Correlation between Mechanical Properties—Structural Characteristics and Cavitation Resistance of Cast Aluminum Alloy Type 5083
Abstract
1. Introduction
2. Materials and Experimental Procedure
3. Experimental Results, Interpretations, and Discussions
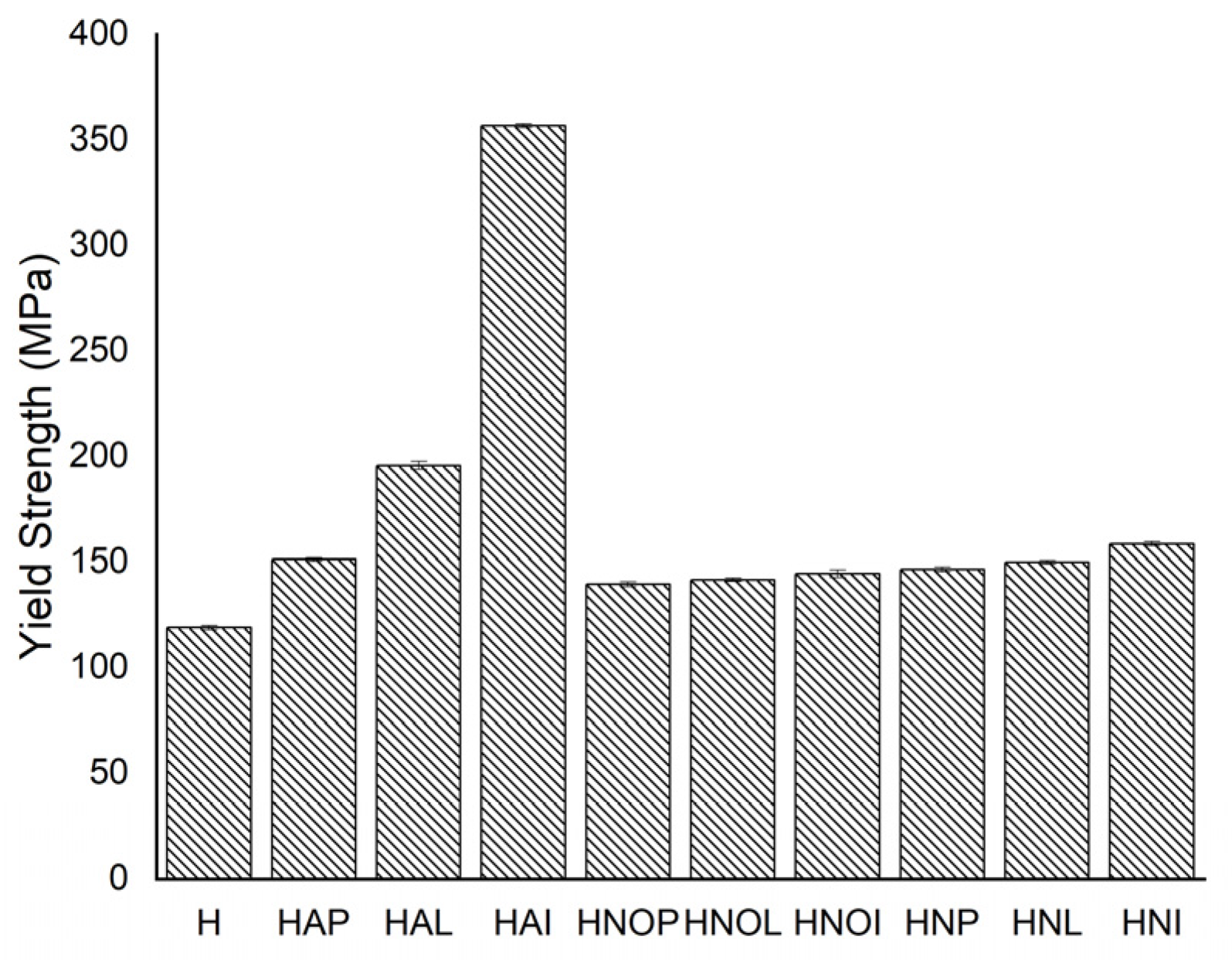
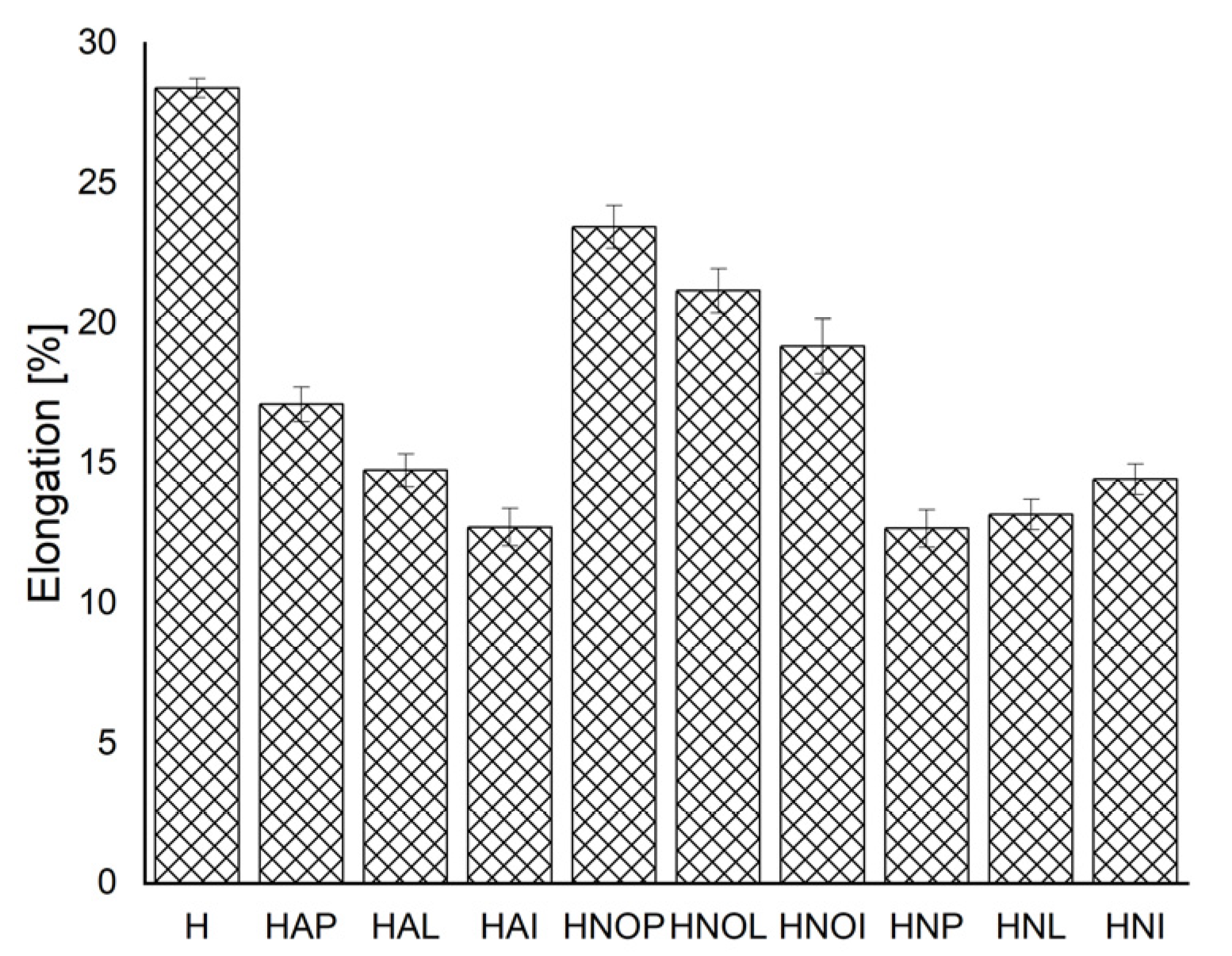
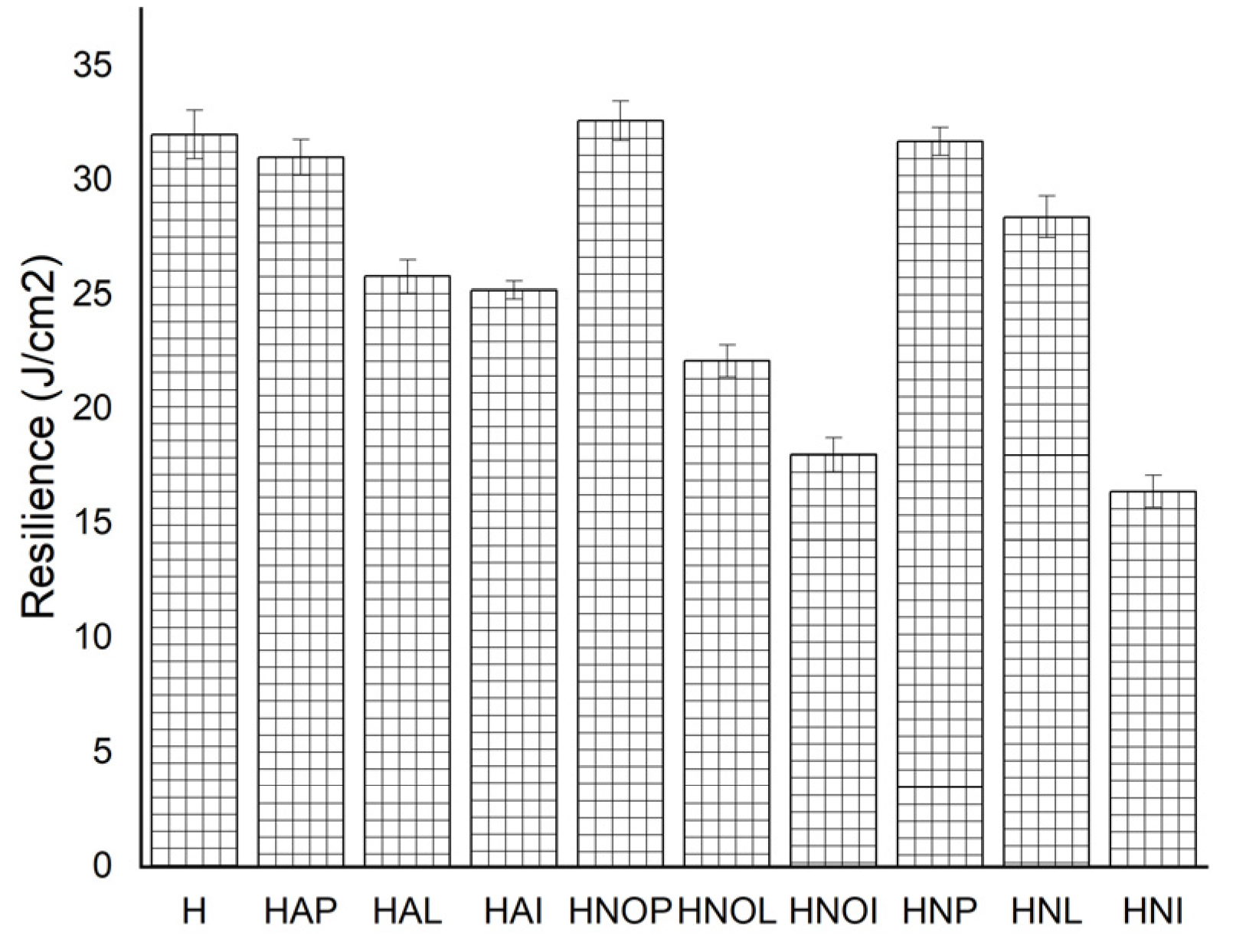
3.1. Results concerning the Mechanical Behavior of the Experimental Samples
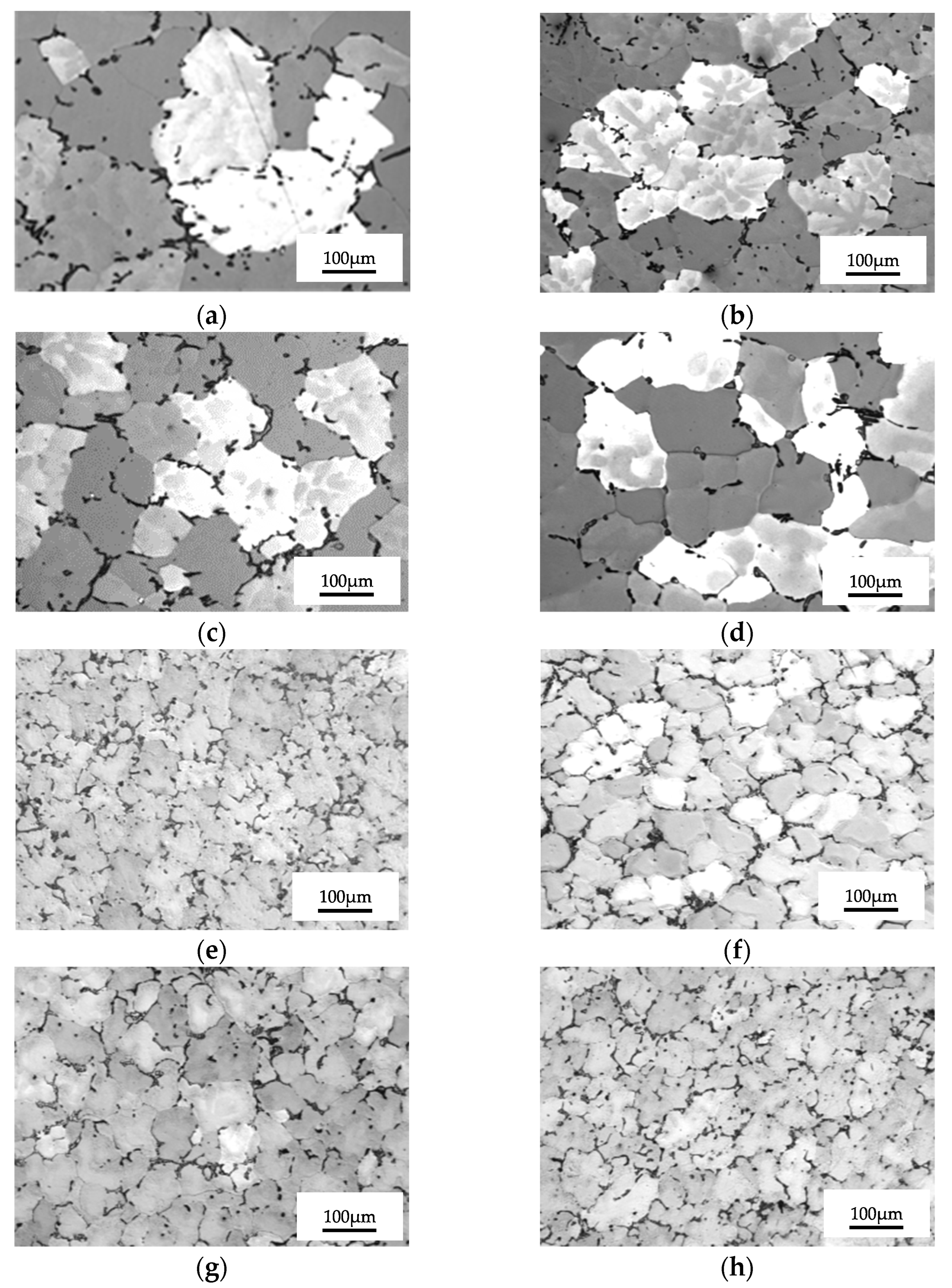
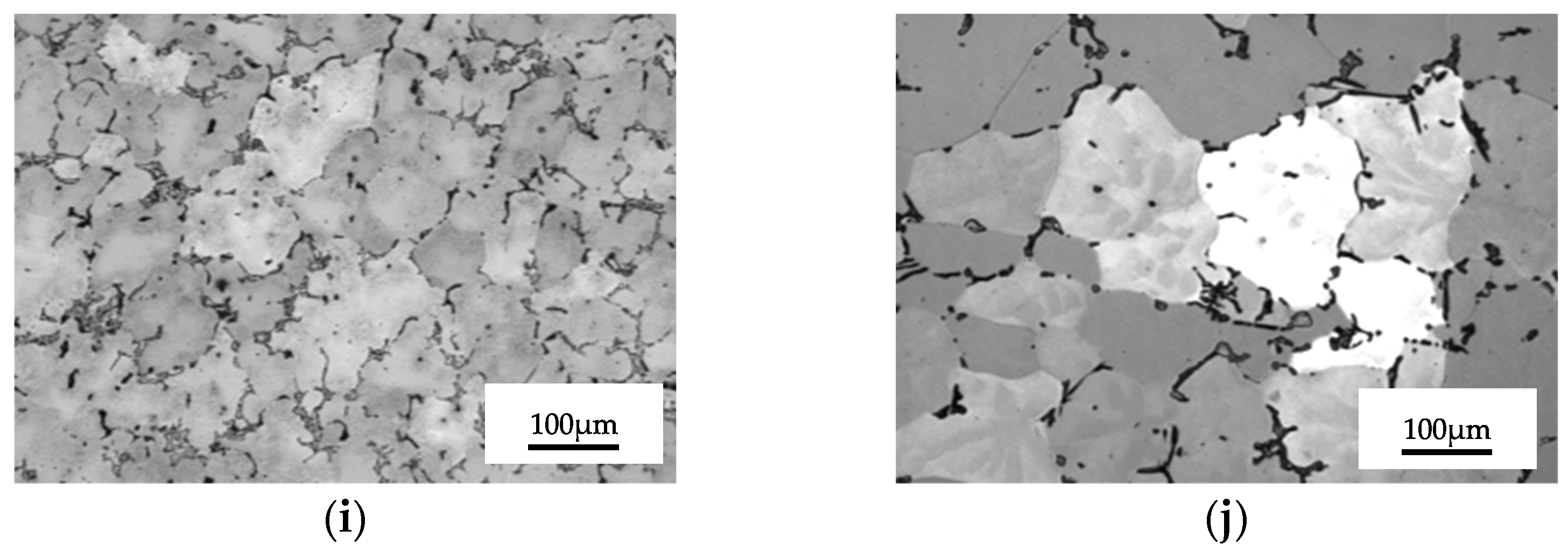
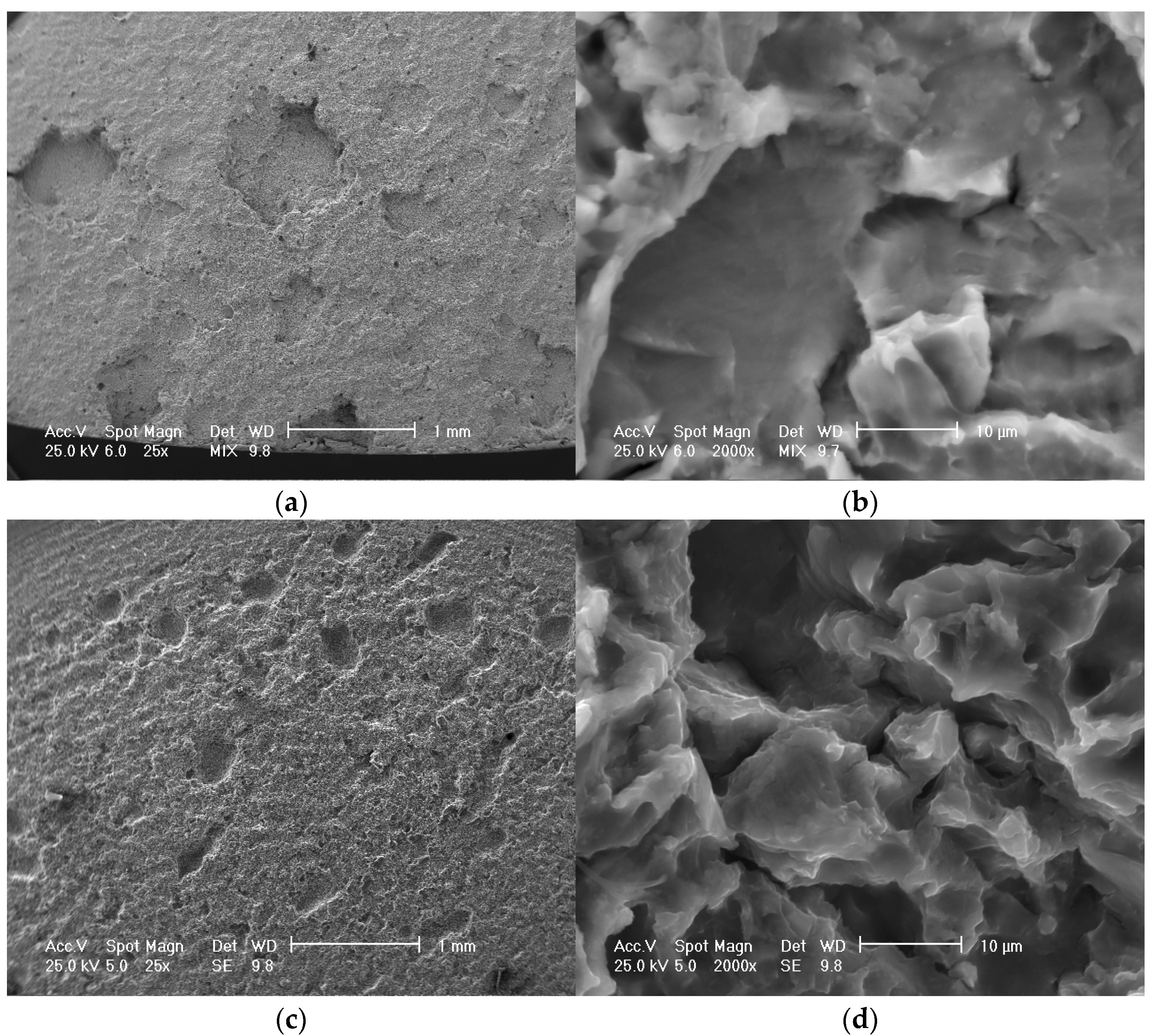
3.2. Results concerning Structural Characterization of the Experimental Samples
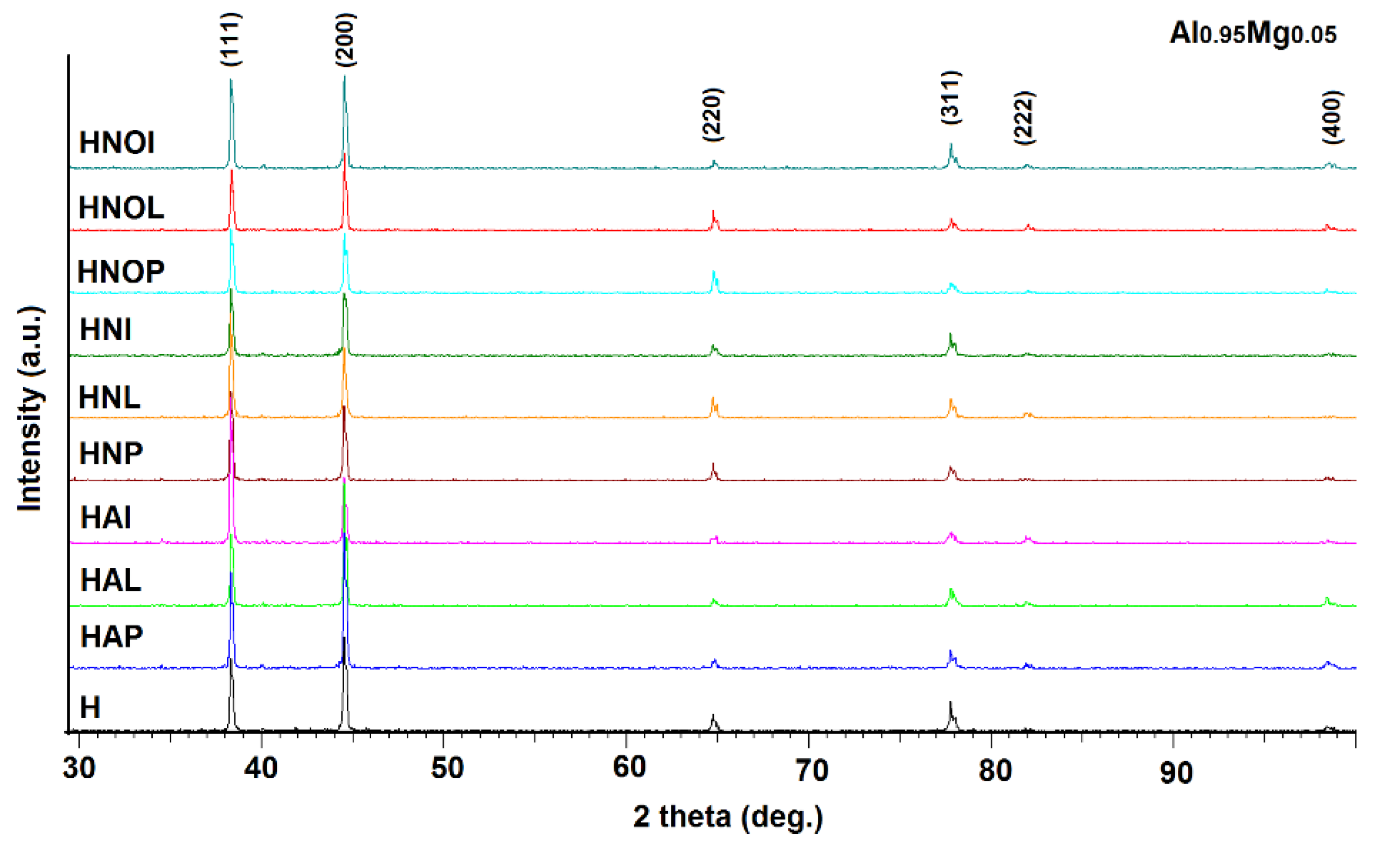
3.3. X-rays Diffraction Analysis
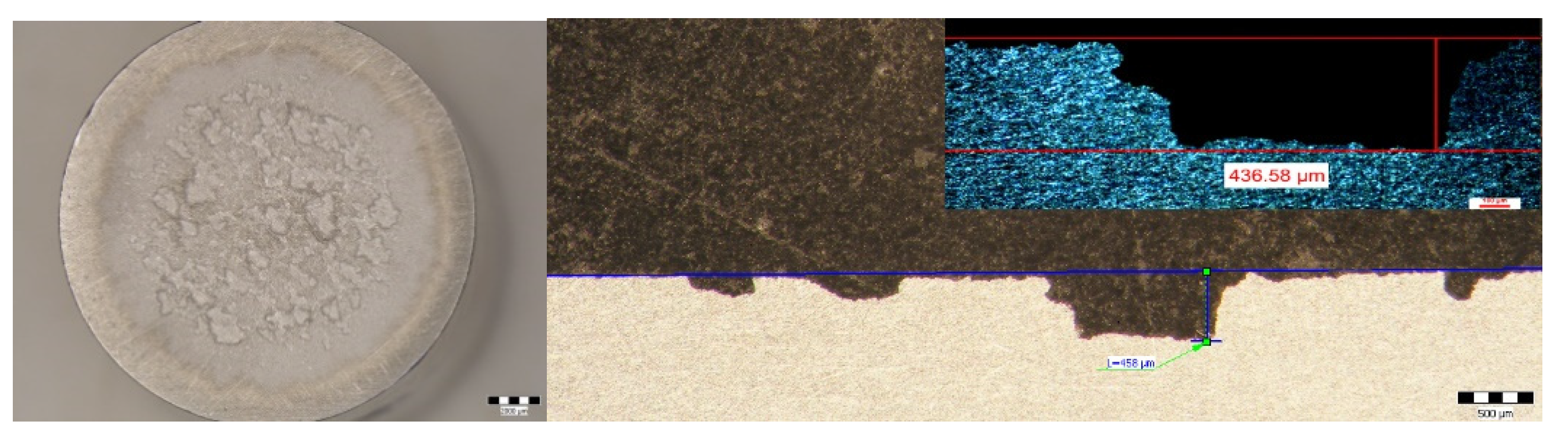
3.4. Determination of the Behavior of Experimental Specimens in Cavitational Corrosion
- The most significant losses, with the development of pinches and caverns in the area of the exposed surface, are registered in the interval 45–120 min (large differences between the experimental, successive values of the measured parameters MDE and MDER, as well as large deviations from the MDE mediation curves (t), respectively, MDER (t)) for all experimental specimens;
- The shape of the approximation/mediation curve of the experimental values has different values between maximum value (MDERmax), and that value which tends to stabilization of the process (final value MDERs) (as can be seen from Table 6, registering differences of about 0–12%). This difference, manifested in all situations, is specific to surfaces with average mechanical properties in a value (with values of hardness of about 80 HB and resilience of about 25 J), which gives this condition a behavior specific to materials with a low resistance to cavitation [20,21,23,27,28,29];
- There are insignificant differences in all heat treatment situations between the experimental values of the erosion rates after 120 min and until the end of the test, which leads to an approximately linear increase in the MDE curve (t) over this time interval and slightly asymptotic stabilization of the MDER curve (t), differences of the structural plastic characteristics which may decrease the resistance to cyclic stresses of cavitation micrometers;
- There is a big difference (which, as indicated in Table 6, is in the range of 8–94%) between the maximum value obtained by the experiment (MDER max.exp) and that defined by the mediation curve (MDER max,m), even if it is recorded at the same duration of the cavitation (90 min). The smallest difference is recorded in the heat-treated sample at 450 °C/100 min/air + 140 °C/12 h/air of about 8%, and the largest difference is recorded in the heat-treated sample at 450 °C/100 min/air + 180 °C/12 h/air of about 94%. In the other heat treatment states, this difference is in the range of 40–65%. This aspect is further proof of the complexity of the mechanism by which the structure responds to the cavitation load and by which the effect of the duration of maintenance at the temperature of the heat treatment on the structure and mechanical properties, as value and mode of distribution in the sample volume.
- By applying heat treatments to 5073 alloy castings, either hardening of the solution at 350 °C/100 min + artificial aging at 180 °C (1 h, 12 h, 24 h), or hardening of the solution at 450 °C/100 min + artificial aging at 140 °C (1 h, 12 h, 24 h) or solution hardening 450 °C/100 min + artificial aging at 180 °C (1 h, 12 h, 24 h) can increase the resistance to cavitational corrosion, in the sense of decreasing the depths of penetration to erosion;
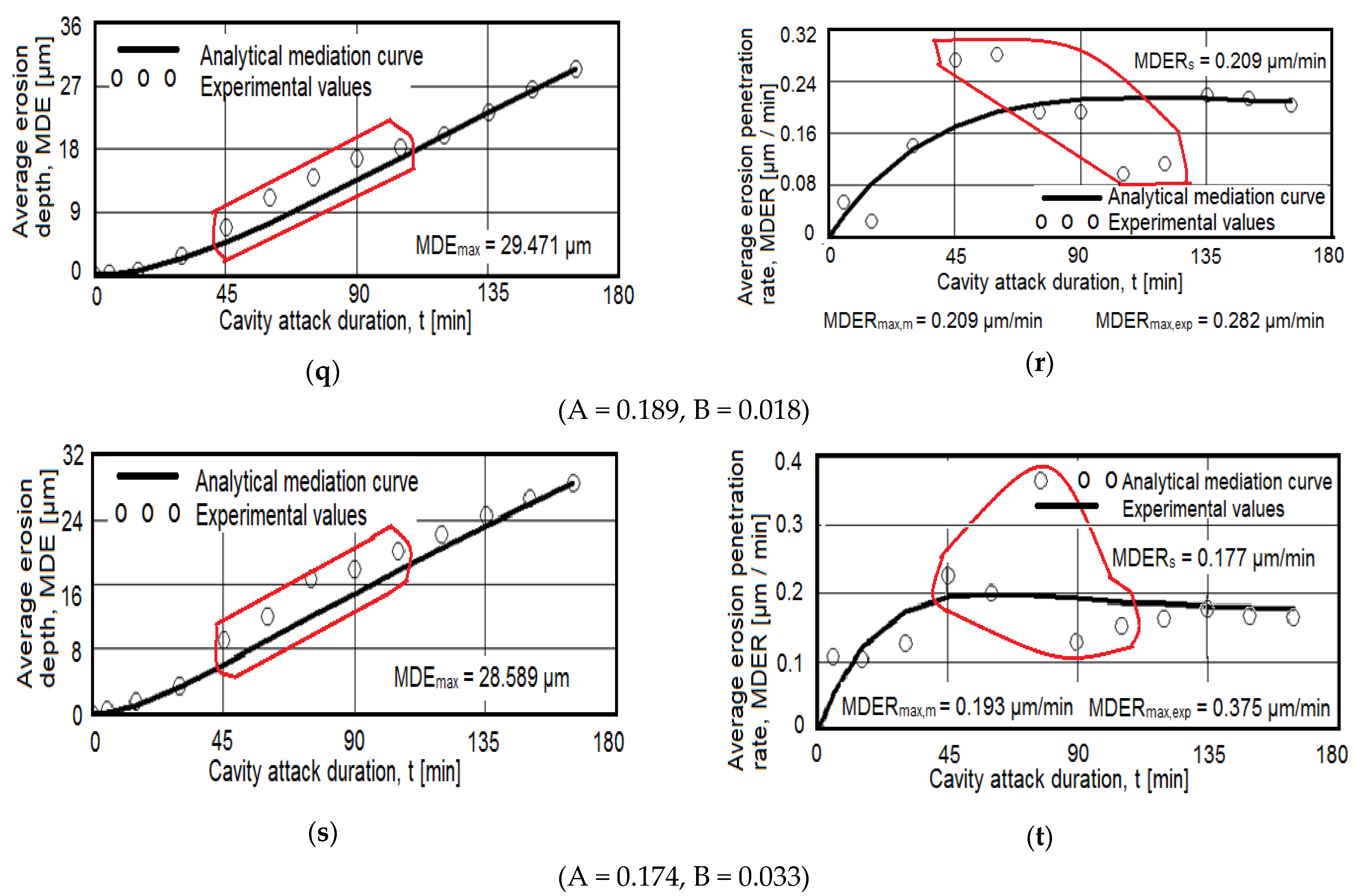
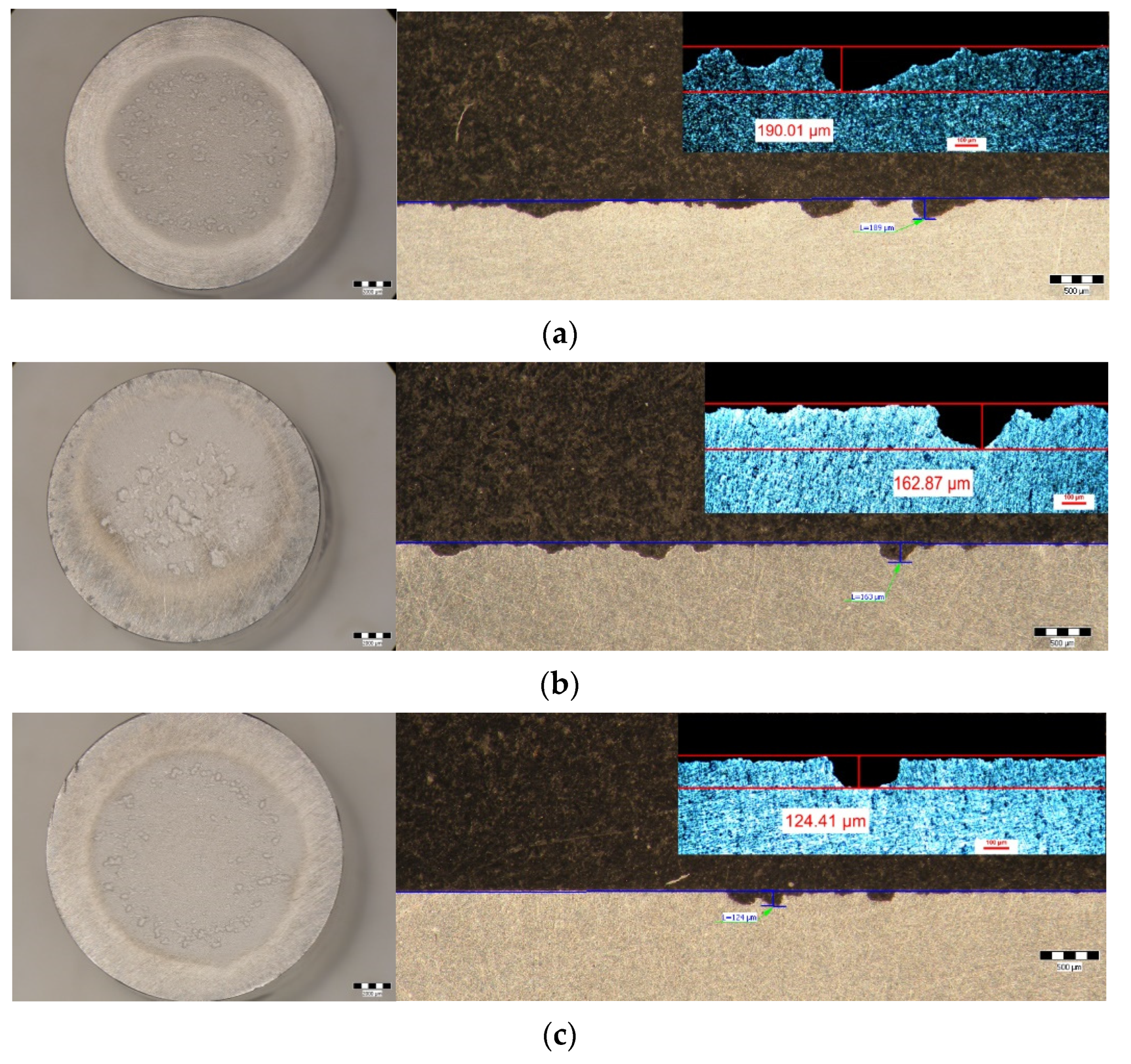
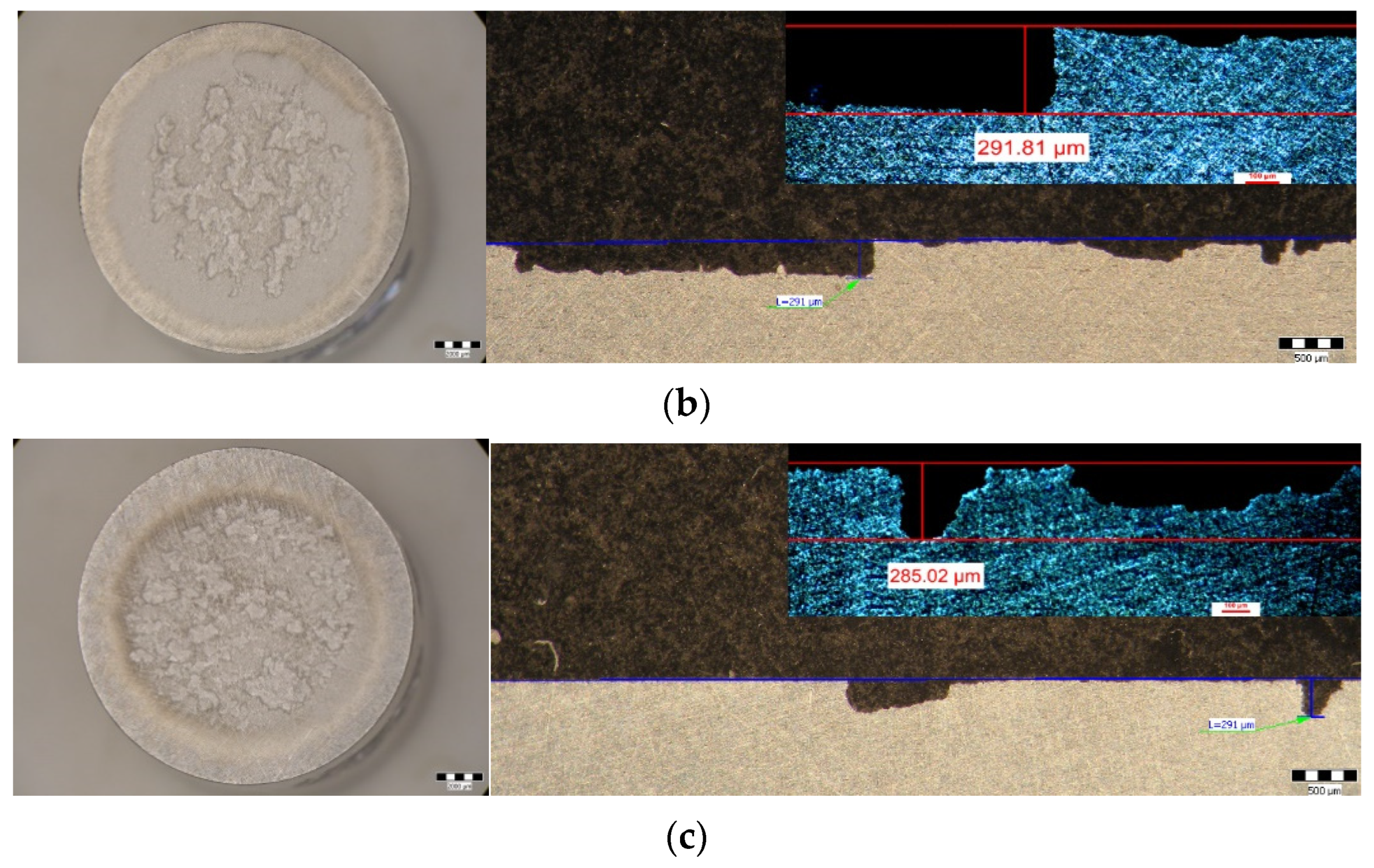
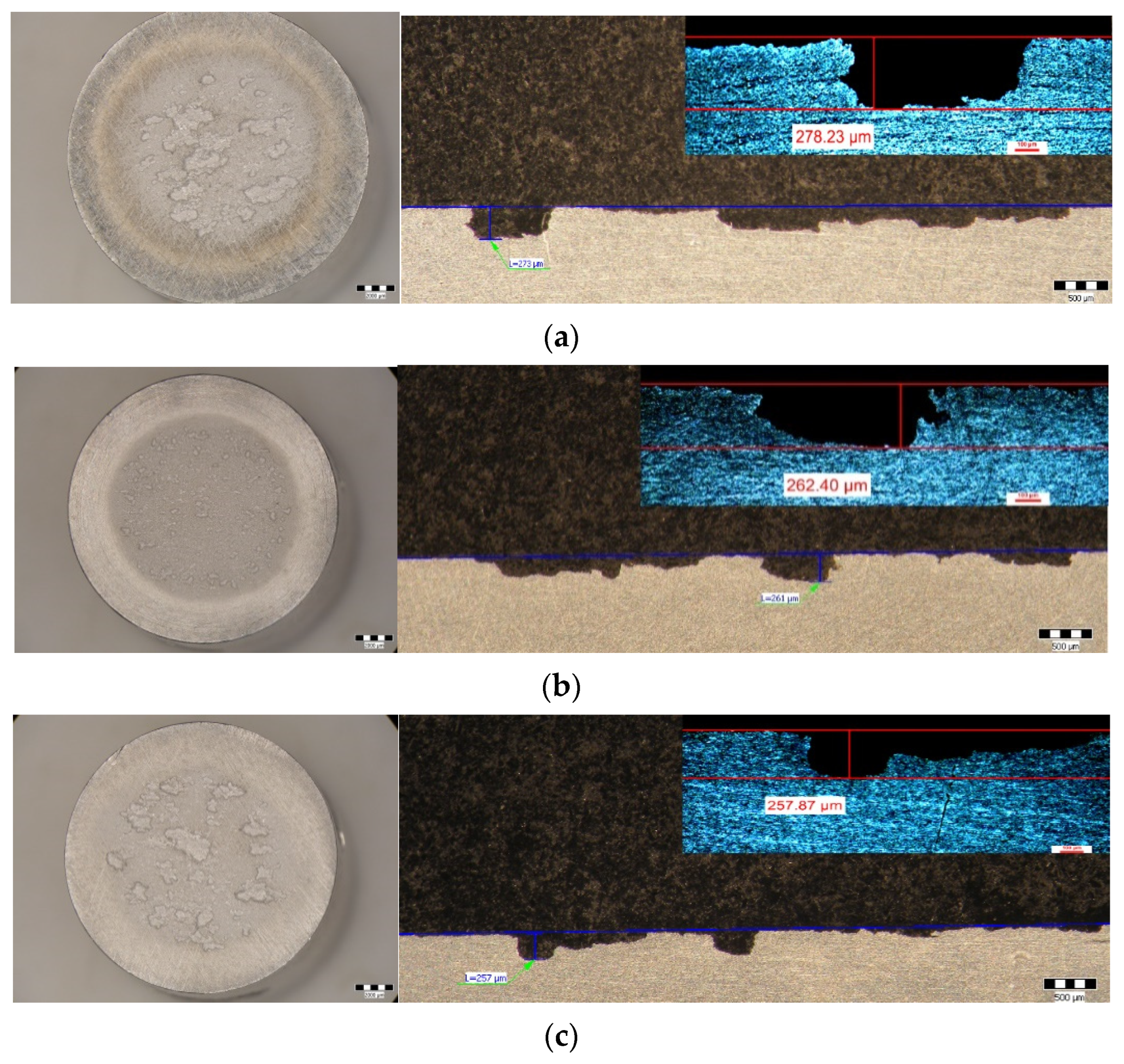
- Within the same combination of heat treatments, increasing the duration of maintenance to artificial aging causes a decrease in the maximum depths of penetration of the cavities. Thus, after hardening of the solution at 350 °C/100 min + 180 °C (Figure 9c–h) the maximum penetration depth of the MDEmax cavity decreases from 17.928 μm (at maintenance 1 h), at 15.128 μm (at maintenance 12 h), reaching 14.572 μm (at maintenance 24 h). After hardening the solution at 450 °C/100 min + 140 °C (Figure 9i–n) the maximum penetration depth of the MDEmax cavity decreases from 52.115 μm (at maintenance 1 h), at 43.017 μm (12 h maintenance), reaching 38.31 μm (24 h maintenance). After hardening the solution at 450 °C/100 min + 180 °C (Figure 9o–t) the maximum penetration depth of the MDEmax cavity decreases from 29.572 μm (at maintenance 1 h), at 29.471 μm (12 h maintenance), reaching 28.589 μm (24 h maintenance);
- After applying a solution hardening at 350 °C/100 min + artificial aging at 180 °C, the lowest penetration depths of the cavity are obtained both compared to the control sample, with 60 μm, and compared to the samples subjected to hardening solution at 450 °C/100 min + artificial aging (either at 140 °C where the maximum depth of penetration is 38–52 μm, or at 180 °C, where the maximum penetration depth is about 29 μm);
- The correlation between the highest mechanical characteristics obtained after applying a solution hardening at 350 °C/100 min + artificial aging at 180 °C (1 h, 12 h, 24 h) and the behavior at cavitational erosion, which is the more favorable to these heat treatments applied to 5083 aluminum alloy castings.
4. Conclusions
- By applying homogenization heat treatments, the mechanical and structural characteristics of 5083 alloy cast products can be modified. So, the values of breaking strength and yield strength change similarly. The elongation values are intermediate for homogenization at 350 °C, compared to homogenization at 450 °C, regardless of the duration of aging. Hardness values decrease slightly after applying homogenization at 350 °C + 180 °C (only 1–4%), compared to homogenization at 450 °C + 140 °C, where the hardness decreases reach up to 10% compared to gauge sample.
- The lowest values of the average grain size are recorded at homogenizations at 350 °C + 180 °C, average sizes for homogenization at 450 °C + 140 °C and the highest grain sizes for 450 °C + 180 °C.
- By applying heat treatments to 5073 alloy castings, we can increase the resistance to cavitational corrosion, in the sense of decreasing the depths of penetration and erosion penetration rate. Within the same combination of heat treatments, increasing the duration of maintenance to artificial aging causes a decrease in the maximum depths of penetration of the cavities. Thus, after hardening of the solution at 350 °C/100 min + 180 °C the maximum penetration depth of the MDEmax cavity decreases from 17.928 μm (for 1 h), at 15.128 μm (for 12 h), reaching 14.572 μm (for 24 h). After hardening the solution at 450 °C/100 min + 140 °C the maximum penetration depth of the MDEmax cavity decreases from 52.115 μm (for 1 h), at 43.017 μm (for 12 h), reaching 38.31 μm (for 24 h). After hardening the solution at 450 °C/100 min + 180 °C the maximum penetration depth of the MDEmax cavity decreases from 29.572 μm (for 1 h), at 29.471 μm (for 12 h), reaching 28.589 μm (for 24 h).
- After applying a solution hardening at 350 °C/100 min + artificial aging at 180 °C, the lowest penetration depths of the cavity are obtained both compared to the control sample, with 60 μm, and compared to the samples subjected to hardening solution at 450 °C/100 min + artificial aging (either at 140 °C where the maximum depth of penetration is 38–52 μm, or at 180 °C, where the maximum penetration depth is about 29 μm).
- The best combination of heat treatments applied to cast aluminum products type 5083 is homogenization at 350 °C followed by artificial aging at 180 °C, at which the highest mechanical characteristics are obtained, a resilience of 25 J/cm2, a grain size of 140–180 μm, and a maximum depth of the erosion MDEm around 14–17 μm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, K.; Lui, T.; Chen, L. Effect of microstructural feature on the tensile properties and vibration fracture resistance of friction stirred 5083 Alloy. J. Alloys Compd. 2011, 509, 7466–7472. [Google Scholar] [CrossRef]
- Bauri, R.; Yadav, D.; Kumar, C.N.S.; Balaji, B. Tungsten particle reinforced Al 5083 composite with high strength and ductility. Mater. Sci. Eng. A 2015, 620, 67–75. [Google Scholar] [CrossRef]
- Newbery, A.P.; Nutt, S.R.; Lavernia, E.J. Multi-scale Al 5083 for military vehicles with improved performance. Jom-Us 2006, 58, 56–61. [Google Scholar] [CrossRef]
- Ke, W.; Bu, X.; Oliveira, J.; Xu, W.; Wang, Z.; Zeng, Z. Modeling and numerical study of keyhole-induced porosity formation in laser beam oscillating welding of 5A06 aluminum alloy. Opt. Laser Technol 2021, 133, 106540. [Google Scholar] [CrossRef]
- Pereira, D.; Oliveira, J.; Santos, T.; Miranda, R.; Lourenço, F.; Gumpinger, J.; Bellarosa, R. Aluminium to Carbon Fibre Reinforced Polymer tubes joints produced by magnetic pulse welding. Compos. Struct. 2019, 230, 111512. [Google Scholar] [CrossRef]
- Torzewski, J.; Grzelak, K.; Wachowski, M.; Kosturek, R. Microstructure and Low Cycle Fatigue Properties of AA5083 H111 Friction Stir Welded Joint. Materials 2020, 13, 2381. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Wang, G.; Zhou, Y.; Liu, K.; Zhao, G.; Zuo, L. Study of the Portevin-Le Chatelier (PLC) Characteristics of a 5083 Aluminum Alloy Sheet in Two Heat Treatment States. States Mater. 2018, 11, 1533. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Obikawa, T.; Nishizaki, I.; Enomoto, M.; Fang, Z. Friction Stir Welding of Non-Heat-Treatable High-Strength Alloy 5083-O. Metals 2018, 8, 208. [Google Scholar] [CrossRef]
- Tamasgavabari, R.; Ebrahimi, A.; Abbasi, S.; Yazdipour, A. The effect of harmonic vibration with a frequency below the resonant range on the mechanical properties of AA-5083-H321 aluminum alloy GMAW welded parts. Mater. Sci. Eng. A 2018, 736, 248. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xie, J.; Sun, S.; Wang, L.; Qian, Y.; Meng, Y.; Wei, Y. Microstructure and mechanical properties of aluminum 5083 weldments by gas tungsten arc and gas metal arc welding. Mater. Sci. Eng. 2012, 549, 7–13. [Google Scholar] [CrossRef]
- Ma, M.; Lai, R.; Qin, J.; Wang, B.; Liu, H.; Yi, D. Effect of weld reinforcement on tensile and fatigue properties of 5083 aluminum metal inert gas (MIG) welded joint: Experiments and numerical simulations. Int. J. Fatigue 2021, 144, 106046. [Google Scholar] [CrossRef]
- Corigliano, P.; Crupi, V.; Pei, X.; Dong, P. DIC-based structural strain approach for low-cycle fatigue assessment of AA 5083 welded joints. Theor. Appl. Fract. Mech. 2021, 116, 103090. [Google Scholar] [CrossRef]
- Mânzână, M.E. Experimental Studies and Investigations Regarding the Structural Modifications Produced through Cavitation-erosion in Different Metallic Materials. Doctoral Thesis, University Politehnica of Bucharest, Bucharest, Romania, 2012. [Google Scholar]
- Guragata, M.C. Studies and Experimental Researches Concerning Plastic Forming and Erosion-Cavitation Behavior of Superalloy Type INCONEL 718. PhD Thesis, University Politehnica of Bucharest, Bucharest, Romania, 2021. [Google Scholar]
- Bordeașu, I. Monograph of the Cavitation Erosion Research Laboratory of the University Politehnica of Timisoara (1960–2020); Politehnica Publishing House: Timisoara, Romania, 2020. [Google Scholar]
- Micu, L.M. Cavitation Erosion Behavior of Duplex Stainless Steels. Doctoral Thesis, University Politehnica of Timisoara, Timisoara, Romania, 2017. [Google Scholar]
- Bordeaşu, I.; Patrăşcoiu, C.; Bădărău, R.; Sucitu, L.; Popoviciu, M.O.; Bălăşoiu, V. New contributions to cavitation erosion curves modeling. FME Trans. 2006, 34, 39–43. [Google Scholar]
- Cornelia Laura, S.; Bordeaşu, I.; Sîrbu, N.A.; Bădărău, R.; Mălaimare, G.; Hluscu, M.; Daniel, O.; Oancă, O.V. Evaluation of the Cavitation Resistance of INCONEL 718, in Delivered and Respectively Heat Treated Condition. Adv. Mater. Res. 2020, 1157, 47–51. [Google Scholar]
- Istrate, D.; Ghera, C.; Sălcianu, L.; Bordeașu, I.; Ghiban, B.; Băzăvan, D.V.; Micu, L.M.; Stroiță, D.C.; Ostoia, D. Heat Treatment Influence of Alloy 5083 on Cavitational Erosion Resistance. Hidraul. Mag. 2021, 3, 15–25. [Google Scholar]
- Bordeasu, I.; Mitelea, I. Cavitation Erosion Behavior of Stainless Steels with Constant Nickel and Variable Chromium Content. Mater. Test. 2012, 54, 53–58. [Google Scholar] [CrossRef]
- Standard G32; Standard Method of Vibratory Cavitation Erosion Test. ASTM: West Conshohocken, PA, USA, 2016.
- Mitelea, I.; Bordeaşu, I.; Riemschneider, E.; Uţu, I.D.; Crăciunescu, C.M. Cavitation erosion improvement following TIG surface-remelting of gray cast iron. Wear 2022, 496, 204282. [Google Scholar] [CrossRef]
- Oanca Victor Octavian Techniques for Optimizing the Resistance to Cavitation Erosion of Some CuAlNiFeMn Alloys Intended for the Execution of Naval Propellers. Doctoral Thesis, University Politehnica of Timisoara, Timisoara, Romania, 2014.
- Steller, K.; Reymann, Z.; Krzysztoowicz, T. Evaluation of the resistance of materials to cavitational erosion. In Proceedings of the Fifth Conference on Fluid Machinery, Akad Kiado, Budapest, 15–20 September 1975; Volume 2. [Google Scholar]
- Sakai, I.; Shima, A. On a New Representative Equation for Cavitation Damage Resistance of Materials; Report, No. 385; Magazine of Hydraulics, Pneumatics, Tribology, Ecology, Sensorics, Mechatronics 24: Tokyo, Japan, 1987. [Google Scholar]
- Micu, L.M.; Bordeasu, I.; Popoviciu, M.O. A New Model for the Equation Describing the Cavitation Mean Depth Erosion Rate Curve. Chem. J. 2017, 4, 894–898. [Google Scholar] [CrossRef]
- Garcia, R. Comprehensive Cavitation Damage Data for Water and Various Liquid Metals Including Correlation with Material and Fluid Properties; Technical Report No. 6; University of Michigan: Ann Arbor, MI, USA, 1966. [Google Scholar]
- Hobbs, J.M. Experience With a 20-kc Cavitation Erosion Test. In Erosion by Cavitation or Impingement; ASTM International: West Conshohocken, PA, USA, 1967; pp. 159–185. [Google Scholar]
- Jean-Pierre, F.; Jean-Louis, K.; Karimi, A.; Fruman, D.-H.; Fréchou, D.; Briançon-Marjollet, L.; Billard, J.-Y.; Belahadji, B.; Avellan, F.; Michel, J.M. Physical Mechanisms and Industrial Aspects; Presses Universitaires de Grenoble: Grenoble, France, 1995. [Google Scholar]
- Bordeasu, I.; Ghera, C.; Istrate, D.; Sălcianu, L.; Ghiban, B.; Băzăvan, D.V.; Micu, L.M.; Stroiţă, D.C.; Suta, A.; Tomoiagă, I. Resistance and Behavior to Cavitation Erosion of Semi-Finished Aluminum Alloy 5083. Hidraul. Mag. 2021, 4, 17–24. [Google Scholar]






| Sample | Chemical Composition, % Rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al | |
| Experimental | 0.41 | 0.29 | 0.106 | 0.52 | 4.21 | 0.12 | 0.16 | 0.028 | Rest |
| SR EN 373- 3 | Max 0.40 | Max 0.40 | Max 0.10 | 0.4–1.0 | 4.0–4.9 | 0.05–0.25 | Max 0.25 | Max 0.15 | Rest |
| State | Mechanical Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tensile Strength | Yield Strength | Elongation | Hardness | μHV | Resilience | ||||||||
| [MPa] | Std Dev | [MPa] | Std Dev | [%] | Std Dev | [daN] | Std Dev | μHV | Std Dev | [J] | Std Dev | ||
| H | Gauge sample | 220.63 | ±0.9959 | 118.84 | ±1.07078 | 28.40 | ±0.34139 | 79.80 | ±0.50992 | 76.97 | ±0.81134 | 32.12 | ±1.05334 |
| HAP | 350 °C/100 min/air + 180 °C/1 h | 318.58 | ±1.00659 | 151.21 | ±0.7681 | 17.11 | ±0.61917 | 76.80 | ±0.8697 | 76.89 | ±1.34867 | 31.00 | ±0.7858 |
| HAL | 350 °C/100 min/air + 180 °C/12 h | 346.20 | ±1.19915 | 195.70 | ±1.92787 | 14.75 | ±0.58161 | 79.00 | ±0.8696 | 79.14 | ±0.83814 | 25.80 | ±0.7305 |
| HAI | 350 °C/100 min/air + 180 °C/24 h | 436.30 | ±1.29539 | 356.68 | ±0.90159 | 12.73 | ±0.66545 | 79.00 | ±0.66545 | 80.01 | ±0.58868 | 25.20 | ±0.38525 |
| HNOP | 450 °C/100 min/air + 140 °C/1 h | 294.82 | ±0.64895 | 139.57 | ±1.13429 | 23.44 | ±0.76294 | 72.80 | ±0.9508 | 74.65 | ±0.79193 | 32.60 | ±0.8542 |
| HNOL | 450 °C/100 min/air + 140 °C/12 h | 307.63 | ±1.25037 | 141.66 | ±0.82781 | 21.17 | ±0.78856 | 76.80 | ±1.01068 | 75.30 | ±1.00258 | 22.10 | ±0.69215 |
| HNOI | 450 °C/100 min/air + 140° C/24 h | 311.21 | ±0.80242 | 144.55 | ±1.86119 | 19.18 | ±0.96929 | 76.80 | ±1.03349 | 80.67 | ±0.72652 | 18.00 | ±0.73799 |
| HNP | 450 °C/100 min/air + 180 °C/1 h | 250.03 | ±0.78143 | 146.50 | ±1.16986 | 12.68 | ±0.6629 | 71.80 | ±0.53337 | 78.12 | ±0.58145 | 31.70 | ±0.62006 |
| HNL | 450 °C/100 min/air + 180 °C/12 h | 315.16 | ±1.02569 | 149.91 | ±0.83089 | 13.18 | ±0.54174 | 77.90 | ±0.62742 | 78.38 | ±0.83996 | 28.40 | ±0.89793 |
| HNI | 450 °C/100 min/air + 180 °C/24 h | 318.34 | ±1.60049 | 158.62 | ±1.08443 | 14.44 | ±0.54551 | 80.70 | ±0.60322 | 79.68 | ±1.09967 | 16.40 | ±0.70432 |
| Code | State | Parameters of the Statistical Analysis | ||||
|---|---|---|---|---|---|---|
| Grain Size Minimum | Grain Size | Grain Size | Standard Deviation | 95% CI | ||
| [μm] | Maximum | Medium | [μm] | [μm] | ||
| [μm] | [μm] | |||||
| H | Gauge sample | 120.10 | 653.51 | 255.95 | 94.17 | 20.17 |
| HAP | 350 °C/100 min/air + 180 °C/1 h | 10.03 | 317.86 | 185.58 | 77.08 | 27.33 |
| HAL | 350 °C/100 min/air + 180 °C/12 h | 41.89 | 302.18 | 164.05 | 52.34 | 16.15 |
| HAI | 350 °C/100 min/air + 180 °C/24 h | 36.89 | 272.76 | 149.69 | 51.21 | 14.78 |
| HNOP | 450 °C/100 min/air + 140 °C/1 h | 20.10 | 425.23 | 212.29 | 83.05 | 12.17 |
| HNOL | 450 °C/100 min/air + 140 °C/12 h | 20.10 | 475.53 | 197.06 | 96.01 | 14.51 |
| HNOI | 450 °C/100 min/air + 140 °C/24 h | 20.10 | 426.54 | 190.38 | 97.38 | 13.94 |
| HNP | 450 °C/100 min/air + 180 °C/1 h | 21.84 | 564.23 | 245.23 | 108.83 | 19.01 |
| HNL | 450 °C/100 min/air + 180 °C/12 h | 49.02 | 418.34 | 225.31 | 43.57 | 18.52 |
| HNI | 450 °C/100 min/air + 180 °C/24 h | 103.10 | 371.86 | 215.21 | 36.28 | 16.83 |
| Test Code | Crystallographic Phase | Elementary Cell Parameters, a [Å] | Crystalline Size |
|---|---|---|---|
| D (nm) | |||
| H | Al0.95Mg0.05 | 4.073 | 163.2 |
| HAP | 4.075 | 150.8 | |
| HAL | 4.074 | 140.3 | |
| HAI | 4.072 | 136.5 | |
| HNOP | 4.073 | 162.2 | |
| HNOL | 4.073 | 145.6 | |
| HNOI | 4.073 | 145.6 | |
| HNP | 4.076 | 158.4 | |
| HNL | 4.075 | 151.2 | |
| HNI | 4.074 | 123.9 |
| Sample | |||||||
|---|---|---|---|---|---|---|---|
| % | % | ||||||
| H | 0.429 | 0.589 | 0.413 | 0.16 | 37 | 0.016 | 5.7 |
| HAP | 0.122 | 0.171 | 0.111 | 0.049 | 40 | 0.011 | 9 |
| HAL | 0.104 | 0.112 | 0.112 | 0.008 | 8 | 0.008 | 8 |
| HAI | 0.101 | 0.166 | 0.092 | 0.065 | 65 | 0.009 | 9 |
| HNOP | 0.101 | 0.166 | 0.092 | 0.065 | 65 | 0.009 | 9 |
| HNOL | 0.359 | 0.385 | 0.317 | 0.03 | 8 | 0.042 | 12 |
| HNOI | 0.296 | 0.426 | 0.262 | 0.13 | 44 | 0.034 | 12 |
| HNP | 0.261 | 0.380 | 0.237 | 0.109 | 46 | 0.014 | 9 |
| HNL | 0.109 | 0.282 | 0.209 | 0.073 | 35 | 0 | 0 |
| HNI | 0.193 | 0.375 | 0.177 | 0.182 | 95 | 0.0.16 | 8 |
| Sample | Maximum Penetration Depth of the Cavitation Attack | ||
|---|---|---|---|
| H | 60.249 | 436.58 | 7 |
| HAP | 17.928 | 190.01 | 11 |
| HAL | 15.128 | 162.87 | 11 |
| HAI | 14.572 | 124.41 | 8 |
| HNOP | 52.115 | 330.26 | 6 |
| HNOL | 43.027 | 291.81 | 7 |
| HNOI | 38.31 | 285.02 | 7 |
| HNP | 29.572 | 278.23 | 9 |
| HNL | 29.471 | 262.4 | 9 |
| HNI | 28.589 | 257.87 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istrate, D.; Sbârcea, B.-G.; Demian, A.M.; Buzatu, A.D.; Salcianu, L.; Bordeasu, I.; Micu, L.M.; Ghera, C.; Florea, B.; Ghiban, B. Correlation between Mechanical Properties—Structural Characteristics and Cavitation Resistance of Cast Aluminum Alloy Type 5083. Crystals 2022, 12, 1538. https://doi.org/10.3390/cryst12111538
Istrate D, Sbârcea B-G, Demian AM, Buzatu AD, Salcianu L, Bordeasu I, Micu LM, Ghera C, Florea B, Ghiban B. Correlation between Mechanical Properties—Structural Characteristics and Cavitation Resistance of Cast Aluminum Alloy Type 5083. Crystals. 2022; 12(11):1538. https://doi.org/10.3390/cryst12111538
Chicago/Turabian StyleIstrate, Dionisie, Beatrice-Gabriela Sbârcea, Alin Mihai Demian, Andreea Daniela Buzatu, Laura Salcianu, Ilare Bordeasu, Lavinia Madalina Micu, Cristian Ghera, Bogdan Florea, and Brândușa Ghiban. 2022. "Correlation between Mechanical Properties—Structural Characteristics and Cavitation Resistance of Cast Aluminum Alloy Type 5083" Crystals 12, no. 11: 1538. https://doi.org/10.3390/cryst12111538
APA StyleIstrate, D., Sbârcea, B.-G., Demian, A. M., Buzatu, A. D., Salcianu, L., Bordeasu, I., Micu, L. M., Ghera, C., Florea, B., & Ghiban, B. (2022). Correlation between Mechanical Properties—Structural Characteristics and Cavitation Resistance of Cast Aluminum Alloy Type 5083. Crystals, 12(11), 1538. https://doi.org/10.3390/cryst12111538






