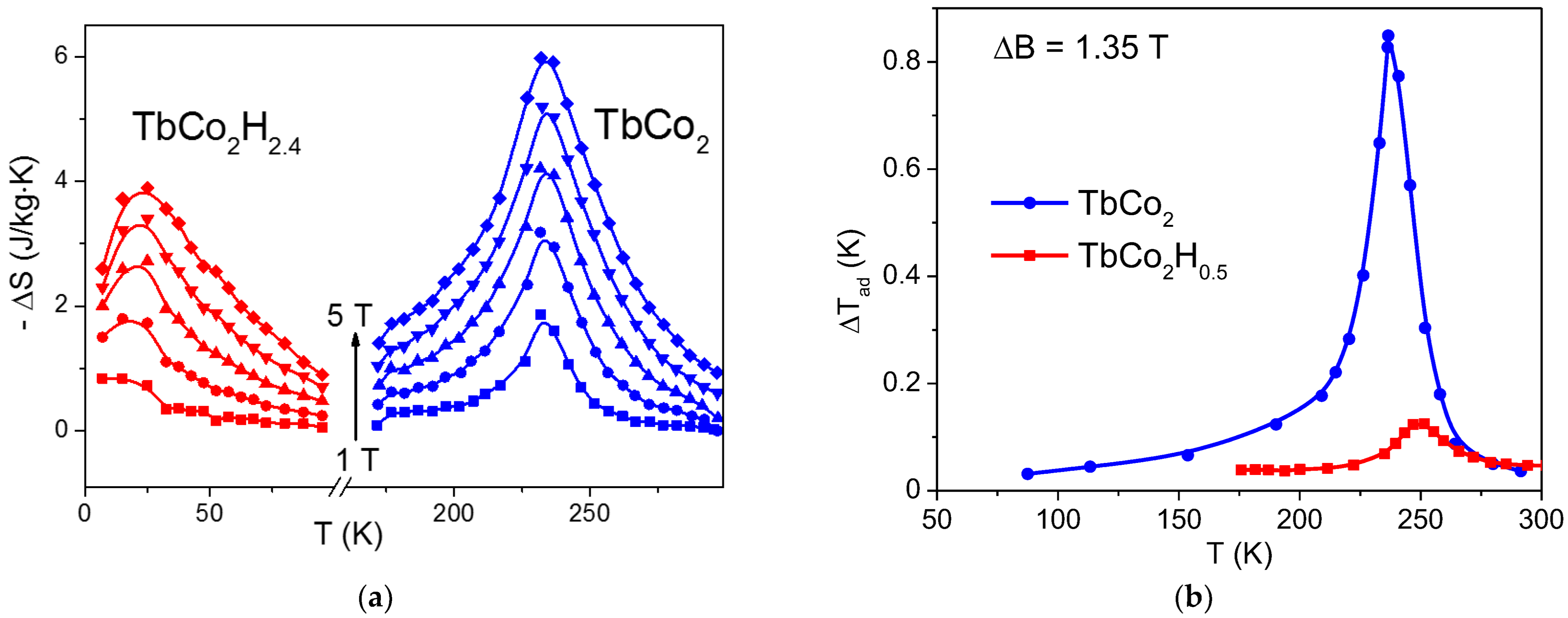
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tishin, A.M.; Spichkin, Y.I. The Magnetocaloric Effect and Its Applications; Institute of Physics Publishing: Bristol, PA, USA, 2003; p. 476. [Google Scholar]
- Kitanovski, A.; Tušek, J.; Tomc, U.; Plaznik, U.; Ožbolt, M.; Poredoš, A. Magnetocaloric Energy Conversion from Theory to Applications; Springer International Publishing: Cham, Switzerland, 2015; p. 456. [Google Scholar]
- Duc, N.H.; Kim, A.D.T.; Brommer, P.E. Metamagnetism, giant magnetoresistance and magnetocaloric effects in RCo2-based compounds in the vicinity of the Curie temperature. Phys. B 2002, 319, 1–8. [Google Scholar] [CrossRef]
- Gschneidner, K.A.; Pecharsky, V.K., Jr. Magnetocaloric materials. Annu. Rev. Mater. Sci. 2000, 30, 387–429. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.K.; Suresh, K.G.; Nigam, A.K.; Malik, S.K.; Coelho, A.A.; Gama, S. Itinerant electron metamagnetism and magnetocaloric effect in RCo2-based Laves phase compounds. J. Magn. Magn. Mater. 2007, 317, 68–79. [Google Scholar] [CrossRef] [Green Version]
- Stein, F.; Leineweber, A. Laves phases: A review of their functional and structural applications and an improved fundamental understanding of stability and properties. J. Mater. Sci. 2021, 56, 5321–5427. [Google Scholar] [CrossRef]
- Gratz, E.; Markosyan, A.S. Physical properties of RCo2 Laves phases. J. Phys. Condens. Matter. 2001, 13, 385–413. [Google Scholar] [CrossRef]
- Zeng, Y.; Tian, F.; Chang, T.; Chen, K.; Yang, S.; Cao, K.; Zhou, C.; Song, X. Large magnetocaloric effect and near-zero thermal hysteresis in the rare earth intermetallic Tb1−xDyxCo2 compounds. J. Phys. Condens. Matter 2017, 29, 055804. [Google Scholar] [CrossRef] [PubMed]
- Balli, M.; Fruchart, D.; Gignoux, D. A study of magnetism and magnetocaloric effect in Ho1-xTbxCo2 compounds. J. Magn. Magn. Mater. 2007, 314, 16–20. [Google Scholar] [CrossRef]
- Cwik, J.; Palewski, T.; Nenkov, K.; Gutfleisch, O.; Klamut, J. The influence of Er substitution on magnetic and magnetocaloric properties of Dy1-xErxCo2 solid solutions. Intermetallics 2011, 19, 1656–1660. [Google Scholar] [CrossRef]
- Zhouv, K.W.; Zhuang, Y.H.; Li, J.Q.; Deng, J.Q.; Zhu, Q.M. Magnetocaloric effects in (Gd1-xTbx)Co2. Solid State Comm. 2006, 137, 275–277. [Google Scholar]
- Tereshina, I.S.; Chzhan, V.B.; Tereshina, E.A.; Khmelevskyi, S.; Burkhanov, G.S.; Ilyushin, A.S.; Paukov, M.A.; Havela, L.; Karpenkov, A.Y.; Cwik, J.; et al. Magnetostructural phase transitions and magnetocaloric effect in Tb-Dy-Ho-Co-Al alloys with a Laves phase structure. J. Appl. Phys. 2016, 120, 013901. [Google Scholar] [CrossRef]
- Politova, G.A.; Tereshina, I.S.; Cwik, J. Multifunctional phenomena in Tb-Dy-Gd(Ho)-Co(Al) compounds with a Laves phase structure: Magnetostriction and magnetocaloric effect. J. Alloys Compd. 2020, 843, 155887. [Google Scholar] [CrossRef]
- Tereshina, I.; Politova, G.; Tereshina, E.; Nikitin, S.; Burkhanov, G.; Chistyakov, O.; Karpenkov, A. Magnetocaloric and magnetoelastic effects in (Tb0.45Dy0.55)1-xErxCo2 multicomponent compounds. J. Phys. Conf. Ser. 2010, 200, 092012. [Google Scholar] [CrossRef]
- De Oliveira, N.A.; von Ranke, P.J. Theoretical aspects of the magnetocaloric effect. Phys. Rep. 2010, 489, 89–159. [Google Scholar] [CrossRef]
- Belov, K.P. Magnetic Transitions; Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Oesterreicher, H.; Parker, F.T. Magnetic cooling near Curie temperatures above 300 K. J. Appl. Phys. 1984, 55, 4334–4338. [Google Scholar] [CrossRef]
- Romanov, A.Y.; Silin, V.P. On the magnetocaloric effect in inhomogeneous ferromagnets. Phys. Met. Metallogr. 1997, 83, 111–115. [Google Scholar]
- Landau, L.D. Theory of Phase Transformations. II. Zh. Eksp. Teor. Fiz. 1937, 7, 627. (In Russian) [Google Scholar]
- Franco, V.; Blázquez, J.; Conde, A. Field dependence of the magnetocaloric effect in materials with a second order phase transition: A master curve for the magnetic entropy change. Appl. Phys. Lett. 2006, 89, 222512. [Google Scholar] [CrossRef]
- Franco, V.; Conde, A.; Pecharsky, V.K.; Gschneidner, K.A. Field dependence of the magnetocaloric effect in Gd and (Er1−xDyx)Al2: Does a universal curve exist. EPL 2007, 79, 47009. [Google Scholar] [CrossRef]
- Franco, V.; Blázquez, J.S.; Ipus, J.J.; Law, J.Y.; Moreno-Ramírez, L.M.; Conde, A. Magnetocaloric effect: From materials research to refrigeration devices. Prog. Mater. Sci. 2018, 93, 112–232. [Google Scholar] [CrossRef]
- Huang, D.; Gao, J.; Lapidus, S.H.; Brown, D.E.; Ren, Y. Exotic hysteresis of ferrimagnetic transition in Laves compound TbCo2. Mater. Res. Lett. 2020, 8, 97–102. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, S.A.; Tskhadadze, G.A.; Ovthenkova, I.A.; Zhukova, D.A.; Ivanova, T.I. The Magnetic Phase Transitions and Magnetocaloric Effect in the Ho(Co1-xAlx)2 and Tb(Co1-xAlx)2 Compounds. Solid State Phenom. 2011, 168–169, 119–121. [Google Scholar]
- Ovchenkova, I.A.; Tskhadadze, G.A.; Zhukova, D.A.; Ivanova, T.I.; Nikitin, S.A. Magnetocaloric effect in RCo2 compounds. Solid State Phenom. 2012, 190, 339–342. [Google Scholar] [CrossRef]
- Janatova, M.; Poltierova, J.; Vejpravova, J.; Javorsky, P.; Prokleska, J.; Svoboda, P.; Danis, S. Effect of Si Substitution and Annealing on Magnetocaloric Properties in TbCo2. Acta Phys. Pol. A 2008, 113, 311–314. [Google Scholar] [CrossRef]
- Gerasimov, E.G.; Inishev, A.A.; Terentev, P.B.; Kazantsev, V.A.; Mushnikov, N.V. Magnetostriction and thermal expansion of nonstoichiometric TbCo2Mnx compounds. J. Magn. Magn. Mater. 2021, 523, 167628. [Google Scholar] [CrossRef]
- Halder, M.; Yusuf, S.M.; Mukadam, M.D. Magnetocaloric effect and critical behavior near the paramagnetic to ferrimagnetic phase transition temperature in TbCo2−xFex. Phys. Rev. B 2010, 81, 174402. [Google Scholar] [CrossRef] [Green Version]
- Fang, C.H. Tuning the magnetic and structural transitions in TbCo2Mnx compounds. Phys. Rev. B 2017, 96, 064425. [Google Scholar] [CrossRef] [Green Version]
- Mushnikov, N.V.; Gaviko, V.S.; Goto, T. Magnetic Properties of Hydrides RCo2Hx with R = Gd, Tb, Dy, Ho, and Er. Phys. Met. Metallogr. 2005, 100, 338–348. [Google Scholar]
- Tereshina, I.S.; Kaminskaya, T.P.; Chzhan, V.B.; Ovchenkova, Y.A.; Trusheva, A.S.; Viryus, A.A. The Influence of Hydrogenation on the Structure, Magnetic and Magnetocaloric Properties of Tb–Dy–Co Alloys with a Laves Phase Structure. Phys. Solid State 2019, 61, 1169–1175. [Google Scholar] [CrossRef]
- Tereshina, I.; Cwik, J.; Tereshina, E.; Politova, G.; Burkhanov, G.; Chzhan, V.; Ilyushin, A.; Miller, M.; Zaleski, A.; Schultz, L.; et al. Multifunctional Phenomena in Rare-Earth Intermetallic Compounds With a Laves Phase Structure: Giant Magnetostriction and Magnetocaloric Effect. IEEE Trans. Magn. 2014, 50, 2504604. [Google Scholar] [CrossRef]
- Nizhankovskii, V.I.; Lugansky, L.B. Vibrating sample magnetometer with a step motor. Meas. Sci. Technol. 2007, 18, 1533–1537. [Google Scholar] [CrossRef]
- Belov, K.P. Magnetic Transformations; FizMatGiz: Moscow, Russia, 1959; p. 260. [Google Scholar]
- Banerjee, S.K. On a generalised approach to first and second order magnetic transitions. Phys. Lett. 1964, 12, 16–17. [Google Scholar] [CrossRef]
- Zhou, C.; Chang, T.; Dai, Z.; Chen, Y.; Guo, C.; Matsushita, Y.; Ke, X.; Murtaza, A.; Zhang, Y.; Tian, F.; et al. Unified understanding of the first-order nature of the transition in TbCo2. Phys. Rev. B. 2022, 106, 064409. [Google Scholar] [CrossRef]
- Khmelevskyi, S.; Mohn, P. The order of the magnetic phase transitions in RCo2 (R = rare earth) intermetallic compounds. J. Phys. Condens. Matter 2000, 12, 9453–9464. [Google Scholar] [CrossRef]
- Burzo, E.; Vlaic, P.; Kozlenko, D.P.; Kichanov, S.E.; Dang, N.T.; Lukin, E.V.; Savenko, B.N. Magnetic properties of TbCo2 compound at high pressures. J. Alloys Compd. 2013, 551, 702–710. [Google Scholar] [CrossRef]
- Brouha, M.; Buschow, K.H.J. The pressure dependence of the Curie temperature of rare earth—Cobalt compounds. J. Phys. F Met. Phys. 1973, 3, 2218–2226. [Google Scholar] [CrossRef]
- Tereshina, E.A.; Khmelevskyi, S.; Politova, G.; Kaminskaya, T.; Drulis, H.; Tereshina, I.S. Magnetic ordering temperature of nanocrystalline Gd: Enhancement of magnetic interactions via hydrogenation-induced “negative” pressure. Sci. Rep. 2016, 6, 22553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tereshina, E.A.; Yoshida, H.; Andreev, A.V.; Tereshina, I.S.; Koyama, K.; Kanomata, T. Magnetism of a Lu2Fe17H Single Crystal under Pressure. J. Phys. Soc. Jpn. 2007, 76 (Suppl. A), 82–83. [Google Scholar] [CrossRef] [Green Version]
- Nikitin, S.A.; Tereshina, I.S.; Verbetsky, V.N.; Salamova, A.A.; Skokov, K.P.; Pankratov, N.Y.; Skourski, Y.V.; Tristan, N.V.; Zubenko, V.V.; Telegina, I.V. Magnetostriction and magnetic anisotropy in TbFe11TiHx single crystal. J. Alloys Compd. 2001, 322, 42–44. [Google Scholar] [CrossRef]
- Shoemaker, D.P.; Shoemaker, C.B. Concerning atomic sites and capacities for hydrogen absorption in the AB2 Friauf-Laves phases. J. Less-Common Met. 1979, 68, 43–58. [Google Scholar] [CrossRef]
- DeSaxce, T.; Berthier, Y.; Fruchart, D. Magnetic and structural properties of the ternary hydrides of ErFe2. J. Less-Common Met. 1985, 107, 35–43. [Google Scholar] [CrossRef]
- Stanley, H.E. Introduction to Phase Transitions and Critical Phenomena; Oxford University Press: New York, NY, USA, 1971. [Google Scholar]
- Stanley, H.E. Scaling, universality, and renormalization: Three pillars of modern critical phenomena. Rev. Mod. Phys. 1999, 71, 358–366. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Midya, A.; Mydeen, K.; Mandal, P.; Loidl, A.; Prabhakaran, D. Critical behavior in single-crystalline La0.67 Sr0.33 CoO3. Phys. Rev. B 2010, 82, 064422. [Google Scholar] [CrossRef]
- Sokolovskiy, V.V.; Miroshkina, O.N.; Buchelnikov, V.D.; Marchenkov, V.V. Magnetocaloric Effect in Metals and Alloys. Phys. Metals Metallogr. 2022, 123, 315–318. [Google Scholar] [CrossRef]


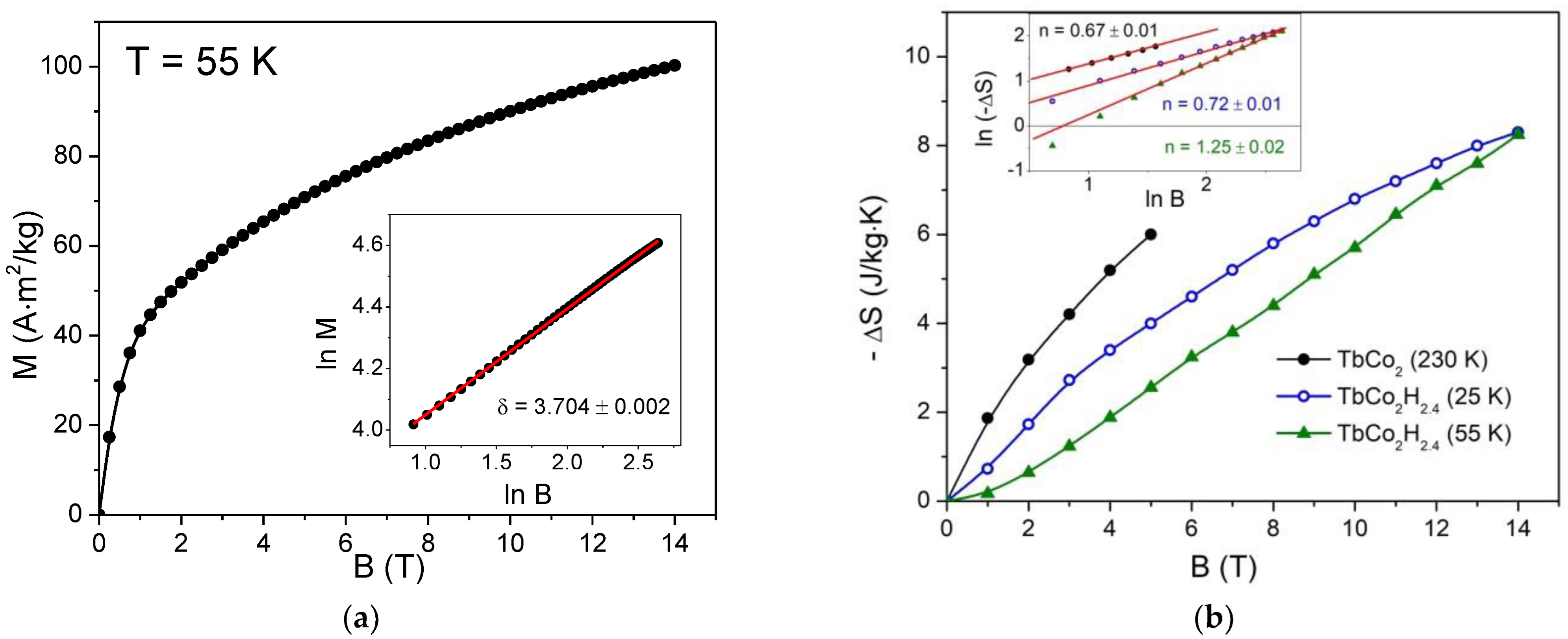
| Compounds | β | δ | n | Tc | −ΔS (0–5 T) | Reference |
|---|---|---|---|---|---|---|
| TbCo2 | 0.386 (5) | 4.83 (4) | 0.67 (1) | 234 | 6 | This work |
| TbCo2 | - | - | 0.67 (1) | 235 | 6 | [25] |
| TbCo2 | 0.380 (4) | 4.85 (3) | 0.65 (1) | 231 | 6.9 | [26] |
| TbCo1.94Fe0.06 | - | - | - | 275 | 3.9 | [25] |
| TbCo1.9Fe0.1 | 0.541 (1) | 2.75 (4) | 0.76 (2) | 303 | 3.7 | [25] |
| TbCo2H2.4 | 0.53 (3) | 3.704 (2) | 0.72 (1) | 55 | 2.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politova, G.; Tereshina, I.; Ovchenkova, I.; Aleroev, A.-R.; Koshkid’ko, Y.; Ćwik, J.; Drulis, H. Investigation of Magnetocaloric Properties in the TbCo2-H System. Crystals 2022, 12, 1783. https://doi.org/10.3390/cryst12121783
Politova G, Tereshina I, Ovchenkova I, Aleroev A-R, Koshkid’ko Y, Ćwik J, Drulis H. Investigation of Magnetocaloric Properties in the TbCo2-H System. Crystals. 2022; 12(12):1783. https://doi.org/10.3390/cryst12121783
Chicago/Turabian StylePolitova, Galina, Irina Tereshina, Ioulia Ovchenkova, Abdu-Rahman Aleroev, Yurii Koshkid’ko, Jacek Ćwik, and Henryk Drulis. 2022. "Investigation of Magnetocaloric Properties in the TbCo2-H System" Crystals 12, no. 12: 1783. https://doi.org/10.3390/cryst12121783
APA StylePolitova, G., Tereshina, I., Ovchenkova, I., Aleroev, A.-R., Koshkid’ko, Y., Ćwik, J., & Drulis, H. (2022). Investigation of Magnetocaloric Properties in the TbCo2-H System. Crystals, 12(12), 1783. https://doi.org/10.3390/cryst12121783







