Study of the Microstructural, Thermal, and Magnetic Properties of High-Energy Ball-Milled Nanocrystalline Fe(Al)
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
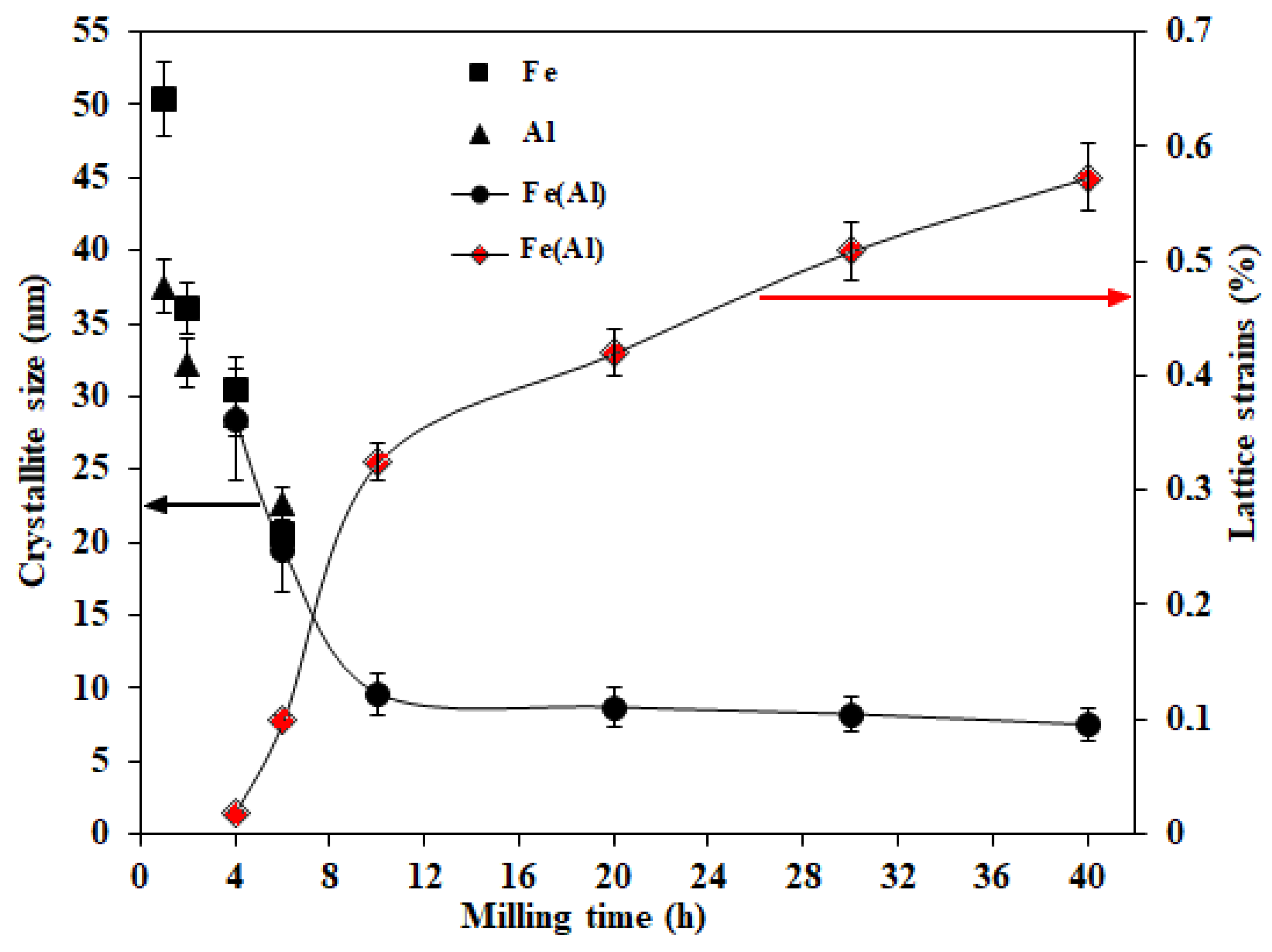
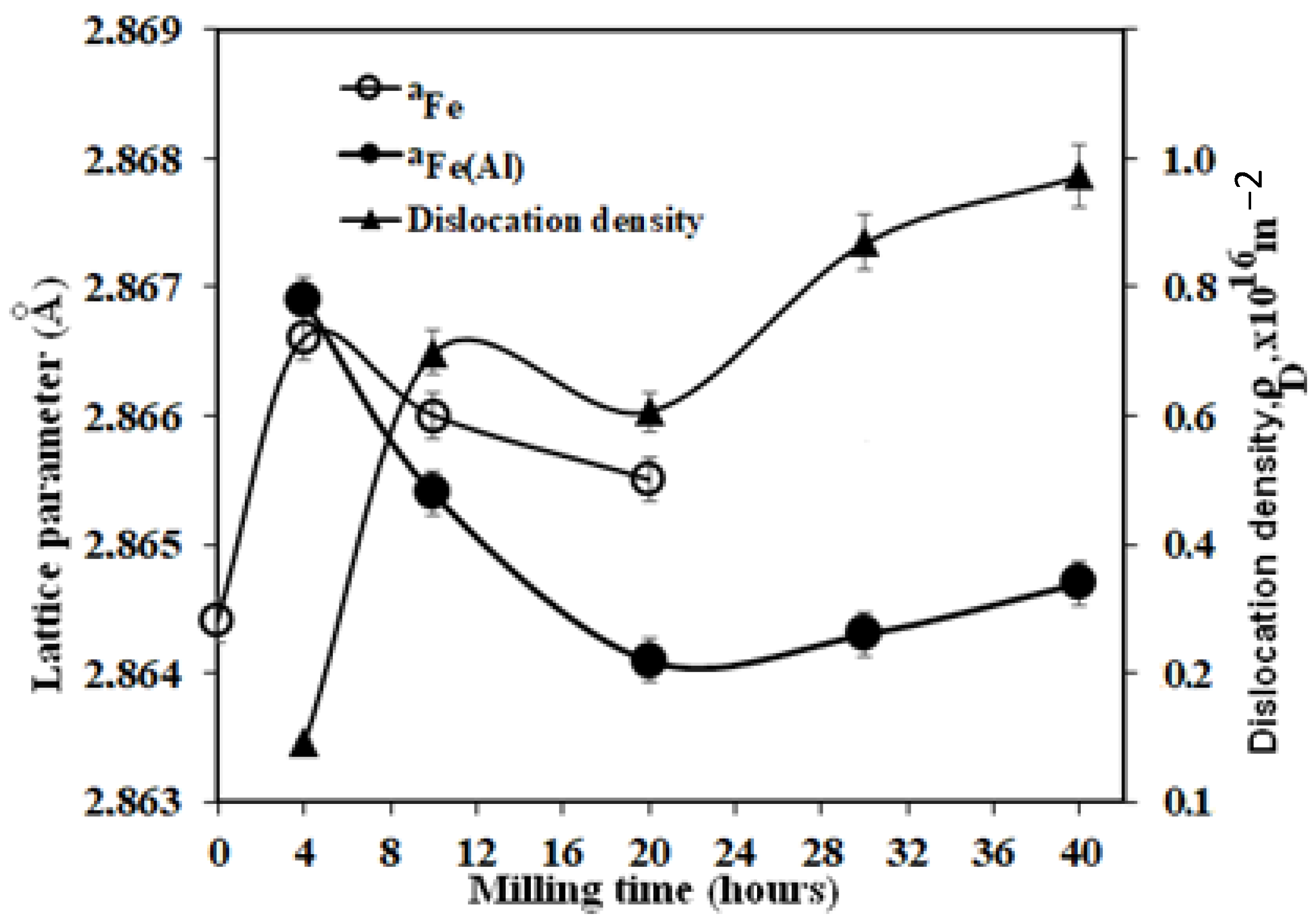
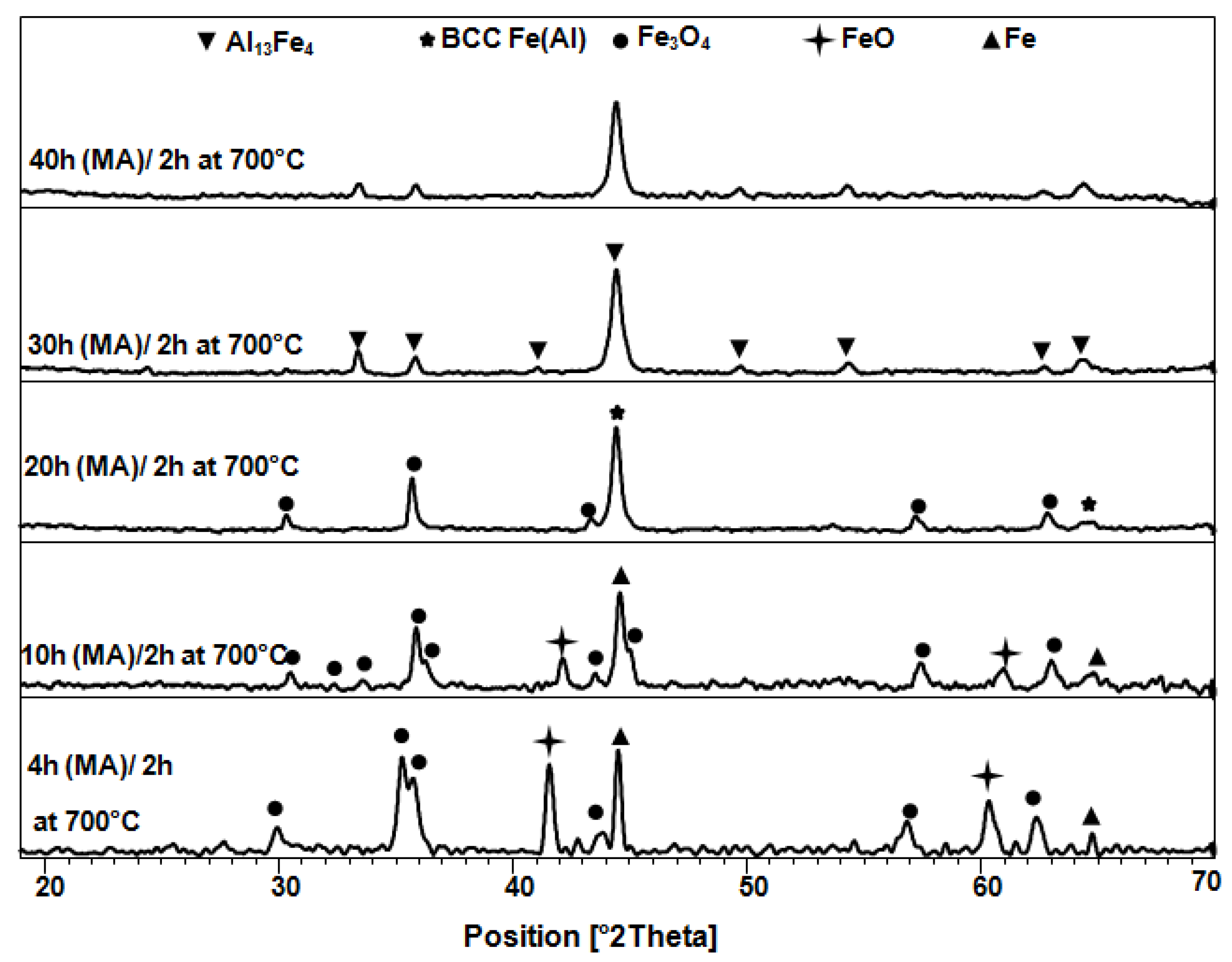
3.1. Structural and Microstructural Analysis
3.2. Thermal Analysis
3.3. Magnetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. 1998, A258, 1–14. [Google Scholar] [CrossRef]
- Deevi, S.C. Advanced intermetallic iron aluminide coatings for high-temperature application. Prog. Mater. Sci. 2021, 118, 100769. [Google Scholar] [CrossRef]
- Durga, P.V.; Nagini, N.; Reddy, A.V.; Bakshi, S.R.; Vijay, R. Effect of grain structure and nano oxide dispersoids on improved strength and ductility of iron aluminide based intermetallics. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2022, 53, 1597–1603. [Google Scholar] [CrossRef]
- Eggersmann, M.; Mehrer, H. Diffusion in intermetallic phases of the FeAl system. Philos. Mag. 2000, A80, 1219–1244. [Google Scholar] [CrossRef]
- Tortorelli, P.F.; DeVan, J.H. Behavior of iron aluminides in oxidizing and oxidizing/sulfidizing environments. Mater. Sci. Eng. 1992, A153, 573–577. [Google Scholar] [CrossRef]
- Nair, V.R.; Maiyalagan, T.; Shendage, S.S. Halloysite clay nanotubes with Fe-Al deposits for the oxidation of benzil alcohol. New J. Chem. 2022, 46, 17213–17222. [Google Scholar] [CrossRef]
- Zagrebin, M.A.; Matyunina, M.V.; Koshkin, A.B.; Sokolovskiy, V.V.; Sokolovskiy, V.V.; Buchelnikov, V.D.; Vasiliy, D. Structural and magnetic properties of Fer-Al alloys: Ab initio studies. J. Magn. Magnntic Mater. 2022, 557, 169437. [Google Scholar] [CrossRef]
- Bormio-Nunes, C.; Dias, M.B.; Ghivelder, L. High magnetostriction of the polycrystalline alloy (Fe0. 8Al0. 2) 97B3. J. Alloy. Compd. 2003, 574, 467–471. [Google Scholar] [CrossRef]
- Han, Y.; Kong, F.L.; Han, F.F.; Inoue, A.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F. New Fe-based soft magnetic amorphous alloys with high saturation magnetization and good corrosion resistance for dust core application. Intermetallics 2016, 76, 18–25. [Google Scholar] [CrossRef]
- Avar, B. Structural, thermal and magnetic characterization of nanocrystalline Co65Ti25W5B5 powders prepared by mechanical alloying. J. Non-Cryst. Solids 2016, 432 Pt B, 246–253. [Google Scholar] [CrossRef]
- Louidi, S.; Bentayeb, F.Z.; Sunol, J.J.; Escoda, L. Formation study of the ball-milled Cr20Co80 alloy. J. Alloy. Compd. 2010, 493, 110–115. [Google Scholar] [CrossRef]
- Noyan, I.C.; Cohen, J.B.; Springer-Verlag New York Inc. Residual Stress Measurement by Diffraction and Interpretation; Ilschner, B., Grant, N.J., Eds.; Springer Series on Materials Research and Engineering; Springer-Verlag: New York, NY, USA; Berlin/Heidelberg, Germany; London, UK; Paris, France; Tokyo, Japan, 1987; 276 Seiten, 160 Bilder, 31 Tabellen, DM 138; ISBN 3-540-96378-2. [Google Scholar]
- Williamson, G.K.; Smallman, R.E. Dislocation densities in some annealed and cold-worked metals from measurements on the X-ray Debye-Scherrer spectrum. Phil. Mag. 1956, 1, 34. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Khitouni, M.; Escoda, L.; Suñol, J.J.; Dammak, M. Characterization of Mechanically Alloyed Nanocrystalline Fe(Al): Crystallite Size and Dislocation Density. J. Nanomater. 2010, 2010, 712407. [Google Scholar] [CrossRef]
- Srivastava, Y.; Srivastava, S. Preparation and properties of Cobalt-based soft magnetic material prepared by novel powder metallurgy. J. Magn. Magn. Mater. 2017, 423, 267–274. [Google Scholar] [CrossRef]
- Morris-Munoz, M.A.; Dodge, A.; Morris, D.G. Structure, strength and toughness of nanocrystalline FeAl. Nano Struct. Mater. 1999, 11, 873–885. [Google Scholar] [CrossRef]
- Krasnowski, M.; Grabias, A.; Kulik, T. Phase transformations during mechanical alloying of Fe–50% Al and subsequent heating of the milling product. J. Alloy. Compd. 2006, 424, 19–127. [Google Scholar] [CrossRef]
- Shi, H.; Guo, D.; Ouyang, Y. Structural evolution of mechanically alloyed nanocrystalline FeAl intermetallics. J. Alloy. Compd. 2008, 455, 207–209. [Google Scholar] [CrossRef]
- Mhadhbi, M.; Khitouni, M.; Escoda, L.; Sunol, J.J.; Dammak, M. Microstructure evolution and mechanical properties of nanocrystalline FeAl obtained by mechanical alloying and cold consolidation. J. Alloy. Compd. 2011, 509, 3293–3298. [Google Scholar] [CrossRef]
- Ikeda, O.; Onuma, I.; Kainuma, R.; Ishida, K. Phase equilibria and stability of ordered BCC phases in the Fe-rich portion of the Fe–Al system. Intermetallics 2001, 9, 755–761. [Google Scholar] [CrossRef]
- Bonetti, E.; Scipione, G.; Valdrf, G.; Enzo, S.; Frattini, R.; Macri, P.P. A study of nanocrystalline iron and aluminium metals and Fe3Al intermetallic by mechanical alloying. J. Mater. Sci. 1995, 30, 2220–2226. [Google Scholar] [CrossRef]
- Zhou, F.; Luck, R.; Scheffer, M.; Lang, D.; Lu, K. The crystallization process of amorphous Al80Fe20 alloy powders prepared by ball milling. J. Non-Cryst. Solids 1999, 250, 704–708. [Google Scholar] [CrossRef]
- Guillemany, J.M.; Cinca, N.; Casas, L.; Molins, E. Ordering and disordering processes in MA and MM intermetallic iron aluminide powders. J. Mater. Sci. 2009, 44, 2152–2161. [Google Scholar] [CrossRef]
- Jette, E.R.; Foote, F. An X-ray Study of the Wüstite (FeO) solid solutions. J. Chem. Phys. 1933, 1, 29. [Google Scholar] [CrossRef]
- Hanawalt, J.D.; Rinn, H.W.; Frevel, L.K. Chemical Analysis by X-ray Diffraction. Anal. Chem. 1938, 10, 475. [Google Scholar] [CrossRef]
- Ibn Gharsallah, H.; Sehri, A.; Aabou, M.; Escoda, L.; Sunol, J.J.; Khitouni, M. Structural and Thermal Study of Nanocrystalline Fe-Al-B Alloy Prepared by Mechanical Alloying. Metall. Mater. Trans. A 2015, 46, 3696–3704. [Google Scholar] [CrossRef]
- Popiel, E.; Tuszynski, M.; Zarek, W.; Rendecki, T. Investigation of Fe3−xVxAl alloys with DO3 type structure by X-ray, magnetostatic and Mössbauer effect methods. J. Less-Common Met. 1989, 146, 127. [Google Scholar] [CrossRef]
- Ellner, M.; Mayer, J. X-ray and electron diffraction investigations on the liquid-quenched Fe2Al5. Scripta Metall. Mater. 1992, 26, 501. [Google Scholar] [CrossRef]
- Nowroozi, M.A.; Shokrollahi, H. The effects of milling time and heat treatment on the micro-structural and magnetic behavior of Fe42Ni28Zr8Ta2B10C10 synthesized by mechanical alloying. J. Magn. Magn. Mater. 2013, 335, 53–58. [Google Scholar] [CrossRef]
- Sharifati, A.; Sharafi, S. Structural and magnetic properties of nanostructured (Fe70Co30)100−xCux alloy prepared by high energy ball milling. Mater. Des. 2012, 41, 8–15. [Google Scholar] [CrossRef]
- Krifa, M.; Mhadhbi, M.; Escoda, L.; Saurina, J.; Suñol, J.J.; Llorca-Isern, N.; Artieda-Guzmán, C.; Khitouni, M. Phase transformations during mechanical alloying of Fe–30% Al–20% Cu. Powder Technol. 2013, 246, 117–124. [Google Scholar] [CrossRef]
- Chen, C.W. Magnetism and Metallurgy of Soft Magnetic Materials; Elsevier B.V.: Amsterdam, The Netherland, 1977. [Google Scholar]
- Herzer, G. Grain size dependence of coercivity and permeability in nanocrystalline ferromagnets. IEEE Trans. Magn. 1990, 26, 1397–1402. [Google Scholar] [CrossRef]
- Herzer, G. Nanocrystalline soft magnetic alloys. In Handbook of Magnetic Materials; Buschow, K.H.L., Ed.; Elsevier: Amsterdam, The Netherland, 1997; Volume 10, pp. 415–462. [Google Scholar]
- Pearson, W.B. Handbook of Lattice Spacing and Structures of Metals and Alloys; Pergamon: Oxford, UK, 1958. [Google Scholar]
- Krifa, M.; Mhadhbi, M.; Escoda, L.; Güell, J.M.; Suñol, J.J.; Llorca-Isern, N.; Artieda-Guzmán, C.; Khitouni, M. Nanocrystalline (Fe60Al40)80Cu20 alloy prepared by mechanical alloying. J. Alloy. Compd. 2013, 554, 51–58. [Google Scholar] [CrossRef]




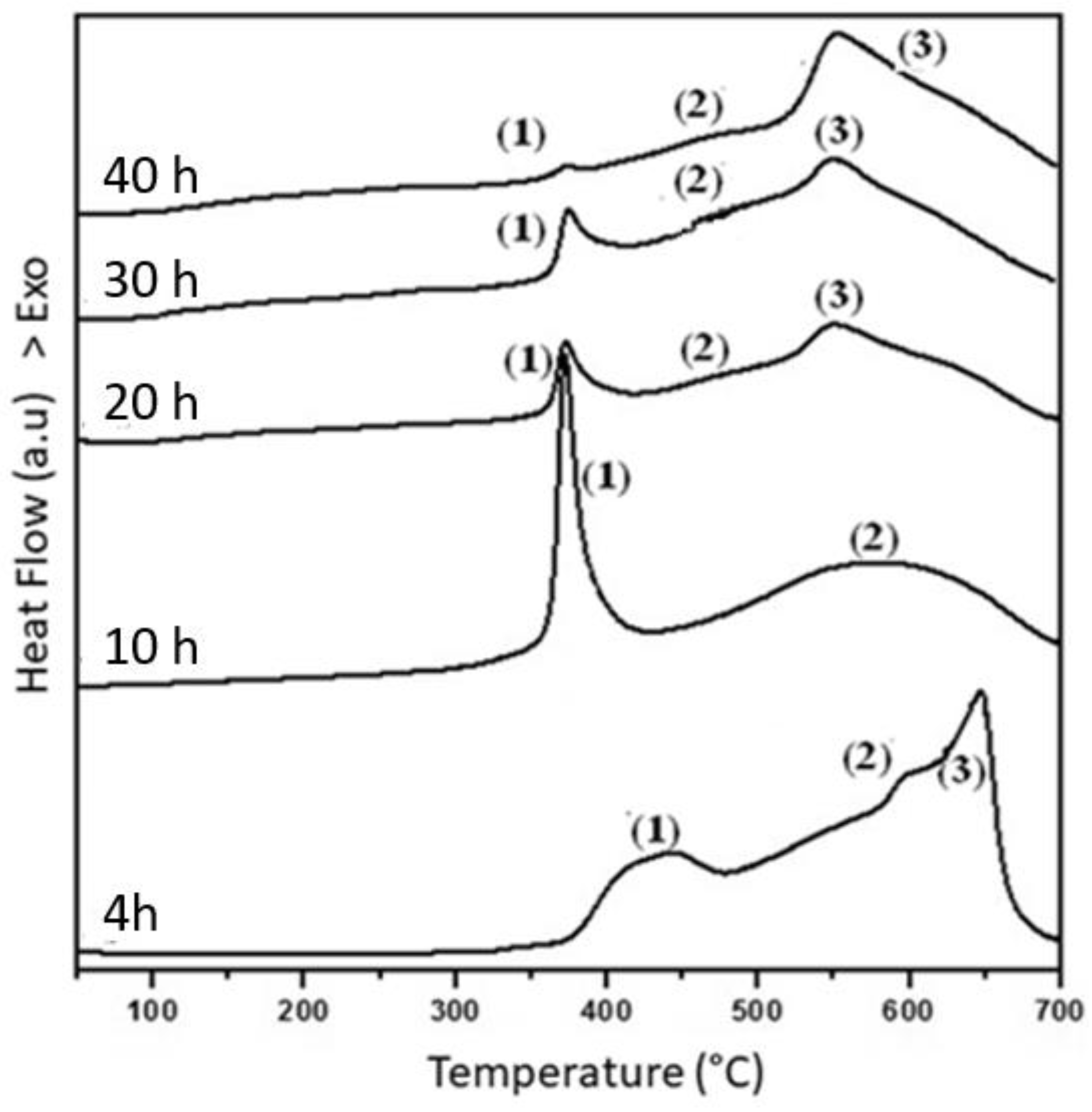

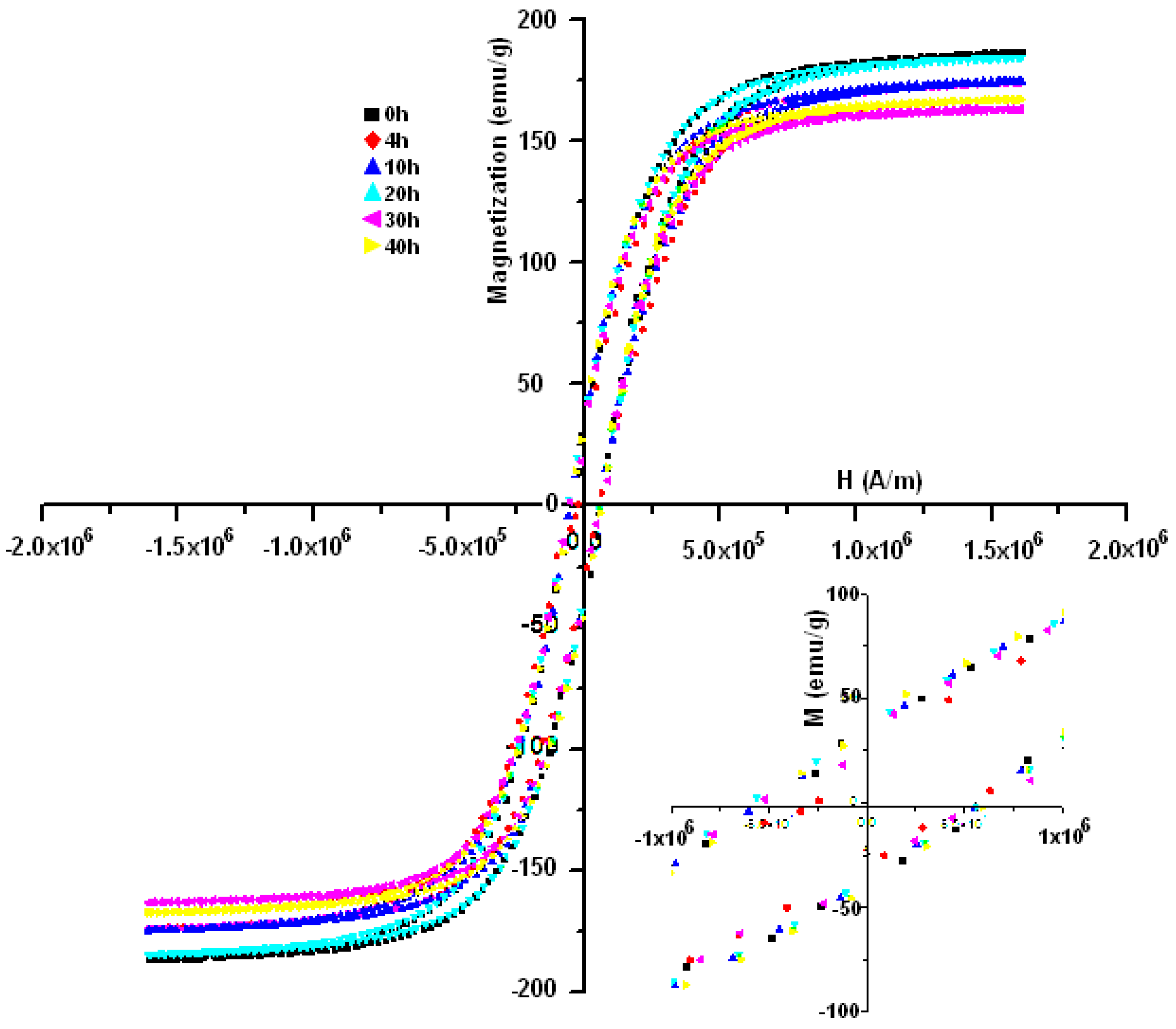
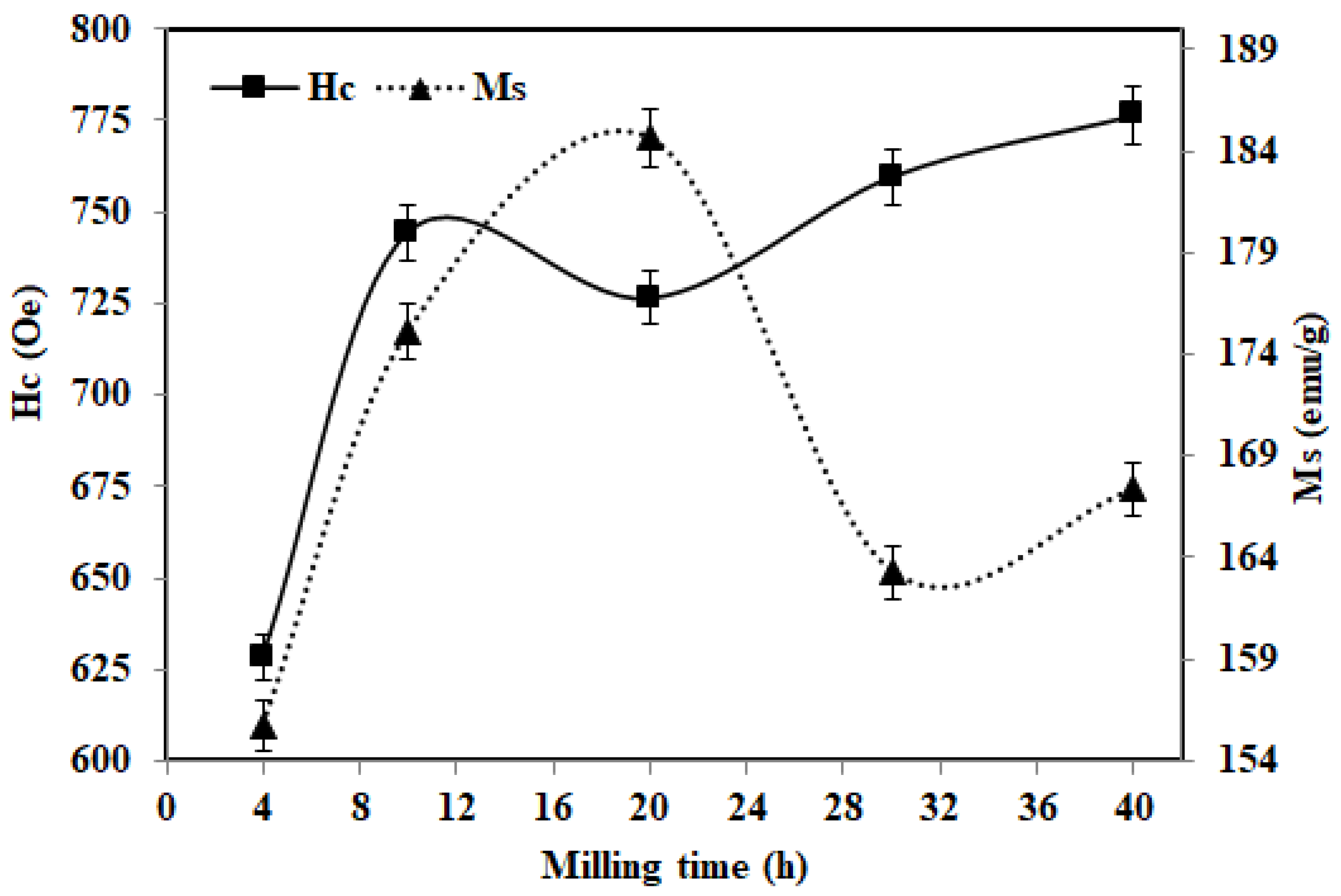
| Time (h) | Peak | T (°C) | ΔH (Jg−1) | Hc (Oe) | Ms (emu/g) | Mr (emu/g) |
|---|---|---|---|---|---|---|
| 4 | (1) | 423 | 75.91 | 628.31 | 155.29 | 24.23 |
| (2) | 700 | 15.21 | ||||
| (3) | 656 | 92.5 | ||||
| 10 | (1) | 377 | 144.5 | 744.44 | 175.3369 | 33.08 |
| (2) | 552 | 295.5 | ||||
| 20 | (1) | 250 | 155.5 | 726.40 | 184.0933 | 16.09 |
| (2) | 380 | 15.5 | ||||
| (3) | 400 | 68.5 | ||||
| (4) | 550 | 295.5 | ||||
| 30 | (1) | 195 | 16.86 | 759.66 | 163.31 | 29.27 |
| (2) | 377 | 31.8 | ||||
| (3) | 575 | 363 | ||||
| 40 | (1) | 250 | 135.6 | 776.42 | 167.37 | 36.51 |
| (2) | 400 | 75.9 | ||||
| (3) | 460 | 25.4 | ||||
| (4) | 560 | 55.4 | ||||
| (5) | 600 | 195.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibn Gharsallah, H.; Azabou, M.; Khitouni, M.; Daza, J.; Suñol, J.-J. Study of the Microstructural, Thermal, and Magnetic Properties of High-Energy Ball-Milled Nanocrystalline Fe(Al). Crystals 2022, 12, 1430. https://doi.org/10.3390/cryst12101430
Ibn Gharsallah H, Azabou M, Khitouni M, Daza J, Suñol J-J. Study of the Microstructural, Thermal, and Magnetic Properties of High-Energy Ball-Milled Nanocrystalline Fe(Al). Crystals. 2022; 12(10):1430. https://doi.org/10.3390/cryst12101430
Chicago/Turabian StyleIbn Gharsallah, Hana, Myriam Azabou, Mohamed Khitouni, Jason Daza, and Joan-Josep Suñol. 2022. "Study of the Microstructural, Thermal, and Magnetic Properties of High-Energy Ball-Milled Nanocrystalline Fe(Al)" Crystals 12, no. 10: 1430. https://doi.org/10.3390/cryst12101430
APA StyleIbn Gharsallah, H., Azabou, M., Khitouni, M., Daza, J., & Suñol, J.-J. (2022). Study of the Microstructural, Thermal, and Magnetic Properties of High-Energy Ball-Milled Nanocrystalline Fe(Al). Crystals, 12(10), 1430. https://doi.org/10.3390/cryst12101430











