Abstract
In order to study the reaction growth process of insensitive JEOL explosive after ignition under cook-off, a series of cook-off tests were carried out on JEOL explosive using a self-designed small cook-off bomb system. A thermocouple was used to measure the internal temperature of the explosive, and a camera recorded macro images of the cook-off process. The temperature change law of JEOL explosive before and after ignition under different heating rates and the smoke ejection caused by the reaction in the slit were studied. The research results showed that the ignition time decreased as the heating rate increased, while the ignition temperature was not sensitive to the heating rate. When the heating rate was faster, the internal temperature gradient of the explosive was larger, and the ignition point appeared at the highest temperature position. As the heating rate decreased, the internal temperature gradient of the explosive decreased, the ignition point appeared random, and multiple ignition points appeared at the same time. The growth process of the ignition point could be divided into severe thermal decomposition, slow combustion, and violent combustion stages. When the heating rate reduced from 7 to 1 °C/min, the burning rate obviously increased.
1. Introduction
Ammunition will go through stages of transportation, storage, maintenance, and launch during its entire life cycle and may experience unexpected stimulation at all stages, such as vibration during transportation; drop during hoisting and transportation; and impact caused by bullets or fragments, fire, heat, etc. These stimuli can cause burning, explosion, or even detonation of ammunition, resulting in loss of personnel and equipment and weakening combat effectiveness. Among the abovementioned types of stimulation, exposure to fire is one of the most important factors affecting the safety of ammunition [1,2]. When ammunition is exposed to fire, two situations are possible: the ammunition is completely immersed in flames or a fire occurs in the nearby environment and high-temperature air heats the ammunition convectively. In the first case, the heating rate of the projectile is extremely fast, and the ammunition reacts very rapidly. In the second case, the heating rate of the projectile is relatively slow. After a long period of heat accumulation, the ammunition reacts more violently than when it heats up quickly. Therefore, studying the reaction mechanism and structural response of ammunition under different heating and thermal stimuli has important guiding significance for the safety design, manufacture, and use of ammunition.
The response of explosives is completely different under the conditions of fast cook-off and slow cook-off. Under fast cook-off, only the outer wall reaches the reaction temperature when the explosive reacts, so the reaction intensity is weaker than slow cook-off [3,4,5]. Therefore, the response of explosives under slow cook-off is a key concern for researchers. HMX-based plastic-bonded explosives have been discussed by many researchers. PBX9501 (95% HMX, 5% binder system) is a high-density pressed explosive [6,7]. Under cook-off, this type of explosive generally does not experience violent reactions above combustion. However, if the explosive is under strong restraint conditions, this kind of explosive may experience deflagration-to-detonation transition (DDT) [8,9,10,11,12,13,14,15,16]. Similarly, other HMX-based plastic-bonded explosives, such as LX-14 (95.5 wt% HMX, 4.5 wt% Estane 5702), LX-10 (94.5 wt% HMX, 5.5 wt% Viton A), and PBX9012 (90 wt% HMX, 10 wt% Viton A), are likely to go through DDT [17,18]. Because HMX will undergo a phase change from β to δ at 160–180 °C, the internal temperature of the PBX explosive will have a plateau during the firing cook-off [19,20,21]. With increase in insensitive components (such as TATB) in the PBX, the ignition temperature of the explosive rises, and the explosive reaction severity decreases. When reaching a certain temperature, the melt cast explosive will melt, so the response of this type of explosive under slow cook-off is also different from that of pressed explosives. Comp-B (63 wt% RDX, 36 wt% TNT, 1 wt% wax) is a widely used melt cast explosive [22,23]. Under cook-off, as the temperature rises, the inside of Comp-B begins to melt, which will affect the temperature distribution inside the explosive, and the internal temperature of the internal explosive measured by the thermocouple will also have a plateau. Due to the sedimentation of the solid, the ignition position of the melt-cast explosive will also appear in the lower part of the explosive. The thermal safety of solid propellants has also been extensively studied [24,25,26,27,28,29,30]. With different energetic subjects, the propellant will have completely different responses at the same heating rate [31]. When the heating rate is 1 K/min, the ignition time of insensitive propellant (AP/HTPE/Al (70/12/18)) is 87% longer than that of traditional propellant (AP/HTPB/Al (70/12/18)).
Regarding the standard cook-off bomb structure with a cylindrical charge and a shell, researcher have previously obtained data including the internal temperature distribution of the explosive, the ignition time, the ignition temperature, the shell flying speed, and the degree of violent reaction through experiments [10,11,12,13,14,15,16,17,18,19]. However, there is little information on the location of the ignition point and subsequent growth. The ignition position characterizes the combustion reaction spreading from there to the surrounding area, and it grows into a more violent reaction as the internal pressure of the ammunition increases. Therefore, the ignition position and the growth of the ignition point determine the final degree of the combustion reaction to a large extent. Determining the location of the ignition point and the growth mechanism and process of the ignition point is of great help to understand the combustion process of explosives after ignition under cook off and can improve the thermal safety of explosives and the viability of ammunition. It is of great significance to study the basic properties of energetic materials and the safe production of ammunition.
JEOL explosive (the composition and mechanical sensitivity as well as detonation parameters are listed in Table 1) is a new type of high-energy insensitive explosive. Although active metal aluminum is added to the explosive, the mechanical sensitivity of the explosive is at a very low level due to the stability of NTO. It has the advantages of high density, detonation heat, and detonation velocity as well as good thermal stability. Its main component, 3-nitro-1,2,4-triazol-5-one (NTO), is a triazolone type of nitrogen heterocyclic compound. Its theoretical density is as high as 1.93 g/cm3, and its detonation performance is close to that of RDX. It has been used in the formulation of insensitive ammunition of multinational armies. Research on the thermal safety of JEOL explosive under cook-off conditions has important guiding significance for the design of insensitive ammunition. However, the ignition characteristics of JEOL explosive in cook-off are rarely reported, and the reaction growth process and mechanism after ignition are still unclear. Therefore, in this study, a small cook-off bomb was used to conduct an experimental study on JEOL explosive. According to the temperature jump time, jump amplitude, and temperature change law at different positions, the ignition position of JEOL explosive under cook off, the rule of ignition point position, ignition characteristics, and subsequent growth process were obtained.

Table 1.
Composition/mechanical sensitivity/detonation parameters of JEOL.
2. Materials and Methods
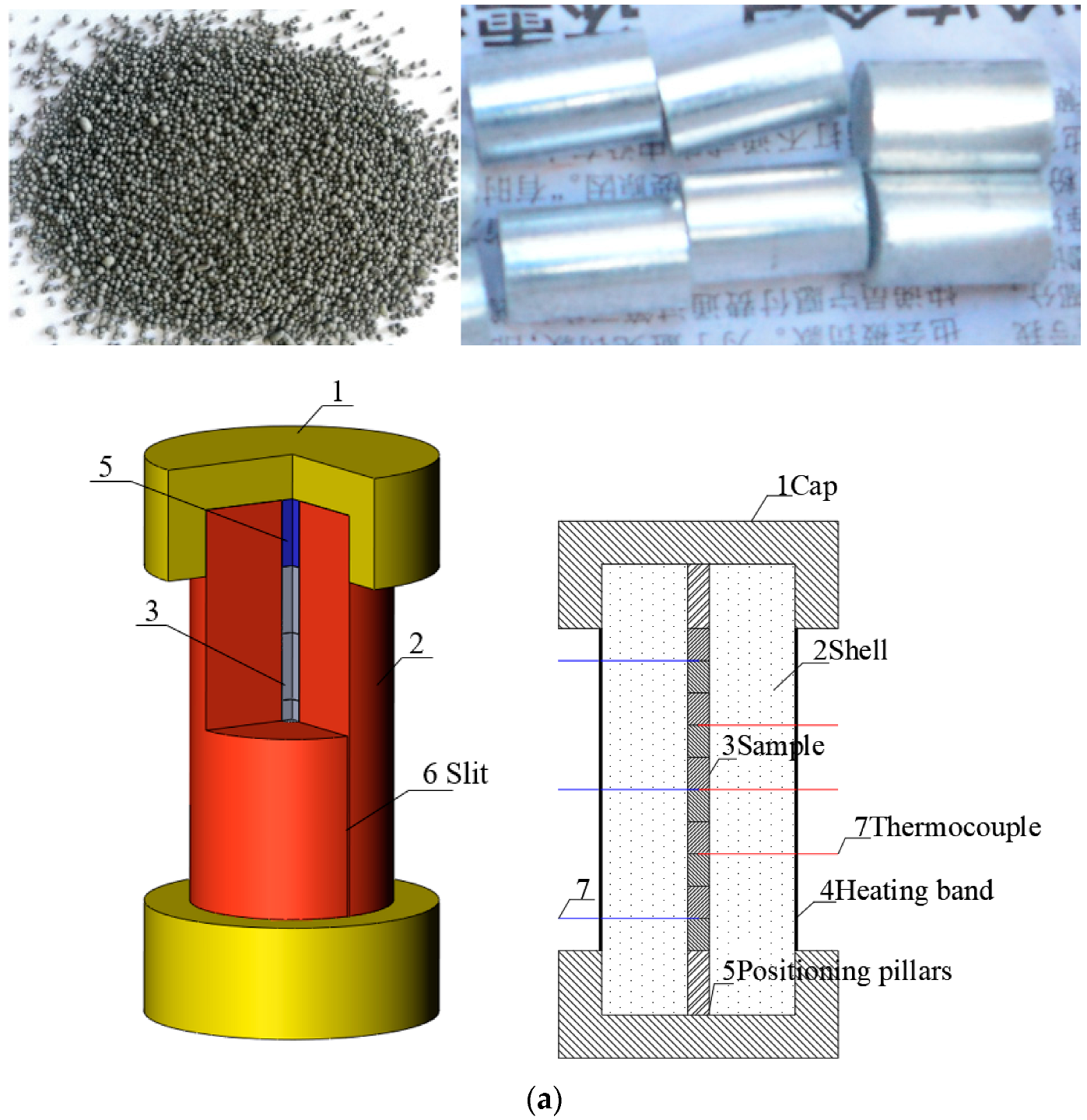
In this paper, JEOL explosive was prepared by the solution water suspension process. The specific process was as follows. A certain amount of NTO, HMX, and Al was weighed according to the formula ratio and placed into a glass beaker. Saturated absolute ethanol solution of NTO was added, a few drops of dispersant were dipped into the beaker with a stirring bar, and a suspension was obtained. The suspension was fully stirred for even dispersion. The beaker was placed in the thermostatic water bath, and heating was commenced. The temperature was raised to 50 °C, and the binder solution was added slowly at a certain stirring speed. After the addition, the temperature was kept constant for 20 min. The temperature of the incubator was adjusted to 60 °C to disperse the solvent. After the solvent was dispersed, the suspension was cooled to 2 °C and filtered, washed, and dried to obtain the explosive molding powder. The obtained molding powder of JEOL explosive is shown in Figure 1. The molding powder was press packed, and the grain is shown in the figure.
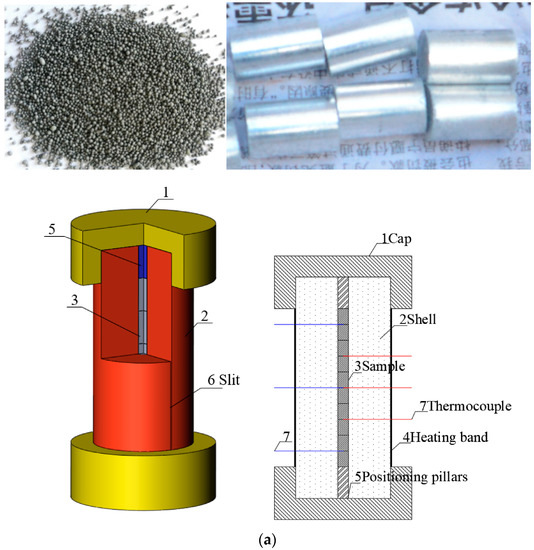
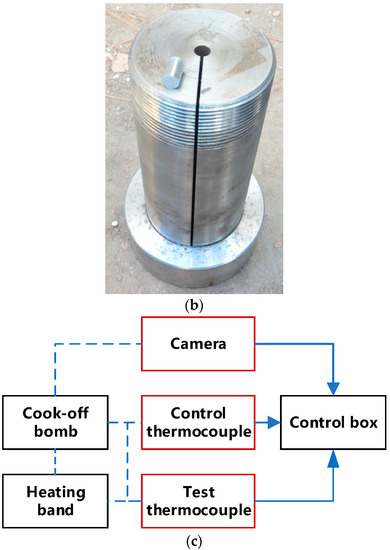
Figure 1.
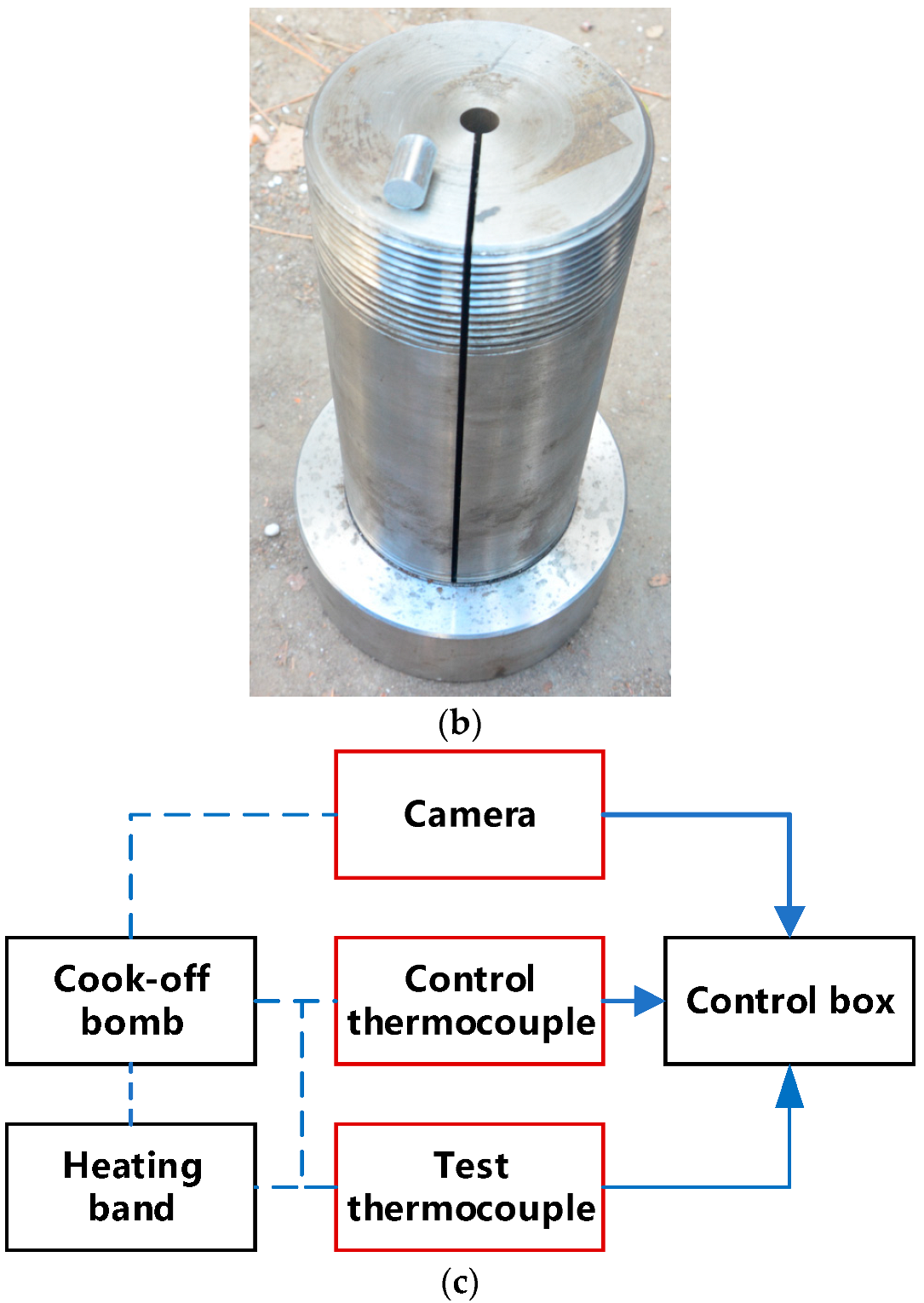
Cook-off experimental system. (a) Cross-section of cook-off bomb. (b) Sample and the shell of cook-off bomb. (c) Test flow chart.
There are two phenomena of sublimation and thermal decomposition of NTO, which is the main component of JEOL, in the process of heating up. Sublimation occurs first at low pressure and low temperature. At this time, the activation energy of explosives is small. Research has shown that the thermal decomposition of NTO occurs in the range of 200–260 °C. At this time, the explosive has a high activation energy under the combined action of sublimation and thermal decomposition, so NTO has high thermal safety. According to the adiabatic decomposition process of JEOL explosive, the explosive also has two exothermic stages, which indicates that its thermal safety is affected by the NTO composition.
The small cook-off bomb used in the present study is presented in Figure 1. The length of the shell was 210 mm, the inner diameter was 10 mm, and the outer diameter was 90 mm. In order to obtain temperature data after igniting the explosive, there were 2 mm slits in the axial direction to ensure that the cook-off bomb does not have a more violent reaction than combustion, which would destroy the thermocouple. The explosive charge was 10 mm in diameter and 15 mm in length. The sample used in each test consisted of 10 such charges stacked on top of each other for the total charge length of 150 mm. The steel positioning pillars with length of 30 mm were placed on the upper and lower sides so that the whole explosive was placed in the middle of the slit. When the explosive starts to react, the slit ensures that the gas produced in the cook-off bomb can be discharged in time and the internal pressure of the device will not be too high. When the cook-off bomb is in a completely airtight and strongly constrained state, the explosive is very likely to experience DDT, and it is impossible to obtain temperature data after the explosive reaction. The outer wall of the shell was wrapped with a heating band, and the asbestos was wrapped around the cook-off bomb for heat preservation. A thermocouple was placed between the samples, and the temperature of the explosive was recorded over time. The sampling frequency was 5 Hz. In order to eliminate the influence of the position of the thermocouple on the reaction, two thermocouple arrangements were used. The first was placing a thermocouple in the center of the charge and placing the other two thermocouples at a distance of 45 mm from the top and bottom of the charge, as shown by the red horizontal line in Figure 1a. The second was placing a thermocouple in the center of the charge and placing the other two thermocouples 15 mm away from the top and bottom of the charge, as shown by the blue horizontal line in Figure 1a. When the cook-off bomb was placed vertically, the thermocouple near the base was the lower thermocouple, and the middle and upper thermocouples were from the bottom to the top. A thermocouple was also placed between the heating band and the shell to record the temperature change of the shell. When the explosive reacted, the ignition sequence was judged according to the sudden jump in temperature, and the image was recorded by a camera. The effect of different heating rates on ignition position and ignition point growth was studied, where shot 1 was rapid heating and shots 2 and 3 were slow heating. The specific shots are shown in Table 2.

Table 2.
Test conditions.
3. Results and Discussion
3.1. Experimental Phenomenon
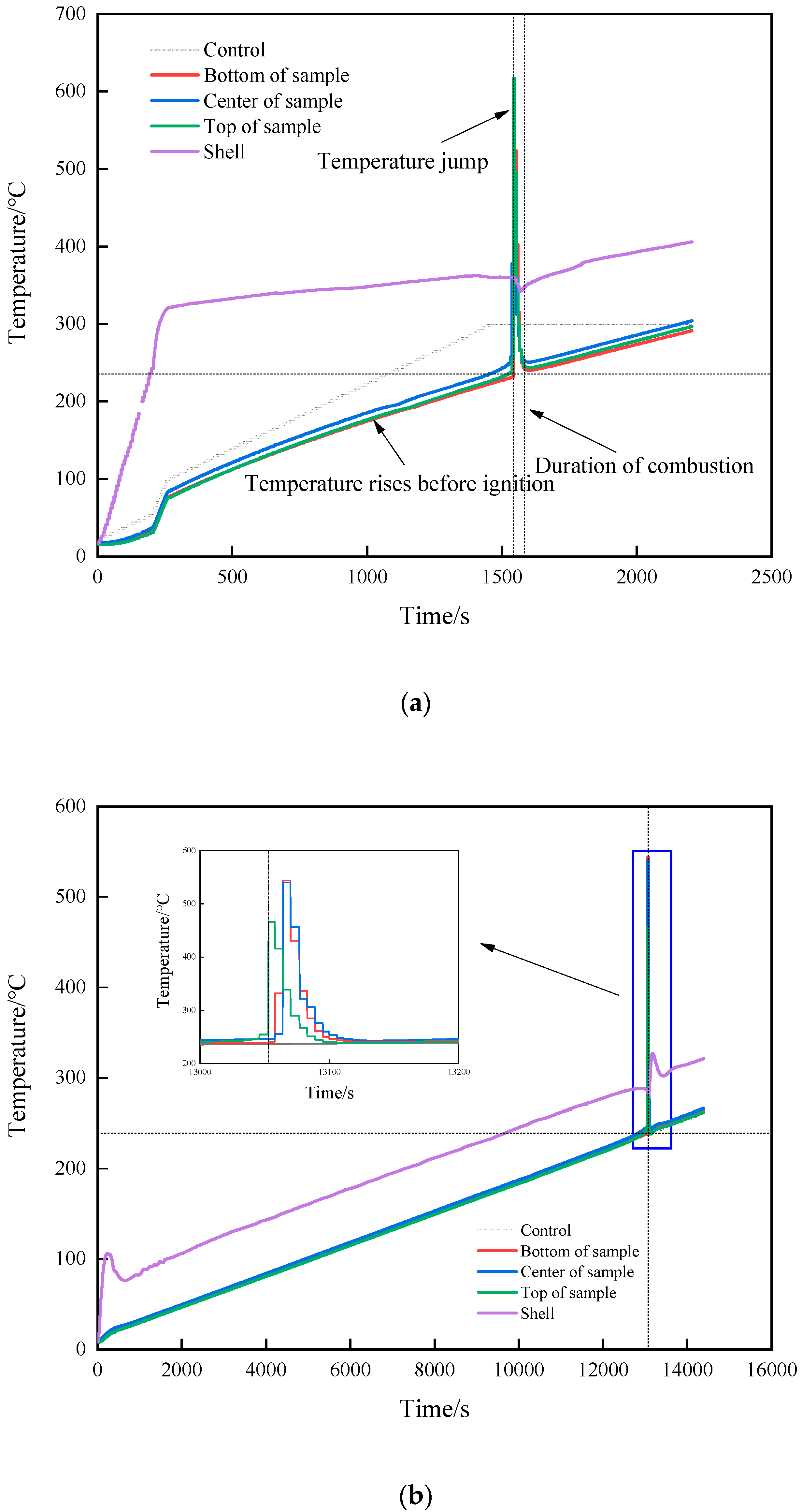
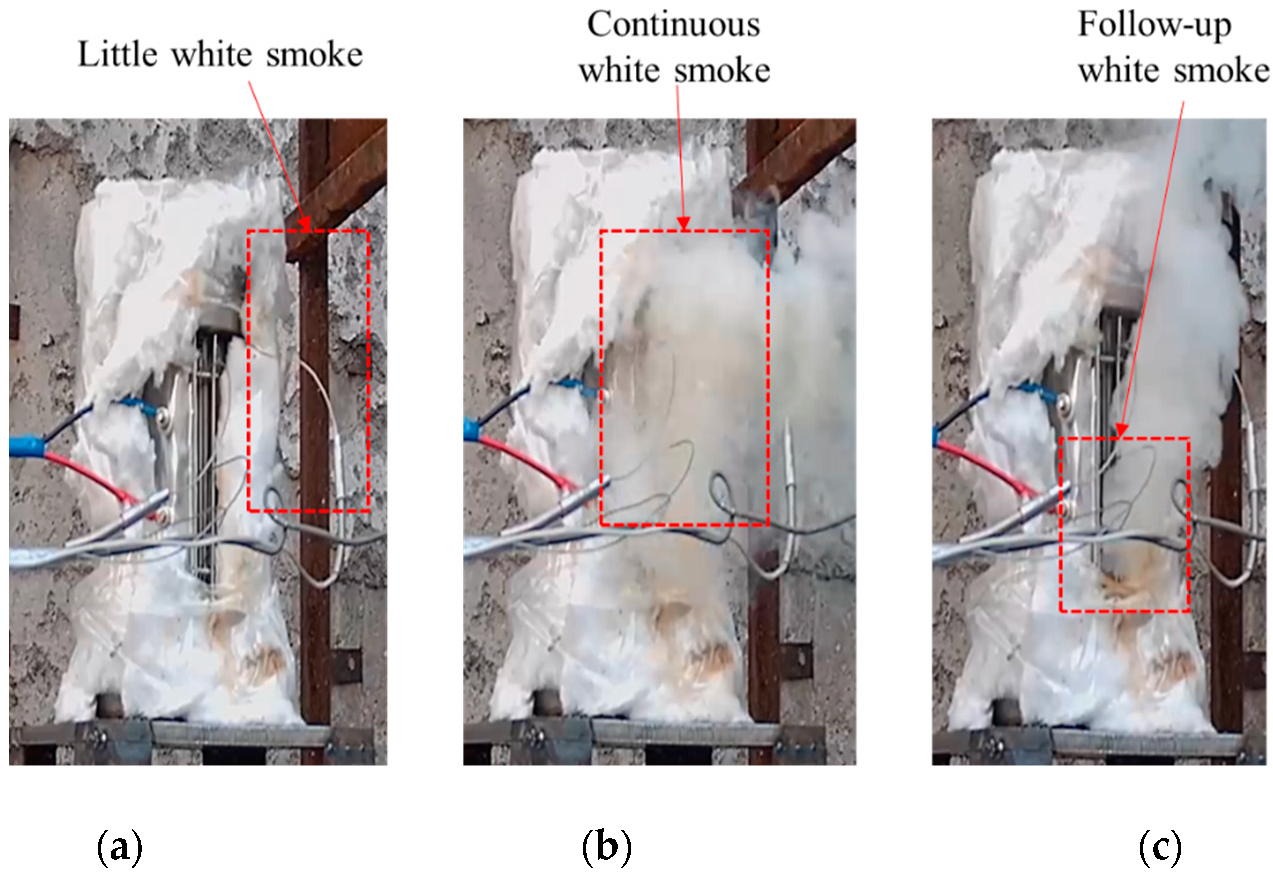
After heating for a certain period of time, strong white smoke was sprayed from the slit in all three shots, as shown in Figure 2. There was no explosion sound, indicating that the explosive did not react violently. After the reaction, the cap and the shell remained intact, as shown in Figure 3. It was judged that the reaction degree of the explosives was combustion of all three shots. The temperature–time curves of the three shots obtained in the experiment are shown in Figure 4. It can be seen from Figure 4 that, except for the warm-up stage and temperature jumps, the overall temperature curve showed a linear upward trend. When there was a sudden jump in temperature as measured by the thermocouple, it was considered that the explosive had ignited there. The test completely recorded the temperature data of the combustion stage after ignition.

Figure 2.
Jetting smoke.

Figure 3.
Cook-off bomb after tests.
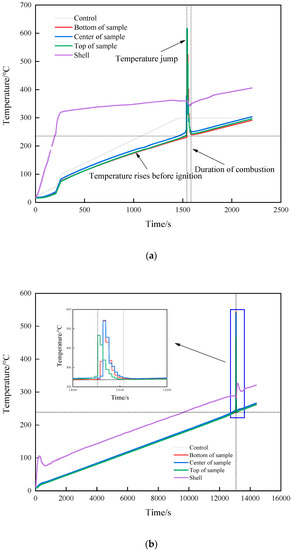
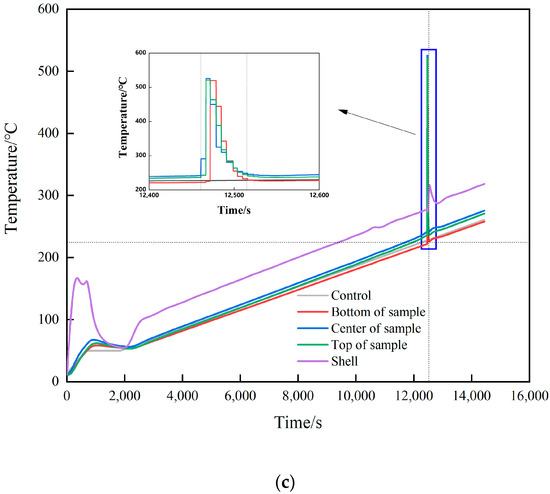
Figure 4.
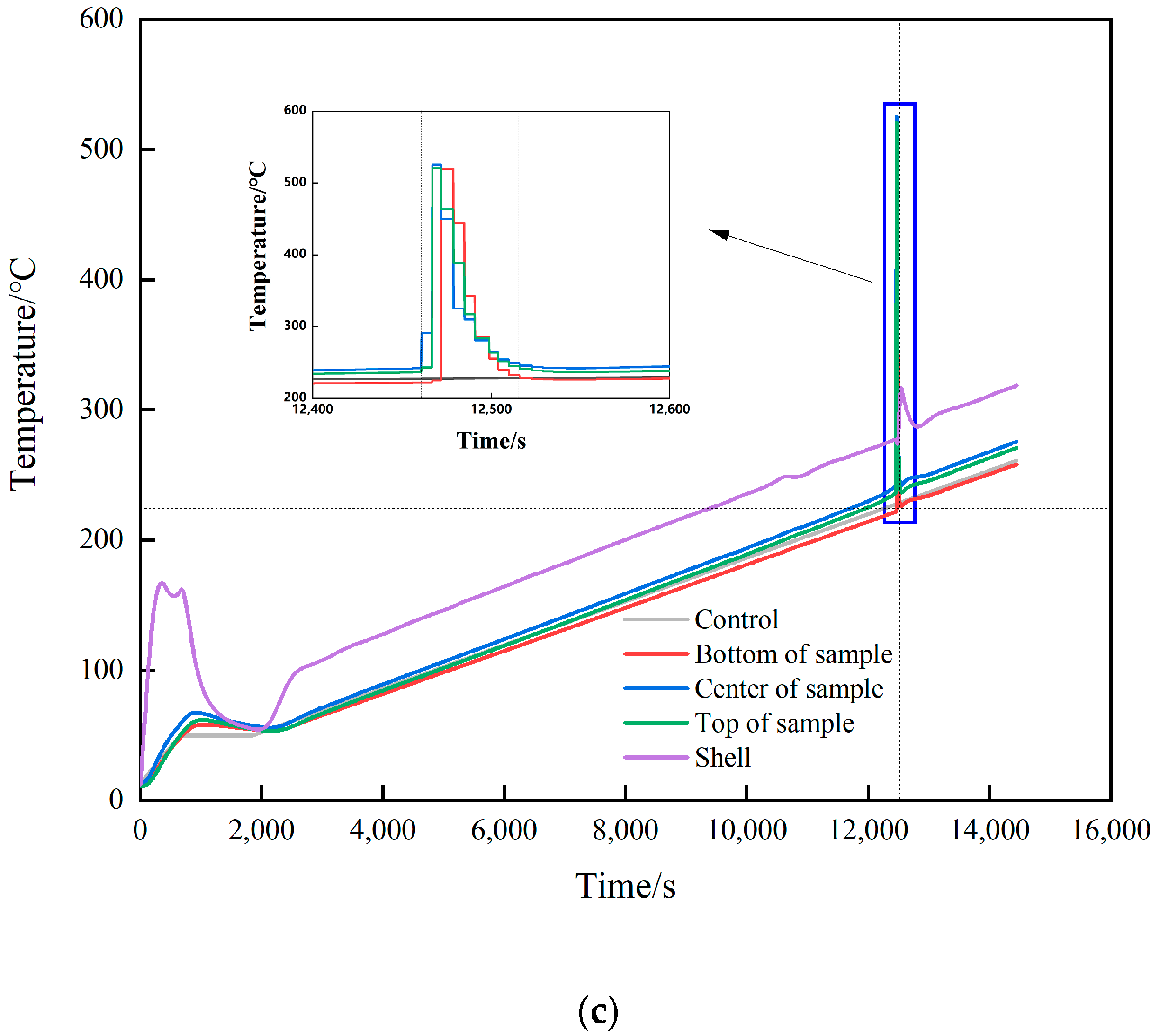
Temperature–time curve. (a) 7 °C/min. (b) 1 °C/min (no temperature preservation). (c) 1 °C/min (temperature preservation for 20 min).
3.2. Analysis of Ignition Time and Ignition Temperature
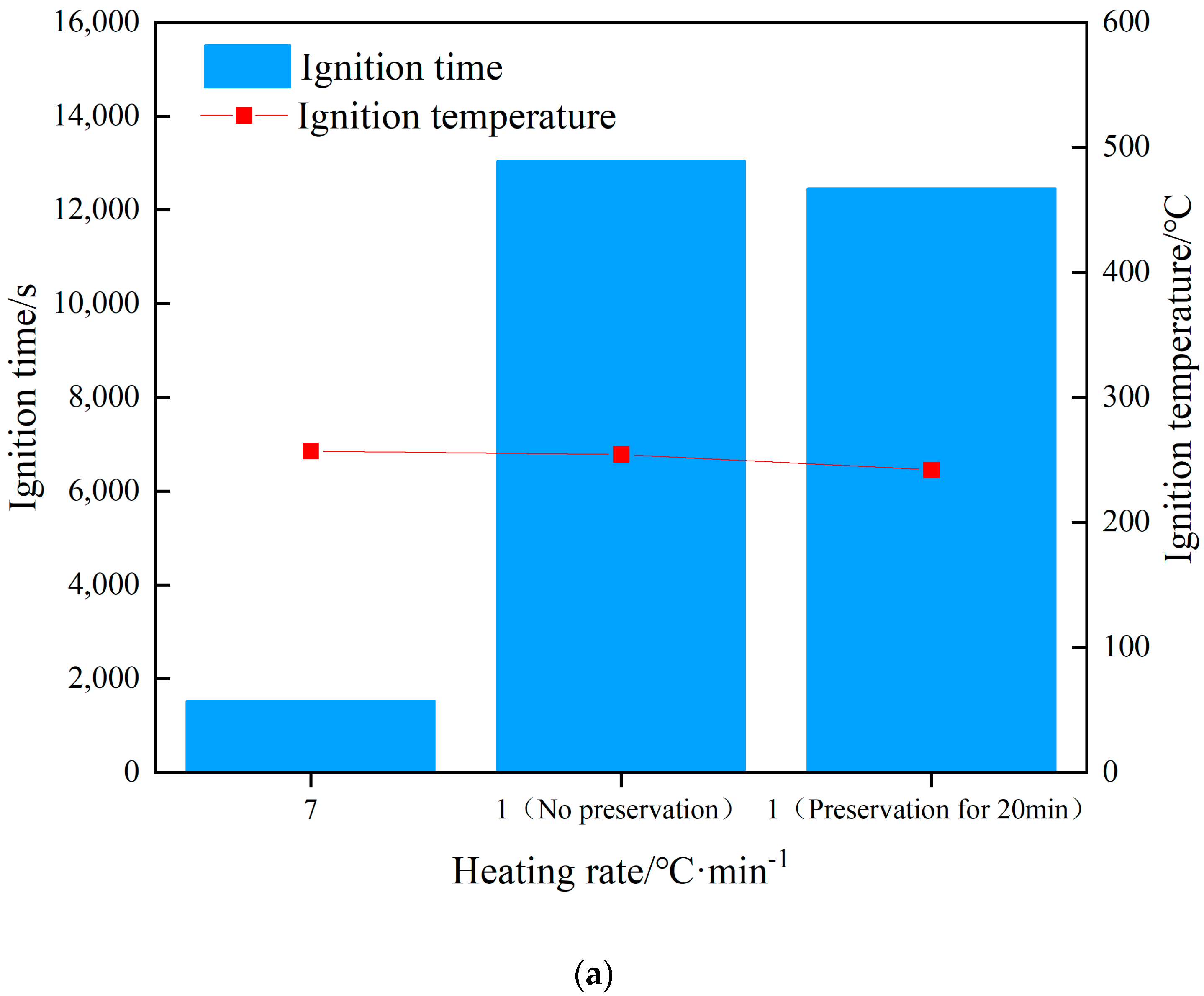
The changes in internal temperature of the explosive with time is shown in Figure 4. The start of heating was recorded as zero time, and the ignition time was recorded when there was a significant jump in temperature as measured by the thermocouple. The rising trend of the temperature curve under the three shots was similar, but with the change in the heating rate, the ignition time was obviously different. Shot 1 heated up rapidly at a rate of 7 °C/min, and the ignition time was 1531 s. The ignition time was 13,046 s when the heating rate was 1 °C/min, and the ignition time was 12,461 s when the heat was preserved for 20 min. The difference between the latter two shots was 585 s, which only accounted for 5% of the entire ignition time. The ignition time of the two could be considered as similar. The increase in the heating rate meant the heat transferred from the outside to the inside of the explosive per unit time increased, and the heating rate of the explosive itself increased accordingly. The self-heating reaction of the explosive followed the Arrhenius law, which led to an increase in the thermal decomposition rate of the explosive. With the combined action of heat and external heat, a local hot spot was formed inside the explosive and ignition occurred. Compared to the experiment of Hobbs et al., the experimental device used in this study was in an open state. Because the thermal decomposition reaction of explosives is related to environmental pressure, the higher the environmental pressure, the higher the thermal decomposition rate of explosives. Therefore, the ignition time of the explosive obtained in this study was relatively increased compared to the cook-off test under closed conditions.
When ignition occurred in shot 1, the ignition temperature was 249.4 °C. As the heating rate decreased, the ignition temperatures of shots 2 and 3 were 245.9 and 236.5 °C, respectively. The ignition temperature of the three shots witnessed little change, the heating rate had no obvious influence on the ignition temperature, and the ignition temperature change caused by the heating rate change was almost negligible. The ignition temperature under heat preservation condition was 9.4 °C lower than that without heat preservation, which was 3.9% of the total temperature rise of the explosive before ignition. The ignition temperature of shots 2 and 3 could be considered as similar. Analysis shows that because the diameter of the explosive used in this study was small, under the condition of low heating rate, the internal temperature of the explosive will be uniform without the insulation stage. Therefore, the temperature field inside the explosive in shots 2 and 3 is the same from the macro perspective. The ignition temperature of a certain energetic material at different heating rates can be roughly estimated based on the ignition temperature at a known heating rate. Analysis shows that whether the explosive ignites or not is determined by the balance between the heat generated by the explosive itself and the heat lost to the outside world. Under the test conditions in this study, the explosive structure was the same, the external environment temperature was the same, and the heat loss could be considered to be the same. Therefore, the ignition of explosives was determined by the heat production. The heat production of explosives is determined by the Arrhenius’ law. In this study, the physicochemical parameters of JEOL explosive did not change significantly with the increase in temperature, so whether the explosives ignited could be determined by the temperature of the explosives. Therefore, the ignition temperature of JEOL explosive was the same at different heating rates. The relationship between ignition time, ignition temperature, and heating rate is shown in Figure 5a.
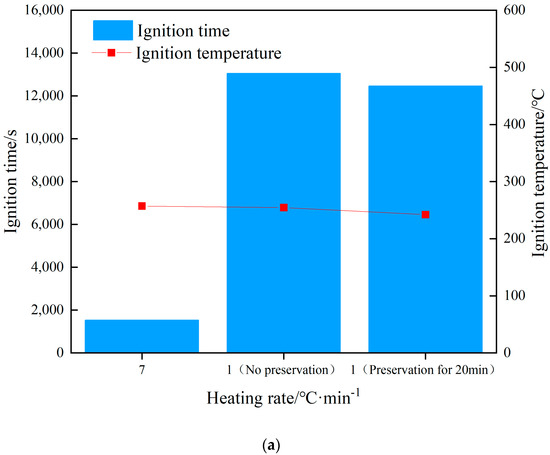
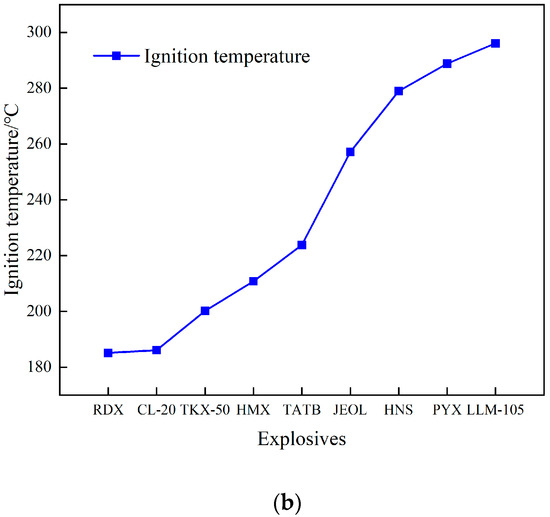
Figure 5.
Ignition time/ignition temperature–heating rate curve. (a) Ignition time/ignition temperature–heating rate curve. (b) Ignition temperature of different explosives.
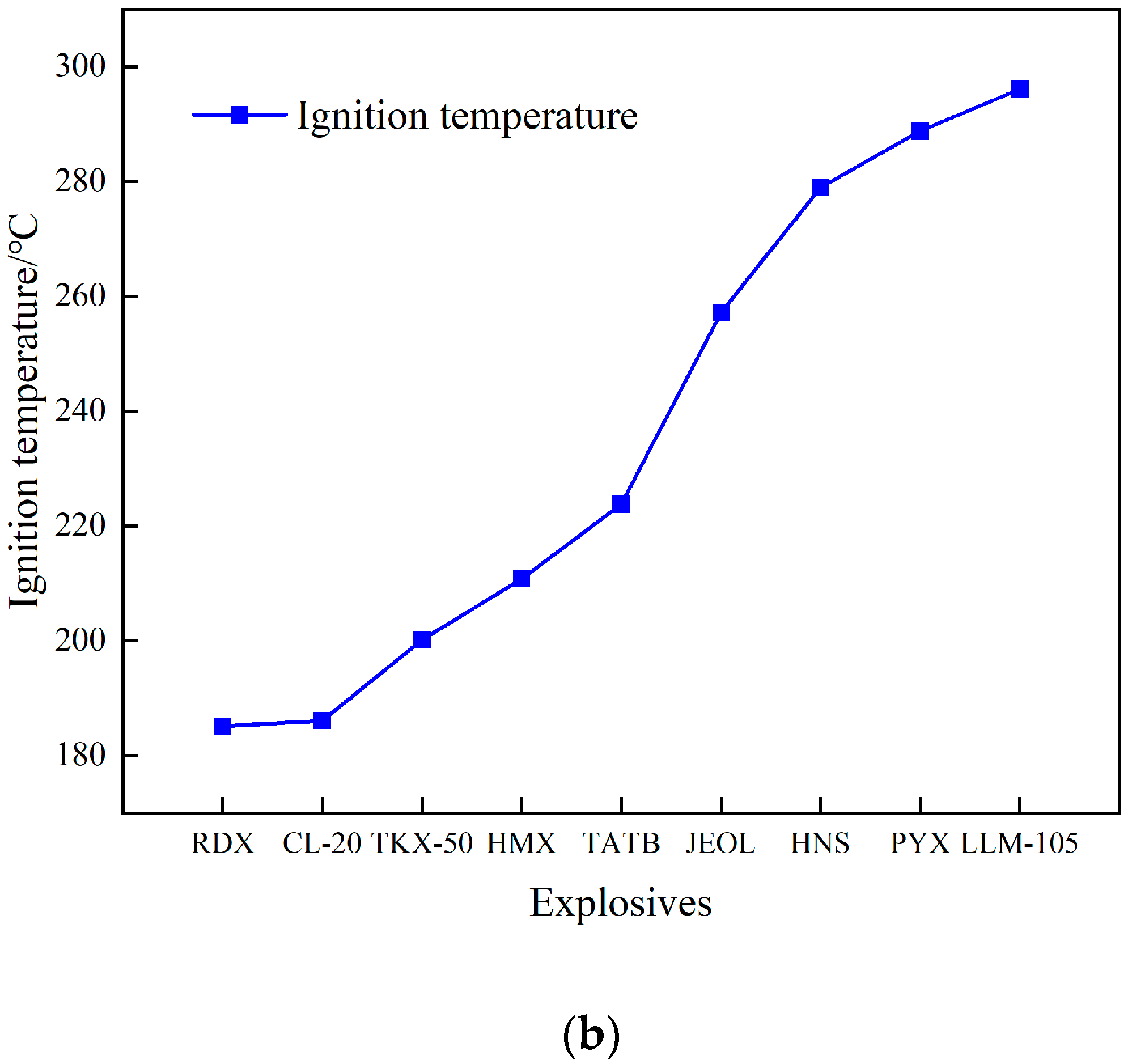
Figure 5b shows the comparison between the ignition temperatures of JEOL and eight other commonly used explosives [12,13,15,18,19]. It can be seen from the figure that the ignition temperature of JEOL is lower than that of heat-resistant explosives HNS, PYX, and LLM-105 but higher than that of high-energy explosives, such as RDX, CL-20, and HMX, indicating that JEOL has high thermal safety.
3.3. Analysis of Ignition Growth and Combustion Process
During the cook-off process, when the explosive did not ignite, the temperature measured by the thermocouple increased linearly. After ignition, the temperature at the ignition point suddenly jumped. Through the temperature data measured by the thermocouple, the ignition point position, the temperature rise of the ignition point, and the combustion duration was obtained. The macro image of the cook-off bomb taken by the camera was used to analyze the state of the explosive in the combustion stage.
3.3.1. Ignition Point Position
According to the temperature jump, the specific location of the ignition point was obtained. The temperature rise at the ignition point is shown in Table 3. The distance between the thermocouples in shot 1 was 30 mm; the center temperature measured before ignition was 249.4 °C; the upper and lower thermocouple temperatures were 230.2 and 236.4 °C, respectively; and the temperature gradients were 19.2 and 13 °C, respectively. The temperature of the center was higher than the two ends because the temperature of the external environment in the test was only 10 °C, and the cook-off bomb was placed on a metal base. Although the thermal insulation asbestos was wrapped, the upper end cover, lower end cover, and metal shelf of the cook-off bomb had the effect of increasing the heat dissipation area, causing the temperature at both ends to be lower than the center. At the next acquisition time, 0.2 s later, the temperature measured by the middle thermocouple was 257.2 °C; the upper and lower thermocouple temperatures were 230.9 and 237.6 °C, respectively; and the temperature increments were 7.8, 0.7, and 0.2 °C, respectively. The increase in the middle was much larger than the changes in the upper and lower positions and the temperature increase preset by the program, so it was judged that the initial ignition position was at the center of the charge axis.

Table 3.
Response characteristics of cook-off.
As the heating rate decreased, the temperature distribution inside the explosive tended to be uniform. For shot 2, the temperatures measured from the top to bottom thermocouples before ignition were 245.9, 245.9, and 238.6 °C. As can be seen, the upper part and the middle part of the explosive had the same temperature. However, due to the heat dissipation effect of the lower end cover and the base, the temperature of the lower part was significantly lower than that of the middle and upper part. The maximum temperature difference between the thermocouples was only 7.3 °C, which was much smaller than the shot of 7 K/min. This conformed to the law that the slower the heating rate, the more uniform the temperature distribution inside the explosive. After ignition, the upper temperature increment was 8.7 °C. According to the temperature change, it was judged that the initial ignition position was in the upper part.
In shot 3, the position of the thermocouples was changed, and the distance between the thermocouples was 45 mm. The temperatures before ignition measured from top to bottom were 236.5, 242.2, and 221.8 °C, respectively. As can be seen, even though the temperature was kept for 20 min, due to the heat dissipation effect of the upper and lower end caps, the farther away from the center of the explosive, the lower was the temperature. At this time, the temperature increments measured at the upper, middle, and lower positions were 6.7, 49, and 0.2 °C, respectively. Both the upper part and the center had a large temperature rise exceeding the preset heating rate, and it was judged that the upper part and the center had ignition points at the same time. Combining the cook-off test of the three shots, it was judged that the initial position of ignition occurred at the position with the highest temperature inside the explosive. When there is a significant temperature gradient, the ignition point only appears in a single position, and there is no situation where multiple ignition points appear at the same time. Analysis shows that when the temperature gradient in the explosive tends to be the same, the whole explosive will approach the critical state with the increase in temperature. The structure of the press-packed explosive itself is uneven, the mixture of the explosive and the additive is not uniform when making the molding powder, and the air gap may be formed between particles during press-packed molding. At the same time, the state of the explosive will also change during the cook-off process, and the explosive volume will expand. At 180 °C, the HMX grain will change phase, and a large number of microcracks will be generated in JEOL explosive. Debonding between particles will also occur. Due to the heterogeneity of the explosive structure in the micro and macro aspects, the conduction of heat in the explosive will be uneven, and the accumulation of heat will also be uneven. Therefore, the occurrence of the ignition position will be random according to the explosive structure. When the temperature gradient is small and tends to be uniform, the position of the ignition point has a certain randomness and multiple initial ignition points may appear at the same time.
3.3.2. Ignition Point Growth Process
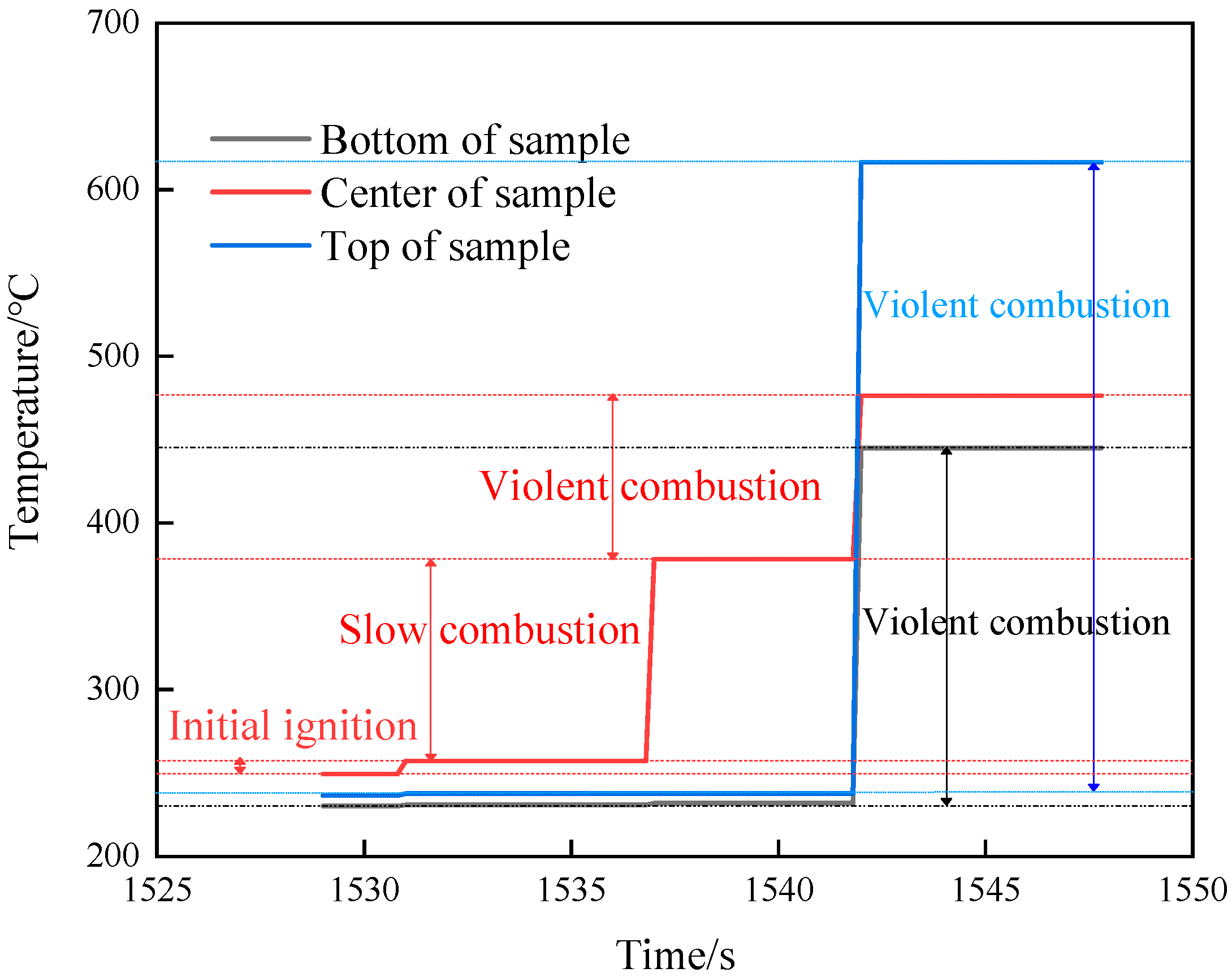
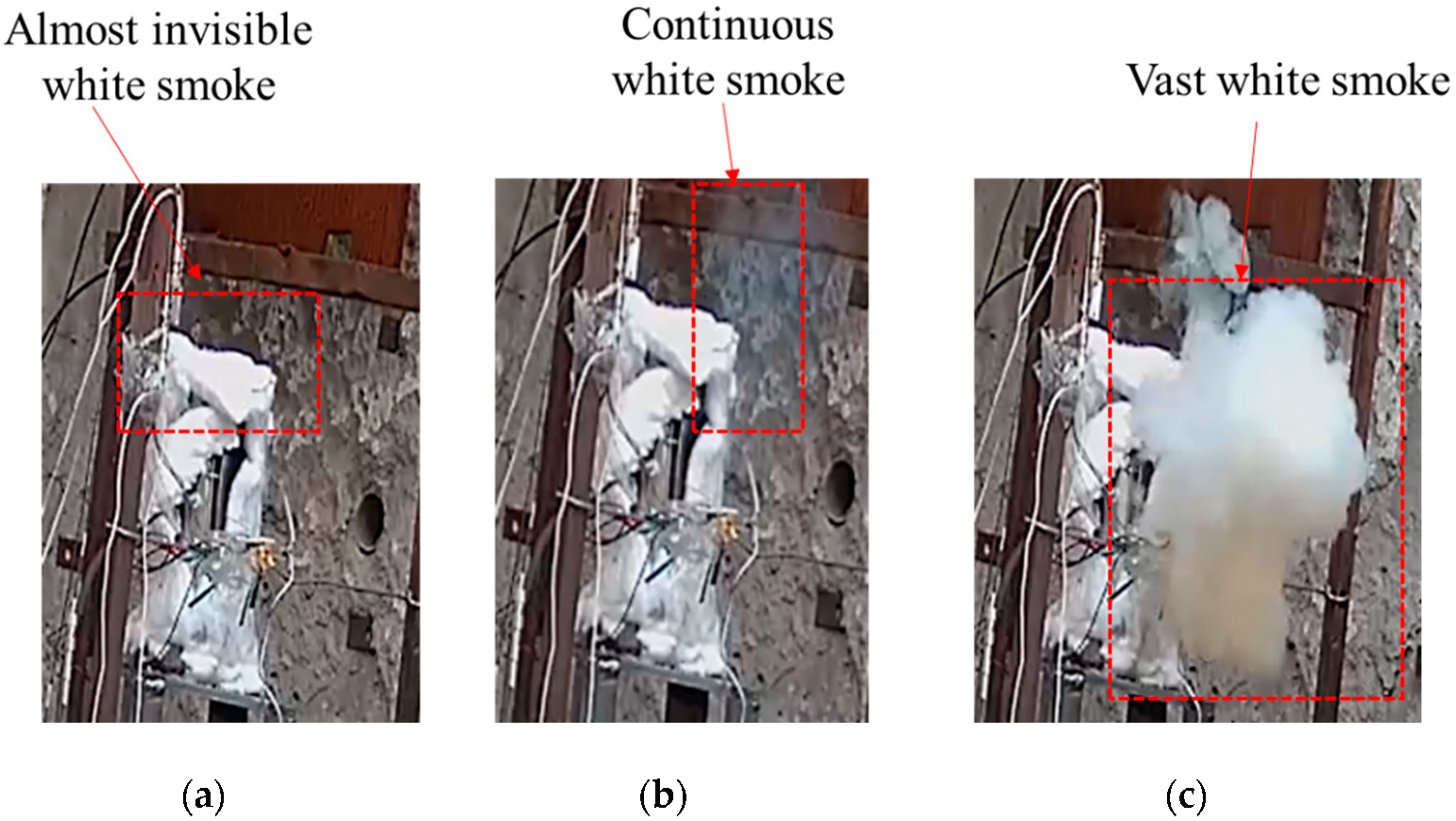
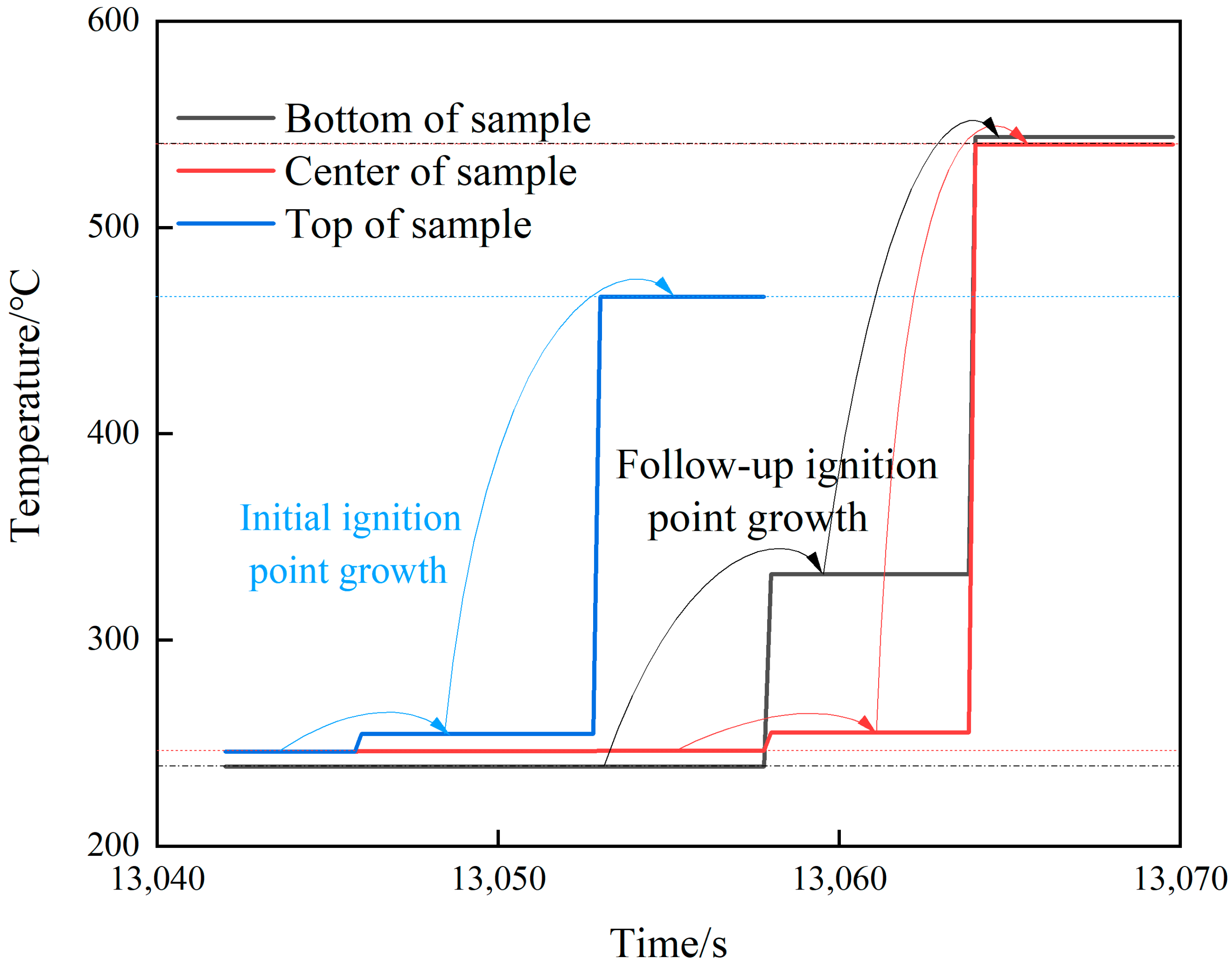
The temperature changes of various parts of shot 1 after ignition are shown in Figure 6. The central thermocouple held for 6 s after the temperature jumped to 257.2 °C. Then, the temperature suddenly changed to 378.2 °C, and the change was as high as 121 °C. The temperature lasted for 5.8 s, and the temperatures at the upper, middle, and lower locations suddenly changed to 616.4, 476.4, and 445 °C at the same time. Combined with the camera recording (see Figure 7), after the initial ignition point appeared, a very small amount of intermittent white smoke was injected into the slit, which was almost undetectable. When the temperature of the ignition point changed again, a small amount of continuous white smoke was emitted from the slit. When the temperature of the thermocouple underwent a sudden jump at the same time, a large amount of continuous white smoke was ejected from the slit. Combining temperature and image data, it was judged that the initial ignition point had a small sudden temperature jump, which was the violent thermal decomposition process of the explosive. After that, the ignition point continued to grow, and the secondary temperature had a large sudden jump, which was the slow combustion process of the explosive. The third temperature jump was a further increase in the flame. Analysis shows that the main reason for this phenomenon is that NTO, the main component of JEOL explosive, has two exothermic stages under adiabatic conditions. Among them, the first, exothermic stage occurs at 194.9 °C and the second, exothermic stage occurs at 220 °C. The heat release and heat release rate in the first stage are very small, and the second stage is the main stage of NTO heat release. The thermal decomposition temperature of HMX, another component of JEOL explosive, is 176 °C, indicating that adding NTO to HMX will greatly improve the thermal safety of HMX itself. We divided the ignition process of explosives into three stages: rapid thermal decomposition, slow combustion, and violent combustion.
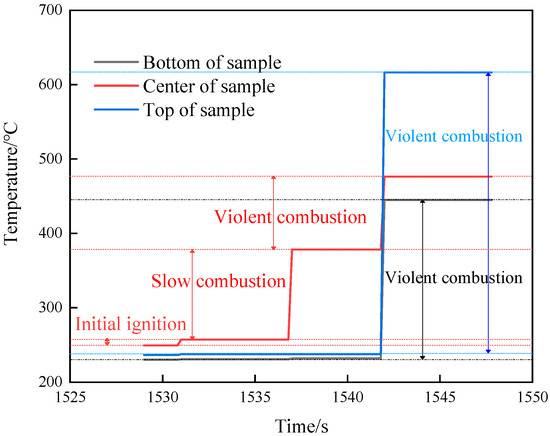
Figure 6.
Temperature–time curve of shot 1.
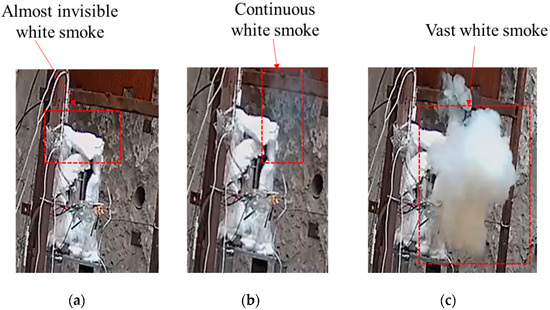
Figure 7.
Response of cook-off bomb of shot 1. (a) Initial ignition. (b) Secondary jump. (c) Third jump.
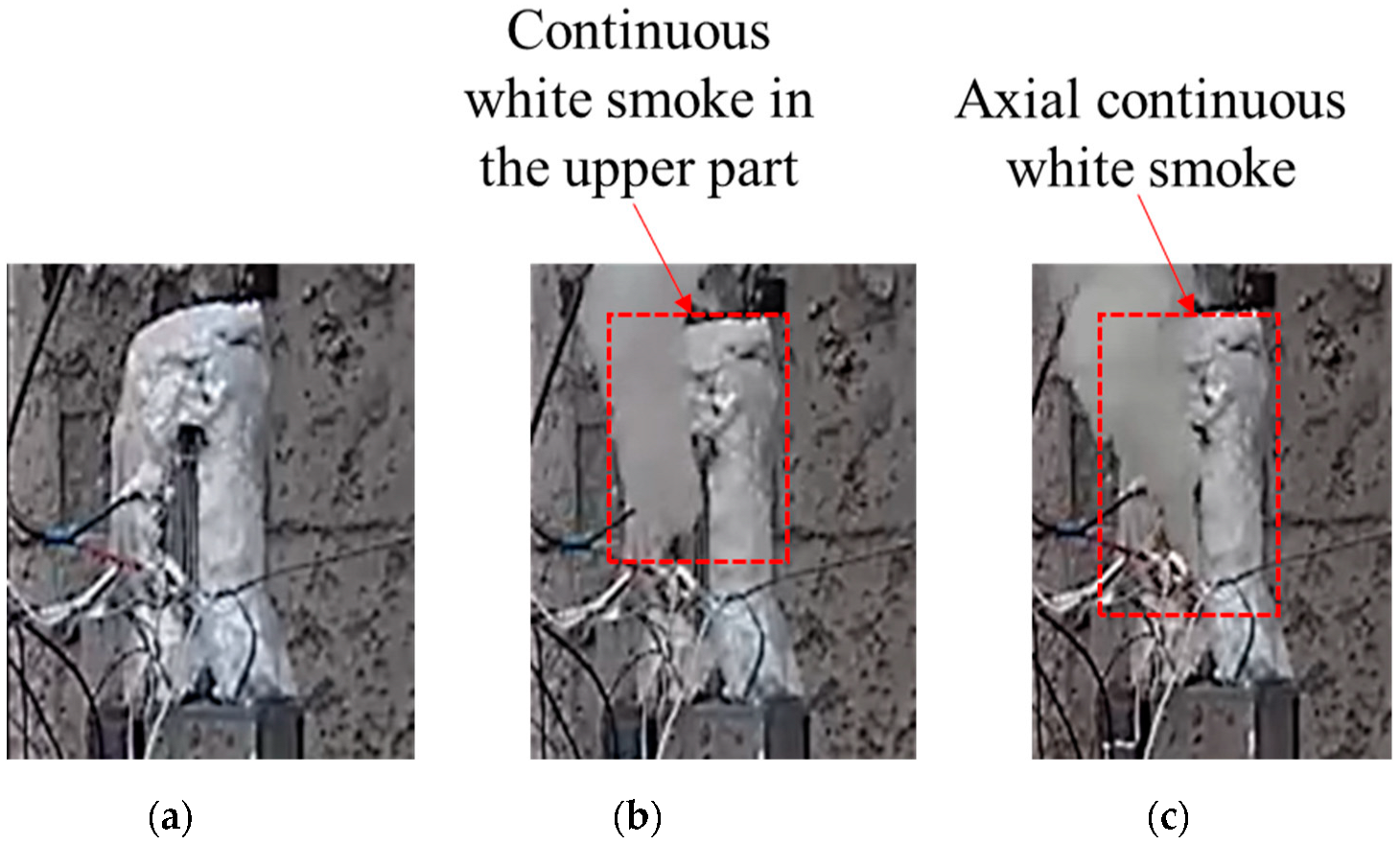
The temperature changes of all parts of shot 2 after ignition are shown in Figure 8. The initial ignition point appeared in the upper part and lasted for 7 s, and then the temperature jumped from 211.7 to 466.3 °C, which lasted for 5s. After that, the temperature in the center and the lower part jumped suddenly, and the temperature increments were 8.8 and 91 °C, respectively. This temperature distribution lasted for 6.2 s, and the central and lower temperatures rose to 540.2 and 543.9 °C, respectively. Combined with the camera image (see Figure 9), after the initial ignition point appeared, the camera did not capture white smoke. When the upper temperature jumped for the second time, it was obvious that white smoke was ejected from the upper half of the slit, and there was no white smoke generated in the lower half. When the temperature of the middle and lower parts suddenly jumped, a large amount of white smoke was sprayed from the middle and lower parts of the slit. Combining the temperature change of this shot, it was found that the internal temperature of the explosive tended to be uniform under the condition of low heating rate, but due to the appearance of the initial ignition point, the explosive entered the first exothermic stage. Even with a temperature rise of only 8.7 °C, the heat released by the explosive was enough to destroy the heat balance in the explosive system. The appearance of the initial ignition point resulted in a temperature rise of more than 200 °C afterwards. However, the area with the same temperature as before did not have the effect of the initial ignition point. Here, the explosive did not enter the first exothermic stage, and the linear heating rate was maintained at the preset rate until the ignition point appeared again in the middle and lower parts.
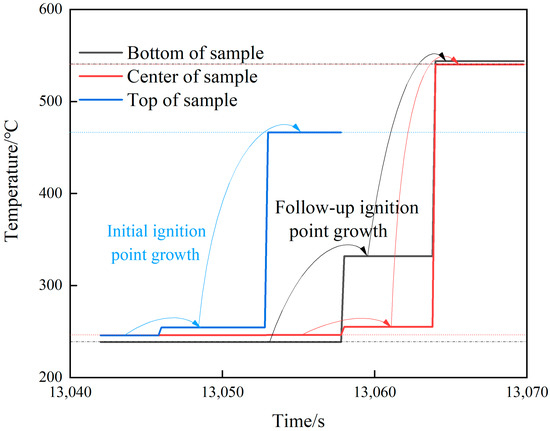
Figure 8.
Temperature–time curve of shot 2.
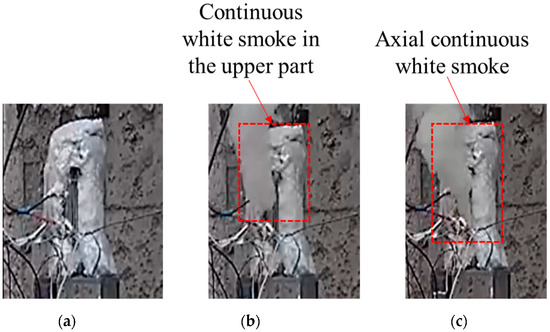
Figure 9.
Response of cook-off bomb of shot 2. (a) Initial ignition. (b) Secondary jump. (c) Third jump.
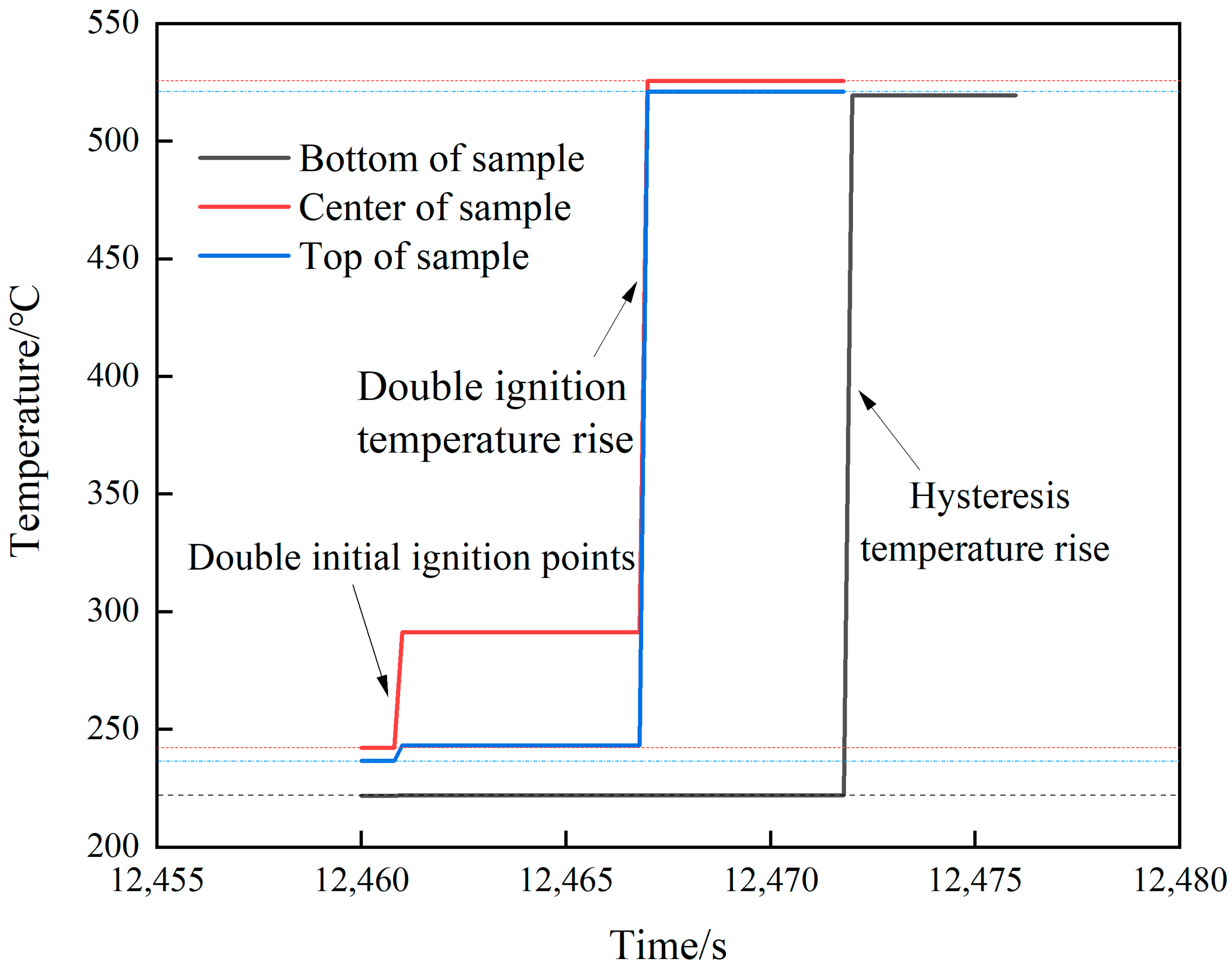
The temperature changes at various locations in shot 3 after ignition are shown in Figure 10. The initial ignition position appeared at the center and upper part at the same time. After 6.2 s, further temperature jumps occurred at both locations with temperature rises of 234.4 and 277.8 °C, respectively. A temperature rise of 294.4 °C appeared in the lower part after 4.6 s. Combined with the camera image (see Figure 11), first, a small amount of intermittent white smoke was sprayed from the upper part of the slit. Then, a large amount of continuous white smoke was obviously sprayed from top to bottom, and the outer flame of the lower part lagged behind the upper middle part. Finally, the spraying was completed. Through shots 2 and 3, it can be seen that, compared to rapid heating, under the condition of slow heating, after the initial ignition point appears, it will directly grow into a violent combustion stage. There is no slow combustion stage, the growth is faster, and the temperature rise rate is higher. Analysis shows that when the heating rate is low, the temperature of JEOL explosive is relatively uniform and the internal energy is high. After the ignition of the explosive enters the first exothermic stage, the superposition of the released heat and the internal energy of the explosive makes the explosive quickly enter the second exothermic stage, and the explosive quickly releases a large amount of heat and enters the stage of intense combustion.
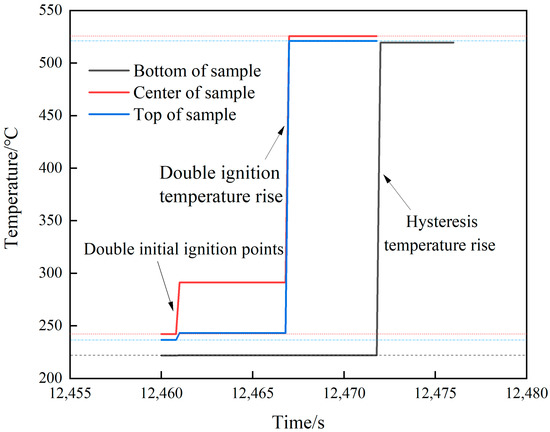
Figure 10.
Temperature–time curve of shot 3.
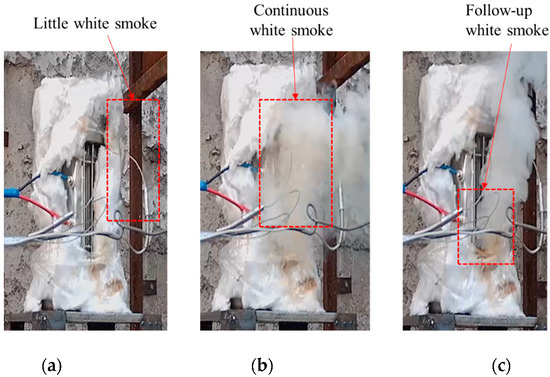
Figure 11.
Response of cook-off bomb of shot 3. (a) Initial ignition. (b) Secondary jump. (c) Hysteretic jump.
3.3.3. Combustion Process
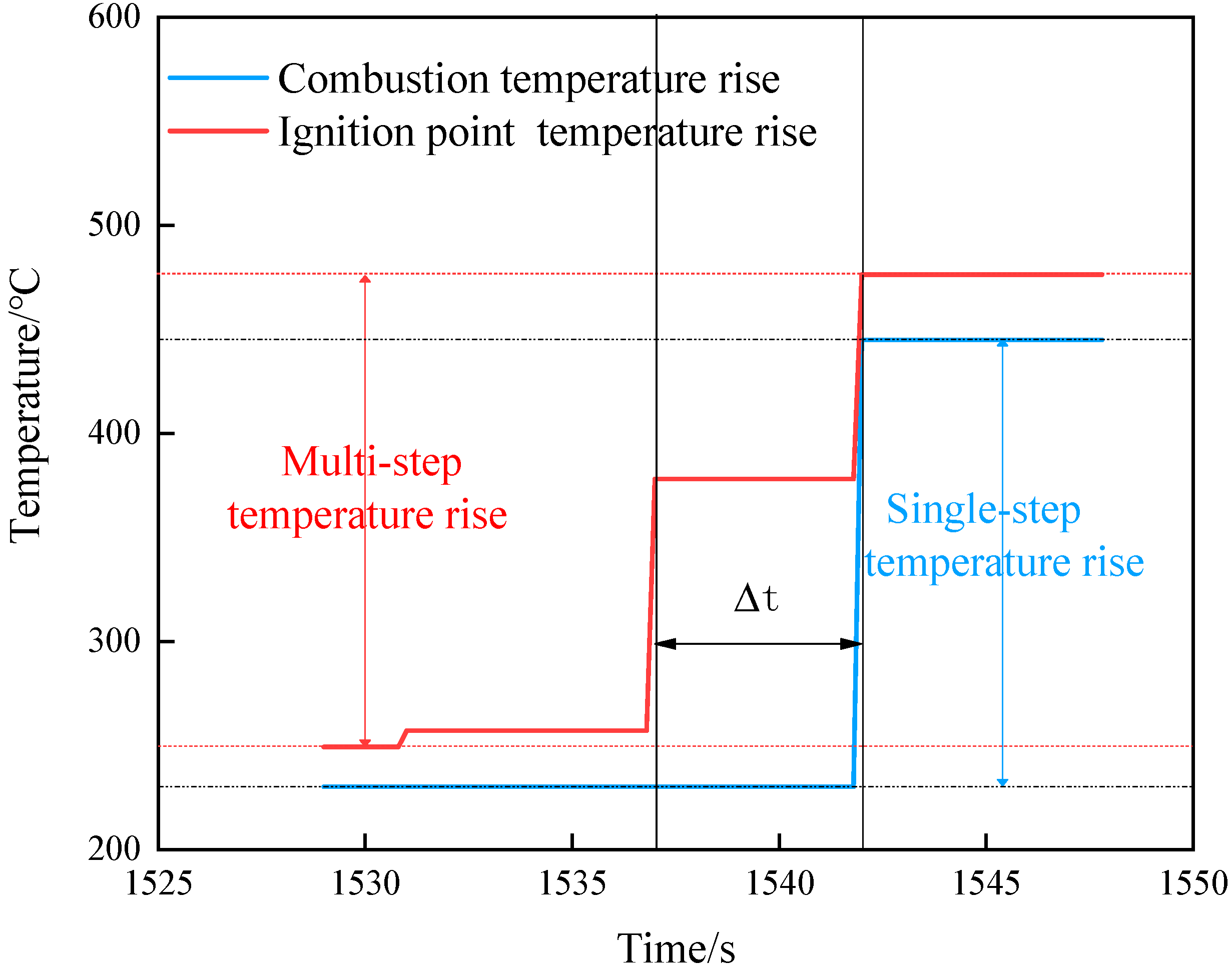
Combining the three shots, the temperature jump can be divided into two forms. One is a multistep jump that finally stabilizes at the combustion temperature. According to the ignition temperature and smoke phenomenon, this situation is judged to be the growth process of ignition. The other is a single-step jump that reaches the combustion temperature, as shown in Figure 12. The situation is the temperature rise caused by flame propagation. Based on this judgment, we further analyzed the temperature changes under the three shots.
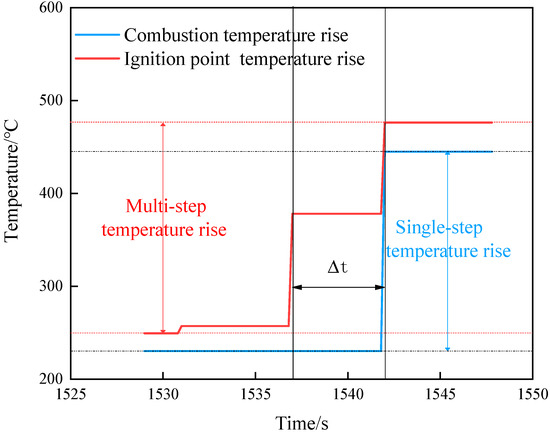
Figure 12.
Temperature rise curve.
The only ignition point appeared in the center of operating shot 1, and then it steadily grew into combustion. The flame propagated from the center to the two ends along the axis, and the upper and lower parts jumped directly from the preignition temperature to the combustion temperature.
The ignition point first appeared in the upper part in shot 2, and then there were multistep temperature jumps in the center and lower part at the same time. This showed that the temperature change in the middle and lower parts was not caused by flame propagation. The ignition point reappeared when the internal temperature field was uniform.
In shot 3, the initial ignition point appeared at the upper and center at the same time and then grew into combustion together. The temperature in the lower part jumped directly to the combustion temperature in one step, indicating that the temperature rise at this place was caused by flame propagation in the center.
The flame propagation speed was calculated according to the temperature change time of shots 1 and 3 and the distance between the thermocouples. The distance between the thermocouples in shot 1 was 30 mm, the time difference between the two temperature jumps was 5 s, and the combustion speed was calculated to be 6mm/s. The distance between the thermocouple in shot 3 was 45 mm, the difference between the two temperature jumps was 4.8 s, and the combustion speed was calculated to be 9.4 mm/s. Low heating rate increases the burning rate by 57% compared to high heating rate. It is believed that thermal damage will occur inside the explosive during the baking process, including porosity increase, cracks, holes, and debonding between the matrix and the binder. When the heating rate is high, the ignition time of the explosive is short, the thermal damage in the explosive is less, and it is not easy for the flame to enter the crack of the explosive when the explosive is burning. The combustion mode is heat conduction combustion. When the heating rate is low, the ignition time of the explosive is long, and macro cracks are easily generated in the explosive (crack width: 200–500 μm). This causes the flame to easily enter the crack of the explosive under the pressure of the combustion product. At this time, the combustion area of the explosive increases rapidly, and the combustion mode changes from heat conduction combustion to convection combustion, resulting in a rapid increase in the combustion rate of the explosive [32,33].
4. Conclusions
In this study, a small cook-off bomb test system was used to study the ignition characteristics and subsequent reaction growth process of JEOL explosive under cook-off conditions. The main conclusions are as follows.
As the heating rate increases, the ignition time of JEOL explosive is significantly shortened, but the ignition temperature does not change significantly.
When the heating rate is high, the internal temperature gradient of JEOL explosive is large, and the ignition point only appears at the highest temperature. With the decrease in heating rate, the internal temperature distribution of the explosive tends to be uniform, the ignition point appears random, and there may be multiple ignition points at the same time.
The reaction growth process of JEOL explosive after ignition can be divided into three stages: violent thermal decomposition, slow combustion, and violent combustion. When the internal temperature of the explosive is uniform, there will be a further temperature jump at the position where the initial ignition point appears, and the other positions will continue to maintain linear heating. Under the condition of slow heating, after the ignition point appears, it is easier to directly grow into violent combustion.
After JEOL explosive is ignited, there are two forms of temperature jump inside the explosive. One is a multistep temperature rise, which characterizes the growth process of the explosive ignition point, while the other is a single step temperature rise, which characterizes the flame propagation process in the explosive. The burning rate at low heating rate is much greater than that at high heating rate.
Although this study took aluminum-containing pressed explosives as the discussion object, the final conclusion was derived from the explosive hot spot ignition mechanism, the Arrhenius chemical reaction mechanism, and the explosive combustion law. Therefore, the conclusion of this article should be applicable to other pressed and cast explosives.
Author Contributions
Conceptualization, X.W. and C.J.; methodology, X.W.; validation, X.W., C.J. and W.L.; formal analysis, Y.F.; investigation, W.L.; resources, C.J. and Z.W.; data curation, X.W.; writing—original draft preparation, X.W.; writing—review and editing, X.W.; visualization, C.J.; supervision, C.J.; project administration, C.J.; funding acquisition, Z.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, G.; Zhao, J.; Du, C. Safety Assessment and Technology of Conventional Missile Ammunition; China Astronautic Publishing House: Beijing, China, 2015; pp. 1–12. [Google Scholar]
- Baalisampang, T.; Abbassi, R.; Garaniya, V.; Khan, F.; Dadashzadeh, M. Review and Analysis of Fire and Explosion Accidents in Maritime Transportation. Ocean Eng. 2018, 158, 350–366. [Google Scholar] [CrossRef]
- Gross, M.L.; Meredith, K.V.; Beckstead, M.W. Fast Cook-off Modeling of HMX. Combust. Flame 2015, 162, 3307–3315. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, S.; Huang, H.; Huang, G.; Wu, F.; Sun, S.; Li, B.; Xu, Z. Numerical and Experimental Study on the Response Characteristics of Warhead in the Fast Cook-off Process. Defence Technol. 2021, 17, 1444–1452. [Google Scholar] [CrossRef]
- Wang, P.; Cheng, L.; Feng, C. Numerical simulation of cook-off for explosive at different heating rates. Chin. J. Energetic Mater. 2009, 17, 46–49. [Google Scholar]
- Berghout, H.L.; Son, S.F.; Skidmore, C.B.; Idar, D.J.; Asay, B.W. Combustion of Damaged PBX 9501 Explosive. Thermochim. Acta 2002, 384, 261–277. [Google Scholar] [CrossRef]
- Berghout, H.L.; Son, S.F.; Hill, L.G.; Asay, B.W. Flame Spread through Cracks of PBX 9501 (a Composite Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine-Based Explosive). J. Appl. Phys. 2006, 99, 114901. [Google Scholar] [CrossRef]
- Herrmann, M.; Engel, W.; Eisenreich, N. Thermal Expansion, Transitions, Sensitivities and Burning Rates of HMX. Propellants Explos. Pyrotech. 1992, 17, 190–195. [Google Scholar] [CrossRef]
- Baer, M.R.; Gross, R.J.; Nunziato, J.W.; Igel, E.A. An Experimental and Theoretical Study of Deflagration-to-Detonation Transition (DDT) in the Granular Explosive, CP. Combust. Flame 1986, 65, 15–30. [Google Scholar] [CrossRef]
- Griffiths, N.; Groocock, J.M. 814. The Burning to Detonation of Solid Explosives. J. Chem. Soc. 1960, 4154–4162. [Google Scholar] [CrossRef]
- Burnham, A.K.; Weese, R.K. Kinetics of Thermal Degradation of Explosive Binders Viton A, Estane, and Kel-F. Thermochim. Acta 2005, 426, 85–92. [Google Scholar] [CrossRef]
- Garcia, F.; Vandersall, K.S.; Forbes, J.W.; Tarver, C.M.; Greenwood, D. Thermal Cook-Off Experiments of the HMX Based High Explosive LX-04 to Characterize Violence with Varying Confinement. AIP Conf. Proc. 2006, 845, 1061–1064. [Google Scholar]
- Parker, G.R.; Heatwole, E.M.; Holmes, M.D.; Asay, B.W.; Dickson, P.M.; McAfee, J.M. Deflagration-to-Detonation Transition in Hot HMX and HMX-Based Polymer-Bonded Explosives. Combust. Flame 2020, 215, 295–308. [Google Scholar] [CrossRef]
- Parker, G.R.; Dickerson, P.M.; Asay, B.W.; Mc Afee, J.M. DDT of Hot, Thermally Damaged PBX 9501 in Heavy Confinement; LA-UR-10-01356; LA-UR-10-1356; Los Alamos National Lab. (LANL): Los Alamos, NM, USA, 2010. [Google Scholar]
- Price, D.; Bernecker, R.R. Effect of Initial Particle Size on the DDT of Pressed Solid Explosives. Propellants Explos. Pyrotech. 1981, 6, 5–10. [Google Scholar] [CrossRef]
- Bernecker, R.R.; Price, D. Studies in the Transition from Deflagration to Detonation in Granular Explosives—II. Transitional Characteristics and Mechanisms Observed in 91/9 RDX/Wax. Combust. Flame 1974, 22, 119–129. [Google Scholar] [CrossRef]
- Hsu, P.C.; DeHaven, M.; McClelland, M.; Maienschein, J.L. Thermal Damage on LX-04 Mock Material and Gas Permeability Assessment. Propellants Explos. Pyrotech. 2006, 31, 56–60. [Google Scholar] [CrossRef]
- Tringe, J.W.; Glascoe, E.A.; McClelland, M.A.; Greenwood, D.; Chambers, R.D.; Springer, H.K.; Levie, H.W. Pre-Ignition Confinement and Deflagration Violence in LX-10 and PBX 9501. J. Appl. Phys. 2014, 116, 054903. [Google Scholar] [CrossRef]
- Tarver, C.M.; Tran, T.D. Thermal Decomposition Models for HMX-Based Plastic Bonded Explosives. Combust. Flame 2004, 137, 50–62. [Google Scholar] [CrossRef]
- Chidester, S.K.; Tarver, C.M.; Green, L.G.; Urtiew, P.A. On the violence of thermal explosion in solid explosives. Combust. Flame 1997, 110, 264–280. [Google Scholar] [CrossRef]
- Glascoe, E.; Springer, H.K.; Tringe, J.W.; Maienschein, J.L. A comparison of deflagration rates at elevated pressures and temperatures with thermal explosion results. In Proceedings of the 17th Biennial International Conference of the APS Topical Group on Shock Compression of Condensed Matter, Chicago, IL, USA, 26 June–1 July 2011; 555–558. [Google Scholar]
- Zhou, J.; Zhi, X.; Wang, S.; Hao, C. Rheological properties of Composition B in slow cook-off process. Explosion Shock Waves 2020, 40, 36–44. [Google Scholar]
- Hobbs, M.L.; Kaneshige, M.J.; Erikson, W.W.; Brown, J.A.; Anderson, M.U.; Todd, S.N.; Moore, D.G. Cookoff experiments of a melt cast explosive (Comp-B3). Combust. Flame 2020, 213, 268–278. [Google Scholar] [CrossRef]
- Kou, Y.; Chen, L.; Lu, J.; Geng, D.; Chen, W.; Wu, J. Assessing the thermal safety of solid propellant charges based on slow cook-off tests and numerical simulations. Combust. Flame 2021, 228, 154–162. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, Y. Numerical Simulation of Cook-off Characteristics for AP/HTPB. Defence Technol. 2018, 14, 451–456. [Google Scholar] [CrossRef]
- Yang, H.-W.; Yu, Y.-G.; Ye, R.; Xue, X.-C.; Li, W.-F. Cook-off Test and Numerical Simulation of AP/HTPB Composite Solid Propellant. J. Loss Prev. Process Ind. 2016, 40, 1–9. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, Y. Numerical Analysis of Cook-off Behavior of Cluster Tubular Double-Based Propellant. Appl. Therm. Eng. 2020, 181, 115972. [Google Scholar] [CrossRef]
- Liu, L.; Li, F.; Tan, L.; Ming, L.; Yi, Y. Effects of Nanometer Ni, Cu, Al and NiCu Powders on the Thermal Decomposition of Ammonium Perchlorate. Propellants Explos. Pyrotech. 2004, 29, 34–38. [Google Scholar] [CrossRef]
- Ye, Q.; Yu, Y.; Li, W. Study on Cook-off Behavior of HTPE Propellant in Solid Rocket Motor. Appl. Therm. Eng. 2020, 167, 114798. [Google Scholar] [CrossRef]
- Ho, S.Y. Thermomechanical Properties of Rocket Propellants and Correlation with Cookoff Behaviour. Propellants Explos. Pyrotech. 1995, 20, 206–214. [Google Scholar] [CrossRef]
- Essel, J.T.; Nelson, A.P.; Smilowitz, L.B.; Henson, B.F.; Merriman, L.R.; Turnbaugh, D.; Gray, C.; Shermer, K.B. Investigating the Effect of Chemical Ingredient Modifications on the Slow Cook-off Violence of Ammonium Perchlorate Solid Propellants on the Laboratory Scale. J. Energetic Mater. 2020, 38, 127–141. [Google Scholar] [CrossRef]
- Dickson, P.M.; Asay, B.W.; Henson, B.F.; Smilowitz, L.B. Thermal cook–off response of confined PBX 9501. Proc. R. Soc. A Math. Phys. Eng. Sci. 2004, 460, 3447–3455. [Google Scholar] [CrossRef]
- Hu, H.; Fu, H.; Li, T.; Shang, H.; Wen, S. Progress in experimental studies on the evolution behaviors of non-shock initiation reaction in low porosity pressed explosive with confinement. Explosion Shock Waves 2021, 40, 011401. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).