Melt-Pool Dynamics and Microstructure of Mg Alloy WE43 under Laser Powder Bed Fusion Additive Manufacturing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Powder Material
2.2. Laser Irradiation for Ex Situ Studies
2.3. Sample Preparation for Microscopy Analysis
2.4. Microscopy
2.5. In Situ X-ray Imaging
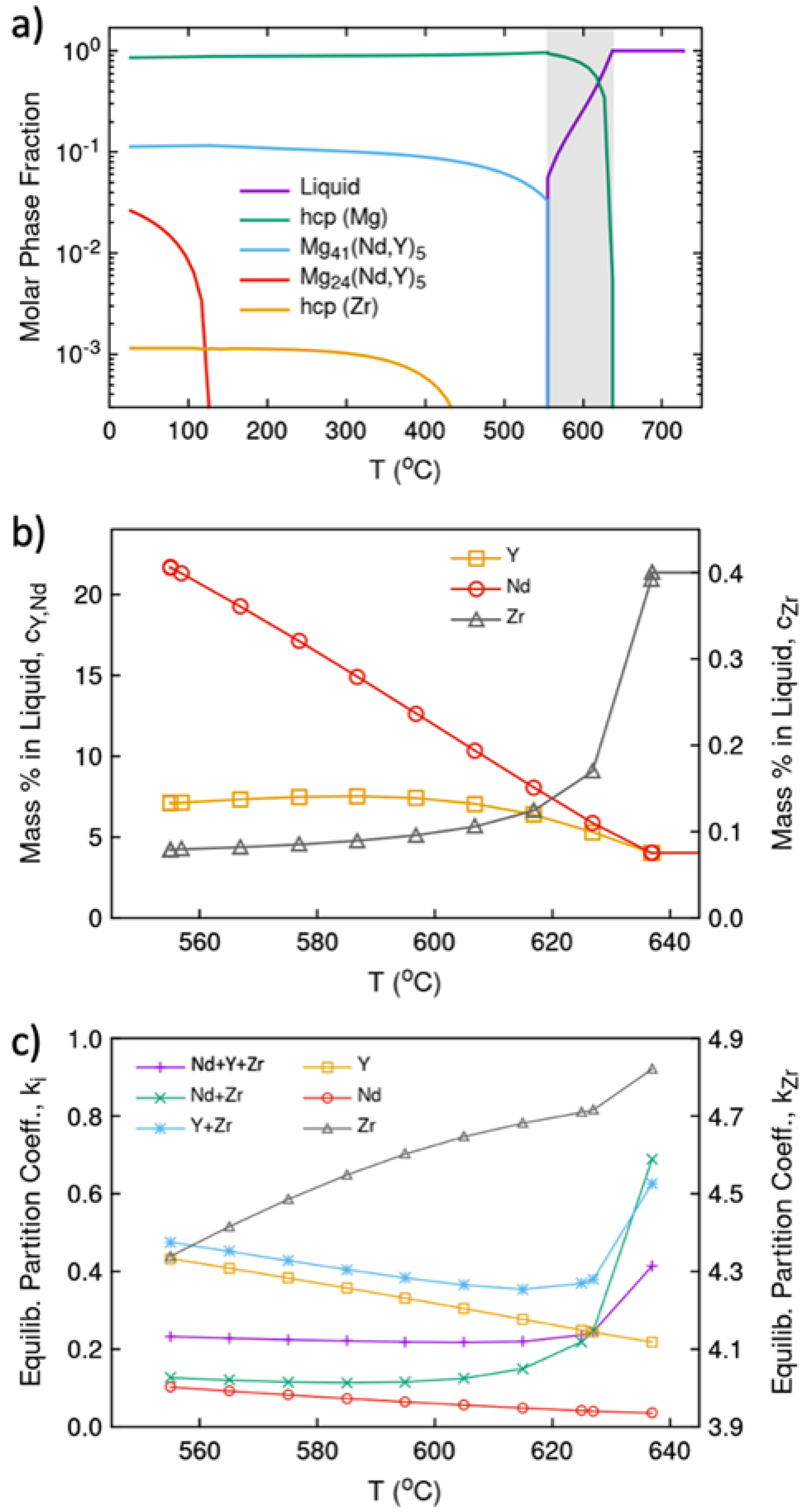
2.6. Thermodynamic and Kinetic Modeling
3. Results and Discussion
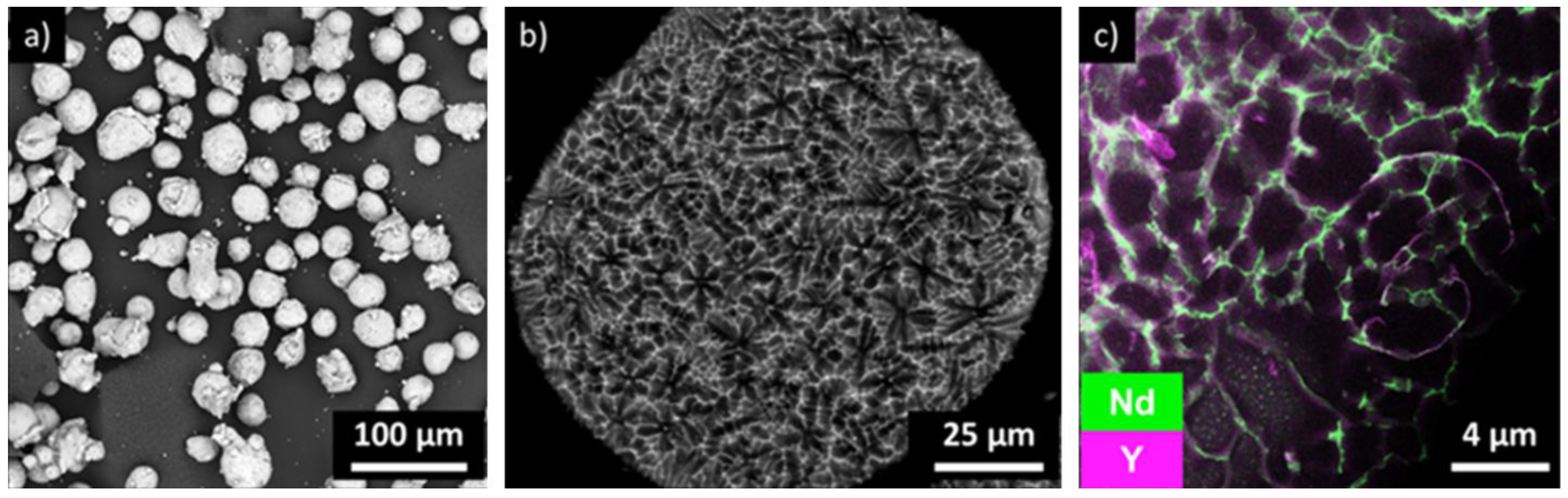
3.1. Powder Characterization
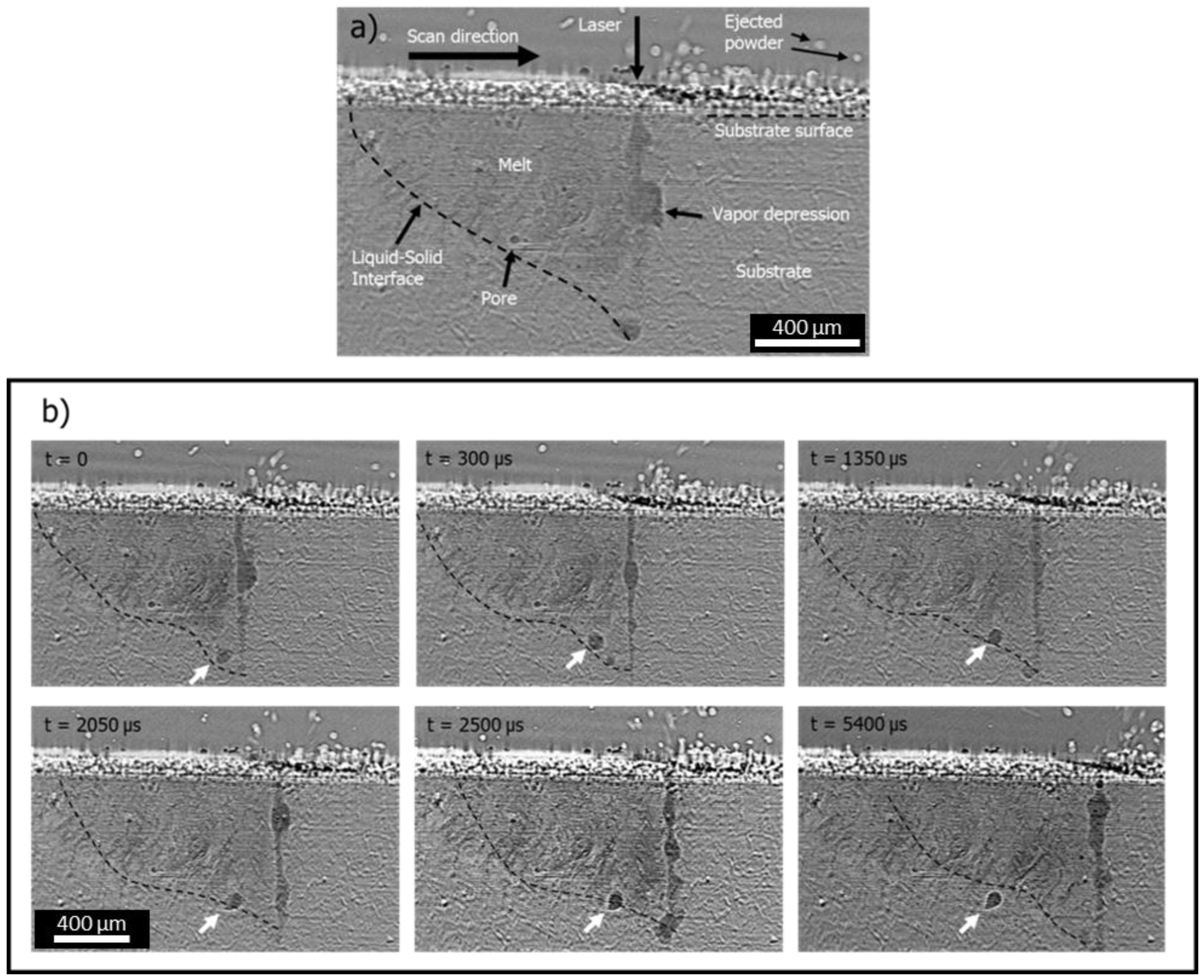
3.2. Vapor Depression Behavior and Geometry
- Movement of the powder particles ahead of the laser, visible as light contrast above the substrate surface (labeled ‘ejected powder’);
- A depression in the surface of the melt-pool due to recoil pressure, appearing as a narrow dark region that protrudes into the substrate following the laser scan (labelled ‘vapor depression’);
- The formation of pores, which appear as variously shaped dark contrast regions.
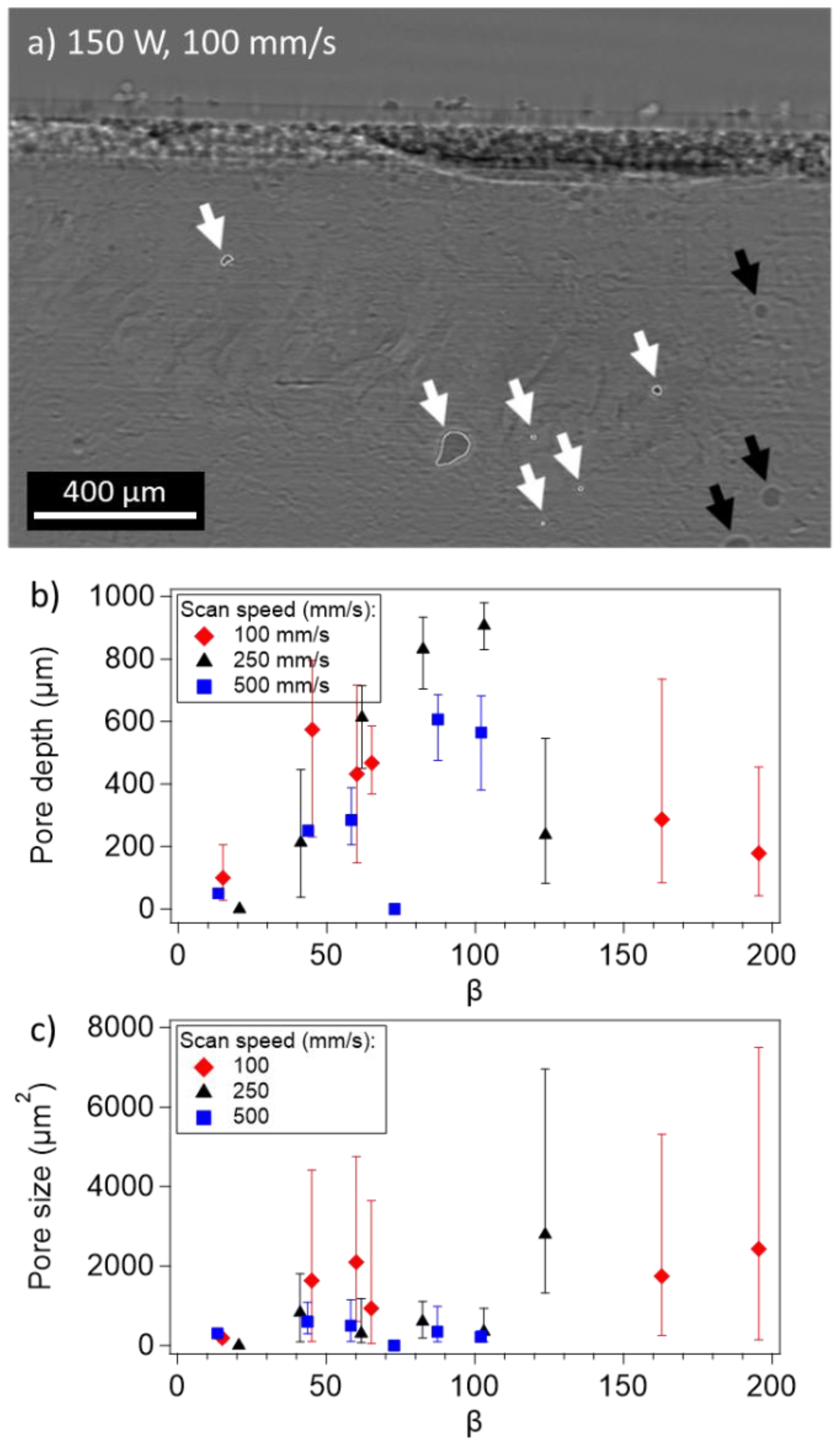
3.3. Pore Formation and Properties
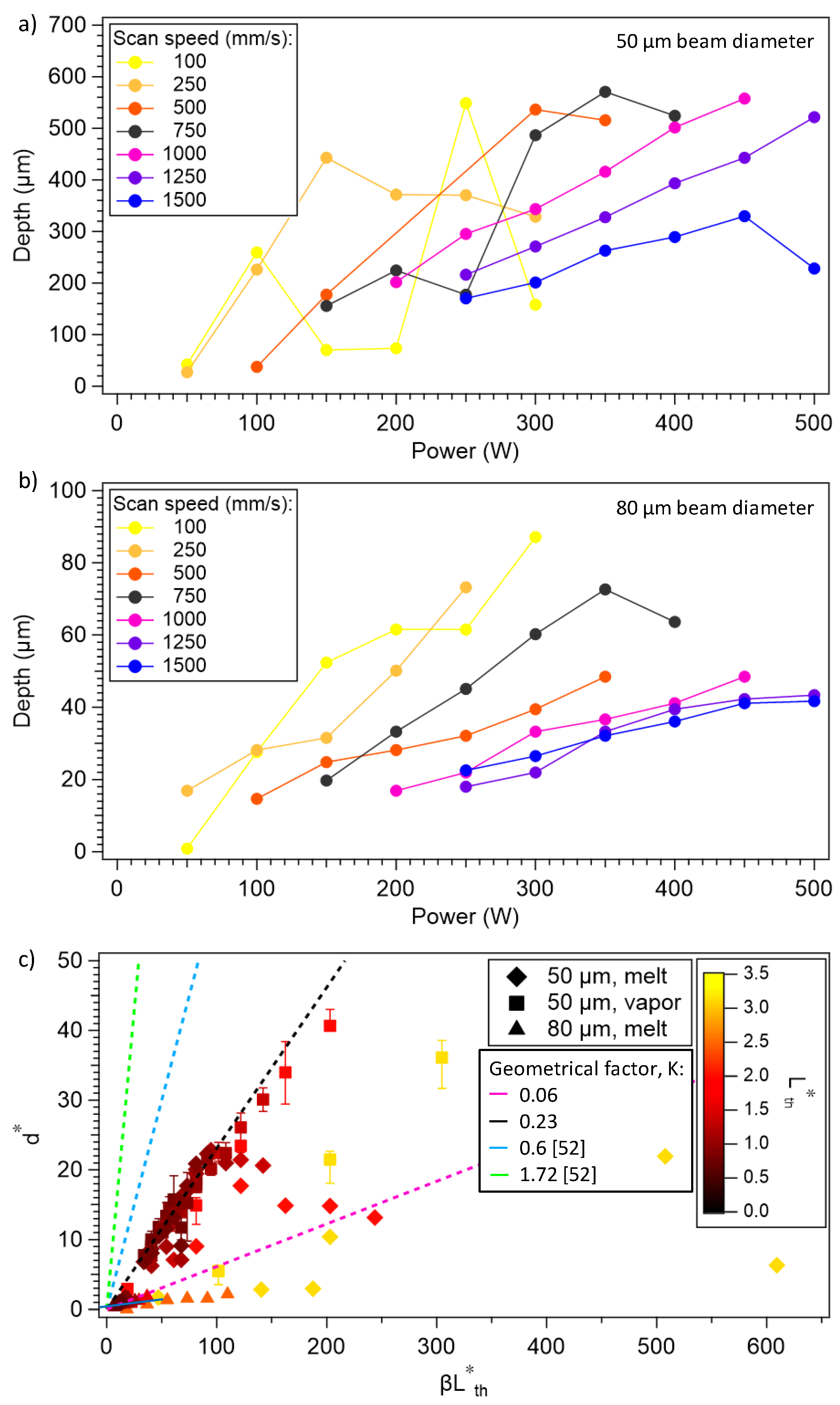
3.4. Melt-pool Depth and Geometry
3.5. Melt-Pool-Shape Regimes
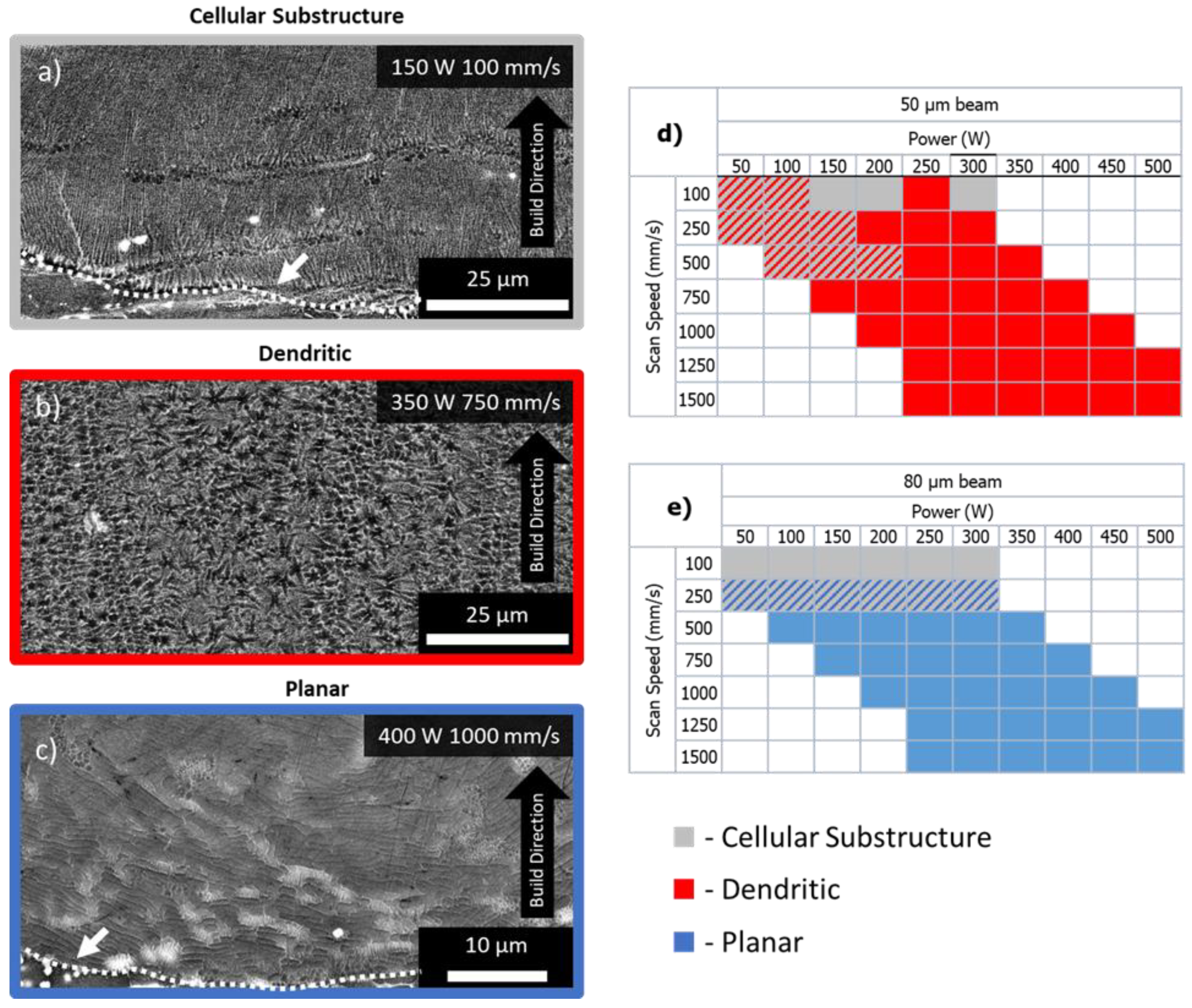
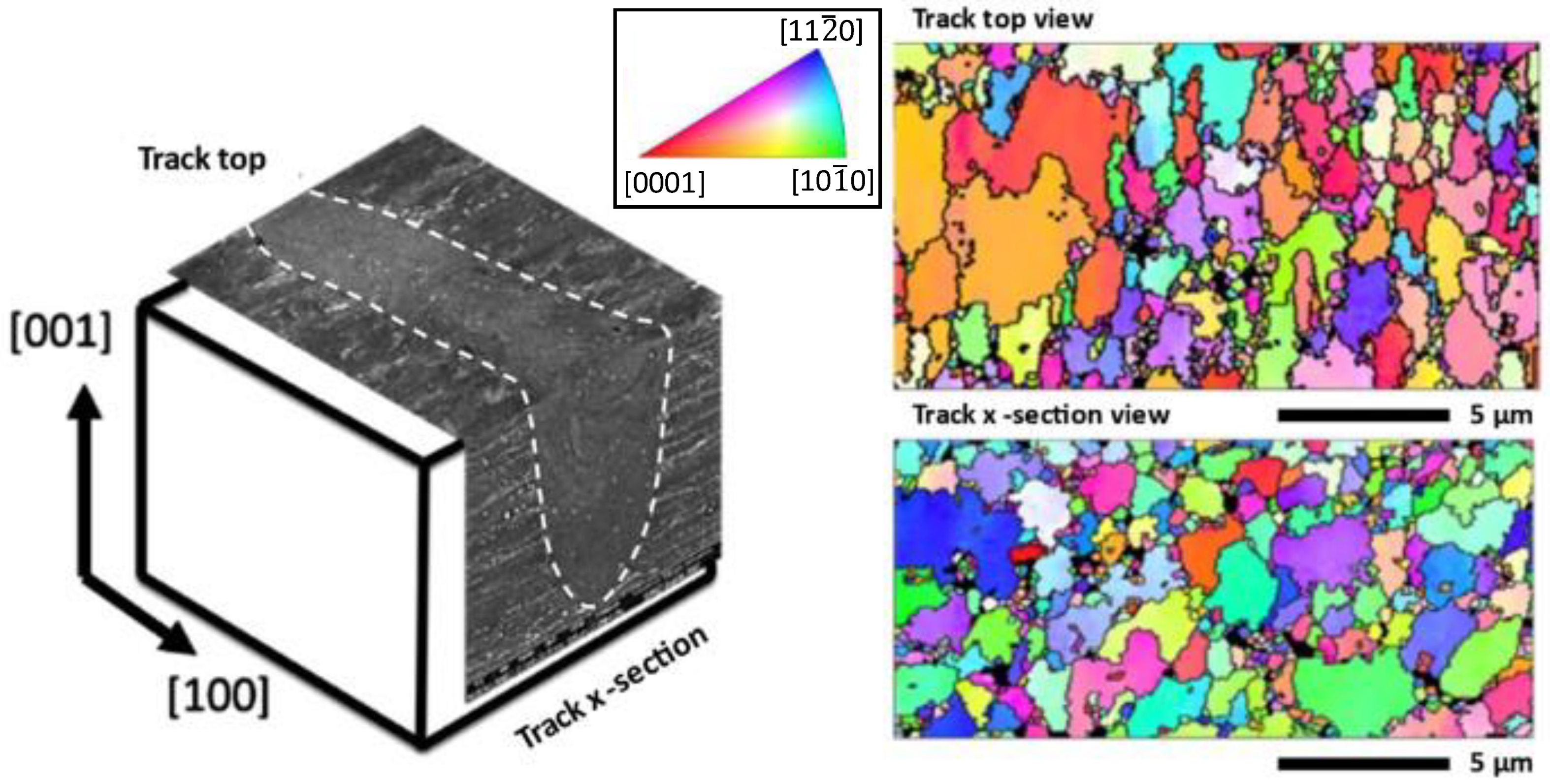
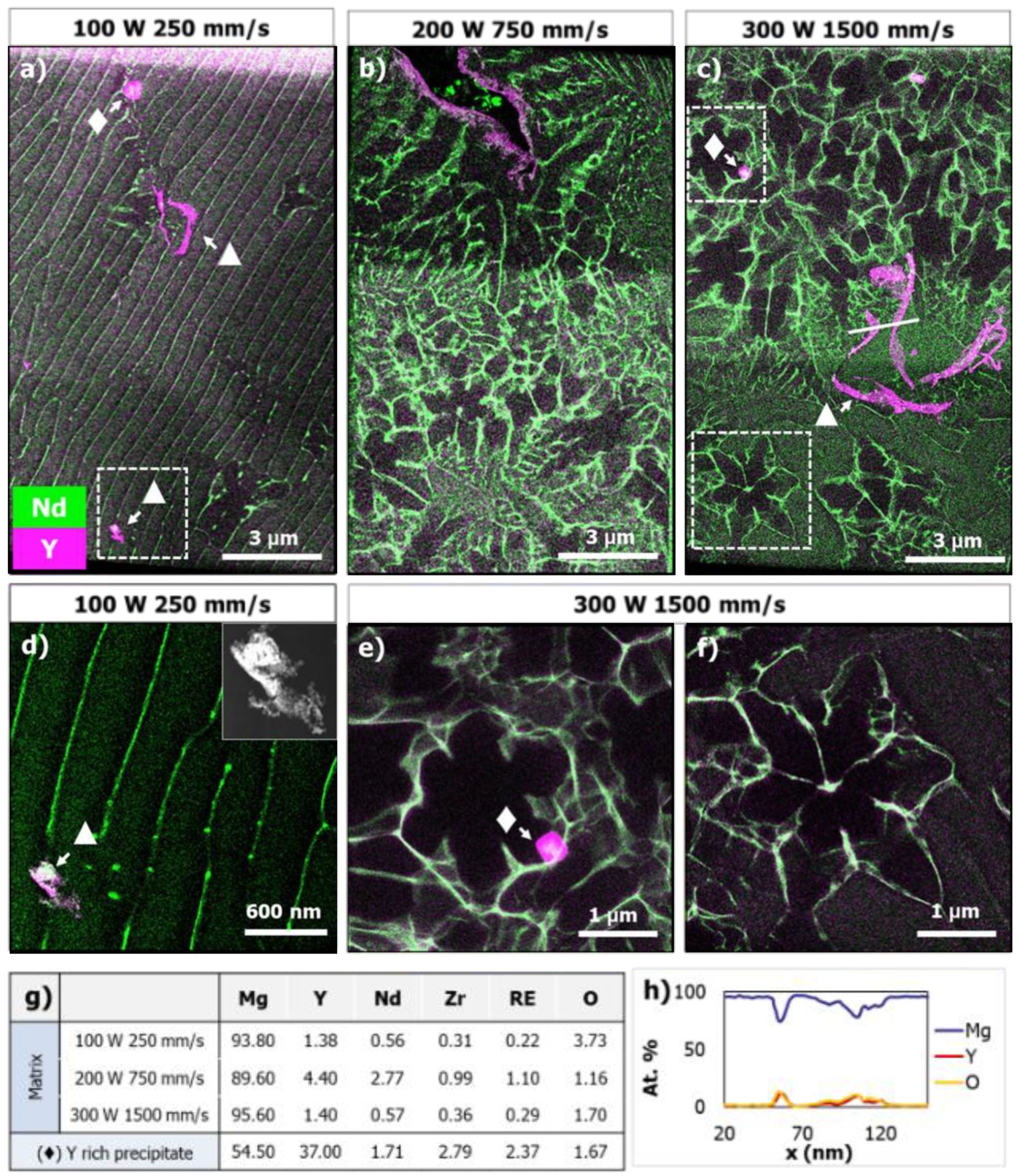
3.6. Microstructure Analysis
3.7. Theoretical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current Status and Outlook on the Clinical Translation of Biodegradable Metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Liu, D.; Ding, Y.; Guo, T.; Qin, X.; Guo, C.; Yu, S.; Lin, S. Influence of Fine-Grain and Solid-Solution Strengthening on Mechanical Properties and in Vitro Degradation of WE43 Alloy. Biomed. Mater. 2014, 9, 015014. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhaut, D.I.; Furrer, A.; Uggowitzer, P.J.; Löffler, J.F. Corrosion Properties of Glassy Mg70Al15Ga15 in 0.1M NaCl Solution. Intermetallics 2009, 17, 811–817. [Google Scholar] [CrossRef]
- Izumi, S.; Yamasaki, M.; Kawamura, Y. Relation between Corrosion Behavior and Microstructure of Mg–Zn–Y Alloys Prepared by Rapid Solidification at Various Cooling Rates. Corros. Sci. 2009, 51, 395–402. [Google Scholar] [CrossRef]
- Kojima, Y.; Aizawa, T.; Higashi, K.; Kamado, S. Corrosion behavior of rapidly solidified Mg-Zn-Y alloy ribbons. Mater. Sci. Forum 2003, 419, 937–942. [Google Scholar]
- Liao, J.; Hotta, M.; Mori, Y. Improved Corrosion Resistance of a High-Strength Mg–Al–Mn–Ca Magnesium Alloy Made by Rapid Solidification Powder Metallurgy. Mater. Sci. Eng. A 2012, 544, 10–20. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion Studies of Rapidly Solidified Magnesium Alloys. J. Electrochem. Soc. 1990, 137, 414. [Google Scholar] [CrossRef]
- Hermann, F.; Sommer, F.; Jones, H.; Edyvean, R.G.J. Corrosion Inhibition in Magnesium-Aluminium-Based Alloys Induced by Rapid Solidification Processing. J. Mater. Sci. 1989, 24, 2369–2379. [Google Scholar] [CrossRef]
- Elsayed, A.; Kondoh, K.; Imai, H.; Umeda, J. Microstructure and Mechanical Properties of Hot Extruded Mg–Al–Mn–Ca Alloy Produced by Rapid Solidification Powder Metallurgy. Mater. Des. 2010, 31, 2444–2453. [Google Scholar] [CrossRef]
- Elsayed, A.; Imai, H.; Umeda, J.; Kondoh, K. Microstructure and Mechanical Properties of Hot Extruded ZK61 Alloy Produced by Rapid Solidified Powder Metallurgy. STEEL Res. Int. 2010, 81, 1304–1307. [Google Scholar]
- Gu, X.N.; Zhou, W.R.; Zheng, Y.F.; Cheng, Y.; Wei, S.C.; Zhong, S.P.; Xi, T.F.; Chen, L.J. Corrosion Fatigue Behaviors of Two Biomedical Mg Alloys—AZ91D and WE43—In Simulated Body Fluid. Acta Biomater. 2010, 6, 4605–4613. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Wu, D.; Chen, R.S.; Han, E.H. Microstructures and Mechanical Properties of the Age Hardened Mg–4.2Y–2.5Nd–1Gd–0.6Zr (WE43) Microalloyed with Zn. J. Magnes. Alloys 2014, 2, 109–115. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly Solidified Powder Metallurgy Mg97Zn1Y2 Alloys with Excellent Tensile Yield Strength above 600 MPa. Mater. Trans. 2001, 42, 1172–1176. [Google Scholar] [CrossRef]
- Soderlind, J.; Cihova, M.; Schäublin, R.; Risbud, S.; Löffler, J.F. Towards Refining Microstructures of Biodegradable Magnesium Alloy WE43 by Spark Plasma Sintering. Acta Biomater. 2019, 98, 67–80. [Google Scholar] [CrossRef]
- Yamashita, A.; Horita, Z.; Langdon, T.G. Improving the Mechanical Properties of Magnesium and a Magnesium Alloy through Severe Plastic Deformation. Mater. Sci. Eng. A 2001, 300, 142–147. [Google Scholar] [CrossRef]
- Kai, M.; Horita, Z.; Langdon, T.G. Developing Grain Refinement and Superplasticity in a Magnesium Alloy Processed by High-Pressure Torsion. Mater. Sci. Eng. A 2008, 488, 117–124. [Google Scholar] [CrossRef]
- Mackenzie, L.W.F.; Davis, B.; Humphreys, F.J.; Lorimer, G.W. The Deformation, Recrystallisation and Texture of Three Magnesium Alloy Extrusions. Mater. Sci. Technol. 2007, 23, 1173–1180. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Vojtěch, D.; Prusa, F.; Nova, K. Preparation of WE43 Using Powder Metallurgy Route. Manuf. Technol. 2016, 16, 680–687. [Google Scholar] [CrossRef]
- Dvorsky, D.; Kubasek, J.; Vojtech, D.; Cavojsky, M. Structure and Mechanical Properties of WE43 Prepared by Powder Metallurgy Route. Manuf. Technol. 2016, 16, 896–902. [Google Scholar] [CrossRef]
- Thampy, V.; Fong, A.Y.; Calta, N.P.; Wang, J.; Martin, A.A.; Depond, P.J.; Kiss, A.M.; Guss, G.; Xing, Q.; Ott, R.T.; et al. Subsurface Cooling Rates and Microstructural Response during Laser Based Metal Additive Manufacturing. Sci. Rep. 2020, 10, 1981. [Google Scholar] [CrossRef] [PubMed]
- Wohlers, T.T.; Associates, W.; Campbell, I.; Caffrey, T.; Diegel, O.; Kowen, J. Wohlers Report 2018: 3D Printing and Additive Manufacturing State of the Industry: Annual Worldwide Progress Report; Wohlers Associates: Washington, DC, USA, 2018; ISBN 978-0-9913332-4-0. [Google Scholar]
- Shi, R.; Khairallah, S.A.; Roehling, T.T.; Heo, T.W.; McKeown, J.T.; Matthews, M.J. Microstructural Control in Metal Laser Powder Bed Fusion Additive Manufacturing Using Laser Beam Shaping Strategy. Acta Mater. 2020, 184, 284–305. [Google Scholar] [CrossRef]
- Roehling, T.T.; Wu, S.S.Q.; Khairallah, S.A.; Roehling, J.D.; Soezeri, S.S.; Crumb, M.F.; Matthews, M.J. Modulating Laser Intensity Profile Ellipticity for Microstructural Control during Metal Additive Manufacturing. Acta Mater. 2017, 128, 197–206. [Google Scholar] [CrossRef]
- Jauer, L.; Kimm, M.; Meiners, W.; Batista, R.S.; Labude, N.; Bienert, M.; Neuss, S.; Lethaus, B.; Müther, M.; Kopp, A.; et al. Additive Manufacturing of Magnesium Alloys. In Proceedings of the 9th Symposium on Biodegradable Metals, Bertinoro, Italy, 27 August–1 September 2017. [Google Scholar]
- Kopp, A.; Derra, T.; Müther, M.; Jauer, L.; Schleifenbaum, J.H.; Voshage, M.; Jung, O.; Smeets, R.; Kröger, N. Influence of Design and Postprocessing Parameters on the Degradation Behavior and Mechanical Properties of Additively Manufactured Magnesium Scaffolds. Acta Biomater. 2019, 98, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Zumdick, N.A.; Jauer, L.; Kersting, L.C.; Kutz, T.N.; Schleifenbaum, J.H.; Zander, D. Additive Manufactured WE43 Magnesium: A Comparative Study of the Microstructure and Mechanical Properties with Those of Powder Extruded and as-Cast WE43. Mater. Charact. 2019, 147, 384–397. [Google Scholar] [CrossRef]
- Cunningham, R.; Zhao, C.; Parab, N.; Kantzos, C.; Pauza, J.; Fezzaa, K.; Sun, T.; Rollett, A.D. Keyhole Threshold and Morphology in Laser Melting Revealed by Ultrahigh-Speed X-Ray Imaging. Science 2019, 363, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.C.; Savalani, M.M.; Lau, M.L.; Man, H.C. Microstructure and Mechanical Properties of Selective Laser Melted Magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Niu, X.; Shen, H.; Fu, J.; Yan, J.; Wang, Y. Corrosion Behaviour of Laser Powder Bed Fused Bulk Pure Magnesium in Hank’s Solution. Corros. Sci. 2019, 157, 284–294. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, Y.; Wu, P.; Lin, X.; Liu, Y.; Zhou, Y.; Feng, P.; Liu, X.; Peng, S. Laser Rapid Solidification Improves Corrosion Behavior of Mg-Zn-Zr Alloy. J. Alloys Compd. 2017, 691, 961–969. [Google Scholar] [CrossRef]
- Bär, F.; Berger, L.; Jauer, L.; Kurtuldu, G.; Schäublin, R.; Schleifenbaum, J.H.; Löffler, J.F. Laser Additive Manufacturing of Biodegradable Magnesium Alloy WE43: A Detailed Microstructure Analysis. Acta Biomater. 2019, 98, 36–49. [Google Scholar] [CrossRef]
- Martin, A.A.; Calta, N.P.; Khairallah, S.A.; Wang, J.; Depond, P.J.; Fong, A.Y.; Thampy, V.; Guss, G.M.; Kiss, A.M.; Stone, K.H.; et al. Dynamics of Pore Formation during Laser Powder Bed Fusion Additive Manufacturing. Nat. Commun. 2019, 10, 1987. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.A.; Calta, N.P.; Hammons, J.A.; Khairallah, S.A.; Nielsen, M.H.; Shuttlesworth, R.M.; Sinclair, N.; Matthews, M.J.; Jeffries, J.R.; Willey, T.M.; et al. Ultrafast Dynamics of Laser-Metal Interactions in Additive Manufacturing Alloys Captured by in Situ X-Ray Imaging. Mater. Today Adv. 2019, 1, 100002. [Google Scholar] [CrossRef]
- Calta, N.P.; Wang, J.; Kiss, A.M.; Martin, A.A.; Depond, P.J.; Guss, G.M.; Thampy, V.; Fong, A.Y.; Weker, J.N.; Stone, K.H.; et al. An Instrument for in Situ Time-Resolved X-Ray Imaging and Diffraction of Laser Powder Bed Fusion Additive Manufacturing Processes. Rev. Sci. Instrum. 2018, 89, 055101. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, H.; Seitz, J.-M.; Açil, Y.; Damm, T.; Möller, I.; Gülses, A.; Wiltfang, J. Osteosynthesis of a Cranio-Osteoplasty with a Biodegradable Magnesium Plate System in Miniature Pigs. Acta Biomater. 2017, 62, 434–445. [Google Scholar] [CrossRef] [PubMed]
- Windhagen, H.; Radtke, K.; Weizbauer, A.; Diekmann, J.; Noll, Y.; Kreimeyer, U.; Schavan, R.; Stukenborg-Colsman, C.; Waizy, H. Biodegradable Magnesium-Based Screw Clinically Equivalent to Titanium Screw in Hallux Valgus Surgery: Short Term Results of the First Prospective, Randomized, Controlled Clinical Pilot Study. Biomed. Eng. OnLine 2013, 12, 62. [Google Scholar] [CrossRef]
- Waksman, R.; Zumstein, P.; Pritsch, M.; Wittchow, E.; Haude, M.; Lapointe-Corriveau, C.; Leclerc, G.; Joner, M. Second-Generation Magnesium Scaffold Magmaris: Device Design and Preclinical Evaluation in a Porcine Coronary Artery Model. Euro Interv. J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2017, 13, 440–449. [Google Scholar] [CrossRef]
- Haude, M.; Ince, H.; Abizaid, A.; Toelg, R.; Lemos, P.A.; von Birgelen, C.; Christiansen, E.H.; Wijns, W.; Neumann, F.-J.; Kaiser, C.; et al. Safety and Performance of the Second-Generation Drug-Eluting Absorbable Metal Scaffold in Patients with de-Novo Coronary Artery Lesions (BIOSOLVE-II): 6 Month Results of a Prospective, Multicentre, Non-Randomised, First-in-Man Trial. Lancet Lond. Engl. 2016, 387, 31–39. [Google Scholar] [CrossRef]
- Biber, R.; Pauser, J.; Geßlein, M.; Bail, H.J. Magnesium-Based Absorbable Metal Screws for Intra-Articular Fracture Fixation. Case Rep. Orthop. 2016, 2016, 9673174. [Google Scholar] [CrossRef]
- Gieseke, M.; Noelke, C.; Kaierle, S.; Wesling, V.; Haferkamp, H. Selective Laser Melting of Magnesium and Magnesium Alloys. In Magnesium Technology 2013; Hort, N., Mathaudhu, S.N., Neelameggham, N.R., Alderman, M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 65–68. ISBN 978-3-319-48150-0. [Google Scholar]
- Liu, C.; Li, Q.; Liang, J.; Zhou, J.; Wang, L. Microstructure and Corrosion Behaviour of Laser Surface Melting Treated WE43 Magnesium Alloy. RSC Adv. 2016, 6, 30642–30651. [Google Scholar] [CrossRef]
- Scipioni Bertoli, U.; Guss, G.; Wu, S.; Matthews, M.J.; Schoenung, J.M. In-Situ Characterization of Laser-Powder Interaction and Cooling Rates through High-Speed Imaging of Powder Bed Fusion Additive Manufacturing. Mater. Des. 2017, 135, 385–396. [Google Scholar] [CrossRef]
- Matthews, M.J.; Guss, G.; Khairallah, S.A.; Rubenchik, A.M.; Depond, P.J.; King, W.E. Denudation of Metal Powder Layers in Laser Powder Bed Fusion Processes. Acta Mater. 2016, 114, 33–42. [Google Scholar] [CrossRef]
- Trapp, J.; Rubenchik, A.M.; Guss, G.; Matthews, M.J. In Situ Absorptivity Measurements of Metallic Powders during Laser Powder-Bed Fusion Additive Manufacturing. Appl. Mater. Today 2017, 9, 341–349. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.I.; Nowell, M.M.; Lindeman, S.P.; Camus, P.P.; De Graef, M.; Jackson, M.A. Introduction and Comparison of New EBSD Post-Processing Methodologies. Ultramicroscopy 2015, 159, 81–94. [Google Scholar] [CrossRef]
- Mathematica; Version 11.1.; Wolfram Research, Inc.: Champaign, IL, USA, 2017.
- Housh, S.; Mikucki, B.; Stevenson, A. Properties of Magnesium Alloys. In Metals Handbook Properties and Selection: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials, 10th ed.; ASM International: Metals Park, OH, USA, 1990; ISBN 978-0-87170-378-1. [Google Scholar] [CrossRef]
- Avedesian, M.M.; Baker, H. ASM Specialty Handbook: Magnesium and Magnesium Alloys; ASM International: Almere, The Netherlands, 1999; ISBN 978-0-87170-657-7. [Google Scholar]
- Lukas, H.; Fries, S.G.; Sundman, B. Computational Thermodynamics: The Calphad Method; Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-86811-2. [Google Scholar]
- Sundman, B.; Jansson, B.; Andersson, J.-O. The Thermo-Calc Databank System. Calphad 1985, 9, 153–190. [Google Scholar] [CrossRef]
- Kurz, W.; Giovanola, B.; Trivedi, R. Theory of Microstructural Development during Rapid Solidification. Acta Metall. 1986, 34, 823–830. [Google Scholar] [CrossRef]
- Liu, Y.; Long, Z.; Wang, H.; Du, Y.; Huang, B. A Predictive Equation for Solute Diffusivity in Liquid Metals. Scr. Mater. 2006, 55, 367–370. [Google Scholar] [CrossRef]
- Spaepen, F.; Meyer, R.B. The Surface Tension in a Structural Model for the Solid-Liquid Interface. Scr. Metall. 1976, 10, 257–263. [Google Scholar] [CrossRef]
- Sun, D.Y.; Mendelev, M.I.; Becker, C.A.; Kudin, K.; Haxhimali, T.; Asta, M.; Hoyt, J.J.; Karma, A.; Srolovitz, D.J. Crystal-Melt Interfacial Free Energies in Hcp Metals: A Molecular Dynamics Study of Mg. Phys. Rev. B 2006, 73, 024116. [Google Scholar] [CrossRef]
- Guo, Q.; Zhao, C.; Qu, M.; Xiong, L.; Hojjatzadeh, S.M.H.; Escano, L.I.; Parab, N.D.; Fezzaa, K.; Sun, T.; Chen, L. In-Situ Full-Field Mapping of Melt Flow Dynamics in Laser Metal Additive Manufacturing. Addit. Manuf. 2020, 31, 100939. [Google Scholar] [CrossRef]
- Ye, J.; Khairallah, S.A.; Rubenchik, A.M.; Crumb, M.F.; Guss, G.; Belak, J.; Matthews, M.J. Energy Coupling Mechanisms and Scaling Behavior Associated with Laser Powder Bed Fusion Additive Manufacturing. Adv. Eng. Mater. 2019, 21, 1900185. [Google Scholar] [CrossRef]
- Machorro, R.; Siqueiros, J.M.; Wang, S. Optical Properties of Mg, from UV to IR, Using Ellipsometry and Reflectometry. Thin Solid Films 1995, 269, 1–5. [Google Scholar] [CrossRef]
- Ly, S.; Rubenchik, A.M.; Khairallah, S.A.; Guss, G.; Matthews, M.J. Metal Vapor Micro-Jet Controls Material Redistribution in Laser Powder Bed Fusion Additive Manufacturing. Sci. Rep. 2017, 7, 4085. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, R. Melt Pool and Keyhole Behaviour Analysis for Deep Penetration Laser Welding. J. Phys. Appl. Phys. 2010, 43, 445501. [Google Scholar] [CrossRef]
- Vrancken, B.; Wauthlé, R.; Kruth, J.-P.; Van Humbeeck, J. Study of the Influence of Material Properties on Residual Stress in Selective Laser Melting. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 12–14 August 2013; pp. 393–407. [Google Scholar]
- Kurz, W.; Trivedi, R. Banded Solidification Microstructures. Metall. Mater. Trans. A 1996, 27, 625–634. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive Manufacturing of Dense WE43 Mg Alloy by Laser Powder Bed Fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Berry, J.; Perron, A.; Fattebert, J.-L.; Roehling, J.D.; Vrancken, B.; Roehling, T.T.; Rosas, D.L.; Turner, J.A.; Khairallah, S.A.; McKeown, J.T.; et al. Toward Multiscale Simulations of Tailored Microstructure Formation in Metal Additive Manufacturing. Mater. Today 2021, 51, 65–86. [Google Scholar] [CrossRef]
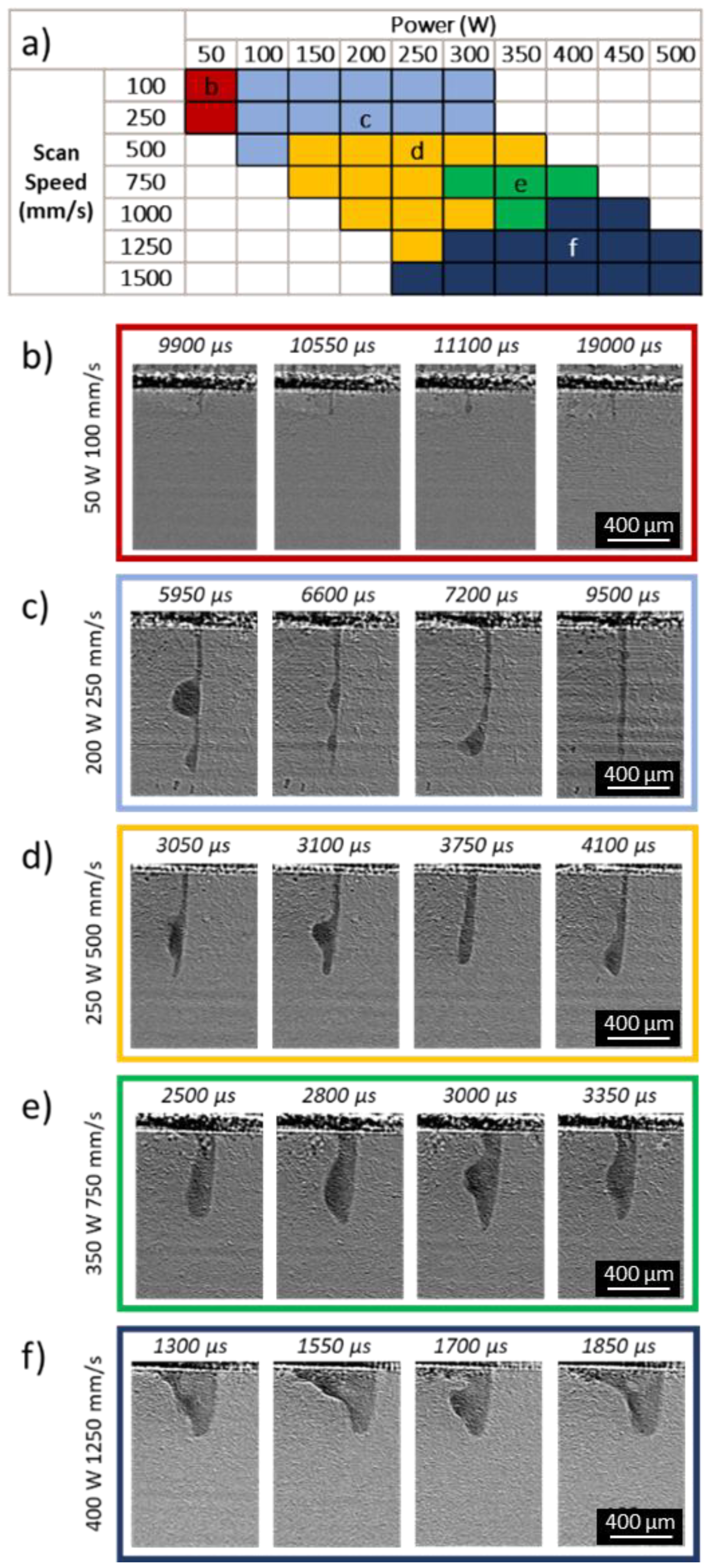


| Power (W) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 150 | 200 | 250 | 300 | 350 | 400 | 450 | 500 | ||
| Scan Speed (mm/s) | 100 | ||||||||||
| 250 | |||||||||||
| 500 | |||||||||||
| 750 | |||||||||||
| 1000 | |||||||||||
| 1250 | |||||||||||
| 1500 | |||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soderlind, J.; Martin, A.A.; Calta, N.P.; DePond, P.J.; Wang, J.; Vrancken, B.; Schäublin, R.E.; Basu, I.; Thampy, V.; Fong, A.Y.; et al. Melt-Pool Dynamics and Microstructure of Mg Alloy WE43 under Laser Powder Bed Fusion Additive Manufacturing Conditions. Crystals 2022, 12, 1437. https://doi.org/10.3390/cryst12101437
Soderlind J, Martin AA, Calta NP, DePond PJ, Wang J, Vrancken B, Schäublin RE, Basu I, Thampy V, Fong AY, et al. Melt-Pool Dynamics and Microstructure of Mg Alloy WE43 under Laser Powder Bed Fusion Additive Manufacturing Conditions. Crystals. 2022; 12(10):1437. https://doi.org/10.3390/cryst12101437
Chicago/Turabian StyleSoderlind, Julie, Aiden A. Martin, Nicholas P. Calta, Philip J. DePond, Jenny Wang, Bey Vrancken, Robin E. Schäublin, Indranil Basu, Vivek Thampy, Anthony Y. Fong, and et al. 2022. "Melt-Pool Dynamics and Microstructure of Mg Alloy WE43 under Laser Powder Bed Fusion Additive Manufacturing Conditions" Crystals 12, no. 10: 1437. https://doi.org/10.3390/cryst12101437
APA StyleSoderlind, J., Martin, A. A., Calta, N. P., DePond, P. J., Wang, J., Vrancken, B., Schäublin, R. E., Basu, I., Thampy, V., Fong, A. Y., Kiss, A. M., Berry, J. M., Perron, A., Nelson Weker, J., Stone, K. H., Tassone, C. J., Toney, M. F., Van Buuren, A., Löffler, J. F., ... Matthews, M. J. (2022). Melt-Pool Dynamics and Microstructure of Mg Alloy WE43 under Laser Powder Bed Fusion Additive Manufacturing Conditions. Crystals, 12(10), 1437. https://doi.org/10.3390/cryst12101437







