Fabrication and Characterization of Clinacanthus nutans Mediated Reduced Graphene Oxide Using a Green Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Graphene Oxide Preparation
2.2. Preparation of Plant Material
2.3. Preparation of C. Nutans Leaf Extract
2.4. Reduced Graphene Oxide Synthesis Using Leaf Extracts
3. Characterization
3.1. Ultraviolet—Visible Spectroscopy
3.2. X-ray Diffraction Spectroscopy
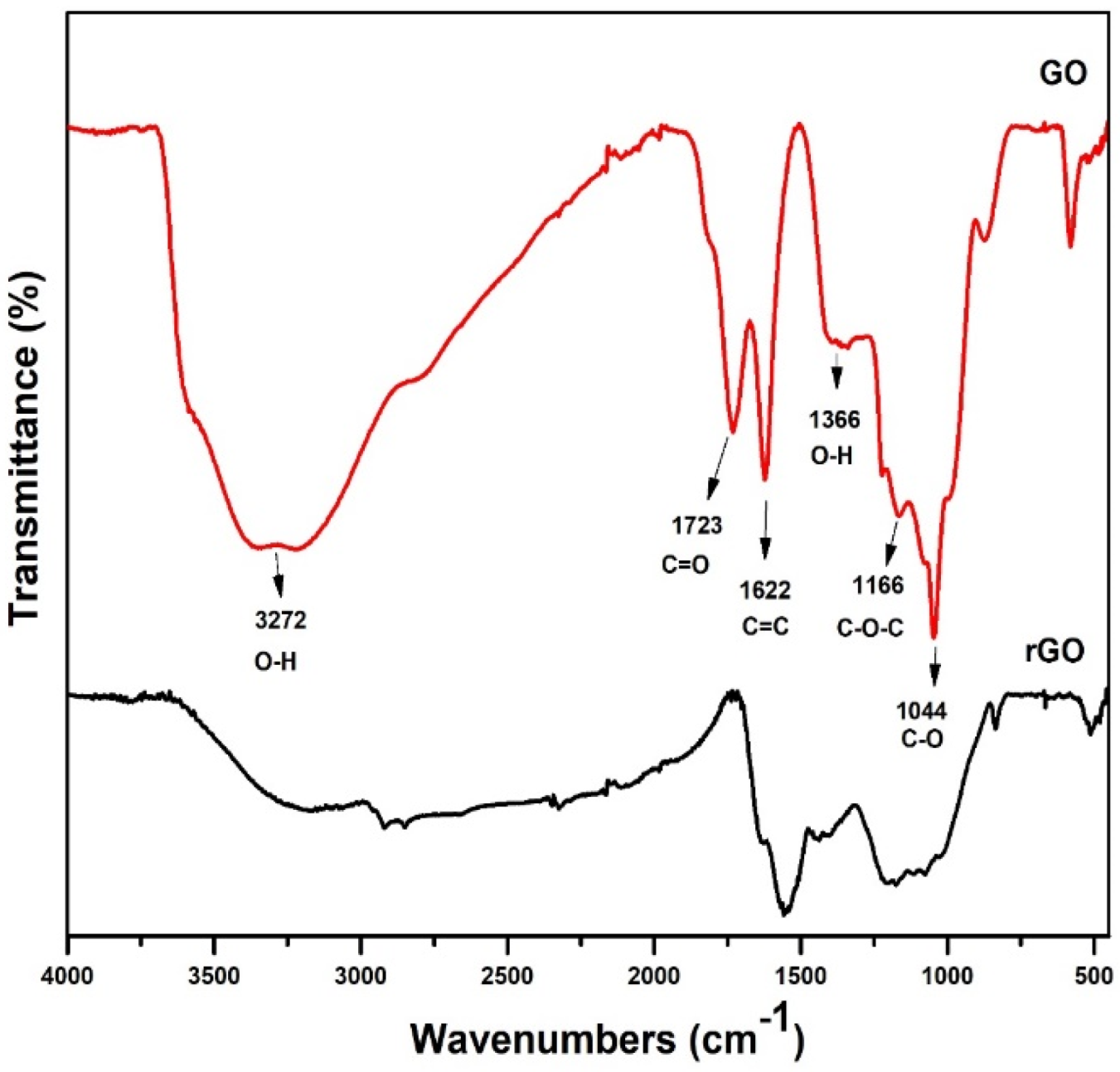
3.3. Fourier Transform Infrared Spectroscopy
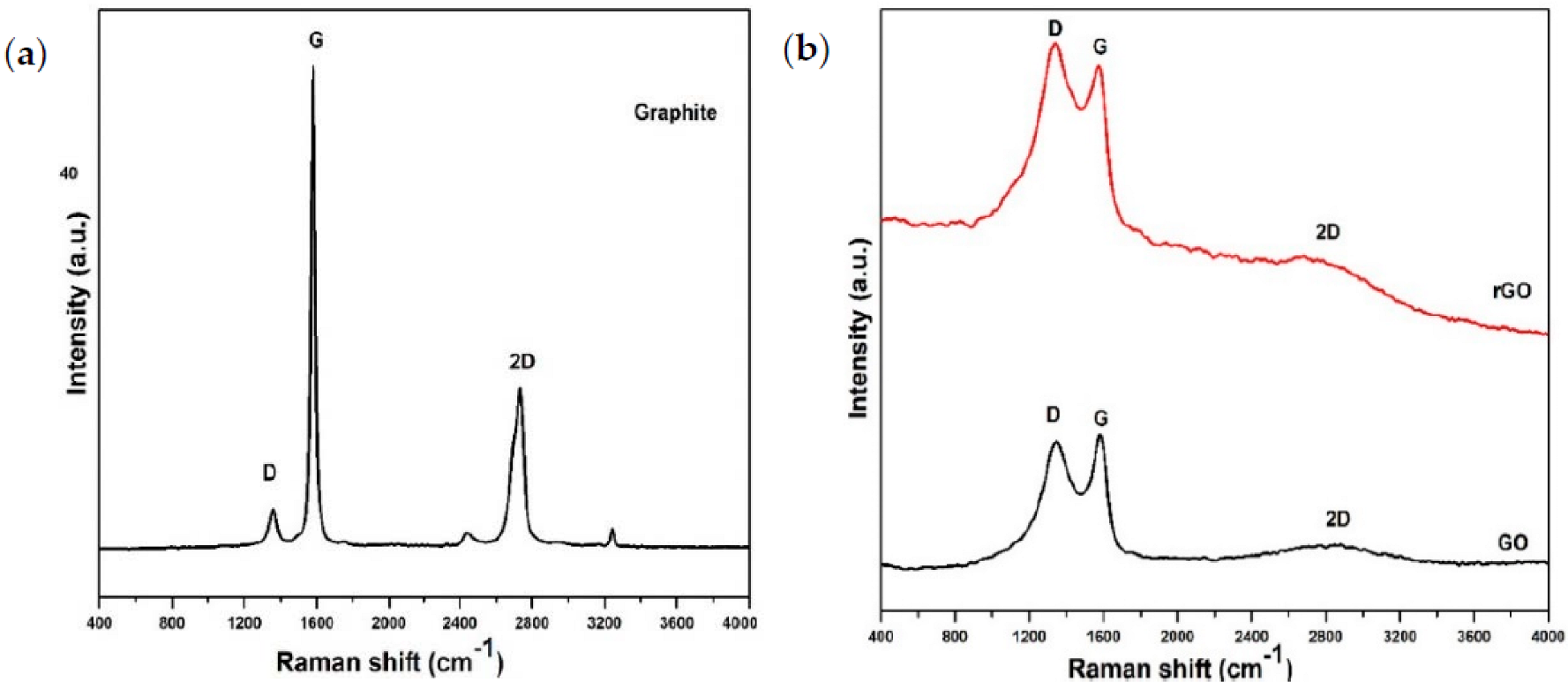
3.4. Raman Spectroscopy
3.5. Field Emission Scanning Electron Microscopy and Energy Dispersive X-ray Spectroscopy
3.6. CHNS-O Elemental Analysis
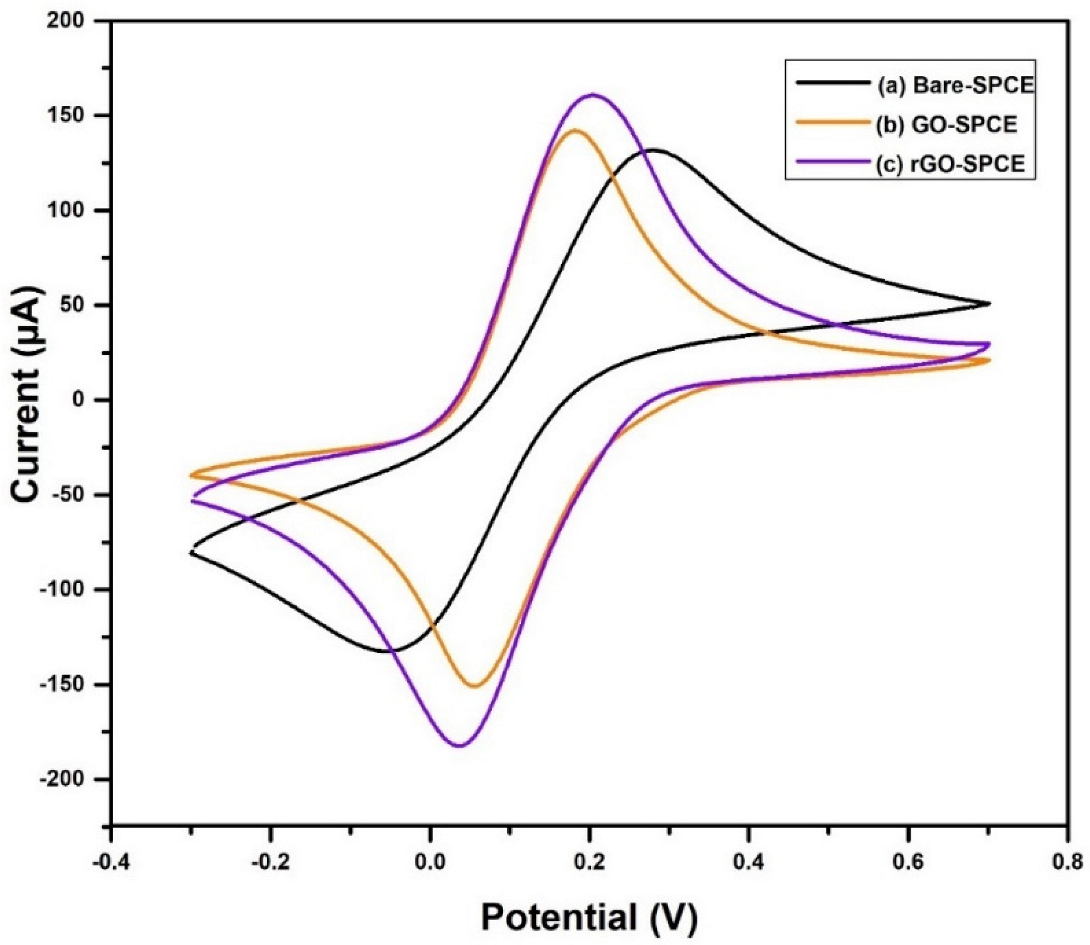
3.7. Electrochemical Measurements
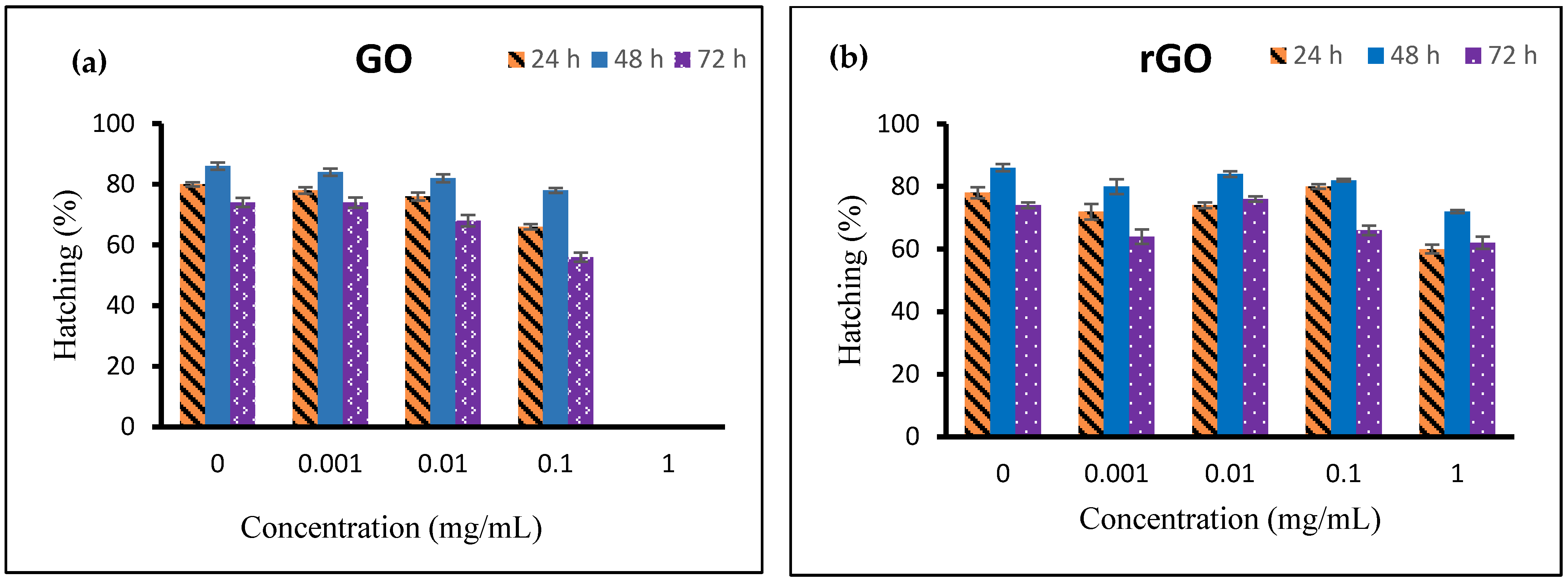
3.8. Hatching Assay
4. Results and Discussion
4.1. Ultraviolet—Visible Analysis
4.2. X-ray Diffraction Analysis
4.3. Fourier Transform Infrared Analysis
4.4. Raman Characterization
4.5. Field Emission Scanning Electron Microscopic Analysis and Energy Dispersive X-ray Spectroscopy Analysis
4.6. CHNS/O Elemental Analysis
4.7. Electrochemical Analysis
4.8. Hatching Assay Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Whitener, K.E.; Sheehan, P.E. Diamond & Related Materials Graphene synthesis. Diam. Relat. Mater. 2014, 46, 25–34. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- La, D.D.; Nguyen, T.A.; Nguyen, T.T.; Ninh, H.D.; Thi, H.P.N.; Nguyen, T.T.; Nguyen, D.A.; Dang, T.D.; Rene, E.R.; Chang, S.W.; et al. Absorption behavior of graphene nanoplates toward oils and organic solvents in contaminated water. Sustainability 2019, 11, 7228. [Google Scholar] [CrossRef]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef]
- Behar, D.; Rajh, T.; Liu, Y.; Connell, J.; Stamenkovic, V.; Rabani, J. Unusual Reduction of Graphene Oxide by Titanium Dioxide Electrons Produced by Ionizing Radiation: Reaction Products and Mechanism. J. Phys. Chem. C 2020, 124, 5425–5435. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon N. Y. 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P.; Fang Liu, C.; Zhan, L.; Fang Li, Y.; Huang, C.Z. Green and easy synthesis of biocompatible graphene for use as an anticoagulant. RSC Adv. 2012, 2, 2322–2328. [Google Scholar] [CrossRef]
- Ismail, Z. Green reduction of graphene oxide by plant extracts: A short review. Ceram. Int. 2019, 45, 23857–23868. [Google Scholar] [CrossRef]
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Highly potent radical scavenging-anti-oxidant activity of biologically reduced graphene oxide using Nettle extract as a green bio-genic amines- based reductants source instead of hazardous hydrazine hydrate. J. Hazard. Mater. 2019, 371, 609–624. [Google Scholar] [CrossRef]
- Lin, S.; Ruan, J.; Wang, S. Biosynthesized of reduced graphene oxide nanosheets and its loading with paclitaxel for their anti cancer effect for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2019, 191, 13–17. [Google Scholar] [CrossRef]
- Jin, X.; Li, N.; Weng, X.; Li, C.; Chen, Z. Green reduction of graphene oxide using Eucalyptus leaf extract and its application to remove dye. Chemosphere 2018, 208, 417–424. [Google Scholar] [CrossRef]
- Chandu, B.; Sai, V.; Mosali, S.; Mullamuri, B.; Bollikolla, H.B. A facile green reduction of graphene oxide using Annona squamosa leaf extract. Carbon Lett. 2017, 21, 74–80. [Google Scholar] [CrossRef]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Raba, S.A.; Ruzniza, M.Z. Low Cost and Green Approach in The Reduction of Graphene Oxide (GO) Using Palm Oil Leaves Extract for Potential in Industrial Applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Mahata, S.; Sahu, A.; Shukla, P.; Rai, A.; Singh, M.; Rai, V.K. The novel and efficient reduction of graphene oxide using Ocimum sanctum L. leaf extract as an alternative renewable bio-resource. N. J. Chem. 2018, 42, 19945–19952. [Google Scholar] [CrossRef]
- Teoh, P.L. A minireview on phytochemical and medicinal properties of Clinacanthus nutans. J. Appl. Pharm. Sci. 2021, 11, 15–21. [Google Scholar] [CrossRef]
- Kuo, X.; Herr, D.R.; Ong, W.Y. Anti-inflammatory and Cytoprotective Effect of Clinacanthus nutans Leaf But Not Stem Extracts on 7-Ketocholesterol Induced Brain Endothelial Cell Injury. NeuroMolecular Med. 2021, 23, 176–183. [Google Scholar] [CrossRef]
- Ismail-Embong, N.; Manan, N.A.; Md Salleh, N.A.; Pa’ee, F. The effect of different growing media on growth performance of Clinacanthus nutans. IOP Conf. Ser. Earth Environ. Sci. 2021, 736, 012026. [Google Scholar] [CrossRef]
- Fong, S.Y.; Piva, T.; Urban, S.; Huynh, T. Genetic homogeneity of vegetatively propagated Clinacanthus nutans (Acanthaceae). J. Med. Plants Res. 2014, 8, 903–914. [Google Scholar] [CrossRef][Green Version]
- Alam, A.; Ferdosh, S.; Ghafoor, K.; Hakim, A.; Juraimi, A.S.; Khatib, A.; Sarker, Z.I. Clinacanthus nutans: A review of the medicinal uses, pharmacology and phytochemistry. Asian Pac. J. Trop. Med. 2016, 9, 402–409. [Google Scholar] [CrossRef]
- Bong, F.J.; Yeou Chear, N.J.; Ramanathan, S.; Mohana-Kumaran, N.; Subramaniam, S.; Chew, B.L. The development of callus and cell suspension cultures of Sabah Snake Grass (Clinacanthus nutans) for the production of flavonoids and phenolics. Biocatal. Agric. Biotechnol. 2021, 33, 101977. [Google Scholar] [CrossRef]
- Chamutpong, S.; Chen, C.J.; Chaiprateep, E.O. Optimization ultrasonic-microwave-assisted extraction of phenolic compounds from Clinacanthus nutans using response surface methodology. J. Adv. Pharm. Technol. Res. 2021, 12, 190–195. [Google Scholar] [CrossRef]
- Kasim, K.F.; Husin, N.; Shamsudin, S.; Amer, N.A.M.; Salleh, N.H.M.; Sofian-Seng, N.S. Impact of commercial cellulase enzyme on the quality of Clinacanthus nutans extracts. IOP Conf. Ser. Earth Environ. Sci. 2021, 765, 012007. [Google Scholar] [CrossRef]
- Kamarudin, M.N.A.; Sarker, M.M.R.; Kadir, H.A.; Ming, L.C. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of Clinacanthus nutans (Burm. f.) Lindau: A comprehensive review. J. Ethnopharmacol. 2017, 206, 245–266. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.; Yusof, N.; Zainal, Z. Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterials 2017, 7, 182. [Google Scholar] [CrossRef]
- Baioun, A.; Kellawi, H.; Falah, A. A modified electrode by a facile green preparation of reduced graphene oxide utilizing olive leaves extract. Carbon Lett. 2017, 24, 47–54. [Google Scholar] [CrossRef]
- Umar, M.F.; Ahmad, F.; Saeed, H.; Usmani, S.A.; Owais, M.; Rafatullah, M. Bio-mediated synthesis of reduced graphene oxide nanoparticles from chenopodium album: Their antimicrobial and anticancer activities. Nanomaterials 2020, 10, 1096. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green Reduction of Graphene Oxide using Kaffir Lime Peel Extract ( Citrus hystrix ) and Its Application as Adsorbent for Methylene Blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, S.; Xie, C.; Liu, Q.; Yang, S. Fabrication of Erythrina senegalensis leaf extract mediated reduced graphene oxide for cardiac repair applications in the nursing care. Inorg. Nano-Metal. Chem. 2021, 51, 143–149. [Google Scholar] [CrossRef]
- Smita, K.M.; Abraham, L.S.; Kumar, V.G.; Vasantharaja, R.; Thirugnanasambandam, R.; Antony, A.; Govindaraju, K.; Velan, T.S. Biosynthesis of reduced graphene oxide using Turbinaria ornata and its cytotoxic effect on MCF-7 cells. IET Nanobiotechnol. 2021, 15, 455–464. [Google Scholar] [CrossRef]
- Chufa, B.M.; Abdisa Gonfa, B.; Yohannes Anshebo, T.; Adam Workneh, G. A Novel and Simplest Green Synthesis Method of Reduced Graphene Oxide Using Methanol Extracted Vernonia Amygdalina: Large-Scale Production. Adv. Condens. Matter Phys. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Lingaraju, K.; Raja Naika, H.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef]
- Shamaila, S.; Khan Leghari Sajjad, A.; Quart-Ul-Ain; Shaheen, S.; Iqbal, A.; Noor, S.; Sughra, G.; Ali, U. A cost effective and eco-friendly green route for fabrication of efficient graphene nanosheets photocatalyst. J. Environ. Chem. Eng. 2017, 5, 5770–5776. [Google Scholar] [CrossRef]
- Coros, M.; Pogacean, F.; Turza, A.; Dan, M.; Berghian-Grosan, C.; Pana, I.O.; Pruneanu, S. Green synthesis, characterization and potential application of reduced graphene oxide. Phys. E Low-Dimens. Syst. Nanostruct. 2020, 119, 113971. [Google Scholar] [CrossRef]
- Parthipan, P.; Cheng, L.; Rajasekar, A.; Govarthanan, M.; Subramania, A. Biologically reduced graphene oxide as a green and easily available photocatalyst for degradation of organic dyes. Environ. Res. 2021, 196, 110983. [Google Scholar] [CrossRef]
- Panicker, N.J.; Sahu, P.P. Green reduction of graphene oxide using phytochemicals extracted from Pomelo Grandis and Tamarindus indica and its supercapacitor applications. J. Mater. Sci. Mater. Electron. 2021, 32, 15265–15278. [Google Scholar] [CrossRef]
- Hidayah, N.M.S.; Liu, W.W.; Lai, C.W.; Noriman, N.Z.; Khe, C.S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar] [CrossRef]
- Gan, L.; Li, B.; Chen, Y.; Yu, B.; Chen, Z. Green synthesis of reduced graphene oxide using bagasse and its application in dye removal: A waste-to-resource supply chain. Chemosphere 2019, 219, 148–154. [Google Scholar] [CrossRef]
- Kumar, S.; Bhorolua, D.; Ojha, A.K.; Kumar, A. Onion Juice Assisted Green Reduction Of Graphene Oxide With Tunable Structural And Optical Properties: Effect Of Onion Juice Concentration And Reaction Temperature. Adv. Mater. Lett. 2019, 10, 58–66. [Google Scholar] [CrossRef]
- Khan, M.; Al-marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-warthan, A.; Tremel, W.; et al. Green Approach for the Effective Reduction of Graphene Oxide Using Salvadora persica L. Root (Miswak) Extract. Nanoscale Res. Lett. 2015, 10, 987. [Google Scholar] [CrossRef]
- Li, C.; Zhuang, Z.; Jin, X.; Chen, Z. A facile and green preparation of reduced graphene oxide using Eucalyptus leaf extract. Appl. Surf. Sci. 2017, 422, 469–474. [Google Scholar] [CrossRef]
- Tai, M.J.Y.; Liu, W.W.; Khe, C.S.; Hidayah, N.M.S.; Teoh, Y.P.; Voon, C.H.; Lee, H.C.; Adelyn, P.Y.P. Green synthesis of reduced graphene oxide using green tea extract. AIP Conf. Proc. 2018, 2045, 020032. [Google Scholar] [CrossRef]
- Deng, S.; Berry, V. Wrinkled, rippled and crumpled graphene: An overview of formation mechanism, electronic properties, and applications. Mater. Today 2016, 19, 197–212. [Google Scholar] [CrossRef]
- Alshamsi, H.A.; Jaber, N.A.A.B.; Altaa, S.H.A. Facile Green Synthesis of Reduced Graphene Oxide in L-cysteine Solution and its Structural, Morphological, Optical and Thermal Characteristics. J. Phys. Conf. Ser. 2021, 1999, 012016. [Google Scholar] [CrossRef]
- Khane, Y.; Benouis, K.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Al Ali, A.; Aouf, D.; Fenniche, F.; Khane, S.; Chaibi, W.; et al. Green Synthesis of Silver Nanoparticles Using Aqueous Citrus limon Zest Extract: Characterization and Evaluation of Their Antioxidant and Antimicrobial Properties. Nanomaterials 2022, 12, 2013. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Kaliannagounder, V.K.; Rajan, R.; Ramesh, T.; Kim, C.S.; Park, C.H.; Liu, B.; Yun, S. Il Silver nanoparticles decorated reduced graphene oxide: Eco-friendly synthesis, characterization, biological activities and embryo toxicity studies. Environ. Res. 2022, 210, 112864. [Google Scholar] [CrossRef]
- Mahendran, G.B.; Ramalingam, S.J.; Rayappan, J.B.B.; Kesavan, S.; Periathambi, T.; Nesakumar, N. Green preparation of reduced graphene oxide by Bougainvillea glabra flower extract and sensing application. J. Mater. Sci. Mater. Electron. 2020, 31, 14345–14356. [Google Scholar] [CrossRef]
- Cavion, F.; Fusco, L.; Sosa, S.; Manfrin, C.; Alonso, B.; Zurutuza, A.; Della Loggia, R.; Tubaro, A.; Prato, M.; Pelin, M. Ecotoxicological impact of graphene oxide: Toxic effects on the model organism: Artemia franciscana. Environ. Sci. Nano 2020, 7, 3605–3615. [Google Scholar] [CrossRef]
- Carballo, J.L.; Hernández-Inda, Z.L.; Pérez, P.; García-Grávalos, M.D. A comparison between two brine shrimp assays to detect in vitro cytotoxicity in marine natural products. BMC Biotechnol. 2002, 2, 17. [Google Scholar] [CrossRef]
- Zhu, S.; Luo, F.; Chen, W.; Zhu, B.; Wang, G. Toxicity evaluation of graphene oxide on cysts and three larval stages of Artemia salin. Sci. Total Environ. 2017, 595, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Xue, M.Y.; Luo, F.; Chen, W.C.; Zhu, B.; Wang, G.X. Developmental toxicity of Fe3O4 nanoparticles on cysts and three larval stages of Artemia salin. Environ. Pollut. 2017, 230, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Albert, E.L.; Aziz, N.A.A.A.; Shaifuddin, M.A.F.; Perumal, D.; Een, L.G.; Abdullah, C.A.C. The effect of various nanoparticles towards Artemia salin cyst. Int. J. Chem. Biochem. Sci. 2021, 20, 35–40. [Google Scholar]
- Murugesan, B.; Sonamuthu, J.; Pandiyan, N.; Pandi, B.; Samayanan, S.; Mahalingam, S. Photoluminescent reduced graphene oxide quantum dots from latex of Calotropis gigantea for metal sensing, radical scavenging, cytotoxicity, and bioimaging in Artemia salin: A greener route. J. Photochem. Photobiol. B Biol. 2018, 178, 371–379. [Google Scholar] [CrossRef]

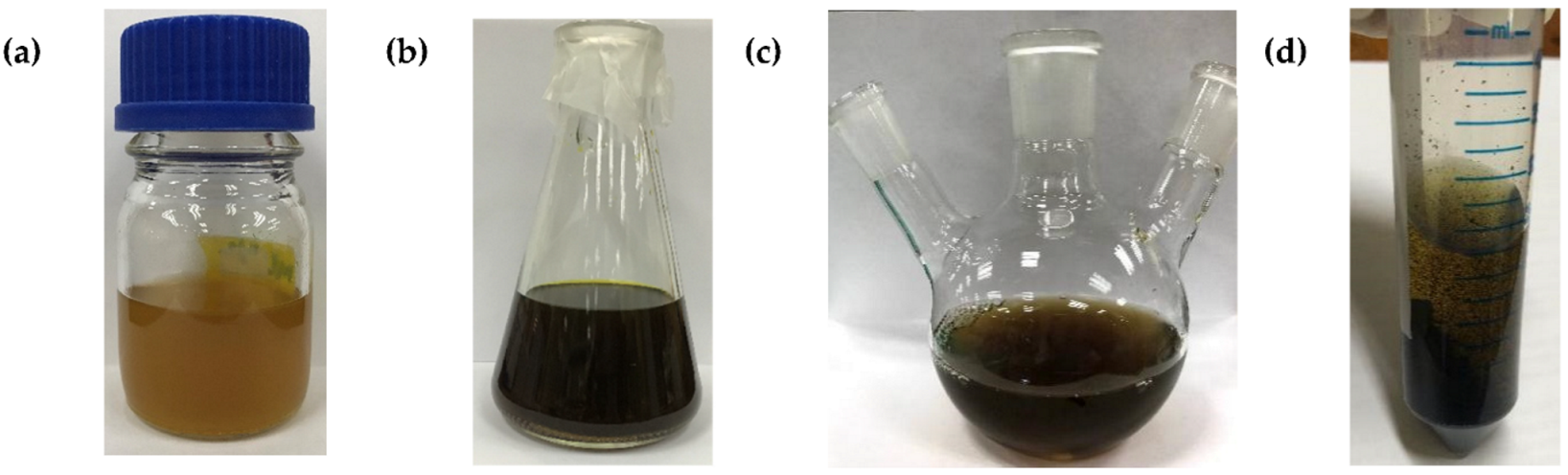
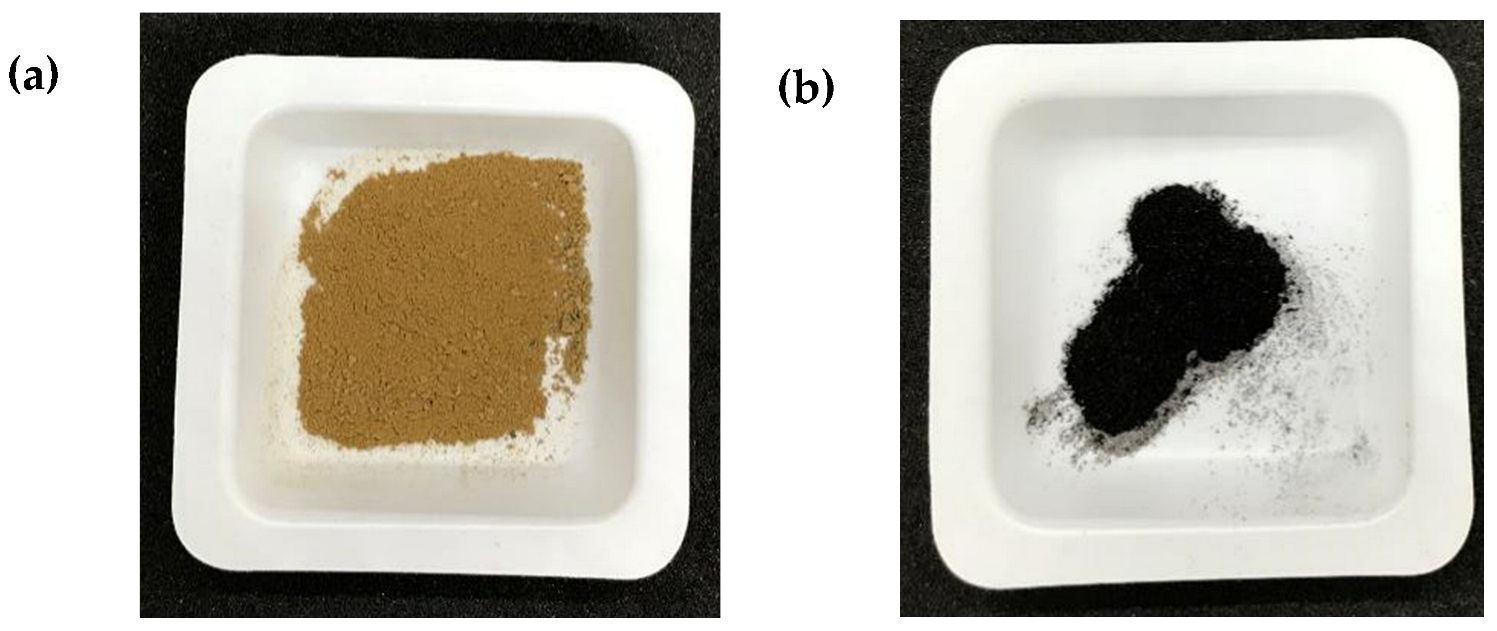
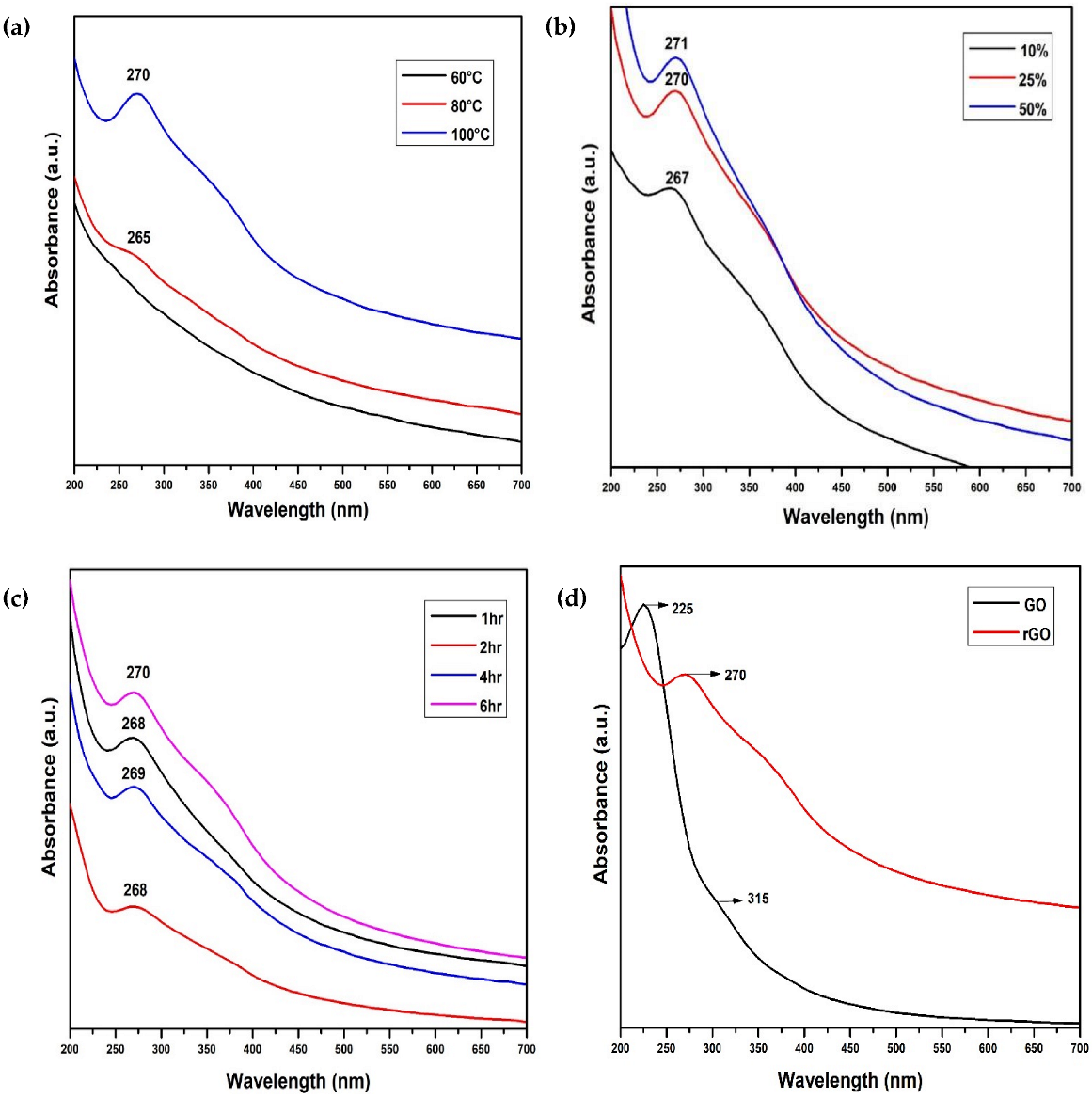
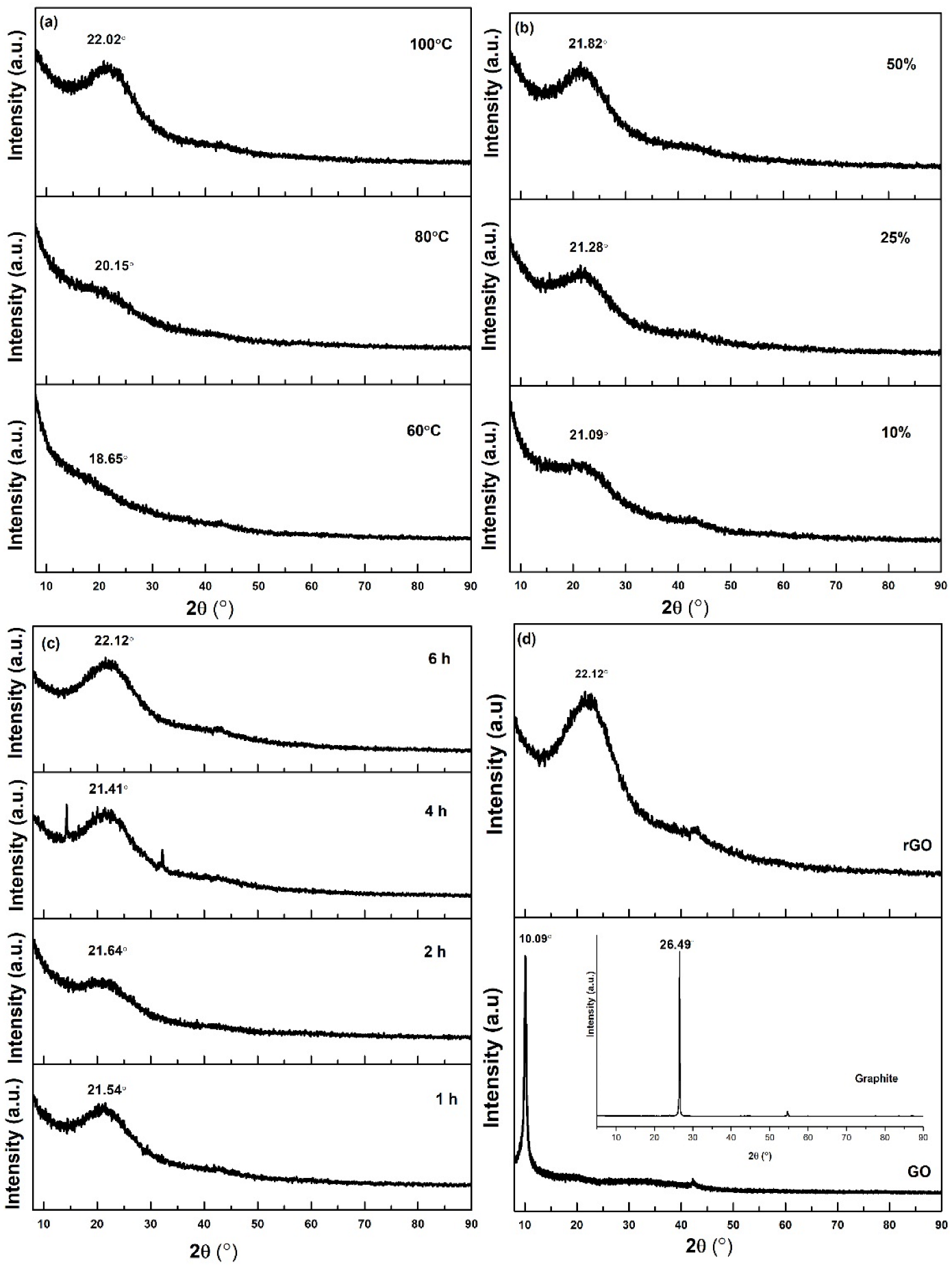

| Parameter | Variable |
|---|---|
| Temperature | 60, 80 and 100 °C |
| Leaf extract concentration | 10, 25, 50% (v/v) |
| Time | 1, 2, 4 and 6 h |
| Sample | 2Ɵ (°) | D—Spacing (nm) |
|---|---|---|
| Graphite | 26.49 | 0.34 |
| GO | 10.09 | 0.88 |
| rGO—60 °C | 18.65 | 0.48 |
| rGO—80 °C | 20.15 | 0.44 |
| rGO—100 °C | 22.02 | 0.40 |
| rGO—10% | 21.09 | 0.42 |
| rGO—25% | 21.28 | 0.42 |
| rGO—50% | 21.82 | 0.41 |
| rGO—1 h | 21.54 | 0.41 |
| rGO—2 h | 21.64 | 0.41 |
| rGO—4 h | 21.41 | 0.41 |
| rGO—6 h | 22.12 | 0.40 |
| Sample | D—Band | G—Band | ID/IG Ratio |
|---|---|---|---|
| Graphite | 1359.55 | 1582.63 | 0.40 |
| GO | 1349.76 | 1576.55 | 1.01 |
| rGO | 1342.19 | 1565.57 | 1.08 |
| Atom | Elemental Compound Weight Percentage (%) | C/O | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | S | Cl | Ca | N | Mg | Si | ||
| GO | 66.8 | 30.3 | 2.0 | 0.9 | 0.0 | 0.0 | 0.0 | 0.0 | 2.2 |
| rGO | 72.7 | 12.4 | 0.8 | 0.0 | 7.7 | 3.6 | 1.6 | 1.1 | 5.9 |
| Sample | Elemental Compound Percentage (%) | C/O | ||||
|---|---|---|---|---|---|---|
| N | C | H | S | O | ||
| GO | 0.00 | 39.10 | 0.00 | 2.26 | 38.92 | 1.00 |
| rGO | 3.72 | 46.32 | 4.35 | 0.30 | 26.90 | 1.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, D.; Albert, E.L.; Saad, N.; Hin, T.Y.Y.; Zawawi, R.M.; Teh, H.F.; Che Abdullah, C.A. Fabrication and Characterization of Clinacanthus nutans Mediated Reduced Graphene Oxide Using a Green Approach. Crystals 2022, 12, 1539. https://doi.org/10.3390/cryst12111539
Perumal D, Albert EL, Saad N, Hin TYY, Zawawi RM, Teh HF, Che Abdullah CA. Fabrication and Characterization of Clinacanthus nutans Mediated Reduced Graphene Oxide Using a Green Approach. Crystals. 2022; 12(11):1539. https://doi.org/10.3390/cryst12111539
Chicago/Turabian StylePerumal, Dharshini, Emmellie Laura Albert, Norazalina Saad, Taufiq Yap Yun Hin, Ruzniza Mohd Zawawi, Huey Fang Teh, and Che Azurahanim Che Abdullah. 2022. "Fabrication and Characterization of Clinacanthus nutans Mediated Reduced Graphene Oxide Using a Green Approach" Crystals 12, no. 11: 1539. https://doi.org/10.3390/cryst12111539
APA StylePerumal, D., Albert, E. L., Saad, N., Hin, T. Y. Y., Zawawi, R. M., Teh, H. F., & Che Abdullah, C. A. (2022). Fabrication and Characterization of Clinacanthus nutans Mediated Reduced Graphene Oxide Using a Green Approach. Crystals, 12(11), 1539. https://doi.org/10.3390/cryst12111539






